Novel Ferrocene Derivatives Induce Apoptosis through Mitochondria-Dependent and Cell Cycle Arrest via PI3K/Akt/mTOR Signaling Pathway in T Cell Acute Lymphoblastic Leukemia
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of F1–F7
2.2. Cell Cultures
2.3. Isolation and Culture of Human Peripheral Normal T Cells
2.4. Cell Viability Analysis
2.5. Hoechst 33258 Fluorescent Staining
2.6. Cell Cycle Analysis
2.7. Assessment of Apoptosis
2.8. ROS Detection
2.9. Mitochondrial Membrane Potential Analysis
2.10. Western Blot Analysis
2.11. Statistical Analysis
3. Results
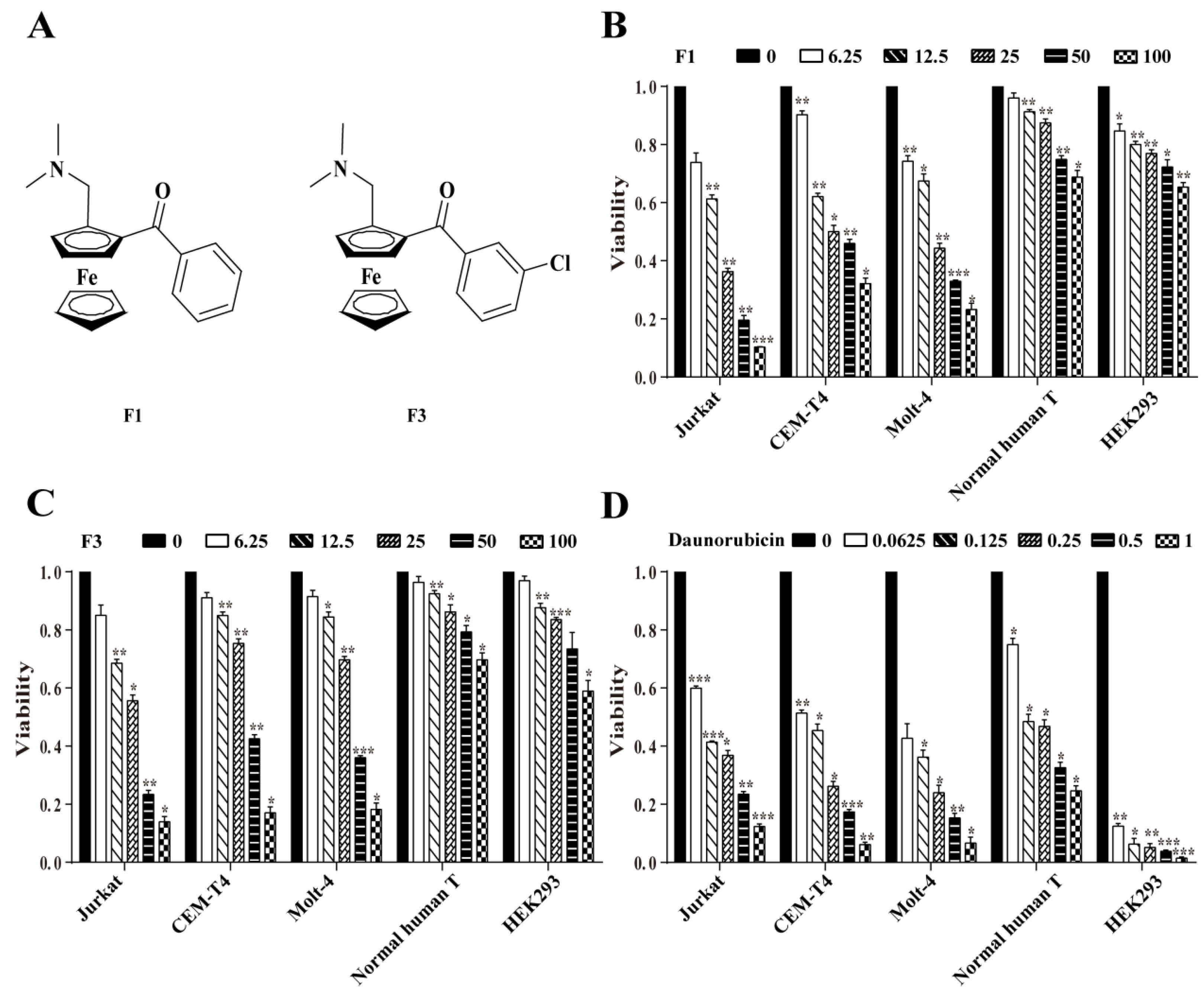
3.1. F1 and F3 Selectively Inhibited the Proliferation of Jurkat Cells
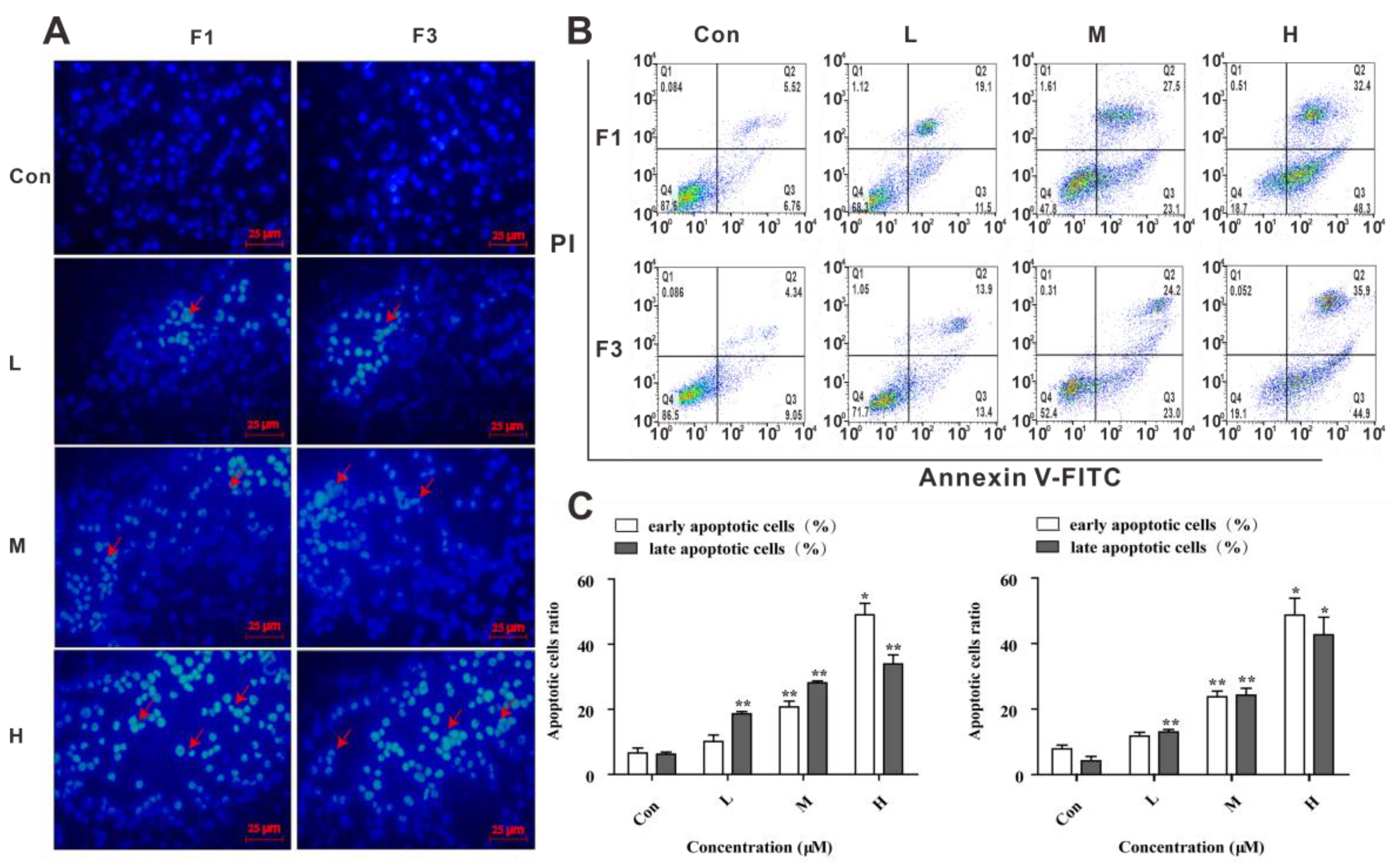
3.2. F1 and F3 Changed the Cell Morphology and Induced Apoptosis in Jurkat Cells
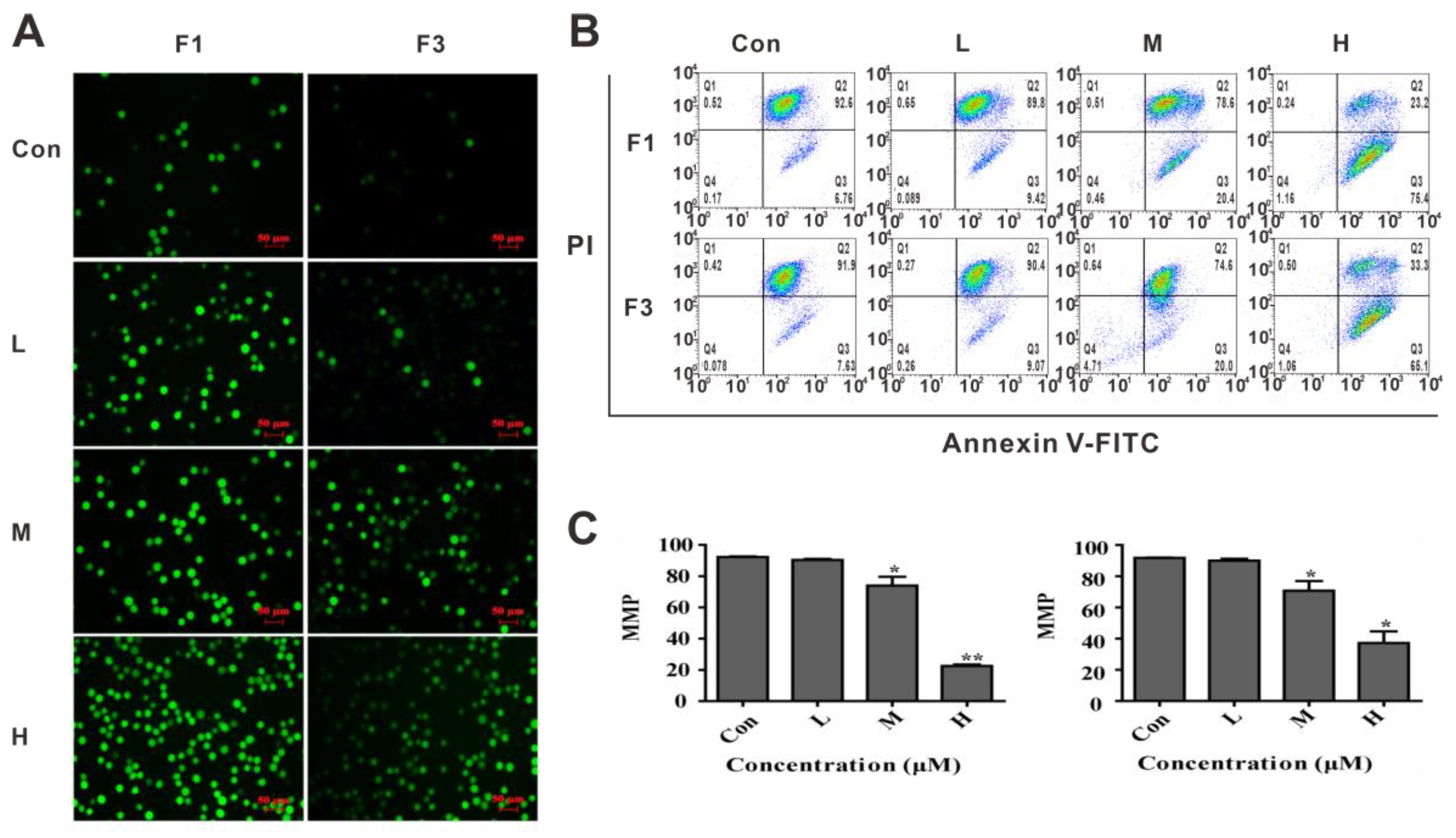
3.3. F1 and F3 Increased Cellular ROS and Down-Regulated the MMP in Jurkat Cells
3.4. F1 and F3 Regulated the Expression of Apoptosis-Related Proteins
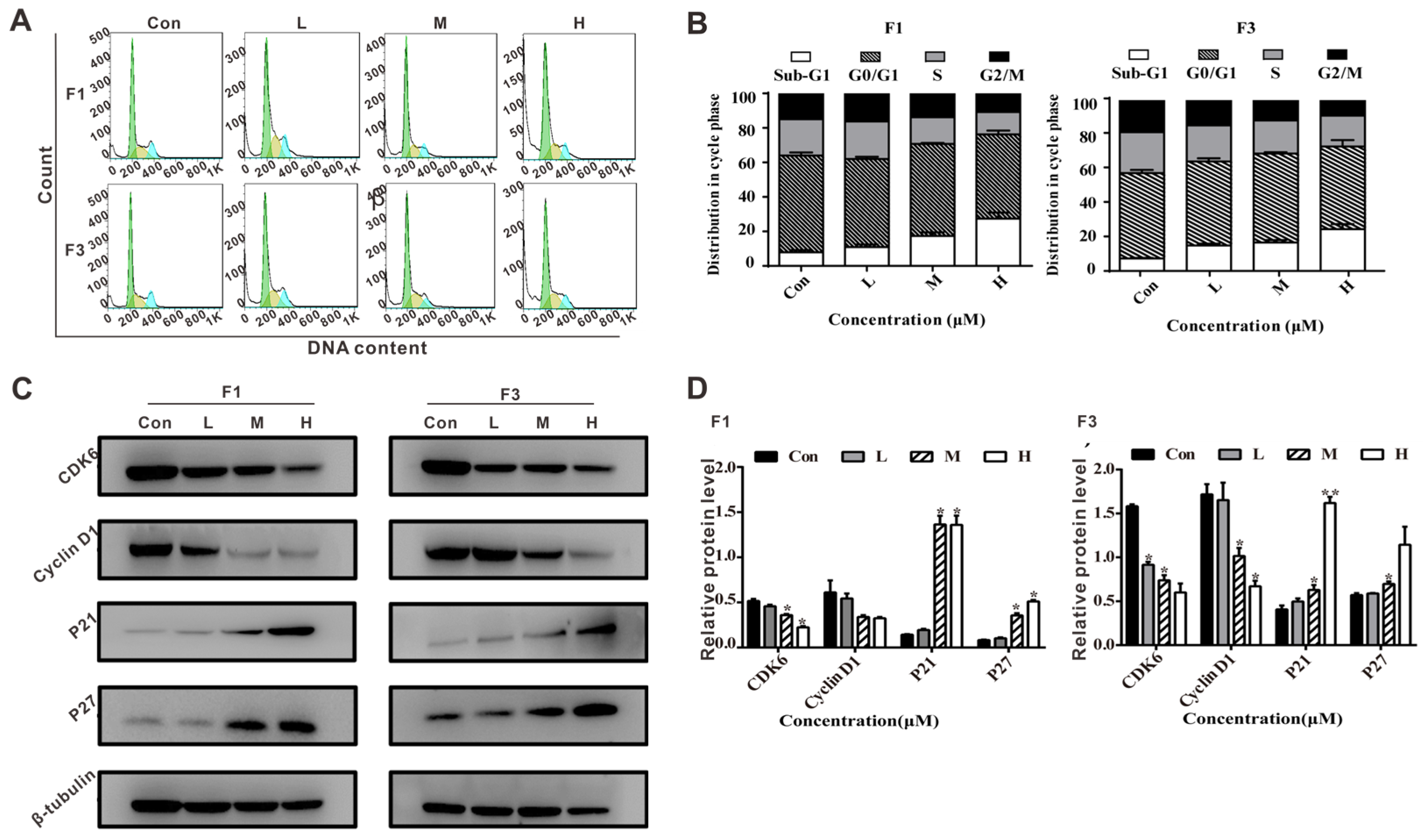
3.5. F1 and F3 Induced Cell Cycle Arrest and Regulated the Expression of Cell Cycle-Related Proteins in Jurkat Cells
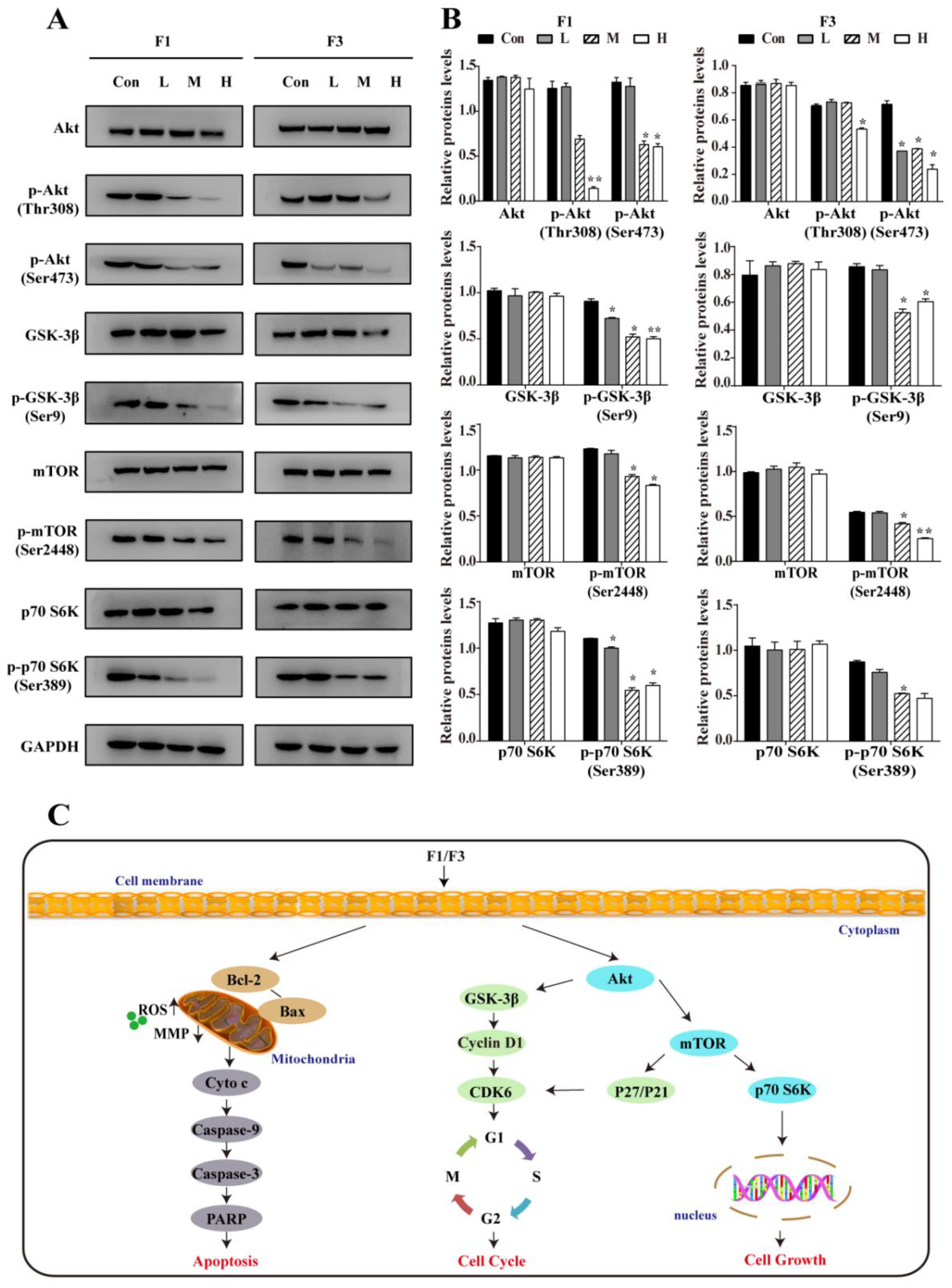
3.6. F1 and F3 Suppressed the PI3K/Akt/mTOR Signaling Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Malard, F.; Mohty, M. Acute lymphoblastic leukaemia. Lancet 2020, 395, 1146–1162. [Google Scholar] [CrossRef]
- Hong, W.H.; Cheng, W.Z.; Zheng, T.J.; Jiang, N.; Xu, R.A. AHR is a tunable knob that controls HTLV-1 latency-reactivation switching. PLoS Pathog. 2020, 16, e1008664. [Google Scholar] [CrossRef]
- Cheng, W.Z.; Zheng, T.J.; Wang, Y.; Cai, K.; Wu, W.C.; Zhao, T.J.; Xu, R.A. Activation of Notch1 signaling by HTLV-1 Tax promotes proliferation of adult T-cell leukemia cells. Biochem. Biophys. Res. Commun. 2019, 512, 598–603. [Google Scholar] [CrossRef]
- Arber, D.A.; Orazi, A.; Hasserjian, R. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 128, 462–463. [Google Scholar] [CrossRef]
- Marks, D.I.; Rowntree, C. Management of adults with T-cell lymphoblastic leukemia. Blood 2017, 129, 1134–1142. [Google Scholar] [CrossRef] [Green Version]
- Pui, C.H.; Evans, W.E. A 50-Year Journey to Cure Childhood Acute Lymphoblastic Leukemia. Semin. Hematol. 2013, 50, 185–196. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Yuan, G.X.; He, Q.H.; Chen, H.W. Rapid analysis of anthracycline antibiotics doxorubicin and daunorubicin by microchip capillary electrophoresis. Microchem. J. 2009, 92, 170–173. [Google Scholar] [CrossRef]
- Evangelisti, C.; Chiarini, F.; McCubrey, J.A.; Martelli, A.M. Therapeutic Targeting of mTOR in T-Cell Acute Lymphoblastic Leukemia: An Update. Int. J. Mol. Sci. 2018, 19, 1878. [Google Scholar] [CrossRef] [Green Version]
- Patra, M.; Gasser, G. The medicinal chemistry of ferrocene and its derivatives. Nat. Rev. Chem. 2017, 1, 0066. [Google Scholar] [CrossRef]
- Plazuk, D.; Top, S.; Vessieres, A.; Plamont, M.A.; Huche, M.; Zakrzewski, J.; Makal, A.; Wozniak, K.; Jaouen, G. Organometallic cyclic polyphenols derived from 1,2-(alpha-keto tri or tetra methylene) ferrocene show strong antiproliferative activity on hormone-independent breast cancer cells. Dalton Trans. 2010, 39, 7444–7450. [Google Scholar] [CrossRef] [PubMed]
- Boros, E.; Dyson, P.J.; Gasser, G. Classification of Metal-Based Drugs according to Their Mechanisms of Action. Chem 2020, 6, 41–60. [Google Scholar] [CrossRef]
- Bruyere, C.; Mathieu, V.; Vessieres, A.; Pigeon, P.; Top, S.; Jaouen, G.; Kiss, R. Ferrocifen derivatives that induce senescence in cancer cells: Selected examples. J. Inorg. Biochem. 2014, 141, 144–151. [Google Scholar] [CrossRef] [Green Version]
- Shagufta; Ahmad,, I. Tamoxifen a pioneering drug: An update on the therapeutic potential of tamoxifen derivatives. Eur. J. Med. Chem. 2018, 143, 515–531. [Google Scholar] [CrossRef]
- Spencer, J.; Amin, J.; Wang, M.H. Synthesis and biological evaluation of JAHAs: Ferrocene-Based Histone Deacetylase Inhibitors. ACS Med. Chem. Lett. 2011, 2, 358–362. [Google Scholar] [CrossRef]
- Sun, A.J.; Lin, J.S.; Pi, C.; Xu, R.A.; Cui, X.L. Biological Evaluation of Ferrocenyl Olefins: Cancer Cell Growth Inhibition, ROS Production, and Apoptosis Activity. Arch. Pharm. 2016, 349, 186–192. [Google Scholar] [CrossRef]
- Zheng, J.R.; Zeng, L.; Tang, M.Q.; Lin, H.J.; Cui, X.L. Novel Ferrocene Derivatives Induce G0/G1 Cell Cycle Arrest and Apoptosis through the Mitochondrial Pathway in Human Hepatocellular Carcinoma. Int. J. Mol. Sci. 2021, 22, 3097. [Google Scholar] [CrossRef]
- Pi, C.; Cui, X.L.; Liu, X.Y.; Guo, M.X.; Zhang, H.Y.; Wu, Y.J. Synthesis of Ferrocene Derivatives with Planar Chirality via Palladium-Catalyzed Enantioselective C-H Bond Activation. Org. Lett. 2014, 16, 5164–5167. [Google Scholar] [CrossRef]
- Gioia, L.; Siddique, A.; Head, S.R.; Salomon, D.R.; Su, A.I. A genome-wide survey of mutations in the Jurkat cell line. BMC Genom. 2018, 19, 334. [Google Scholar] [CrossRef] [Green Version]
- Ratiani, L.; Sanikidze, T.; Sulakvelidze, M.; Bejitashvili, N.; Meladze, K. Jurkat cell as an appropriate model for drug investigation. Georgian Med. News 2009, 168, 117–120. [Google Scholar]
- Kowalski, K.; Hikisz, P.; Szczupak, L.; Therrien, B.; Koceva-Chyla, A. Ferrocenyl and dicobalt hexacarbonyl chromones-New organometallics inducing oxidative stress and arresting human cancer cells in G2/M phase. Eur. J. Med. Chem. 2014, 81, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Novgorodov, S.A.; Voltin, J.R.; Gooz, M.A.; Li, L.; Lemasters, J.J.; Gudz, T.I. Acid sphingomyelinase promotes mitochondrial dysfunction due to glutamate-induced regulated necrosis. J. Lipid Res. 2018, 59, 312–329. [Google Scholar] [CrossRef] [Green Version]
- Cassidy-Stone, A.; Chipuk, J.; Ingerman, E. Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Dev. Cell 2008, 14, 193–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez, J.; Tait, S. Mitochondrial apoptosis: Killing cancer using the enemy within. Brit. J. Cancer 2015, 112, 957–962. [Google Scholar] [CrossRef] [Green Version]
- Aoki, M.; Fujishita, T. Oncogenic Roles of the PI3K/AKT/mTOR Axis. Curr. Top. Microbiol. 2017, 407, 153–189. [Google Scholar]
- Buttrick, G.; Wakefield, J. PI3K and GSK-3: Akting together with microtubules. Cell Cycle 2008, 7, 2621–2625. [Google Scholar] [CrossRef] [Green Version]
- Shams, H.Z.; Mohareb, R.M.; Helal, M.H. Design and Synthesis of Novel Antimicrobial Acyclic and Heterocyclic Dyes and Their Precursors for Dyeing and/or Textile Finishing Based on 2-N-Acylamino-4,5,6,7-tetrahydrobenzo [b] thiophene Systems. Molecules 2011, 16, 6271–6305. [Google Scholar] [CrossRef] [Green Version]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Lam, M.; Oleinick, N.L.; Nieminen, A.L. Photodynamic therapy-induced apoptosis in epidermoid carcinoma cells-Reactive oxygen species and mitochondrial inner membrane permeabilization. J. Biol. Chem. 2001, 276, 47379–47386. [Google Scholar] [CrossRef] [Green Version]
- Shi, M.C.; Zhou, L.N.; Zhao, L.; Shang, M.; He, T.T.; Tang, Z.L.; Sun, H.C.; Ren, P.L.; Lin, Z.P.; Chen, T.J.; et al. Csseverin inhibits apoptosis through mitochondria-mediated pathways triggered by Ca2+ dyshomeostasis in hepatocarcinoma PLC cells. PLoS Negl. Trop. Dis. 2017, 11, e0006074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, W.C.; Wang, X.Q.; Tu, Y.C.; Masaki, H.; Tanaka, S.; Onda, K.; Sugiyama, K.; Yamada, H.; Hirano, T. Tetrandrine and cepharanthine induce apoptosis through caspase cascade regulation, cell cycle arrest, MAPK activation and PI3K /Akt / mTOR signal modification in glucocorticoid resistant human leukemia Jurkat T cells. Chem.-Biol. Interact. 2019, 310, 108726. [Google Scholar] [CrossRef]
- Soriano, M.E.; Scorrano, L. Traveling Bax and forth from mitochondria to control apoptosis. Cell 2011, 145, 15–17. [Google Scholar] [CrossRef]
- Li, P.; Nijhawan, D.; Budihardjo, I.; Srinivasula, S.M.; Ahmad, M.; Alnemri, E.S.; Wang, X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 1997, 91, 479–489. [Google Scholar] [CrossRef] [Green Version]
- Koh, D.W.; Dawson, T.M.; Dawson, V.L. Mediation of cell death by poly (ADP-ribose) polymerase-1. Pharmacol. Res. 2005, 52, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Stockwell, B.R. The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 2014, 10, 9–17. [Google Scholar] [CrossRef]
- Huang, H.; Chen, J.; Lu, H.; Zhou, M.; Chai, Z.; Hu, Y. Iron-induced generation of mitochondrial ROS depends on AMPK activity. Biometals 2017, 30, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.C.; Pellecchia, M. Review in translational hematology Apoptosis-based therapies for hematologic malignancies. Cell 2005, 106, 408–418. [Google Scholar]
- Adjei, A. Blocking Oncogenic Ras signaling for Cancer Therapy. J. Natl. Cancer Inst. 2001, 93, 1062. [Google Scholar] [CrossRef] [Green Version]
- Malumbres, M. Cyclin-dependent kinases. Genome Biol. 2014, 15, 122. [Google Scholar] [CrossRef] [Green Version]
- Starostina, N.G.; Kipreos, E.T. Multiple degradation pathways regulate versatile CIP/KIP CDK inhibitors. Trends Cell Biol. 2012, 22, 33–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riccardi, C.; Nicoletti, I. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat. Protoc. 2006, 1, 1458–1461. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yang, Y. TRIM44 is indispensable for glioma cell proliferation and cell cycle progression through AKT/p21/p27 signaling pathway. J. Neuro-Oncol. 2019, 145, 211–222. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. mTOR signaling in growth control and disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bongiovanni, D.; Saccomani, V.; Piovan, E. Aberrant signaling pathways in T-cell acute lymphoblastic leukemia. Int. J. Mol. Sci. 2017, 18, 1904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, M.; Akkapeddi, P. From the outside, from within: Biological and therapeutic relevance of signal transduction in T-cell acute lymphoblastic leukemia. Cell. Signal. 2017, 38, 10–25. [Google Scholar] [CrossRef]
- Bowles, D.; Jimeno, A. New phosphatidylinositol 3-kinase inhibitors for cancer. Expert Opin. Investig. Drugs 2011, 20, 507–518. [Google Scholar] [CrossRef]
- Baer, R.; Cintas, C.; Dufresne, M. Pancreatic cell plasticity and cancer initiation induced by oncogenic Kras is completely dependent on wild-type PI 3-kinase p110α Genes. Gene. Dev. 2014, 28, 2621–2635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhat, M.; Robichaud, N.; Hulea, L. Targeting the translation machinery in cancer. Nat. Rev. Drug Discov. 2015, 14, 261–278. [Google Scholar] [CrossRef]
- Ben-Sahra, I.; Hoxhaj, G.; Ricoult, S.J.H. mTORC1 induces purine synthesis through control of the mitochondrial tetrahydrofolate cycle. Science 2016, 351, 728–733. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, L.; Tang, M.; Pi, C.; Zheng, J.; Gao, S.; Chabanne, T.; Chauvin, R.; Cheng, W.; Lin, H.; Xu, R.; et al. Novel Ferrocene Derivatives Induce Apoptosis through Mitochondria-Dependent and Cell Cycle Arrest via PI3K/Akt/mTOR Signaling Pathway in T Cell Acute Lymphoblastic Leukemia. Cancers 2021, 13, 4677. https://doi.org/10.3390/cancers13184677
Zeng L, Tang M, Pi C, Zheng J, Gao S, Chabanne T, Chauvin R, Cheng W, Lin H, Xu R, et al. Novel Ferrocene Derivatives Induce Apoptosis through Mitochondria-Dependent and Cell Cycle Arrest via PI3K/Akt/mTOR Signaling Pathway in T Cell Acute Lymphoblastic Leukemia. Cancers. 2021; 13(18):4677. https://doi.org/10.3390/cancers13184677
Chicago/Turabian StyleZeng, Liao, Mingqing Tang, Chao Pi, Jianrong Zheng, Sanxing Gao, Titaua Chabanne, Remi Chauvin, Wenzhao Cheng, Hongjun Lin, Ruian Xu, and et al. 2021. "Novel Ferrocene Derivatives Induce Apoptosis through Mitochondria-Dependent and Cell Cycle Arrest via PI3K/Akt/mTOR Signaling Pathway in T Cell Acute Lymphoblastic Leukemia" Cancers 13, no. 18: 4677. https://doi.org/10.3390/cancers13184677
APA StyleZeng, L., Tang, M., Pi, C., Zheng, J., Gao, S., Chabanne, T., Chauvin, R., Cheng, W., Lin, H., Xu, R., & Cui, X. (2021). Novel Ferrocene Derivatives Induce Apoptosis through Mitochondria-Dependent and Cell Cycle Arrest via PI3K/Akt/mTOR Signaling Pathway in T Cell Acute Lymphoblastic Leukemia. Cancers, 13(18), 4677. https://doi.org/10.3390/cancers13184677




