EBV-Driven Lymphoproliferative Disorders and Lymphomas of the Gastrointestinal Tract: A Spectrum of Entities with a Common Denominator (Part 1)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Epstein–Barr Virus Biology
3. EBVMCU
3.1. General Features and Etiology
3.2. EBVMCU and GIT
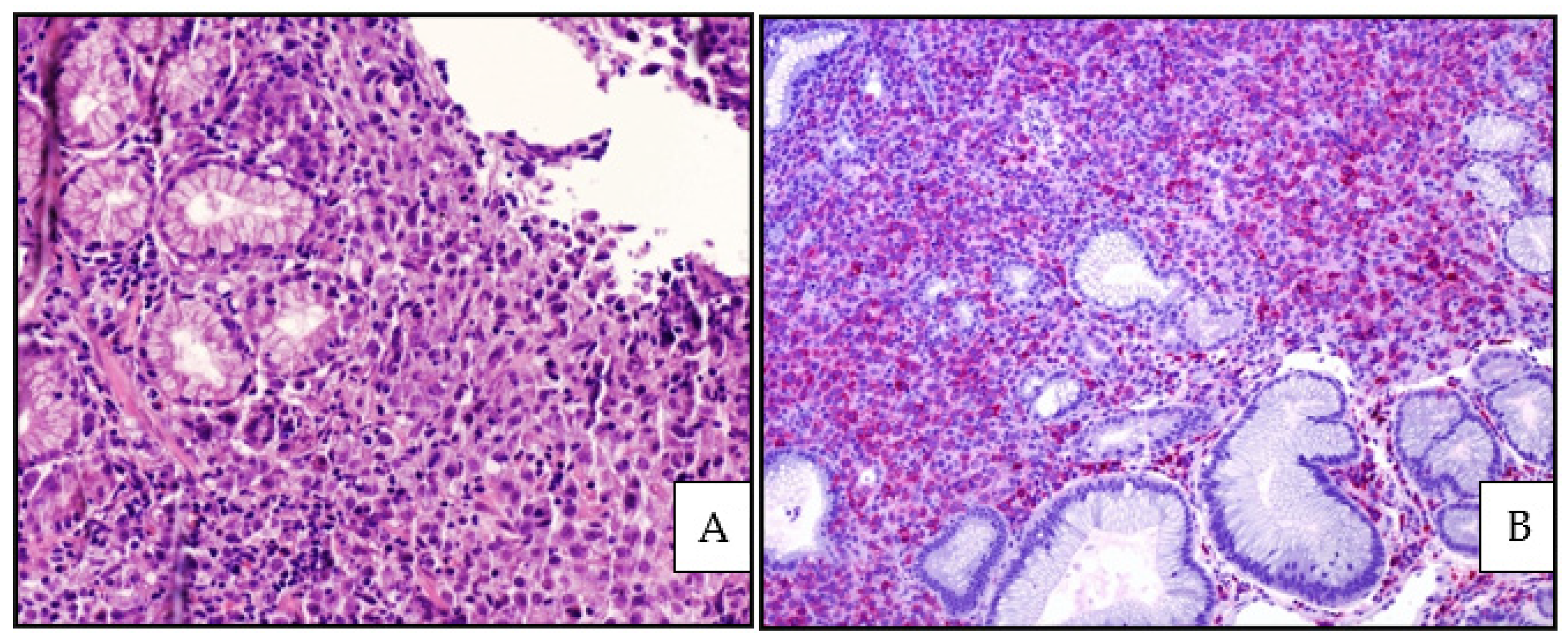
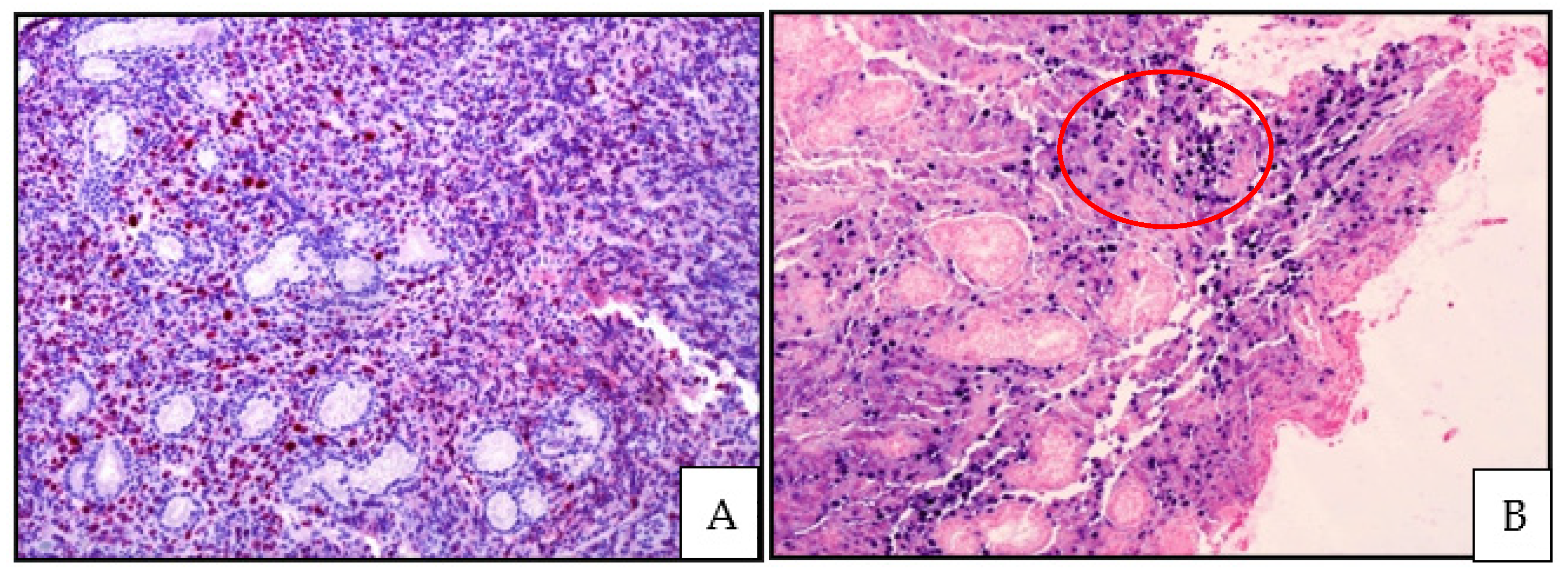
3.3. Histology, Immunophenotype and Genetic Profile
3.4. Differential Diagnosis
3.5. Treatment and Outcome
4. EBV-Positive DLBCL, NOS
4.1. General Features and Etiology
4.2. EBV-Positive DLBCL, NOS and GIT
4.3. Histology, Immunophenotype and Genetic Profile
4.4. PD-L1 Expression
4.5. Differential Diagnosis
4.6. Treatment and Oucome
5. cHL
5.1. General Features and Etiology
5.2. cHL and GIT
5.3. Histology, Immunophenotype and Genetic Profile
5.4. Differential Diagnosis
5.5. Treatment and Outcome
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taylor, G.S.; Long, H.M.; Brooks, J.M.; Rickinson, A.B.; Hislop, A.D. The immunology of Epstein-Barr virus induced disease. Annu. Rev. Immunol. 2015, 33, 787–821. [Google Scholar] [CrossRef] [PubMed]
- Young, L.S.; Yap, L.F.; Murray, P.G. Epstein-Barr virus: More than 50 years old and still providing surprises. Nat. Rev. Cancer 2016, 16, 789–802. [Google Scholar] [CrossRef]
- Resk, S.A.; Weiss, L.M. Epstein-Barr virus-associated lymphoproliferative disorders. Hum. Pathol. 2007, 38, 1293–1304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanner, J.E.; Alfieri, C. The Epstein-Barr virus and post-transplant lymphoproliferative disease: Interplay of immunosuppression, EBV, and the immune system in disease pathogenesis. Transpl. Infect. Dis. 2001, 3, 60–69. [Google Scholar] [CrossRef]
- Hakim, F.T.; Gress, R.E. Immunosenescence: Deficits in adaptative immunity in the elderly. Tissue Antigens 2007, 70, 179–189. [Google Scholar] [CrossRef]
- Ghia, P.; Prato, G.; Stella, S.; Scielzo, C.; Geuna, M.; Caligaris-Cappio, F. Age-dependent accumulation of monoclonal CD4+CD8+ double positive T lymphocytes in the peripheral blood of the elderly. Br. J. Haematol. 2007, 139, 780–790. [Google Scholar] [CrossRef]
- Dolcetti, R.; Dal Col, J.; Martorelli, D.; Carbone, A.; Klein, E. Interplay among viral antigens, cellular pathways and tumor microenvironment in the pathogenesis of EBV-driven lymphoma. Semin. Cancer Biol. 2013, 13, 441–456. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial Board (Ed.) WHO Classification of Tumours Haematopoietic and Lymphoid Tissues, Revised 4th ed.IARC: Lyon, France, 2017. [Google Scholar]
- Dojcinov, S.D.; Fend, F.; Quintanilla-Martinez, L. EBV-positive lymphoproliferation of B- T- and NK-cell derivation in non-immunocompromised hosts. Pathogens 2021, 7, 28. [Google Scholar] [CrossRef] [Green Version]
- Dunmire, S.K.; Hogquist, K.A.; Balfour, H.H. Infectious mononucleosis. Curr. Top. Microbiol. 2015, 390, 151–209. [Google Scholar]
- Rickinson, A.B. Co-infections, inflammation and oncogenesis: Future directions for EBV research. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2014; pp. 99–115. [Google Scholar]
- Price, A.M.; Luftig, M.A. To be or not IIb: A multi-step process for Epstein-Barr virus latency establishment and consequences for B cell tumorigenesis. PLoS Pathog. 2015, 11, e1004656. [Google Scholar] [CrossRef] [PubMed]
- Dojcinov, S.D.; Venkataraman, G.; Raffeld, M.; Pittaluga, S.; Jaffe, E.S. EBV positive mucocutaneous ulcer. A study of 26 cases associated with various sources of immunosuppression. Am. J. Surg. Pathol. 2010, 34, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Gion, Y.; Yoshino, T.; Sato, Y. A review of EBV-positive mucocutaneous ulcers focusing on clinical and pathological aspects. J. Clin. Exp. Hematopathol. 2019, 59, 64–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikeda, T.; Gion, Y.; Nishimura, M.F.; Yoshino, T.; Sato, Y. Epstein-Barr Virus-positive mucocutaneous ulcer: A unique and curious disease entity. Int. J. Mol. Sci. 2021, 22, 1053. [Google Scholar] [CrossRef] [PubMed]
- Natkunam, Y.; Goodlad, J.R.; Chadburn, A.; Jong, D.; Gratzinger, D.; Chan, J.K.C.; Said, J.; Jaffe, E.S. EBV-positive B-cell proliferations of varied malignant potential. Am. J. Clin. Pathol. 2017, 147, 129–152. [Google Scholar] [CrossRef] [Green Version]
- Hart, M.; Thakral, B.; Yohe, S.; Balfour, H.H., Jr.; Singh, C.; Speras, M.; McKenna, R.W. EBV-positive mucocutaneous ulcer in organ transplant recipients: A localized indolent posttransplant lymphoproliferative disorder. Am. J. Surg. Pathol. 2014, 38, 1522–1529. [Google Scholar] [CrossRef] [PubMed]
- Matnani, R.; Peker, D. Azathioprine induced Epstein Barr virus-positive mucocutaneous ulcer arising in perianal fistula and abscess associated with Crohn’s disease. J. Crohn’s Colitis 2014, 8, 1747–1748. [Google Scholar] [CrossRef] [Green Version]
- Moran, N.R.; Webster, B.; Lee, K.M.; Trotman, J.; Kwan, Y.-L.; Napoli, J.; Leong, R.W. Epstein Barr virus-positive mucocutaneous ulcer of the colon associated Hodgkin lymphoma in Crohn’s disease. World J. Gastroenterol. 2015, 21, 6072–6076. [Google Scholar] [CrossRef]
- Juan, A.; Lobaton, T.; Tapja, G.; Manosa, M.; Cabrè, E. Epstein-Barr virus-positive mucocutaneous ulcer in Crohn’s disease. A condition to consider in immunosuppressed IBD patients. Dig. Liver Dis. 2017, 49, 934–937. [Google Scholar] [CrossRef]
- Zanelli, M.; Mengoli, M.C.; Valli, R.; Froio, E.; Bisagni, A.; Zizzo, M.; De Marco, L.; Ascani, S. Primary classic Hodgkin lymphoma of the ileum and Epstein-Barr virus mucocutaneous ulcer of the colon: Two entities compared. Virchows Archiv. 2019, 474, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Kleinman, S.; Jhaveri, D.; Caimi, P.; Cameron, R.; Lemonovich, T.; Meyerson, H.; Hostoffer, R.; Tcheurekdjian, H. A rare presentation of EBV+ mucocutaneous ulcer that led to the diagnosis of hypogammaglobulinemia. J. Allergy Clin. Immunol. Pract. 2014, 2, 810–812. [Google Scholar] [CrossRef]
- Osman, M.; Al Salihi, M.; Abu Sitta, E.; Al Hadidi, S. A rare case of Epstein-Barr virus mucocutaneous ulcer of the colon. BMJ Case Rep. 2017, 2017, bcr-2017-220717. [Google Scholar] [CrossRef]
- Zanelli, M.; Zizzo, M.; Foroni, M.; De Marco, L.; Martino, G.; Ascani, S. EBV-positive mucocutaneous ulcer within colonic diverticulitis mimicking diffuse large B-cell lymphoma. Ann. Hematol. 2019, 98, 1795–1797. [Google Scholar] [CrossRef]
- Volaric, A.K.; Singh, K.; Gru, A.A. Rare EBV-associated B cell neoplasms of the gastrointestinal tract. Semin. Diagn. Pathol. 2021, 38, 38–45. [Google Scholar] [CrossRef]
- Ikeda, T.; Gion, Y.; Sakamoto, M.; Tachibana, T.; Nishikori, A.; Nishimura, M.F.; Yoshino, T.; Sato, Y. Clinicopathological analysis of 34 Japanese patients with EBV-positive mucocutaneous ulcer. Mod. Pathol. 2020, 33, 2437–2448. [Google Scholar] [CrossRef]
- Prieto-Torres, L.; Erana, I.; Gil-Redondo, R.; de la Riva, I.G.; Manso, R.; Pajares, R.; Cordoba, R.; Machan, S.; Ara, M.; Requena, L.; et al. The spectrum of EBV-positive mucocutaneous ulcer: A study of 9 cases. Am. J. Surg. Pathol. 2019, 43, 201–210. [Google Scholar] [CrossRef]
- Goetgebuer, R.L.; van der Woude, C.J.; de Ridder, L.; Doukas, M.; de Vries, A.C. Clinical and endoscopic complications of Epstein-Barr virus in inflammatory bowel disease: An illustrative case series. Int. J. Colorectal. Dis. 2019, 34, 923–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pugh, M.R.; Leopold, G.D.; Morgan, M.; Christian, A.D.; Hewett, R.; Durai, D.; Wagstaff, J.; Harris, D.; Dojcinov, S.D. Epstein Barr virus-positive mucocutaneous ulcers complicate colitis caused by immune checkpoint regulator therapy and associate with colon perforation. Clin. Gastroenterol. Hepatol. 2020, 18, 1785. [Google Scholar] [CrossRef]
- Daroontum, T.; Kohno, K.; Eladi, A.E.; Satou, A.; Sakakibara, A.; Matsukage, S.; Yakushiji, N.; Ya-In, C.; Nakamura, S.; Asano, N.; et al. Comparison of Epstein-Barr virus-positive mucocutaneous ulcer associated with treated lymphoma or methotrexate in Japan. Histopathology 2018, 72, 1115–1127. [Google Scholar] [CrossRef] [PubMed]
- Morita, N.; Okuse, C.; Suetani, K.; Nakano, H.; Hiraishi, T.; Ishigooka, S.; Mori, S.; Shimamura, T.; Asakura, T.; Koike, J.; et al. A rare case of Epstein-Barr virus-positive mucocutaneous ulcer that developed into an intestinal obstruction: A case report. BMC Gastroenterol. 2020, 20, 9. [Google Scholar] [CrossRef]
- Di Napoli, A.; Giubettini, M.; Duranti, E.; Ferrari, A.; Guglielmi, C.; Uccini, S.; Ruco, L. Iatrogenic EBV-positive lymphoprolifeartive disorder with features of EBV plus mucocutaneous ulcer: Evidence for concomitantTCR gamma/IGH rearrangements in the Hodgkin-like neoplastic cells. Virchows Archiv. 2011, 458, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Karube, K.; Takatori, M.; Kohno, K.; Tomoyose, T.; Ohshiro, K.; Nakazato, I. Co-occurrence of EBV-positive classic Hodgkin lymphoma and B-cell lymphomas of different clonal origins: A case report and literature review. Pathol. Int. 2020, 70, 893–898. [Google Scholar] [CrossRef]
- Nomura, M.; Sumya, R.; Ono, H.; Nagai, T.; Kumazawa, K.; Shimizu, A.; Endo, D.; Aoyanagi, N. Cessation of methotrexate and a small intestine resection provide a good clinical course for a patient with a jejunum perforation induced by a methotrexate-associated lymphoproliferative disorder: A case report. World J. Surg. Oncol. 2021, 19, s12957. [Google Scholar] [CrossRef] [PubMed]
- Isnard, P.; Bruneau, J.; Sberro-Soussan, R.; Wendum, D.; Legendre, C.; Molina, T.; Chatenoud, L.; Hermine, O.; Rossignol, J. Dissociation of humoral and cellular immune responses in kidney transplant recipients with EBV mucocutaneous ulcer. Transpl. Infect. Dis. 2021, 23, e13552. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Chapman, J.R.; Vega, F. A case of EBV-associated blastic lymphoplasmacytic proliferation in an oesophageal ulcer with a self-limiting course: Overlapping lesion between EBV mucocutaneous ulcer and polymorphic lymphoplasmocytic disorder. Histopathology 2019, 74, 964–966. [Google Scholar] [CrossRef]
- Ishikawa, E.; Satou, A.; Nakamura, M.; Nakamura, S.; Fujishiro, M. Epstein-Barr virus positive B-cell lymphoproliferative disorder of the gastrointestinal tract. Cancers 2021, 13, 3815. [Google Scholar] [CrossRef]
- Sinit, R.B.; Horan, K.L.; Dorer, R.K.; Aboulafia, D.M. Epstein-Barr virus-positive mucocutaneous ulcer: Case report and review of the first 100 published cases. Clin. Lymphoma Myeloma Leuk. 2019, 19, E81–E92. [Google Scholar] [CrossRef]
- Oyama, T.; Yamamoto, K.; Asano, N.; Oshiro, A.; Suzuki, R.; Kagami, Y.; Morishima, Y.; Takeuki, K.; Izumo, T.; Mori, S.; et al. Age-related EBV-associated B-cell lymphoproliferative disorders constitute a distinct clinicopathologic group: A study of 96 patients. Clin. Cancer Res. 2007, 13, 5124–5132. [Google Scholar] [CrossRef] [Green Version]
- Dojcinov, S.D.; Venkataraman, G.; Pittaluga, S.; Wlodarska, I.; Schrager, J.A.; Raffeld, M.; Hills, R.K.; Jaffe, E.S. Age-related EBV associated lymphoproliferative disorders in the western population: A spectrum of reactive lymphoid hyperplasia and lymphoma. Blood 2011, 117, 4726–4735. [Google Scholar] [CrossRef] [Green Version]
- Swerdlow, S.H.; Campo, E.; Harris, N.L.; Jaffe, E.S.; Pileri, S.A.; Stein, H.; Thiele, J.; Vardiman, J.W. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissue; IARC: Lyon, France, 2008. [Google Scholar]
- Nicolae, A.; Pittaluga, S.; Abdullah, S.; Steinberg, S.M.; Pham, T.A.; Davies-Hill, T.; Xi, L.-Q.; Raffeld, M.; Jaffe, E.S. EBV-positive large B-cell lymphomas in young patients: A nodal lymphoma with evidence for a tolerogenic immune environment. Blood 2015, 126, 863–872. [Google Scholar] [CrossRef] [Green Version]
- Uccini, S.; Al-Jadiry, M.F.; Scarpino, S.; Ferraro, D.; Alsaadawi, A.R.; Al-Darraji, A.F.; Moletti, M.L.; Testi, A.M.; Al-Hadad, S.A.; Ruco, L. Epstein-Barr virus-positive diffuse large B-cell lymphoma in children: A disease reminiscent of Epstein Barr virus-positive diffuse large B-cell lymphoma of the elderly. Hum. Pathol. 2015, 46, 716–724. [Google Scholar] [CrossRef]
- Miyagi, S.; Ishikawa, E.; Nakamura, M.; Shimada, K.; Yamamura, T.; Furukawa, K.; Tanaka, T.; Mabuchi, S.; Tsuyuki, Y.; Kohno, K.; et al. Reappraisal of primary Epstein-Barr virus (EBV)-positive diffuse large B-cell lymphoma of the gastrointestinal tract. Am. J. Surg. Pathol. 2020, 44, 1173–1183. [Google Scholar] [CrossRef]
- Ishikawa, E.; Kato, S.; Shimada, K.; Tanaka, T.; Suzuki, Y.; Satou, A.; Kohno, K.; Sakakibara, A.; Yamamura, T.; Nakamura, M.; et al. Clinicopathological analysis of primary intestinal diffuse large B-cell lymphoma: Prognostic evaluation of CD5, PD-L1 and Epstein-Barr virus on tumor cells. Cancer Med. 2018, 7, 6051–6063. [Google Scholar] [CrossRef]
- Kleinschmidt-De Masters, B.K.; Damek, D.M.; Lillehei, K.O.; Dogan, A.; Giannini, C. Epstein-Barr virus-associated primary CNS lymphomas in elderly patients on immunosuppressive medications. J. Neuropathol. Exp. Neurol. 2008, 67, 1103–1111. [Google Scholar] [CrossRef] [Green Version]
- Castillo, J.J.; Beltran, B.E.; Miranda, R.N.; Young, K.H.; Chavez, J.C.; Sotomayor, E.M. EBV-positive large B-cell lymphoma, not otherwise specified: 2018 update on diagnosis, risk-stratification and management. Am. J. Hematol. 2018, 93, 953–962. [Google Scholar] [CrossRef] [Green Version]
- Kato, H.; Karube, K.; Yamamoto, K.; Takizawa, J.; Tsuzuki, S.; Yatabe, Y.; Kanda, T.; Katayama, M.; Ozawa, Y.; Ishitsuka, K.; et al. Gene expression profiling of Epstein-Barr virus-positive diffuse large B-cell lymphoma of the elderly reveals characteristic oncogenic pathways. Cancer Sci. 2014, 105, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Sanguedolce, F.; Zanelli, M.; Ascani, S.; Zizzo, M.; Tortorella, S.; Soriano, A.; Fiorelli, A.; Cocco, G.; Ardò, N.; Sollitto, F.; et al. PD-L1 as a prognostic and predictive biomarker in neuroendocrine tumors of the lung: State of the art and future perspectives. Minerva Respir. Med. 2021, 60, 36–51. [Google Scholar] [CrossRef]
- Hu, L.Y.; Xu, X.L.; Rao, H.L.; Chen, J.; Lai, R.C.; Huang, H.Q.; Jiang, W.Q.; Lin, T.Y.; Xia, Z.J.; Cai, Q.Q. Expression and clinical value of programmed cell death-ligand 1 (PD-L1) in diffuse large B-cell lymphoma: A retrospective study. Clin. J. Cancer 2017, 36, s40880. [Google Scholar] [CrossRef]
- Georgiou, K.; Chen, L.; Berglund, M.; Ren, W.; de Miranda, N.; Lisboa, S.; Fangazio, M.; Zhu, S.D.; Hou, Y.; Wu, K.; et al. Genetic basis of PD-L1 overexpression in diffuse large B-cell lymphoma. Blood 2016, 127, 3026–3034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCord, R.; Bolen, C.R.; Koeppen, H.; Kadel, E.E.; Oestergaard, M.Z.; Nielsen, T.; Sehn, L.H.; Venstrom, J.M. PD-L1 and tumor-associated macrophages in de novo DLBCL. Blood Adv. 2019, 3, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Kiyasu, J.; Miyoshi, H.; Hirata, A.; Arakawa, F.; Ichikawa, A.; Niino, D.; Sugita, Y.; Yufu, Y.; Choi, I.; Abe, Y.; et al. Expression of programmed cell death ligand 1 is associated with poor overall survival in patients with diffuse large B-cell lymphoma. Blood 2015, 126, 2193–2201. [Google Scholar] [CrossRef]
- Green, M.R.; Rodig, S.; Juszczynski, P.; Ouyang, J.; Sinha, P.; O’Donnell, E.; Neuberg, D.; Shipp, M.A. Constitutive AP-1 activity and EBV infection induce PD-L1 in Hodgkin lymphomas and posttransplant lymphoproliferative disorders: Implications for targeted therapy. Clin. Cancer Res. 2012, 18, 1611–1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishikawa, E.; Nakamura, M.; Shimada, K.; Tanaka, T.; Satou, A.; Kohno, K.; Sakakibara, A.; Furukawa, K.; Yamamura, T.; Miyahara, R. Prognostic impact of PD-L1 expression in primary gastric and intestinal diffuse large B-cell lymphoma. J. Gastroenterol. 2020, 55, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Hyeon, J.; Cho, I.; Ko, Y.H.; Kim, W.S. Comparison of efficacy of Pembrolizumab between Epstein-Barr virus-positive and -negative relapsed or refractory non-Hodgkin lymphomas. Cancer Res. Treat. 2019, 51, 611–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanelli, M.; Mengoli, M.C.; Del Sordo, R.; Cagini, A.; De Marco, L.; Simonetti, E.; Martino, G.; Zizzo, M.; Ascani, S. Intravascular NK/T-cell lymphoma, Epstein-Barr virus positive with multiorgan involvement: A clinical dilemma. BMC Cancer 2018, 18, 1115. [Google Scholar] [CrossRef]
- Anastasiadou, E.; Stroopinsky, D.; Alimperti, S.; Jiao, A.L.; Pyzer, A.R.; Cippitelli, C.; Pepe, G.; Severa, M.; Rosenblatt, J.; Etna, M.P.; et al. Epstein-Barr virus-encoded EBNA2 alters immune checkpoint PD-L1 expression by downregulating miR-34a in B-cell lymphomas. Leukemia 2019, 33, 132–147. [Google Scholar] [CrossRef] [Green Version]
- Zanelli, M.; Ragazzi, M.; Valli, R.; De Marco, L.; Cecinato, P.; Azzolini, F.; Ferrari, A.; Bacci, F.; Ascani, S. Unique presentation of a plasmablastic lymphoma superficially involving the entire large bowel. Pathol. Res. Pract. 2015, 211, 1030–1033. [Google Scholar] [CrossRef]
- Sanguedolce, F.; Zanelli, M.; Zizzo, M.; Martino, G.; Rossi, C.; Parente, P.; Ascani, S. Clinical, pathological and molecular features of plasmablastic lymphoma arising in the gastrointestinal tract: A review and reappraisal. Pathol. Res. Pract. 2020, 216, 152973. [Google Scholar] [CrossRef] [PubMed]
- Zanelli, M.; Sanguedolce, F.; Zizzo, M.; Palicelli, A.; Bassi, M.C.; Santandrea, G.; Martino, G.; Soriano, A.; Caprera, C.; Corsi, M.; et al. Primary effusion lymphoma occurring in the setting of transplanted patients: A systematic review of a rare, life-threatening post-transplantation occurrence. BMC Cancer 2021, 21, 468. [Google Scholar] [CrossRef] [PubMed]
- Zanelli, M.; Bertuzzi, C.; Zizzo, M.; Martino, G.; Sabattini, E.; Ascani, S. Extracavitary primary effusion lymphoma in a post-transplantation patient. Br. J. Haematol. 2019, 187, 555. [Google Scholar] [CrossRef]
- Sanguedolce, F.; Zanelli, M.; Zizzo, M.; Bisagni, A.; Soriano, A.; Cocco, G.; Palicelli, A.; Santandrea, G.; Caprera, C.; Corsi, M.; et al. Primary Pulmonary B-cell lymphoma: A review and update. Cancers 2021, 13, 415. [Google Scholar] [CrossRef]
- Tagliavini, E.; Rossi, G.; Valli, R.; Zanelli, M.; Cadioli, A.; Mengoli, M.C.; Bisagni, A.; Cavazza, A.; Gardini, G. Lymphomatoid granulomatosis: A practical review for pathologists dealing with this rare pulmonary lymphoproliferative process. Pathologica 2013, 105, 111–116. [Google Scholar]
- Song, J.Y.; Pittaluga, S.; Duleavy, K.; Grant, N.; White, T.; Jiang, L.; Davies-Hill, T.; Raffeld, M.; Wilson, W.H.; Jaffe, E.S. Lymphomatoid granulomatosis—A single institute experience: Pathologic findings and clinical correlations. Am. J. Surg. Pathol. 2015, 39, 141–156. [Google Scholar] [CrossRef] [Green Version]
- Zanelli, M.; Zizzo, M.; De Marco, L.; Bisagni, A.; Ascani, S. Fibrin-associated diffuse large B-cell lymphoma. Br. J. Haematol. 2019, 185, 397. [Google Scholar] [CrossRef]
- Zanelli, M.; Zizzo, M.; Montanaro, M.; Gomes, V.; Martino, G.; De Marco, L.; Fraternali Orcioni, G.; Martelli, M.P.; Ascani, S. Fibrin-associated large B-cell lymphoma: First cases report within a cerebral artery aneurysm and literature review. BMC Cancer 2019, 19, 916. [Google Scholar] [CrossRef] [Green Version]
- Parente, P.; Zanelli, M.; Zizzo, M.; Carosi, I.; Di Candia, L.; Sperandeo, M.; Lacedonia, D.; Fesce, V.F.; Ascani, S.; Graziano, P. Primary pulmonary Hodgkin lymphoma presenting as multiple cystic lung lesions: Diagnostic usefulness of cell block. Cytopathology 2020, 31, 236–239. [Google Scholar] [CrossRef]
- Devaney, K.; Jaffe, E.S. The surgical pathology of gastrointestinal Hodgkin’s disease. Am. J. Clin. Pathol. 1991, 95, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, Y.; Zhao, H.; Liu, S.; Li, Q.; Lin, L.; Yue, Y.; Wang, X.; Zhao, Z.; Yu, Y.; et al. Clinical characteristics of 26 patients with primary extranodal Hodgkin lymphoma. Int. J. Clin. Exp. Pathol. 2014, 7, 5045–5050. [Google Scholar] [PubMed]
- Morgan, P.B.; Kessel, I.L.; Xiao, S.Y.; Colman, M. Uncommon presentations of Hodgkin’s disease. Case 1. Hodgkin’s disease of the jejunum. J. Clin. Oncol. 2004, 22, 193–195. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, A.; Flora, A.C.; Soares, F.A.; de Lima, V.C.C. Primary Hodgkin lymphoma of the rectum: An unusual presentation. J. Clin. Oncol. 2011, 29, 268–270. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Rana, S.; Kapur, S.; Jairajpuri, Z.S. Primary intestinal Hodgkin lymphoma: An uncommon presentation. J. Lab. Physicians 2013, 5, 124–126. [Google Scholar]
- Vaduvesan, J.A.; Nair, R.A.; Nambiar, K.R. Primary classical Hodgkin lymphoma of rectum: Report of an extremely rare case and review of the literature. Indian J. Pathol. Microbiol. 2017, 60, 412–414. [Google Scholar]
- Kumar, S.; Fend, F.; Quintanilla-Martinez, L.; Kingma, D.; Sorbara, L.; Raffeld, M.; Banks, P.M.; Jaffe, E.S. Epstein-Barr virus-positive primary gastrointestinal Hodgkin’s disease: Association with inflammatory bowel disease and immunosuppression. Am. J. Surg. Pathol. 2000, 24, 66. [Google Scholar] [CrossRef]
- Barzilai, M.; Polliack, A.; Avivi, I.; Herishanu, Y.; Ram, R.; Tang, C.; Perry, C.; Sarid, N. Hodgkin lymphoma of the gastrointestinal tract in patients with inflammatory bowel disease: Portrait of a rare entity. Leuk. Res. 2018, 71, 1–5. [Google Scholar] [CrossRef]
- Gibson, B.; Bajramovic Podoli, M.; Baumgartner, E.M.; Haninger Maley, D. Syncitial variant of nodular sclerosis classical Hodgkin lymphoma of the terminal ileum in a patient with longstanding Crohn’s disease. Ann. Clin. Lab. Sci. 2016, 46, 219–221. [Google Scholar]
- Gualco, G.; Ortega, V.; Chioato, L.; Musto, M.L.; Bacchi, L.M.; Weiss, L.M.; Bacchi, C.E. Hodgkin’s lymphoma presenting as dominant gastric lesion in immunocompetent patients: Report of 5 cases with EBV analysis. Int. J. Surg. Pathol. 2011, 19, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Parente, P.; Zanelli, M.; Sanguedolce, F.; Mastracci, L.; Graziano, P. Hodgkin Reed-Sternberg-like cells in non-Hodgkin lymphoma. Diagnostics 2020, 10, 1019. [Google Scholar] [CrossRef] [PubMed]
- Ansell, S.M. Hodgkin lymphoma: A 2020 update on diagnosis, risk stratification and management. Am. J. Haematol. 2020, 95, 978–989. [Google Scholar] [CrossRef]
- Hu, B.; Jacobs, R.; Ghosh, N. Checkpoint inhibitors in Hodgkin lymphoma and non-Hodgkin lymphoma. Curr. Hematol. Malig. Rep. 2018, 13, 543–554. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanelli, M.; Sanguedolce, F.; Palicelli, A.; Zizzo, M.; Martino, G.; Caprera, C.; Fragliasso, V.; Soriano, A.; Valle, L.; Ricci, S.; et al. EBV-Driven Lymphoproliferative Disorders and Lymphomas of the Gastrointestinal Tract: A Spectrum of Entities with a Common Denominator (Part 1). Cancers 2021, 13, 4578. https://doi.org/10.3390/cancers13184578
Zanelli M, Sanguedolce F, Palicelli A, Zizzo M, Martino G, Caprera C, Fragliasso V, Soriano A, Valle L, Ricci S, et al. EBV-Driven Lymphoproliferative Disorders and Lymphomas of the Gastrointestinal Tract: A Spectrum of Entities with a Common Denominator (Part 1). Cancers. 2021; 13(18):4578. https://doi.org/10.3390/cancers13184578
Chicago/Turabian StyleZanelli, Magda, Francesca Sanguedolce, Andrea Palicelli, Maurizio Zizzo, Giovanni Martino, Cecilia Caprera, Valentina Fragliasso, Alessandra Soriano, Luca Valle, Stefano Ricci, and et al. 2021. "EBV-Driven Lymphoproliferative Disorders and Lymphomas of the Gastrointestinal Tract: A Spectrum of Entities with a Common Denominator (Part 1)" Cancers 13, no. 18: 4578. https://doi.org/10.3390/cancers13184578
APA StyleZanelli, M., Sanguedolce, F., Palicelli, A., Zizzo, M., Martino, G., Caprera, C., Fragliasso, V., Soriano, A., Valle, L., Ricci, S., Cavazza, A., Merli, F., Pileri, S. A., & Ascani, S. (2021). EBV-Driven Lymphoproliferative Disorders and Lymphomas of the Gastrointestinal Tract: A Spectrum of Entities with a Common Denominator (Part 1). Cancers, 13(18), 4578. https://doi.org/10.3390/cancers13184578







