Endometrial Cancer Incidence in Endometriosis and Adenomyosis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. Cohort Characteristics
2.2. Endometrial Cancer
2.3. Odds Ratio of Endometrial Cancer
2.4. Histological Distribution of Endometrial Cancer Subtypes
3. Discussion
3.1. Principal Findings
3.2. Results of the Study in the Context of Other Observations
3.3. Endometrial Cancer Subtypes
3.4. Age at Endometrial Cancer Diagnosis and Combined Endometriosis and Adenomyosis
3.5. Possible Key Factors in the Malignant Transformation of Endometriosis/Adenomyosis
3.6. Strengths and Limitations
4. Future
5. Material and Methods
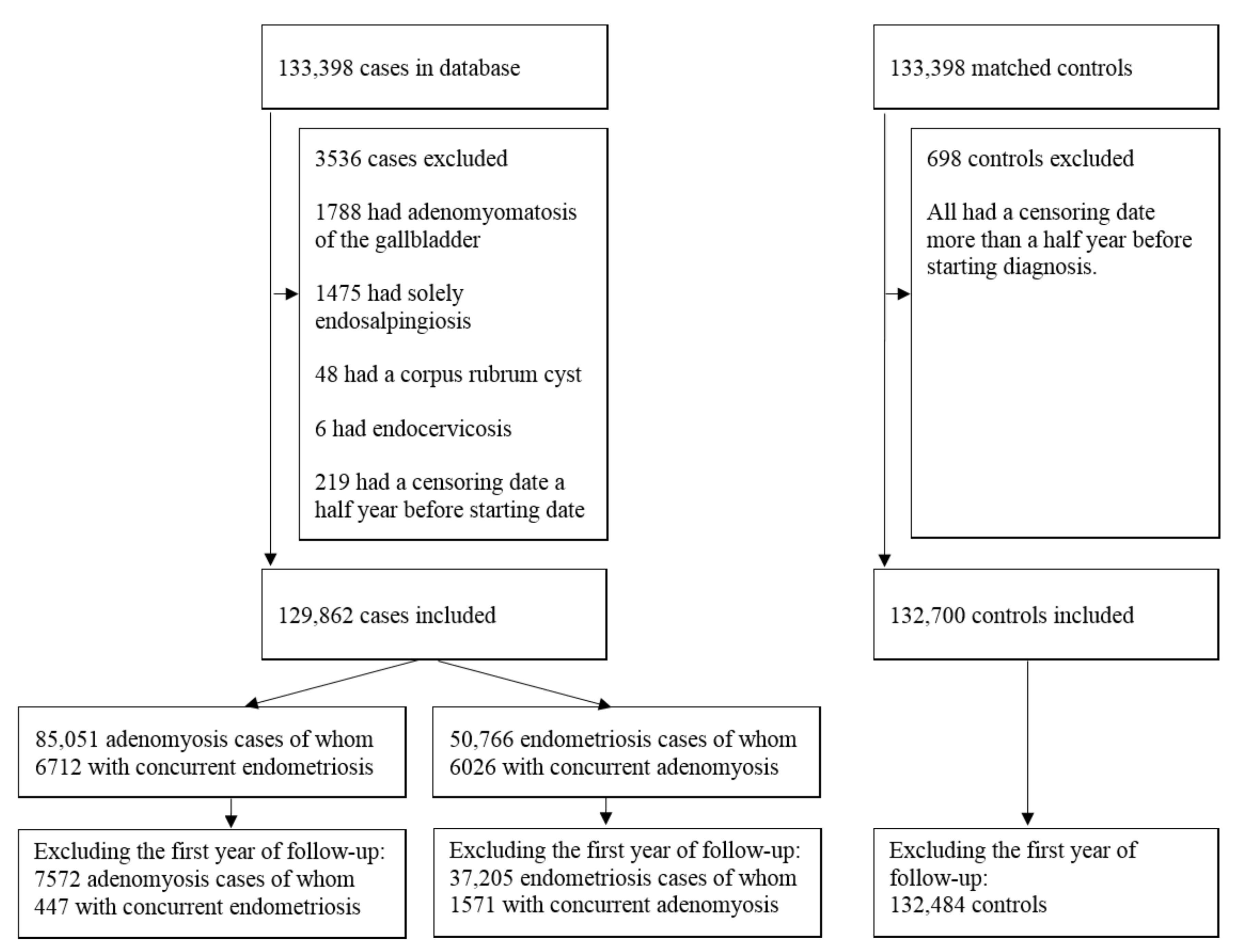
5.1. Study Population and Design
5.2. Statistical Analysis
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gordts, S.; Grimbizis, G.; Campo, R. Symptoms and classification of uterine adenomyosis, including the place of hysteroscopy in diagnosis. Fertil. Steril. 2018, 109, 380–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunselman, G.A.; Vermeulen, N.; Becker, C.; Calhaz-Jorge, C.; D’Hooghe, T.; De Bie, B.; Heikinheimo, O.; Horne, A.W.; Kiesel, L.; Nap, A.; et al. ESHRE guideline: Management of women with endometriosis. Hum. Reprod. Oxf. Engl. 2014, 29, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Eskenazi, B.; Warner, M.L. Epidemiology of endometriosis. Obstet. Gynecol. Clin. N. Am. 1997, 24, 235–258. [Google Scholar] [CrossRef]
- Adamson, G.D.; Kennedy, S.H.; Hummelshoj, L. Creating Solutions in Endometriosis: Global Collaboration through the World Endometriosis Research Foundation. J. Endometr. 2010, 2, 3–6. [Google Scholar] [CrossRef] [Green Version]
- Mao, X.; Zheng, W.; Mao, W. Malignant changes in adenomyosis in patients with endometrial adenocarcinoma: A case series. Medicine 2017, 96, e8336. [Google Scholar] [CrossRef] [PubMed]
- Pollacco, J.; Sacco, K.; Portelli, M.; Schembri-Wismayer, P.; Calleja-Agius, J. Molecular links between endometriosis and cancer. Gynecol. Endocrinol. 2012, 28, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Amant, F.; Mirza, M.R.; Koskas, M.; Creutzberg, C.L. Cancer of the corpus uteri. Int. J. Gynaecol. Obstet. 2018, 143 (Suppl. 2), 37–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hermens, M.; van Altena, A.M.; Nieboer, T.E.; Schoot, B.C.; van Vliet, H.; Siebers, A.G.; Bekkers, R.L.M. Incidence of endometrioid and clear-cell ovarian cancer in histological proven endometriosis: The ENOCA population-based cohort study. Am. J. Obstet. Gynecol. 2020, 223, 107.e1–107.e11. [Google Scholar] [CrossRef]
- Pearce, C.L.; Templeman, C.; Rossing, M.A.; Lee, A.; Near, A.M.; Webb, P.M.; Nagle, C.M.; Doherty, J.A.; Cushing-Haugen, K.L.; Wicklund, K.G.; et al. Association between endometriosis and risk of histological subtypes of ovarian cancer: A pooled analysis of case-control studies. Lancet Oncol. 2012, 13, 385–394. [Google Scholar] [CrossRef] [Green Version]
- Kvaskoff, M.; Mahamat-Saleh, Y.; Farland, L.V.; Shigesi, N.; Terry, K.L.; Harris, H.R.; Roman, H.; Becker, C.M.; As-Sanie, S.; Zondervan, K.T.; et al. Endometriosis and cancer: A systematic review and meta-analysis. Hum. Reprod. Update 2020, 27, 393–420. [Google Scholar] [CrossRef]
- Kalaitzopoulos, D.R.; Mitsopoulou, A.; Iliopoulou, S.M.; Daniilidis, A.; Samartzis, E.P.; Economopoulos, K.P. Association between endometriosis and gynecological cancers: A critical review of the literature. Arch. Gynecol. Obstet. 2020, 301, 355–367. [Google Scholar] [CrossRef]
- Raffone, A.; Seracchioli, R.; Raimondo, D.; Maletta, M.; Travaglino, A.; Raimondo, I.; Giaquinto, I.; Orsini, B.; Insabato, L.; Pellicano, M.; et al. Prevalence of adenomyosis in endometrial cancer patients: A systematic review and meta-analysis. Arch. Gynecol. Obstet. 2020, 295, 1459–1468. [Google Scholar] [CrossRef]
- Shen, F.; Liu, Y.; Lin, L.; Zhao, M.; Chen, Q. Association of benign gynaecological diseases and risk of endometrial and ovarian cancers. J. Cancer 2020, 11, 3186–3191. [Google Scholar] [CrossRef] [PubMed]
- Kok, V.C.; Tsai, H.J.; Su, C.F.; Lee, C.K. The Risks for Ovarian, Endometrial, Breast, Colorectal, and Other Cancers in Women With Newly Diagnosed Endometriosis or Adenomyosis: A Population-Based Study. Int. J. Gynecol. Cancer 2015, 25, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Saavalainen, L.; Lassus, H.; But, A.; Tiitinen, A.; Härkki, P.; Gissler, M.; Pukkala, E.; Heikinheimo, O. Risk of Gynecologic Cancer According to the Type of Endometriosis. Obstet. Gynecol. 2018, 131, 1095–1102. [Google Scholar] [CrossRef]
- Saraswat, L.; Ayansina, D.; Cooper, K.G.; Bhattacharya, S.; Horne, A.W.; Bhattacharya, S. Impact of endometriosis on risk of further gynaecological surgery and cancer: A national cohort study. Bjog 2018, 125, 64–72. [Google Scholar] [CrossRef] [Green Version]
- Mueck, A.O.; Seeger, H.; Rabe, T. Hormonal contraception and risk of endometrial cancer: A systematic review. Endocr. Relat. Cancer 2010, 17, R263–R271. [Google Scholar] [CrossRef] [Green Version]
- Mogensen, J.B.; Kjær, S.K.; Mellemkjær, L.; Jensen, A. Endometriosis and risks for ovarian, endometrial and breast cancers: A nationwide cohort study. Gynecol. Oncol. 2016, 143, 87–92. [Google Scholar] [CrossRef]
- Clement, P.B.; Young, R.H. Non-endometrioid carcinomas of the uterine corpus: A review of their pathology with emphasis on recent advances and problematic aspects. Adv. Anat. Pathol. 2004, 11, 117–142. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, D.; Raffone, A.; Travaglino, A.; Maletta, M.; Casadio, P.; Ambrosio, M.; Chiara Aru, A.; Santoro, A.; Franco Zannoni, G.; Insabato, L.; et al. Impact of adenomyosis on the prognosis of patients with endometrial cancer. Int. J. Gynaecol. Obstet. 2021. [Google Scholar] [CrossRef]
- Hermens, M.; van Altena, A.M.; van der Aa, M.; Bulten, J.; van Vliet, H.; Siebers, A.G.; Bekkers, R.L.M. Endometrial cancer prognosis in women with endometriosis and adenomyosis. A retrospective nationwide cohort study of 40,847 women. Unpublished work. 2021. [Google Scholar]
- Burg, L.; Timmermans, M.; van der Aa, M.; Boll, D.; Rovers, K.; de Hingh, I.; van Altena, A. Incidence and predictors of peritoneal metastases of gynecological origin: A population-based study in the Netherlands. J. Gynecol. Oncol. 2020, 31, e58. [Google Scholar] [CrossRef]
- Yu, O.; Schulze-Rath, R.; Grafton, J.; Hansen, K.; Scholes, D.; Reed, S.D. Adenomyosis incidence, prevalence and treatment: United States population-based study 2006–2015. Am. J. Obstet. Gynecol. 2020, 223, 94.e1–94.e10. [Google Scholar] [CrossRef]
- Ignatov, A.; Ortmann, O. Endocrine Risk Factors of Endometrial Cancer: Polycystic Ovary Syndrome, Oral Contraceptives, Infertility, Tamoxifen. Cancers 2020, 12, 1766. [Google Scholar] [CrossRef]
- Felix, A.S.; Weissfeld, J.L.; Stone, R.A.; Bowser, R.; Chivukula, M.; Edwards, R.P.; Linkov, F. Factors associated with Type I and Type II endometrial cancer. Cancer Causes Control 2010, 21, 1851–1856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riccio, L.; Santulli, P.; Marcellin, L.; Abrão, M.S.; Batteux, F.; Chapron, C. Immunology of endometriosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 50, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Bourdon, M.; Santulli, P.; Jeljeli, M.; Vannuccini, S.; Marcellin, L.; Doridot, L.; Petraglia, F.; Batteux, F.; Chapron, C. Immunological changes associated with adenomyosis: A systematic review. Hum. Reprod. Update 2020. [Google Scholar] [CrossRef] [PubMed]
- Karin, M.; Greten, F.R. NF-kappaB: Linking inflammation and immunity to cancer development and progression. Nat. Rev. Immunol. 2005, 5, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Hever, A.; Roth, R.B.; Hevezi, P.A.; Lee, J.; Willhite, D.; White, E.C.; Marin, E.M.; Herrera, R.; Acosta, H.M.; Acosta, A.J.; et al. Molecular characterization of human adenomyosis. Mol. Hum. Reprod. 2006, 12, 737–748. [Google Scholar] [CrossRef]
- Bilyk, O.; Coatham, M.; Jewer, M.; Postovit, L.M. Epithelial-to-Mesenchymal Transition in the Female Reproductive Tract: From Normal Functioning to Disease Pathology. Front. Oncol. 2017, 7, 145. [Google Scholar] [CrossRef] [Green Version]
- Vannuccini, S.; Tosti, C.; Carmona, F.; Huang, S.J.; Chapron, C.; Guo, S.W.; Petraglia, F. Pathogenesis of adenomyosis: An update on molecular mechanisms. Reprod. Biomed. Online 2017, 35, 592–601. [Google Scholar] [CrossRef] [Green Version]
- Toumpeki, C.; Liberis, A.; Tsirkas, I.; Tsirka, T.; Kalagasidou, S.; Inagamova, L.; Anthoulaki, X.; Tsatsaris, G.; Kontomanolis, E.N. The Role of ARID1A in Endometrial Cancer and the Molecular Pathways Associated With Pathogenesis and Cancer Progression. In Vivo 2019, 33, 659–667. [Google Scholar] [CrossRef]
- Kyo, S.; Sato, S.; Nakayama, K. Cancer-associated mutations in normal human endometrium: Surprise or expected? Cancer Sci. 2020, 111, 3458–3467. [Google Scholar] [CrossRef]
- Anglesio, M.S.; Papadopoulos, N.; Ayhan, A.; Nazeran, T.M.; Noë, M.; Horlings, H.M.; Lum, A.; Jones, S.; Senz, J.; Seckin, T.; et al. Cancer-Associated Mutations in Endometriosis without Cancer. N. Engl. J. Med. 2017, 376, 1835–1848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kvaskoff, M.; Han, J.; Qureshi, A.A.; Missmer, S.A. Pigmentary traits, family history of melanoma and the risk of endometriosis: A cohort study of US women. Int. J. Epidemiol. 2014, 43, 255–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kvaskoff, M.; Mesrine, S.; Clavel-Chapelon, F.; Boutron-Ruault, M.C. Endometriosis risk in relation to naevi, freckles and skin sensitivity to sun exposure: The French E3N cohort. Int. J. Epidemiol. 2009, 38, 1143–1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapron, C.; Vannuccini, S.; Santulli, P.; Abrão, M.S.; Carmona, F.; Fraser, I.S.; Gordts, S.; Guo, S.W.; Just, P.A.; Noël, J.C.; et al. Diagnosing adenomyosis: An integrated clinical and imaging approach. Hum. Reprod. Update 2020, 26, 392–411. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Endometriosis/Adenomyosis Combined | Endometriosis | Adenomyosis | Nevus |
|---|---|---|---|---|
| Total Group | ||||
| Included patients in analysis | 129,862 | 50,766 | 85,051 | 132,700 |
| Age at study inclusion (IQR) | 44 (38–50) 1 | 39 (32–45) 3 | 47 (42–52) 3 | 45 (38–51) |
| Median inclusion year | 2001 (1995–2008) 1 | 2002 (1996–2009) 3 | 2000 (1994–2007) 3 | 2001 (1995–2008) |
| Median follow up (range) | 0 (0–27) 2 | 8 (0–27) 3 | 0 (0–27) 3 | 16 (0–27) |
| Person years | 606,083 | 492,278 | 119,465 | 2,029,597 |
| Hysterectomy (%) | 88,112 (67.9%) 2 | 15,695 (30.9%) 3 | 77,993 (91.7%) 3 | 1982 (1.5%) |
| Number of endometrial cancer cases (%) | 1827 (1.4%) 2 | 519 (1.0%) 3 | 1455 (1.7%) 3 | 771 (0.6%) |
| Age at diagnosis of endometrial cancer (IQR) | 61 (55–69) 1 | 59 (52–67) 3 | 61 (55–69) 4 | 62 (56–68) |
| Metachronous group | ||||
| Included patients in analysis | 44,377 | 37,205 | 7572 | 132,484 |
| Age at study inclusion (IQR) | 37 (31–44) 2 | 36 (30–42) 3 | 45 (40–50) 3 | 45 (38–51) |
| Median inclusion year | 2003 (1996–2009) 2 | 2004 (1997–2009) 3 | 1999 (1994–2007) 3 | 2001 (1995–2008) |
| Median follow up (range) | 13 (1–27) 2 | 12 (1–27) 3 | 17 (1–27) 3 | 16 (1–27) |
| Person years | 605,716 | 492,074 | 119,294 | 2,029,570 |
| Hysterectomy (%) | 2679 (6.0%) 2 | 2155 (5.8%) 3 | 551 (7.3%) 3 | 1778 (1.3%) |
| Number of endometrial cancer cases (%) | 143 (0.3%) 2 | 98 (0.3%) 3 | 46 (0.6%) 4 | 726 (0.5%) |
| Age at diagnosis of endometrial cancer (IQR) | 59 (52–65) 2 | 56 (51–63) 3 | 64 (57–70) 4 | 62 (56–68) |
| Endometrial Cancer Versus No Endometrial Cancer | Endometriosis | Adenomyosis | Nevus |
|---|---|---|---|
| Endometrial cancer (n = 2413) | 480 (3.1%) | 1408 (1.8%) | 666 (33.6%) |
| Endometrial cancer diagnosis at time of hysterectomy (n = 451) | 109 (22.7%) | 275 (19.5%) | 103 (15.5%) |
| Prior endometrial hyperplasia, atypia or polyp in (micro)curettage or cervical smear (n = 274) 2 | 60 (55.0%) | 165 (60.0%) | 69 (67.0%) |
| Prior benign endometrium or invalid (micro)curettage or cervical smear (n = 30) 2 | 8 (7.3%) | 20 (7.3%) | 5 (4.9%) |
| No previous endometrial sampling (n = 147) 2 | 41 (37.6%) | 90 (32.7%) | 29 (28.2%) |
| Hysterectomy after endometrial cancer diagnosis (n = 1962) | 371 (77.3%) | 1133 (80.5%) | 563 (84.5%) |
| No endometrial cancer (n = 87,680) | 15,215 (96.9%) | 76,585 (98.2%) | 1316 (66.4%) |
| Total (n = 90,093) | 15,695 (100%) | 77,993 (100%) | 1982 (100%) |
| Endometrial Cancer Subtypes Per Cohort | Total Group | Metachronous Group | ||||
|---|---|---|---|---|---|---|
| ON | Crude OR (95%CI) | Age-Adjusted OR (95%CI) | ON | Crude OR (95%CI) | Age-Adjusted OR (95%CI) | |
| Endometrioid | ||||||
| Cases combined | 1118 | 1.98 (1.79–2.19) | 2.06 (1.86–2.28) | 114 | 0.61 (0.50–0.74) | 0.77 (0.63–0.95) |
| Endometriosis | 327 | 1.48 (1.29–1.70) | 2.08 (1.80–2.39) | 80 | 0.51 (0.40–0.65) | 0.68 (0.53–0.86) |
| Adenomyosis | 885 | 2.40 (2.16–2.67) | 2.11 (1.90–2.34) | 34 | 1.07 (0.76–1.51) | 1.07 (0.75–1.51) |
| Nevus | 579 | ref | ref | 557 | ref | ref |
| Clear cell | ||||||
| Cases combined | 28 | 2.86 (1.39–5.89) | 3.02 (1.47–6.23) | 3 | 1.00 (0.27–3.68) | 1.45 (0.38–5.56) |
| Endometriosis | 5 | 1.31 (0.45–3.82) | 2.15 (0.72–6.40) | 2 | 0.79 (0.17–3.66) | 1.25 (0.26–6.13) |
| Adenomyosis | 24 | 3.75 (1.79–7.83) | 3.25 (1.56–6.81) | 1 | 1.94 (0.25–15.35) | 1.99 (0.25–15.72) |
| Nevus | 10 | ref | ref | 9 | ref | ref |
| Serous | ||||||
| Cases combined | 98 | 2.09 (1.48–2.95) | 2.20 (1.55–3.10) | 5 | 0.35 (0.14–0.88) | 0.50 (0.20–1.27) |
| Endometriosis | 26 | 1.42 (0.88–2.28) | 2.24 (1.37–3.64) | 2 | 0.17 (0.04–0.68) | 0.24 (0.06–1.02) |
| Adenomyosis | 81 | 2.63 (1.84–3.77) | 2.29 (1.60–3.27) | 3 | 1.22 (0.38–3.94) | 1.24 (0.38–3.99) |
| Nevus | 48 | ref | ref | 43 | ref | ref |
| Mucinous | ||||||
| Cases combined | 9 | 1.84 (0.62–5.49) | 2.02 (0.68–6.04) | 0 | NA | NA |
| Endometriosis | 6 | 3.14 (0.96–10.28) | 5.90 (1.77–19.63) | 0 | NA | NA |
| Adenomyosis | 5 | 1.56 (0.45–5.39) | 1.36 (0.39–4.70) | 0 | NA | NA |
| Nevus | 5 | ref | ref | 5 | ref | ref |
| Adenocarcinoma NOS | ||||||
| Cases combined | 574 | 4.56 (3.77–5.52) | 4.84 (3.99–5.86) | 21 | 0.56 (0.35–0.89) | 0.76 (0.47–1.23) |
| Endometriosis | 155 | 3.15 (2.49–3.98) | 5.21 (4.10–6.62) | 14 | 0.45 (0.26–0.78) | 0.64 (0.36–1.13) |
| Adenomyosis | 460 | 5.59 (4.60–6.80) | 4.89 (4.02–5.95) | 8 | 1.25 (0.61–2.56) | 1.26 (0.61–2.57) |
| Nevus | 129 | ref | ref | 112 | ref | ref |
| All endometrial cancers | ||||||
| Cases combined | 1827 | 2.44 (2.24–2.66) | 2.58 (2.37–2.81) | 143 | 0.59 (0.49–0.70) | 0.76 (0.63–0.92) |
| Endometriosis | 519 | 1.77 (1.58–1.98) | 2.63 (2.35–2.95) | 98 | 0.48 (0.39–0.59) | 0.65 (0.52–0.81) |
| Adenomyosis | 1455 | 2.98 (2.73–3.25) | 2.63 (2.40–2.87) | 46 | 1.11 (0.82–1.50) | 1.11 (0.82–1.50) |
| Nevus | 771 | ref | ref | 726 | ref | ref |
| Histological Type | Endometriosis/Adenomyosis Combined | Endometriosis | Adenomyosis | Nevus |
|---|---|---|---|---|
| Total group 1 | ||||
| Endometrioid | 1118 (61.2%) | 327 (63.0%) | 885 (60.8%) | 579 (75.1%) |
| Clear cell | 28 (1.5%) | 5 (1.0%) | 24 (1.6%) | 10 (1.3%) |
| Serous | 98 (5.4%) | 26 (5.0%) | 81 (5.6%) | 48 (6.2%) |
| Mucinous | 9 (0.5%) | 6 (1.2%) | 5 (0.3%) | 5 (0.6%) |
| Adenocarcinoma NOS | 574 (31.4%) | 155 (29.9%) | 460 (31.6%) | 129 (16.7%) |
| Total | 1827 (100%) | 519 (100%) | 1455 (100%) | 771 (100%) |
| Metachronous group 2 | ||||
| Endometrioid | 114 (79.7%) | 80 (81.6%) | 34 (73.9%) | 557 (76.7%) |
| Clear cell | 3 (2.1%) | 2 (2.0%) | 1 (2.2%) | 9 (1.2%) |
| Serous | 5 (3.5%) | 2 (2.0%) | 3 (6.5%) | 43 (5.9%) |
| Mucinous | 0 (0%) | 0 (0%) | 0 (0%) | 5 (0.7%) |
| Adenocarcinoma NOS | 21 (14.7%) | 14 (14.3%) | 8 (17.4%) | 112 (15.4%) |
| Total | 143 (100%) | 98 (100%) | 46 (100%) | 726 (100%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hermens, M.; van Altena, A.M.; Velthuis, I.; van de Laar, D.C.M.; Bulten, J.; van Vliet, H.A.A.M.; Siebers, A.G.; Bekkers, R.L.M. Endometrial Cancer Incidence in Endometriosis and Adenomyosis. Cancers 2021, 13, 4592. https://doi.org/10.3390/cancers13184592
Hermens M, van Altena AM, Velthuis I, van de Laar DCM, Bulten J, van Vliet HAAM, Siebers AG, Bekkers RLM. Endometrial Cancer Incidence in Endometriosis and Adenomyosis. Cancers. 2021; 13(18):4592. https://doi.org/10.3390/cancers13184592
Chicago/Turabian StyleHermens, Marjolein, Anne M. van Altena, Iris Velthuis, Danielle C. M. van de Laar, Johan Bulten, Huib A. A. M. van Vliet, Albert G. Siebers, and Ruud L. M. Bekkers. 2021. "Endometrial Cancer Incidence in Endometriosis and Adenomyosis" Cancers 13, no. 18: 4592. https://doi.org/10.3390/cancers13184592
APA StyleHermens, M., van Altena, A. M., Velthuis, I., van de Laar, D. C. M., Bulten, J., van Vliet, H. A. A. M., Siebers, A. G., & Bekkers, R. L. M. (2021). Endometrial Cancer Incidence in Endometriosis and Adenomyosis. Cancers, 13(18), 4592. https://doi.org/10.3390/cancers13184592






