CYP3A Activity in End-of-Life Cancer Patients Measured by 4β-Hydroxycholesterol/cholesterol Ratio, in Men and Women
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patient Study Cohort
3.2. Study Controls: Young and Elderly
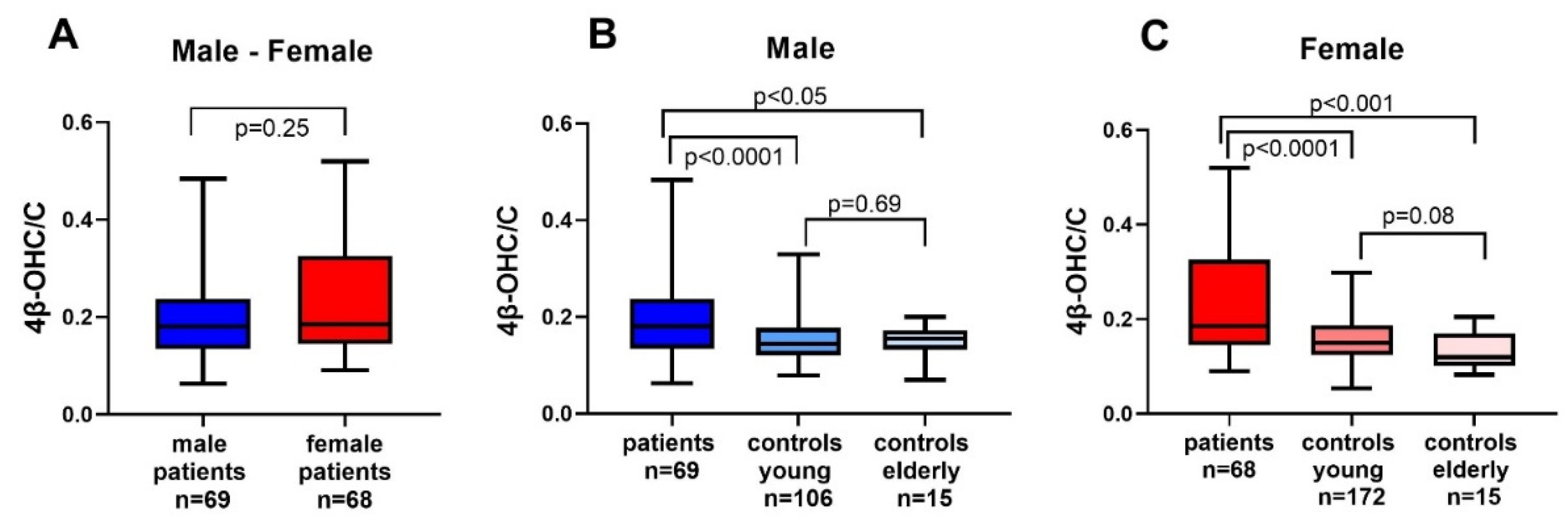
3.3. 4β-OCH/C Ratio in Patients, Young and Elderly Controls
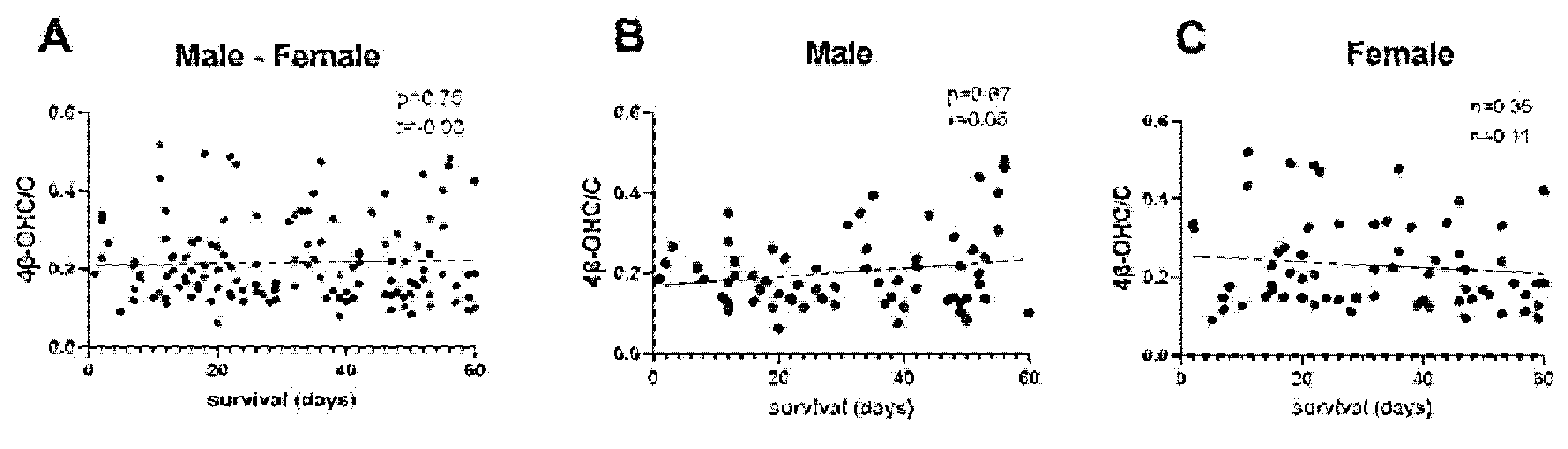
3.4. Correlation Analysis 4β-OHC/C
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zanger, U.M.; Schwab, M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharm. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef]
- Fuhr, U.; Jetter, A.; Kirchheiner, J. Appropriate phenotyping procedures for drug metabolizing enzymes and transporters in humans and their simultaneous use in the “cocktail” approach. Clin. Pharm. Ther. 2007, 81, 270–283. [Google Scholar] [CrossRef] [PubMed]
- Björkhem-Bergman, L.; Bäckström, T.; Nylén, H.; Ronquist-Nii, Y.; Bredberg, E.; Andersson, T.B.; Bertilsson, L.; Diczfalusy, U. Comparison of endogenous 4β-hydroxycholesterol with midazolam as markers for CYP3A4 induction by rifampicin. Drug Metab. Dispos. 2013, 41, 1488–1493. [Google Scholar] [CrossRef] [PubMed]
- Tomalik-Scharte, D.; Lütjohann, D.; Doroshyenko, O.; Frank, D.; Jetter, A.; Fuhr, U. Plasma 4β-hydroxycholesterol: An endogenous CYP3A metric? Clin. Pharm. Ther. 2009, 86, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Gjestad, C.; Hole, K.; Haslemo, T.; Diczfalusy, U.; Molden, E. Effect of Grapefruit Juice Intake on Serum Level of the Endogenous CYP3A4 Metabolite 4β-Hydroxycholesterol—An Interaction Study in Healthy Volunteers. AAPS J. 2019, 21, 58. [Google Scholar] [CrossRef]
- Diczfalusy, U.; Nylén, H.; Elander, P.; Bertilsson, L. 4β-Hydroxycholesterol, an endogenous marker of CYP3A4/5 activity in humans. Br. J. Clin. Pharm. 2011, 71, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Lütjohann, D.; Marinova, M.; Schneider, B.; Oldenburg, J.; von Bergmann, K.; Bieber, T.; Björkhem, I.; Diczfalusy, U. 4β-hydroxycholesterol as a marker of CYP3A4 inhibition in vivo-effects of itraconazole in man. Int. J. Clin. Pharm. Ther. 2009, 47, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Geist, M.J.P.; Siller, N.; Egerer, G.; Bardenheuer, H.; Burhenne, J.; Mikus, G. Decreased Cytochrome P450 3A activity in palliative patients with haematological diseases: Potential impact on supportive drug therapies. Basic Clin. Pharmacol. Toxicol. 2019. [Google Scholar] [CrossRef]
- Rivory, L.P.; Slaviero, K.; Seale, J.P.; Hoskins, J.M.; Boyer, M.; Beale, P.J.; Millward, M.J.; Bishop, J.F.; Clarke, S.J. Optimizing the erythromycin breath test for use in cancer patients. Clin. Cancer Res. 2000, 6, 3480–3485. [Google Scholar] [PubMed]
- Sadeghi, M.; Keshavarz-Fathi, M.; Baracos, V.; Arends, J.; Mahmoudi, M.; Rezaei, N. Cancer cachexia: Diagnosis, assessment, and treatment. Crit. Rev. Oncol. Hematol. 2018, 127, 91–104. [Google Scholar] [CrossRef]
- Huijberts, S.; Buurman, B.M.; de Rooij, S.E. End-of-life care during and after an acute hospitalization in older patients with cancer, end-stage organ failure, or frailty: A sub-analysis of a prospective cohort study. Palliat. Med. 2016, 30, 75–82. [Google Scholar] [CrossRef]
- de Graan, A.J.; Sparreboom, A.; de Bruijn, P.; de Jonge, E.; van der Holt, B.; Wiemer, E.A.; Verweij, J.; Mathijssen, R.H.; van Schaik, R.H. 4β-hydroxycholesterol as an endogenous CYP3A marker in cancer patients treated with taxanes. Br. J. Clin. Pharm. 2015, 80, 560–568. [Google Scholar] [CrossRef]
- Ogasawara, K.; LoRusso, P.M.; Olszanski, A.J.; Rixe, O.; Xu, C.; Yin, J.; Palmisano, M.; Krishna, G. Assessment of effects of repeated oral doses of fedratinib on inhibition of cytochrome P450 activities in patients with solid tumors using a cocktail approach. Cancer Chemother Pharm. 2020, 86, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Björkhem-Bergman, L.; Nylén, H.; Norlin, A.C.; Lindh, J.D.; Ekström, L.; Eliasson, E.; Bergman, P.; Diczfalusy, U. Serum levels of 25-hydroxyvitamin D and the CYP3A biomarker 4β-hydroxycholesterol in a high-dose vitamin D supplementation study. Drug Metab. Dispos. 2013, 41, 704–708. [Google Scholar] [CrossRef] [PubMed]
- Lindh, J.D.; Björkhem-Bergman, L.; Eliasson, E. Vitamin D and drug-metabolising enzymes. Photochem. Photobiol. Sci. 2012, 11, 1797–1801. [Google Scholar] [CrossRef] [PubMed]
- Bergman, P.; Sperneder, S.; Höijer, J.; Bergqvist, J.; Björkhem-Bergman, L. Low vitamin D levels are associated with higher opioid dose in palliative cancer patients--results from an observational study in Sweden. PLoS ONE 2015, 10, e0128223. [Google Scholar] [CrossRef]
- Helde-Frankling, M.; Bergqvist, J.; Klasson, C.; Nordstrom, M.; Höijer, J.; Bergman, P.; Björkhem-Bergman, L. Vitamin D supplementation to palliative cancer patients: Protocol of a double-blind, randomised controlled trial ‘Palliative-D’. BMJ Supportive Palliat. Care 2017, 7, 458–463. [Google Scholar] [CrossRef]
- Helde Frankling, M.; Klasson, C.; Sandberg, C.; Nordström, M.; Warnqvist, A.; Bergqvist, J.; Bergman, P.; Björkhem-Bergman, L. ‘Palliative-D’-Vitamin D Supplementation to Palliative Cancer Patients: A Double Blind, Randomized Placebo-Controlled Multicenter Trial. Cancers 2021, 13, 3707. [Google Scholar] [CrossRef]
- Klasson, C.; Helde-Frankling, M.; Sandberg, C.; Nordström, M.; Lundh-Hagelin, C.; Björkhem-Bergman, L. Vitamin D and Fatigue in Palliative Cancer: A Cross-Sectional Study of Sex Difference in Baseline Data from the Palliative D Cohort. J. Palliat. Med. 2021, 24, 433–437. [Google Scholar] [CrossRef]
- Bodin, K.; Bretillon, L.; Aden, Y.; Bertilsson, L.; Broomé, U.; Einarsson, C.; Diczfalusy, U. Antiepileptic drugs increase plasma levels of 4β-hydroxycholesterol in humans: Evidence for involvement of cytochrome p450 3A4. J. Biol. Chem. 2001, 276, 38685–38689. [Google Scholar] [CrossRef]
- Diczfalusy, U.; Miura, J.; Roh, H.K.; Mirghani, R.A.; Sayi, J.; Larsson, H.; Bodin, K.G.; Allqvist, A.; Jande, M.; Kim, J.W.; et al. 4β-hydroxycholesterol is a new endogenous CYP3A marker: Relationship to CYP3A5 genotype, quinine 3-hydroxylation and sex in Koreans, Swedes and Tanzanians. Pharm. Genom. 2008, 18, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Sevrioukova, I. Interaction of Human Drug-Metabolizing CYP3A4 with Small Inhibitory Molecules. Biochemistry 2019, 58, 930–939. [Google Scholar] [CrossRef]
- Lütjohann, D.; Stellaard, F.; Kerksiek, A.; Lötsch, J.; Oertel, B.G. Serum 4β-hydroxycholesterol increases during fluconazole treatment. Eur. J. Clin. Pharm. 2021, 77, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Bergström, H.; Ekström, L.; Warnqvist, A.; Bergman, P.; Björkhem-Bergman, L. Variations in biomarkers of dyslipidemia and dysbiosis during the menstrual cycle: A pilot study in healthy volunteers. BMC Womens Health 2021, 21, 166. [Google Scholar] [CrossRef]
- Uno, Y.; Takata, R.; Kito, G.; Yamazaki, H.; Nakagawa, K.; Nakamura, Y.; Kamataki, T.; Katagiri, T. Sex- and age-dependent gene expression in human liver: An implication for drug-metabolizing enzymes. Drug Metab. Pharm. 2017, 32, 100–107. [Google Scholar] [CrossRef]
- Butler, L.M.; Perone, Y.; Dehairs, J.; Lupien, L.E.; de Laat, V.; Talebi, A.; Loda, M.; Kinlaw, W.B.; Swinnen, J.V. Lipids and cancer: Emerging roles in pathogenesis, diagnosis and therapeutic intervention. Adv. Drug Deliv. Rev. 2020, 159, 245–293. [Google Scholar] [CrossRef]
- Rivory, L.P.; Slaviero, K.A.; Clarke, S.J. Hepatic cytochrome P450 3A drug metabolism is reduced in cancer patients who have an acute-phase response. Br. J. Cancer 2002, 87, 277–280. [Google Scholar] [CrossRef]
- Kacevska, M.; Robertson, G.R.; Clarke, S.J.; Liddle, C. Inflammation and CYP3A4-mediated drug metabolism in advanced cancer: Impact and implications for chemotherapeutic drug dosing. Expert Opin. Drug Metab. Toxicol. 2008, 4, 137–149. [Google Scholar] [CrossRef]
- Alexandre, J.; Rey, E.; Girre, V.; Grabar, S.; Tran, A.; Montheil, V.; Rabillon, F.; Dieras, V.; Jullien, V.; Hérait, P.; et al. Relationship between cytochrome 3A activity, inflammatory status and the risk of docetaxel-induced febrile neutropenia: A prospective study. Ann. Oncol. 2007, 18, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Charles, K.A.; Rivory, L.P.; Brown, S.L.; Liddle, C.; Clarke, S.J.; Robertson, G.R. Transcriptional repression of hepatic cytochrome P450 3A4 gene in the presence of cancer. Clin. Cancer Res. 2006, 12, 7492–7497. [Google Scholar] [CrossRef]
- Morgan, E.T.; Skubic, C.; Lee, C.M.; Cokan, K.B.; Rozman, D. Regulation of cytochrome P450 enzyme activity and expression by nitric oxide in the context of inflammatory disease. Drug Metab. Rev. 2020, 52, 455–471. [Google Scholar] [CrossRef]
- Dvorak, Z.; Pavek, P. Regulation of drug-metabolizing cytochrome P450 enzymes by glucocorticoids. Drug Metab. Rev. 2010, 42, 621–635. [Google Scholar] [CrossRef]
- Meijer, O.C.; Koorneef, L.L.; Kroon, J. Glucocorticoid receptor modulators. Ann. Endocrinol. 2018, 79, 107–111. [Google Scholar] [CrossRef]
- Watanabe, M.; Tateishi, T.; Asoh, M.; Nakura, H.; Tanaka, M.; Kumai, T.; Kobayashi, S. Effects of glucocorticoids on pharmacokinetics and pharmacodynamics of midazolam in rats. Life Sci. 1998, 63, 1685–1692. [Google Scholar] [CrossRef]
- Meduri, G.U.; Chrousos, G.P. General Adaptation in Critical Illness: Glucocorticoid Receptor-alpha Master Regulator of Homeostatic Corrections. Front. Endocrinol. 2020, 11, 161. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S.; Mellman, I. Oncology meets immunology: The cancer-immunity cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef]
- Ishida, T.; Naito, T.; Sato, H.; Kawakami, J. Relationship between the plasma fentanyl and serum 4β-hydroxycholesterol based on CYP3A5 genotype and gender in patients with cancer pain. Drug Metab. Pharm. 2016, 31, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Pavek, P.; Pospechova, K.; Svecova, L.; Syrova, Z.; Stejskalova, L.; Blazkova, J.; Dvorak, Z.; Blahos, J. Intestinal cell-specific vitamin D receptor (VDR)-mediated transcriptional regulation of CYP3A4 gene. Biochem. Pharm. 2010, 79, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Goh, B.C.; Fan, L.; Khoo, Y.M.; Wang, L.; Lim, R.; Ong, A.B.; Chua, C. Phenotyping CYP3A using midazolam in cancer and noncancer Asian patients. Br. J. Clin. Pharm. 2003, 55, 270–277. [Google Scholar] [CrossRef][Green Version]
- Lepper, E.R.; Baker, S.D.; Permenter, M.; Ries, N.; van Schaik, R.H.; Schenk, P.W.; Price, D.K.; Ahn, D.; Smith, N.F.; Cusatis, G.; et al. Effect of common CYP3A4 and CYP3A5 variants on the pharmacokinetics of the cytochrome P450 3A phenotyping probe midazolam in cancer patients. Clin. Cancer Res. 2005, 11, 7398–7404. [Google Scholar] [CrossRef] [PubMed]
- Hole, K.; Gjestad, C.; Heitmann, K.M.; Haslemo, T.; Molden, E.; Bremer, S. Impact of genetic and nongenetic factors on interindividual variability in 4beta-hydroxycholesterol concentration. Eur J. Clin. Pharm. 2017, 73, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.D.; van Schaik, R.H.; Rivory, L.P.; Ten Tije, A.J.; Dinh, K.; Graveland, W.J.; Schenk, P.W.; Charles, K.A.; Clarke, S.J.; Carducci, M.A.; et al. Factors affecting cytochrome P-450 3A activity in cancer patients. Clin. Cancer Res. 2004, 10, 8341–8350. [Google Scholar] [CrossRef] [PubMed]
- Mannheimer, B.; Wagner, H.; Östenson, C.G.; Diczfalusy, U. No impact of vitamin D on the CYP3A biomarker 4β-hydroxycholesterol in patients with abnormal glucose regulation. PLoS ONE 2015, 10, e0121984. [Google Scholar] [CrossRef] [PubMed]
| Patient Characteristics | Men (n = 69) | Women (n = 68) | p-Value |
| Age (years) | 70 | 71 | 0.90 |
| (63–78) | (62–78) | ||
| CRP (mg/L) | 45 | 37.5 | 0.45 |
| (20–91) | (10–81) | ||
| Albumin (g/L) | 24.5 | 25.5 | 0.87 |
| (21–29) | (21–29) | ||
| Creatinine (mmol/L) | 72 (59.93) | 58 (49–78) | <0.01 |
| Cholesterol (mmol/L) | 4.2 (3.6–5.6) | 5 (3.6–5.9) | 0.22 |
| 25-OHD (nmol/L) | 41 (27–56) | 45 (27–61) | 0.64 |
| Survival (days) | 31 (16–48) | 32 (18–47) | 0.64 |
| 4β-OHC/C × 104 | 0.18 (0.13–0.24) | 0.19 (0.14–0.32) | 0.25 |
| Type of Cancer | Men (n = 69) | Women (n = 68) | p-Value |
| Lung | 12 | 15 | ns |
| Gastrointestinal | 12 | 7 | ns |
| Pancreas, liver, gallbladder | 9 | 7 | ns |
| Breast | 0 | 14 | NA |
| Urological | 5 | 6 | ns |
| Gynecological | NA | 10 | NA |
| Prostate | 7 | NA | NA |
| Hematological | 5 | 4 | ns |
| Head-Neck | 3 | 1 | ns |
| Brain tumor | 1 | 1 | ns |
| Esophageal | 5 | 1 | ns |
| Melanoma | 5 | 2 | ns |
| Other (mesothelioma n = 4, unknown n = 1) | 5 | 0 | ns |
| n = 137 | 69 | 68 |
| Controls: Young | Men (n = 107) | Women (n = 173) | p-Value |
|---|---|---|---|
| Age (years) | 27 | 28 | <0.01 |
| (24–32) | (24–37) | ||
| Cholesterol (mmol/L) | 4.1 | 4.5 | <0.01 |
| (3.7–4.7) | (4.1–5) | ||
| 4β-OHC/C × 104 | 0.14 | 0.15 | 0.31 |
| (0.12–0.18) | (0.12–0.19) | ||
| Controls: Elderly | Men | Women | |
| (n = 15) | (n = 15) | ||
| Age (years) | 74 (66–79) | 66 (62–74) | <0.01 |
| CRP (mg/L) | 1 (1–3) | 1 (1–3) | 0.57 |
| Albumin (g/L) | 37 (34–40) | 37 (35–40) | 0.57 |
| Creatinine (mmol/L) | 89 (81–101) | 63 (57–71) | <0.01 |
| Cholesterol (mmol/L) | 4,5 (3.8–5) | 5.2 (4.5–5.5) | 0.12 |
| 25-OHD (nmol/L) | 70 (66–92) | 79 (73–92) | 0.21 |
| 4β-OHC/C × 104 | 0.16(0.13–0.17) | 0.12(0.10–0.17) | 0.53 |
| Correlation with 4β-OHC/C | All (n = 137) | Men (n = 69) | Women (n = 68) |
|---|---|---|---|
| Age (years) | r = 0.06 | r = 0.20 | r = −0.08 |
| p = 0.47 | p = 0.09 | p = 0.52 | |
| Albumin (g/L) | r = −0.02 | r = −0.00 | r = −0.06 |
| p = 0.78 | p = 0.98 | p = 0.64 | |
| CRP (mg/L) | r = 0.08 | r = −0.03 | r = 0.21 |
| p = 0.33 | p = 0.77 | p = 0.08 | |
| 25-OHC (nmol/L) | r = −0.05 | r = −0.04 | r = −0.10 |
| p = 0.56 | p = 0.75 | p = 0.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bergström, H.; Helde Frankling, M.; Klasson, C.; Lövgren Sandblom, A.; Diczfalusy, U.; Björkhem-Bergman, L. CYP3A Activity in End-of-Life Cancer Patients Measured by 4β-Hydroxycholesterol/cholesterol Ratio, in Men and Women. Cancers 2021, 13, 4689. https://doi.org/10.3390/cancers13184689
Bergström H, Helde Frankling M, Klasson C, Lövgren Sandblom A, Diczfalusy U, Björkhem-Bergman L. CYP3A Activity in End-of-Life Cancer Patients Measured by 4β-Hydroxycholesterol/cholesterol Ratio, in Men and Women. Cancers. 2021; 13(18):4689. https://doi.org/10.3390/cancers13184689
Chicago/Turabian StyleBergström, Helena, Maria Helde Frankling, Caritha Klasson, Anita Lövgren Sandblom, Ulf Diczfalusy, and Linda Björkhem-Bergman. 2021. "CYP3A Activity in End-of-Life Cancer Patients Measured by 4β-Hydroxycholesterol/cholesterol Ratio, in Men and Women" Cancers 13, no. 18: 4689. https://doi.org/10.3390/cancers13184689
APA StyleBergström, H., Helde Frankling, M., Klasson, C., Lövgren Sandblom, A., Diczfalusy, U., & Björkhem-Bergman, L. (2021). CYP3A Activity in End-of-Life Cancer Patients Measured by 4β-Hydroxycholesterol/cholesterol Ratio, in Men and Women. Cancers, 13(18), 4689. https://doi.org/10.3390/cancers13184689







