Efficacy and Safety of Lenvatinib in Hepatocellular Carcinoma Patients with Liver Transplantation: A Case-Control Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient
2.2. Lenvatinib Treatment and Safety Assessment
2.3. Staging and Efficacy Assessment
2.4. Statistical Analysis
2.5. Ethics Statement
3. Results
3.1. Patient Characteristics
3.2. Lenvatinib Treatment and Efficacy
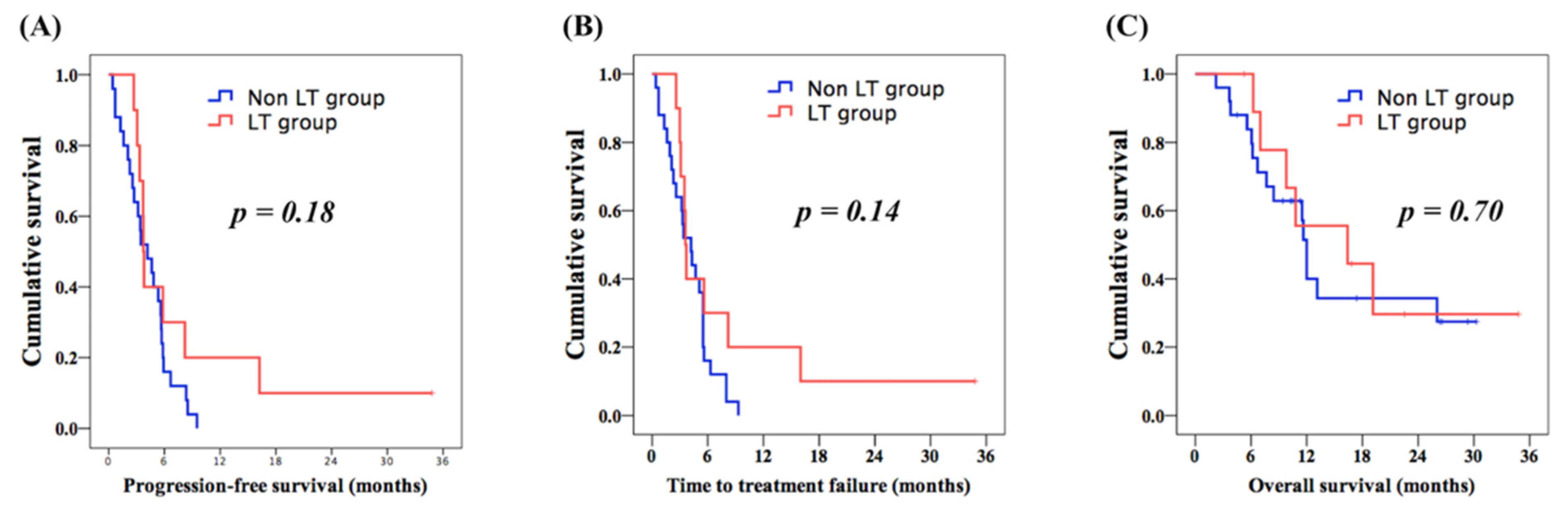
3.3. Comparison of Prognosis between LT and Control Groups
3.4. Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ministry of Health and Welfare of Taiwan. Cancer Registry Annual Report 1972–2015; Health Promotion Administration, Ministry of Health and Welfare: Taipei, Taiwan, 2015.
- Forner, A.; Llovet, J.M.; Bruix, J. Hepatocellular carcinoma. Lancet 2012, 379, 1245–1255. [Google Scholar] [CrossRef]
- Hung, T.H.; Liang, C.M.; Hsu, C.N.; Tai, W.C.; Tsai, K.L.; Ku, M.K.; Wang, J.W.; Tseng, K.L.; Yuan, L.T.; Nguang, S.H.; et al. Association between complicated liver cirrhosis and the risk of hepatocellular carcinoma in Taiwan. PLoS ONE 2017, 12, e0181858. [Google Scholar] [CrossRef] [PubMed]
- Su, S.Y.; Lee, W.C. Mortality trends of liver diseases from 1981 to 2016 and the projection to 2035 in Taiwan: An age-period-cohort analysis. Liver Int. 2019, 39, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, V.; Regalia, E.; Doci, R.; Andreola, S.; Pulvirenti, A.; Bozzetti, F.; Montalto, F.; Ammatuna, M.; Morabito, A.; Gennari, L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N. Engl. J. Med. 1996, 334, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Figueras, J.; Jaurrieta, E.; Valls, C.; Benasco, C.; Rafecas, A.; Xiol, X.; Fabregat, J.; Casanovas, T.; Torras, J.; Baliellas, C.; et al. Survival after liver transplantation in cirrhotic patients with and without hepatocellular carcinoma: A comparative study. Hepatology 1997, 25, 1485–1489. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Sposito, C.; Zhou, J.; Pinna, A.D.; De Carlis, L.; Fan, J.; Cescon, M.; Di Sandro, S.; He, Y.; Lauterio, A.; et al. Metroticket 2.0 Model for Analysis of Competing Risks of Death After Liver Transplantation for Hepatocellular Carcinoma. Gastroenterology 2018, 154, 128–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roayaie, S.; Schwartz, J.D.; Sung, M.W.; Emre, S.H.; Miller, C.M.; Gondolesi, G.E.; Krieger, N.R.; Schwartz, M.E. Recurrence of hepatocellular carcinoma after liver transplant: Patterns and prognosis. Liver Transpl. 2004, 10, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.; Roayaie, S.; Llovet, J. How should patients with hepatocellular carcinoma recurrence after liver transplantation be treated? J. Hepatol. 2005, 43, 584–589. [Google Scholar] [CrossRef]
- Toso, C.; Cader, S.; Mentha-Dugerdil, A.; Meeberg, G.; Majno, P.; Morard, I.; Giostra, E.; Berney, T.; Morel, P.; Mentha, G.; et al. Factors predicting survival after post-transplant hepatocellular carcinoma recurrence. J. Hepatobiliary Pancreat. Sci. 2013, 20, 342–347. [Google Scholar] [CrossRef] [Green Version]
- Cheng, A.L.; Kang, Y.K.; Chen, Z.; Tsao, C.J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.S.; et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009, 10, 25–34. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Schwartz, L.; Ricci, S.; Amadori, D.; Santoro, A.; Figer, A.; De Greve, J.; Douillard, J.Y.; Lathia, C.; Schwartz, B.; et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J. Clin. Oncol. 2006, 24, 4293–4300. [Google Scholar] [CrossRef] [Green Version]
- Alsina, A.E.; Makris, A.; Nenos, V.; Sucre, E.; Arrobas, J.; Franco, E.; Kemmer, N. Can sorafenib increase survival for recurrent hepatocellular carcinoma after liver transplantation? A pilot study. Am. Surg. 2014, 80, 680–684. [Google Scholar] [CrossRef] [PubMed]
- Pinero, F.; Thompson, M.; Marin, J.I.; Silva, M. Lenvatinib as first-line therapy for recurrent hepatocellular carcinoma after liver transplantation: Is the current evidence applicable to these patients? World J. Transplant. 2020, 10, 297–306. [Google Scholar] [CrossRef]
- Sposito, C.; Mariani, L.; Germini, A.; Flores Reyes, M.; Bongini, M.; Grossi, G.; Bhoori, S.; Mazzaferro, V. Comparative efficacy of sorafenib versus best supportive care in recurrent hepatocellular carcinoma after liver transplantation: A case-control study. J. Hepatol. 2013, 59, 59–66. [Google Scholar] [CrossRef]
- Staufer, K.; Fischer, L.; Seegers, B.; Vettorazzi, E.; Nashan, B.; Sterneck, M. High toxicity of sorafenib for recurrent hepatocellular carcinoma after liver transplantation. Transpl. Int. 2012, 25, 1158–1164. [Google Scholar] [CrossRef]
- Waghray, A.; Balci, B.; El-Gazzaz, G.; Kim, R.; Pelley, R.; Narayanan Menon, K.V.; Estfan, B.; Romero-Marrero, C.; Aucejo, F. Safety and efficacy of sorafenib for the treatment of recurrent hepatocellular carcinoma after liver transplantation. Clin. Transplant. 2013, 27, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Zavaglia, C.; Airoldi, A.; Mancuso, A.; Vangeli, M.; Vigano, R.; Cordone, G.; Gentiluomo, M.; Belli, L.S. Adverse events affect sorafenib efficacy in patients with recurrent hepatocellular carcinoma after liver transplantation: Experience at a single center and review of the literature. Eur. J. Gastroenterol. Hepatol. 2013, 25, 180–186. [Google Scholar] [CrossRef]
- Roh, Y.N.; David Kwon, C.H.; Song, S.; Shin, M.; Man Kim, J.; Kim, S.; Joh, J.W.; Lee, S.K. The prognosis and treatment outcomes of patients with recurrent hepatocellular carcinoma after liver transplantation. Clin. Transplant. 2014, 28, 141–148. [Google Scholar] [CrossRef]
- Sapisochin, G.; Goldaracena, N.; Astete, S.; Laurence, J.M.; Davidson, D.; Rafael, E.; Castells, L.; Sandroussi, C.; Bilbao, I.; Dopazo, C.; et al. Benefit of Treating Hepatocellular Carcinoma Recurrence after Liver Transplantation and Analysis of Prognostic Factors for Survival in a Large Euro-American Series. Ann. Surg. Oncol. 2015, 22, 2286–2294. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef] [Green Version]
- Al-Salama, Z.T.; Syed, Y.Y.; Scott, L.J. Lenvatinib: A Review in Hepatocellular Carcinoma. Drugs 2019, 79, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Schlumberger, M.; Tahara, M.; Wirth, L.J.; Robinson, B.; Brose, M.S.; Elisei, R.; Habra, M.A.; Newbold, K.; Shah, M.H.; Hoff, A.O.; et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N. Engl. J. Med. 2015, 372, 621–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.Y.; Wang, C.C.; Liu, Y.W.; Li, W.F.; Chen, Y.H. Clinical impact of lenvatinib in patients with unresectable hepatocellular carcinoma who received sorafenib. PeerJ 2020, 8, e10382. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Qin, S.; Merle, P.; Granito, A.; Huang, Y.H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Breder, V.; et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 389, 56–66. [Google Scholar] [CrossRef] [Green Version]
- Zhu, A.X.; Kang, Y.K.; Yen, C.J.; Finn, R.S.; Galle, P.R.; Llovet, J.M.; Assenat, E.; Brandi, G.; Pracht, M.; Lim, H.Y.; et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased alpha-fetoprotein concentrations (REACH-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 282–296. [Google Scholar] [CrossRef]
- Sangro, B.; Sarobe, P.; Hervas-Stubbs, S.; Melero, I. Advances in immunotherapy for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 525–543. [Google Scholar] [CrossRef]
- Rizzo, A.; Dadduzio, V.; Ricci, A.D.; Massari, F.; Di Federico, A.; Gadaleta-Caldarola, G.; Brandi, G. Lenvatinib plus pembrolizumab: The next frontier for the treatment of hepatocellular carcinoma? Expert Opin. Investig. Drugs 2021, 1–8. [Google Scholar] [CrossRef]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef] [Green Version]
- Omata, M.; Cheng, A.L.; Kokudo, N.; Kudo, M.; Lee, J.M.; Jia, J.; Tateishi, R.; Han, K.H.; Chawla, Y.K.; Shiina, S.; et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: A 2017 update. Hepatol. Int. 2017, 11, 317–370. [Google Scholar] [CrossRef] [Green Version]
- Common Terminology Criteria for Adverse Events v4.0 (CTCAE) May 28, 2009 NIH Publication No. 03-5410. Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50 (accessed on 1 July 2021).
- Llovet, J.M.; Bru, C.; Bruix, J. Prognosis of hepatocellular carcinoma: The BCLC staging classification. Semin. Liver Dis. 1999, 19, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 2010, 30, 52–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, A.; Moriguchi, M.; Seko, Y.; Ishikawa, H.; Yo, T.; Kimura, H.; Fujii, H.; Shima, T.; Mitsumoto, Y.; Ishiba, H.; et al. Impact of Relative Dose Intensity of Early-phase Lenvatinib Treatment on Therapeutic Response in Hepatocellular Carcinoma. Anticancer Res. 2019, 39, 5149–5156. [Google Scholar] [CrossRef] [PubMed]
- Weinmann, A.; Niederle, I.M.; Koch, S.; Hoppe-Lotichius, M.; Heise, M.; Duber, C.; Schuchmann, M.; Otto, G.; Galle, P.R.; Worns, M.A. Sorafenib for recurrence of hepatocellular carcinoma after liver transplantation. Dig. Liver Dis. 2012, 44, 432–437. [Google Scholar] [CrossRef]
- Yoon, D.H.; Ryoo, B.Y.; Ryu, M.H.; Lee, S.G.; Hwang, S.; Suh, D.J.; Lee, H.C.; Kim, T.W.; Ahn, C.S.; Kim, K.H.; et al. Sorafenib for recurrent hepatocellular carcinoma after liver transplantation. Jpn. J. Clin. Oncol. 2010, 40, 768–773. [Google Scholar] [CrossRef]
- Gomez-Martin, C.; Bustamante, J.; Castroagudin, J.F.; Salcedo, M.; Garralda, E.; Testillano, M.; Herrero, I.; Matilla, A.; Sangro, B. Efficacy and safety of sorafenib in combination with mammalian target of rapamycin inhibitors for recurrent hepatocellular carcinoma after liver transplantation. Liver Transpl. 2012, 18, 45–52. [Google Scholar] [CrossRef]
- Bruix, J.; Cheng, A.L.; Meinhardt, G.; Nakajima, K.; De Sanctis, Y.; Llovet, J. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: Analysis of two phase III studies. J. Hepatol. 2017, 67, 999–1008. [Google Scholar] [CrossRef] [Green Version]


| Patient Number | Age/Sex | Body Weight (kg) | ECOG PS | Child-Pugh Classification | BCLC Classification | Viral Hepatitis Status | Immunosuppressant Agents | Duration from LT to Tumor Recurrence (Months) | Recurrent Status | Extrahepatic Spread at the Time of Recurrence | Metastatic Site at the Time of Recurrence | Duration of Prior Sorafenib (Months) | Best Response to Sorafenib | Main PVT at the Time of Lenvatinib | Macrovascular Invasion at the Time of Lenvatinib | Extrahepatic Spread at the Time of Lenvatinib | Metastatic Site at the Time of Lenvatinib | LN Involvement at the Time of Lenvatinib | AFP at the Time of Lenvatinib | Best Response to Lenvatinib |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 39/M | 67.0 | 0 | A | C | hepatitis B | Everolimus Tacrolimus Prolonged-release | 22.0 | Hepatic combined with extrahepatic | Yes | Regional lymph nodes | 4.6 | PR | No | No | Yes | Regional lymph nodes | Yes | <2.0 | PR |
| 2 | 37/M | 65.7 | 1 | A | C | hepatitis B | Sirolimus Tacrolimus (FK506) | 49.2 | Extrahepatic | Yes | lung | 3.0 | SD | No | No | Yes | Lung and regional lymph nodes | Yes | 3.8 | SD |
| 3 | 54/M | 66.6 | 1 | A | C | hepatitis B | Tacrolimus (FK506) Sirolimus | 10.2 | Hepatic combined with extrahepatic | Yes | lung | 30.7 | PR | No | No | Yes | Lung | No | 78.2 | SD |
| 4 | 62/M | 78.2 | 1 | A | C | hepatitis B | Tacrolimus (FK506) | 18.3 | Extrahepatic | Yes | bone | 3.1 | SD | No | No | Yes | Bone | No | <3.0 | SD |
| 5 | 63/M | 61.0 | 1 | A | C | hepatitis C | Everolimus Tacrolimus Prolonged-release | 23.4 | Extrahepatic | Yes | Peritoneal seeding | 2.5 | SD | No | No | Yes | Lung and peritoneal seeding | No | 196.1 | SD |
| 6 | 39/M | 67.6 | 0 | A | C | hepatitis B | Tacrolimus (FK506) Sirolimus | 14.9 | Hepatic combined with extrahepatic | Yes | lung | 5.1 | PD | No | No | Yes | Lung | No | 830.4 | PD |
| 7 | 58/M | 73.1 | 0 | A | C | hepatitis C | Sirolimus Mycophenolate mofetil | 54.2 | Extrahepatic | Yes | bone, lung | 18.9 | PR | No | No | Yes | Lung and bone | No | 2942.0 | PR |
| 8 | 49/M | 66.9 | 0 | A | C | hepatitis B | Everolimus Tacrolimus (FK506) | 34.0 | Extrahepatic | Yes | lung | 12.9 | SD | No | No | Yes | Lung | No | 2.7 | SD |
| 9 | 57/M | 63.5 | 1 | A | B | hepatitis C | Tacrolimus (FK506) Sirolimus | 13.8 | Hepatic | No | – | 4.7 | PD | No | No | No | – | No | 57.9 | PD |
| 10 | 57/M | 71.0 | 1 | A | C | hepatitis C | Tacrolimus (FK506) Mycophenolate mofetil | 13.0 | Extrahepatic | Yes | bone | 4.2 | PD | No | No | Yes | Bone | No | 46,142.3 | PD |
| Characteristics | Liver Transplantation Group # (n = 10) | Control Group (n = 25) | p Value |
|---|---|---|---|
| Age | |||
| <60 years | 8 (80.0%) | 11 (44.0%) | 0.05 |
| ≥60 years | 2 (20.0%) | 14 (56.0%) | |
| ECOG performance status | |||
| 0 | 3 (30.0%) | 4 (16.0%) | 0.35 |
| 1 | 7 (70.0%) | 21 (84.0%) | |
| Sex | |||
| Male | 9 (90.0%) | 22 (88.0%) | 0.87 |
| Female | 1 (10.0%) | 3 (12.0%) | |
| Child–Pugh classification | |||
| A | 10 (100.0%) | 25 (100.0%) | 1.0 |
| BCLC staging classification | |||
| B | 2 (20.0%) | 1 (4.0%) | 0.13 |
| C | 8 (80.0%) | 24 (96.0%) | |
| Hepatitis B | |||
| Yes | 6 (60.0%) | 17 (68.0%) | 0.65 |
| No | 4 (40.0%) | 8 (32.0%) | |
| Hepatitis C | |||
| Yes | 4 (40.0%) | 2 (8.0%) | 0.023 * |
| No | 6 (60.0%) | 23 (92.0%) | |
| Macrovascular invasion (including main PVT) at the time of lenvatinib | |||
| Yes | 0 (0%) | 9 (36.0%) | 0.042 * |
| No | 10 (100.0%) | 16 (64.0%) | |
| Hepatectomy before lenvatinib treatment | |||
| Yes | 10 (100.0%) | 15 (60.0%) | 0.018 * |
| No | 0 (0%) | 10 (40.0%) | |
| Extrahepatic spread at the time of lenvatinib | |||
| Yes | 9 (90.0%) | 20 (80.0%) | 0.48 |
| No | 1 (10.0%) | 5 (20.0%) | |
| AFP level > 400 at the time of lenvatinib | |||
| Yes | 2 (20.0%) | 13 (52.0%) | 0.08 |
| No | 8 (80.0%) | 12 (48.0%) |
| Category | Liver Transplantation Group # (n = 10) | Control Group (n = 25) |
|---|---|---|
| Any post-lenvatinib anti-cancer treatment | 8 (80%) | 17 (68%) |
| TACE/TAE | 3 (30%) | 3 (12%) |
| Chemotherapy | 4 (40%) | 7 (28%) |
| Radiotherapy | 1 (10%) | 1 (4%) |
| Clinical trials | 0 | 2 (8%) |
| Palliative metastasectomy | 2 (20%) | 0 |
| Target Therapy | 4 (40%) | 7 (28%) |
| Carbozantinib | 2 (20%) | 0 |
| Regorafenib | 2 (20%) | 2 (8%) |
| Ramucirumab | 0 | 1 (4%) |
| Sorafenib | 0 | 1 (4%) |
| Thalidomide | 1 (10%) | 4 (16%) |
| Immune checkpoint inhibitors | 0 | 6 (24%) |
| Atezolizumab plus bevacizumab | 0 | 1 (4%) |
| Atezolizumab | 0 | 1 (4%) |
| Nivolumab | 0 | 3 (12%) |
| Pembrolizumab | 0 | 1 (4%) |
| Adverse Event | Liver Transplantation Group # (n = 10) | Control Group (n = 25) | p Value | ||
|---|---|---|---|---|---|
| Any Grades | Grade 3 | Any Grades | Grade 3 | ||
| Hypertension | 4 (40.0%) | 1 (10.0%) | 12 (48.0%) | 3 (12.0%) | 0.98 |
| Diarrhea | 3 (30.0%) | 0 (0%) | 7 (28.0%) | 1 (4%) | 0.78 |
| Decreased appetite | 2 (20.0%) | 0 (0%) | 4 (16.0%) | 0 (0%) | 0.78 |
| Decreased body weight | 1 (10.0%) | 0 (0%) | 3 (12.0%) | 0 (0%) | 0.87 |
| Fatigue | 3 (30.0%) | 0 (0%) | 8 (32.0%) | 1 (4.0%) | 0.91 |
| Palmar-plantar erythrodysesthesia | 2 (20.0%) | 0 (0%) | 6 (24.0%) | 0 (0%) | 0.80 |
| Nausea | 1 (10.0%) | 0 (0%) | 3 (12.0%) | 0 (0%) | 0.87 |
| Vomiting | 1 (10.0%) | 0 (0%) | 2 (8.0%) | 0 (0%) | 0.85 |
| Skin rash | 1 (10.0%) | 0 (0%) | 3 (12.0%) | 0 (0%) | 0.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-Y.; Chen, C.-L.; Lin, C.-C.; Wang, C.-C.; Liu, Y.-W.; Li, W.-F.; Chen, Y.-H. Efficacy and Safety of Lenvatinib in Hepatocellular Carcinoma Patients with Liver Transplantation: A Case-Control Study. Cancers 2021, 13, 4584. https://doi.org/10.3390/cancers13184584
Chen Y-Y, Chen C-L, Lin C-C, Wang C-C, Liu Y-W, Li W-F, Chen Y-H. Efficacy and Safety of Lenvatinib in Hepatocellular Carcinoma Patients with Liver Transplantation: A Case-Control Study. Cancers. 2021; 13(18):4584. https://doi.org/10.3390/cancers13184584
Chicago/Turabian StyleChen, Yen-Yang, Chao-Long Chen, Chih-Che Lin, Chih-Chi Wang, Yueh-Wei Liu, Wei-Feng Li, and Yen-Hao Chen. 2021. "Efficacy and Safety of Lenvatinib in Hepatocellular Carcinoma Patients with Liver Transplantation: A Case-Control Study" Cancers 13, no. 18: 4584. https://doi.org/10.3390/cancers13184584
APA StyleChen, Y.-Y., Chen, C.-L., Lin, C.-C., Wang, C.-C., Liu, Y.-W., Li, W.-F., & Chen, Y.-H. (2021). Efficacy and Safety of Lenvatinib in Hepatocellular Carcinoma Patients with Liver Transplantation: A Case-Control Study. Cancers, 13(18), 4584. https://doi.org/10.3390/cancers13184584






