Elderly Patients with Locally Advanced and Unresectable Non-Small-Cell Lung Cancer May Benefit from Sequential Chemoradiotherapy
Abstract
Simple Summary
Abstract
1. Introduction
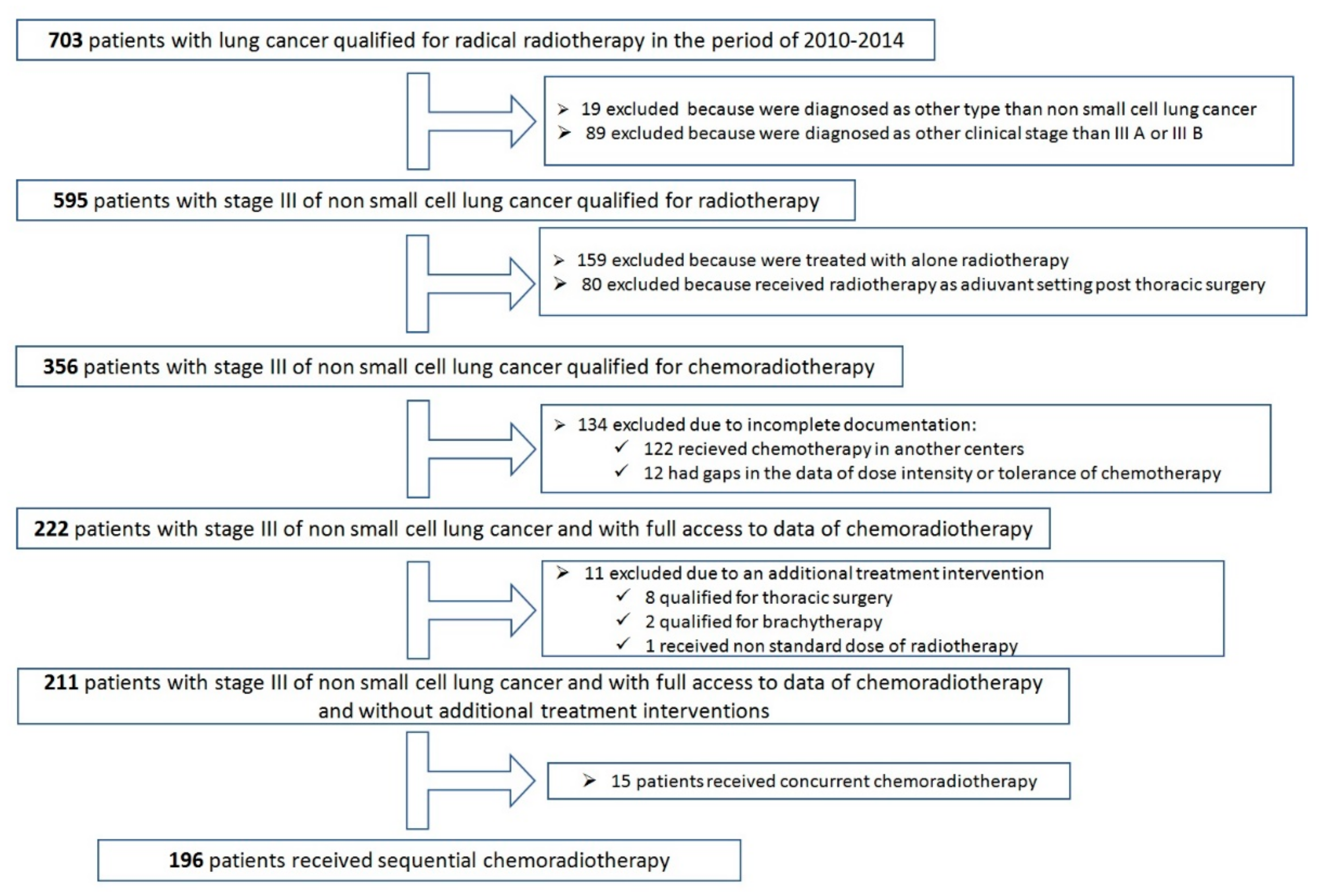
2. Results
- ➢
- 5880 cGy—administered in 94 (48%) patients
- ➢
- 6600 cGy—administered in 87 (44.4%) patients
- ➢
- 6000 cGy—administered in 9 (4.6%) patients
- ➢
- 6400 cGy—administered in 2 (1%) patients
- ➢
- 6200 cGy—administered in 1 patient
- ➢
- 5040 cGy—administered in 1 patient
- ➢
- 5040 cGy + BOOST brachytherapy 7.5 Gy—administered in 1 patient
- ➢
- 6200 cGy (4200 + BOOST to 6200)—administered in 1 patient
- complete response (CR) in 15 (7.7%) patients;
- partial response (PR) in 123 (62.8%) patients;
- stable disease (SD) in 44 (22.4%) patients;
- progressive disease (PD) in 14 (7.1%) patients.
3. Discussion
4. Limitations of the Study
5. Materials and Methods
- (1)
- diagnosis of C34 according to ICD 10;
- (2)
- number of fractions of radiotherapy—21, 30 or 33;
- (3)
- the period of using radiotherapy: 2010–2014.
- patient-related: gender, BMI/obesity, smoking, fitness status, weight loss within the 3 months prior to starting chemotherapy;
- cancer-related: stage IIIA vs. IIIB, feature N 3, feature T 4;
- comorbidities-related expressed by the Charlson Comorbidity Index (CCI) and the Simplified Charlson Comorbidity Index (SCCI).
- occurrence of adverse events grade 3 or higher according to CTCAE;
- deterioration of performance status confirmed by the Karnofsky Performance Score (KPS);
- response to treatment according to RECIST 1.1, assessed 6 weeks after the end of radiotherapy;
- type of the cancer disease progression (recurrence or distant metastases);
- progression-free survival (PFS)
6. Statistical Analysis
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Non-small Cell Lung Cancer Collaborative Group. Chemotherapy in non-small cell lung cancer: A meta-analysis using updated data on individual patients from 52 randomised clinical trials. BMJ 1995, 311, 899–909. [Google Scholar] [CrossRef]
- Auperin, A.; Le Pechoux, C.; Rolland, E.; Curran, W.J.; Furuse, K.; Fournel, P.; Belderbos, J.; Clamon, G.; Ulutin, H.C.; Paulus, R.; et al. Meta-analysis of concomitant versus sequential radiochemiotherapy in locally advanced non-small-cell lung cancer. J. Clin. Oncol. 2010, 28, 2181–2190. [Google Scholar] [CrossRef] [PubMed]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N. Engl. J. Med. 2018, 379, 2342–2350. [Google Scholar] [CrossRef]
- Gray, J.E.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; Cho, B.C.; et al. Three-Year Overall Survival with Durvalumab after chemoradiotherapy in stage III NSCLC- Update from PACIFIC. J. Thorac. Oncol. 2020, 15, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Pentheroudakis, G.; ESMO Guidelines Committee. Recent eUpdate to the ESMO Clinical Practice Guidelines on early and locally advanced non-small-cell lung cancer (NSCLC). Ann. Oncol. 2020, 31, 1265–1266. [Google Scholar] [CrossRef]
- Park, K.; Vansteenkiste, J.; Lee, K.H.; Pentheroudakis, G.; Zhou, C.; Prabhash, K.; Seto, T.; Voon, P.J.; Tan, D.S.W.; Yang, J.C.H.; et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with locally-advanced unresectable non-small-cell lung cancer: A KSMO-ESMO initiative endorsed by CSCO, ISMPO, JSMO, MOS, SSO and TOS. Ann. Oncol. 2020, 31, 191–201. [Google Scholar] [CrossRef]
- Cardenal, F.; Nadal, E.; Jové, M.; Faivre-Finn, C. Concurrent systemic therapy with radiotherapy for the treatment of poor-risk patients with unresectable stage III non-small-cell lung cancer: A review of the literature. Ann. Oncol. 2015, 26, 278–288. [Google Scholar] [CrossRef] [PubMed]
- De Ruysscher, D.; Botterweck, A.; Dirx, M.; Pijls-Johannesma, M.; Wanders, R.; Hochstenbag, M.; Dingemans, A.M.; Bootsma, G.; Geraedts, W.; Simons, J.; et al. Eligibility for concurrent chemotherapy and radiotherapy of locally advanced lung cancer patients: A prospective, population-based study. Ann. Oncol. 2009, 20, 98–102. [Google Scholar] [CrossRef]
- Atagi, S.; Kawahara, M.; Tamura, T.; Noda, K.; Watanabe, K.; Yokoyama, A.; Sugiura, T.; Senba, H.; Ishikura, S.; Ikeda, H.; et al. Standard thoracic radiotherapy with or without concurrent daily low-dose carboplatin in elderly patients with locally advanced non-small cell lung cancer: A Phase III trial of the Japan Clinical Oncology Group (JCOG9812). Jpn. J. Clin. Oncol. 2005, 35, 195–201. [Google Scholar] [CrossRef]
- Stinchcombe, T.E.; Zhang, Y.; Vokes, E.E.; Schiller, J.H.; Bradley, J.D.; Kelly, K.; Curran, W.J., Jr.; Schild, S.E.; Movsas, B.; Clamon, G.; et al. Pooled analysis of individual patient data on concurrent chemoradiotherapy for stage III non-small-cell lung cancer in elderly patients compared with younger patients who participated in US National Cancer Institute cooperative group studies. J. Clin. Oncol. 2017, 35, 2885–2892. [Google Scholar] [CrossRef]
- Miller, E.D.; Fisher, J.L.; Haglund, K.E.; Grecula, J.C.; Xu-Welliver, M.; Bertino, E.M.; He, K.; Shields, P.G.; Carbone, D.P.; Williams, T.M.; et al. The Addition of Chemotherapy to Radiation Therapy Improves Survival in Elderly Patients with Stage III Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2018, 13, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Laine, A.M.; Westover, K.D.; Choy, H. Radiation therapy as a backbone of treatment of locally advanced non-small cell lung cancer. Semin. Oncol. 2014, 41, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Maguire, J.; Khan, I.; McMenemin, R.; O’Rourke, N.; McNee, S.; Kelly, V.; Peedell, C.; Snee, M. SOCCAR: A randomised phase II trial comparing sequential versus concurrent chemotherapy and radical hypofractionated radiotherapy in patients with inoperable stage III Non-Small Cell Lung Cancer and good performance status. Eur. J. Cancer 2014, 50, 2939–2949. [Google Scholar] [CrossRef]
- Walraven, I.; Damhuis, R.A.; Ten Berge, M.G.; Rosskamp, M.; van Eycken, L.; de Ruysscher, D.; Belderbos, J.S.A. Treatment Variation of Sequential versus Concurrent Chemoradiotherapy in Stage III Non-Small Cell Lung Cancer Patients in the Netherlands and Belgium. Clin. Oncol. 2017, 29, e177–e185. [Google Scholar] [CrossRef]
- Firat, S.; Byhardt, R.W.; Gore, E. Comorbidity and Karnofksy performance score are independent prognostic factors in stage III non-small-cell lung cancer: An institutional analysis of patients treated on four RTOG studies. Radiation Therapy Oncology Group. Int. J. Radiat. Oncol. Biol. Phys. 2002, 54, 357–364. [Google Scholar] [CrossRef]
- Mohile, S.G.; Dale, W.; Somerfield, M.R.; Schonberg, M.A.; Boyd, C.M.; Burhenn, P.S.; Canin, B.; Cohen, H.J.; Holmes, H.M.; Hopkins, J.O.; et al. Practical Assessment and Management of Vulnerabilities in Older Patients Receiving Chemotherapy: ASCO Guideline for Geriatric Oncology. J. Clin. Oncol. 2018, 36, 2326–2347. [Google Scholar] [CrossRef] [PubMed]
- Locher, C.; Pourel, N.; Le Caer, H.; Berard, H.; Auliac, J.B.; Monnet, I.; Descourt, R.; Vergnenègre, A.; Lafay, I.M.; Greillier, L.; et al. Impact of a comprehensive geriatric assessment to manage elderly patients with locally advanced non-small-cell lung cancers: An open phase II study using concurrent cisplatin-oral vinorelbine and radiotherapy (GFPC 08-06). Lung Cancer 2018, 121, 25–29. [Google Scholar] [CrossRef]
- Antonio, M.; Saldaña, J.; Linares, J.; Ruffinelli, J.C.; Palmero, R.; Navarro, A.; Arnaiz, M.D.; Brao, I.; Aso, S.; Padrones, S.; et al. Geriatric assessment may help decision-making in elderly patients with inoperable, locally advanced non-small-cell lung cancer. Br. J. Cancer 2018, 118, 639–647. [Google Scholar] [CrossRef]
- Postmus, P.E.; Kerr, K.M.; Oudkerk, M.; Senan, S.; Waller, D.A.; Vansteenkiste, J.; Escriu, C.; Peters, S.; ESMO Guidelines Committee. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28 (Suppl. S4), iv1–iv21. [Google Scholar]
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a combined comorbidity index. J. Clin. Epidemiol. 1994, 47, 1245–1251. [Google Scholar] [CrossRef]
- Colinet, B.; Jacot, W.; Bertrand, D.; Lacombe, S.; Bozonnat, M.C.; Daurès, J.P.; Pujol, J.L.; oncoLR health network. A new simplified comorbidity score as a prognostic factor in non-small-cell lung cancer patients: Description and comparison with the Charlson’s index. Br. J. Cancer 2005, 93, 1098–1105. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Wu, H.G.; Kim, H.J.; Kim, D.W.; Lee, S.H.; Kim, T.M.; Kim, Y.W.; Heo, D.S. Influence of Comorbidities on the Efficacy of Radiotherapy with or without Chemotherapy in Elderly Stage III Non-small Cell Lung Cancer Patients. Cancer Res. Treat. 2012, 44, 242–250. [Google Scholar] [CrossRef]
- Blanco, R.; Maestu, I.; de la Torre, M.G.; Cassinello, A.; Nuñez, I. A review of the management of elderly patients with non-small-cell lung cancer. Ann. Oncol. 2015, 26, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Coate, L.E.; Massey, C.; Hope, A.; Sacher, A.; Barrett, K.; Pierre, A.; Leighl, N.; Brade, A.; de Perrot, M.; Waddell, T.; et al. Treatment of the elderly when cure is the goal: The influence of age on treatment selection and efficacy for stage III non-small cell lung cancer. J. Thorac. Oncol. 2011, 6, 537–544. [Google Scholar] [CrossRef]
- Eberhardt, W.E.; De Ruysscher, D.; Weder, W.; Le Péchoux, C.; De Leyn, P.; Hoffmann, H.; Westeel, V.; Stahel, R.; Felip, E.; Peters, S.; et al. 2nd ESMO Consensus Conference in Lung Cancer: Locally advanced stage III non-small-cell lung cancer. Ann. Oncol. 2015, 26, 1573–1588. [Google Scholar] [CrossRef]
- Hayakawa, K.; Mitsuhashi, N.; Katano, S.; Saito, Y.; Nakayama, Y.; Sakurai, H.; Akimoto, T.; Hasegawa, M.; Yamakawa, M.; Niibe, H. High-dose radiation therapy for elderly patients with inoperable or unresectable non-small cell lung cancer. Lung Cancer 2001, 32, 81–88. [Google Scholar] [CrossRef]
- Dillman, R.O.; Seagren, S.L.; Propert, K.J.; Guerra, J.; Eaton, W.L.; Perry, M.C.; Carey, R.W.; Frei, E.F., 3rd; Green, M.R. A randomized trial of induction chemotherapy plus high-dose radiation versus radiation alone in stage III non-small-cell lung cancer. N. Engl. J. Med. 1990, 323, 940–945. [Google Scholar] [CrossRef]
- Pallis, A.G.; Gridelli, C.; van Meerbeeck, J.P.; Greillier, L.; Wedding, U.; Lacombe, D.; Welch, J.; Belani, C.P.; Aapro, M. EORTC Elderly Task Force and Lung Cancer Group and International Society for Geriatric Oncology (SIOG) experts’ opinion for the treatment of non-small-cell lung cancer in an elderly population. Ann. Oncol. 2010, 21, 692–706. [Google Scholar] [CrossRef] [PubMed]
- Schild, S.E.; Stella, P.J.; Geyer, S.M.; Bonner, J.A.; McGinnis, W.L.; Mailliard, J.A.; Brindle, J.; Jatoi, A.; Jett, J.R.; North Central Cancer Treatment Group. The outcome of combined-modality therapy for stage III non-small-cell lung cancer in the elderly. J. Clin. Oncol. 2003, 21, 3201–3206. [Google Scholar] [CrossRef]
- Jalal, S.I.; Riggs, H.D.; Melnyk, A.; Richards, D.; Agarwala, A.; Neubauer, M.; Ansari, R.; Govindan, R.; Bruetman, D.; Fisher, W.; et al. Updated survival and outcomes for older adults with inoperable stage III non-small-cell lung cancer treated with cisplatin, etoposide, and concurrent chest radiation with or without consolidation docetaxel: Analysis of a phase III trial from the Hoosier Oncology Group (HOG) and US Oncology. Ann. Oncol. 2012, 23, 1730–1738. [Google Scholar] [PubMed]
- Semrau, S.; Bier, A.; Thierbach, U.; Virchow, C.; Ketterer, P.; Klautke, G.; Fietkau, R. 6-year experience of concurrent radiochemotherapy with vinorelbine plus a platinum compound in multimorbid or aged patients with inoperable non-small cell lung cancer. Strahlenther. Onkol. 2007, 183, 30–35. [Google Scholar] [CrossRef]
- Atagi, S.; Kawahara, M.; Yokoyama, A.; Okamoto, H.; Yamamoto, N.; Ohe, Y.; Sawa, T.; Ishikura, S.; Shibata, T.; Fukuda, H.; et al. Thoracic radiotherapy with or without daily low-dose carboplatin in elderly patients with non-small-cell lung cancer: A randomised, controlled, phase 3 trial by the Japan Clinical Oncology Group (JCOG0301). Lancet Oncol. 2012, 13, 671–678. [Google Scholar] [CrossRef]
- Movsas, B.; Scott, C.; Sause, W.; Byhardt, R.; Komaki, R.; Cox, J.; Johnson, D.; Lawton, C.; Dar, A.R.; Wasserman, T.; et al. The benefit of treatment intensification is age and histology-dependent in patients with locally advanced non-small cell lung cancer (NSCLC): A quality-adjusted survival analysis of radiation therapy oncology group (RTOG) chemoradiation studies. Int. J. Radiat. Oncol. Biol. Phys. 1999, 45, 1143–1149. [Google Scholar] [CrossRef]
- Gaspar, L.E.; Chansky, K.; Albain, K.S.; Vallieres, E.; Rusch, V.; Crowley, J.J.; Livingston, R.B.; Gandara, D.R. Time from treatment to subsequent diagnosis of brain metastases in stage III non-small-cell lung cancer: A retrospective review by the Southwest Oncology Group. J. Clin. Oncol. 2005, 23, 2955–2961. [Google Scholar] [CrossRef] [PubMed]
- De Leyn, P.; Vansteenkiste, J.; Lievens, Y.; Van Raemdonck, D.; Nafteux, P.; Decker, G.; Coosemans, W.; Decaluwé, H.; Moons, J.; Lerut, T. Survival after trimodality treatment for superior sulcus and central T4 non-small cell lung cancer. J. Thorac. Oncol. 2009, 4, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Goldstraw, P.; Crowley, J.; Chansky, K.; Giroux, D.J.; Groome, P.A.; Rami-Porta, R.; Postmus, P.E.; Rusch, V.; Sobin, L.; International Association for the Study of Lung Cancer International Staging Committee; et al. The IASLC Lung Cancer Staging Project: Proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J. Thorac. Oncol. 2007, 2, 706–714. [Google Scholar] [CrossRef]
- Brunelli, A.; Charloux, A.; Bolliger, C.T.; Rocco, G.; Sculier, J.P.; Varela, G.; Licker, M.; Ferguson, M.K.; Faivre-Finn, C.; Huber, R.M.; et al. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy). Eur. Respir. J. 2009, 34, 17–41. [Google Scholar] [CrossRef] [PubMed]
- Stinchcombe, T.E.; Bogart, J.A. Novel approaches of chemoradiotherapy in unresectable stage IIIA and stage IIIB non-small cell lung cancer. Oncologist 2012, 17, 682–693. [Google Scholar] [CrossRef][Green Version]
- Zaborowska-Szmit, M.; Krzakowski, M.; Kowalski, D.M.; Szmit, S. Cardiovascular Complications of Systemic Therapy in Non-Small-Cell Lung Cancer. J. Clin. Med. 2020, 9, 1268. [Google Scholar] [CrossRef]
- Łazar-Poniatowska, M.; Bandura, A.; Dziadziuszko, R.; Jassem, J. Concurrent chemoradiotherapy for stage III non-small-cell lung cancer: Recent progress and future perspectives (a narrative review). Transl. Lung Cancer Res. 2021, 10, 2018–2031. [Google Scholar] [CrossRef] [PubMed]
- Bezjak, A.; Temin, S.; Franklin, G.; Giaccone, G.; Govindan, R.; Johnson, M.L.; Rimner, A.; Schneider, B.J.; Strawn, J.; Azzoli, C.G. Definitive and Adjuvant Radiotherapy in Locally Advanced Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Endorsement of the American Society for Radiation Oncology Evidence-Based Clinical Practice Guideline. J. Clin. Oncol. 2015, 33, 2100–2105. [Google Scholar] [CrossRef] [PubMed]
- Faivre-Finn, C.; Vicente, D.; Kurata, T.; Planchard, D.; Paz-Ares, L.; Vansteenkiste, J.F.; Spigel, D.R.; Garassino, M.C.; Reck, M.; Senan, S.; et al. Four-Year Survival with Durvalumab After Chemoradiotherapy in Stage III NSCLC-an Update From the PACIFIC Trial. J. Thorac. Oncol. 2021, 16, 860–867. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Number (%) |
|---|---|
| Sex | Female—69 (35.2) |
| Male—127 (64.8) | |
| Age | |
| ≤65 | 144 (73.5) |
| >65 | 52 (26.5) |
| BMI (kg/m2) <25 kg/m2 | 77 (39.3) |
| Overweight (BMI: ≥ 25 and <30 kg/m2) | 68 (34.7) |
| Obesity (BMI ≥ 30kg/m2) | 51 (26) |
| Lack of weight loss | 107 (54.6) |
| Weight loss <10% | 65 (33. 2) |
| Weight loss ≥10% | 24 (12. 2) |
| Pathology | |
| Squamous-cell carcinoma | 91 (46.4) |
| Adenocarcinoma | 47 (24.0) |
| Other types | 58 (29.6) |
| Clinical Stage | III A–94 (48) |
| III B–102 (52) | |
| TNM | |
| TX | 2 (1) |
| T0 | 2 (1) |
| T1 | 14 (7.1) |
| T2 | 70 (35.7) |
| T3 | 44 (22.4) |
| T4 | 64 (32.7) |
| N0 | 13 (6.6) |
| N1 | 9 (4.6) |
| N2 | 154 (78.6) |
| N3 | 20 (10. 2) |
| Declared smoking status | |
| never smokers | 16 (8.16) |
| <20 pack years | 41 (20.92) |
| 20–50 pack years | 94 (47.96) |
| ≥50 pack years | 45 (22.96) |
| Karnofsky performance status (KPS) | |
| 100 | 67 (34.2) |
| 90 | 101 (51.5) |
| 80 | 28 (14.3) |
| Charlson Comorbidity Index (CCI) | |
| ≤3 | 56 (28.6) |
| 4 | 55 (28.1) |
| 5 | 44 (22.4) |
| ≥6 | 41 (20.9) |
| Simplified Charlson Comorbidity Index (SCCI) | |
| ≥7 | 72 (36.7) |
| 8 | 65 (33.2) |
| ≥9 | 59 (30.1) |
| Type of Cancer Disease Progression | Number (%) |
|---|---|
| Local recurrence | 87 (44.4%) |
| Distant metastases | 81 (41.3%) |
| nodal metastases (N feature) | 23 (11.7%) |
| pleural effusion | 12 (6.1%) |
| metastases to the second lung | 21 (10.7%) |
| metastases to the central nervous system | 32 (16.3%) |
| bone metastases | 11 (5.6%) |
| liver metastases | 10 (5.1%) |
| adrenal metastases | 6 (3.1%) |
| Patients with a Possible Predictor Compared to Patients without Such Feature | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Women | 0.78 | 0.56–1.08 | 0.13 | 0.79 | 0.56–1.11 | 0.18 |
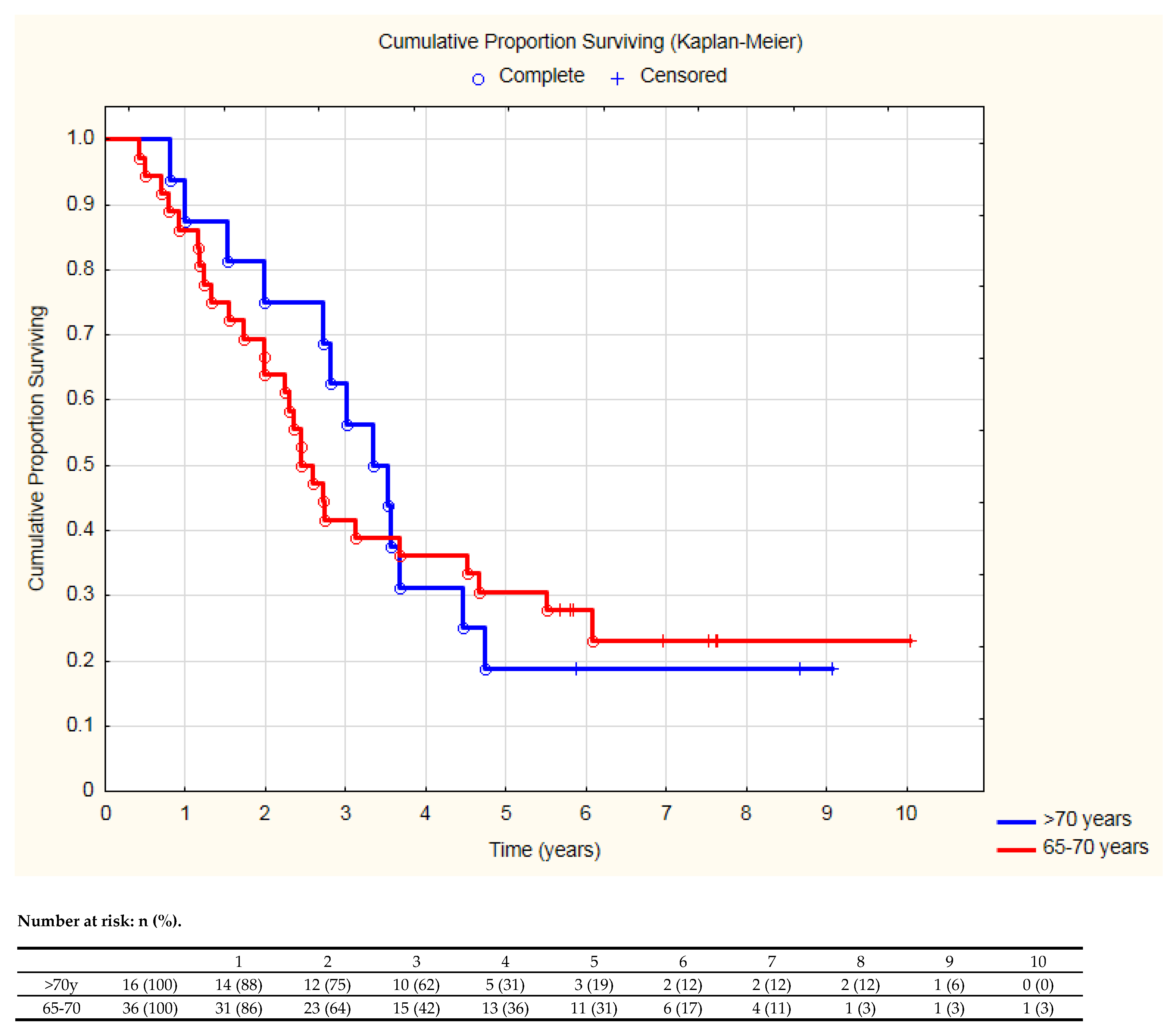
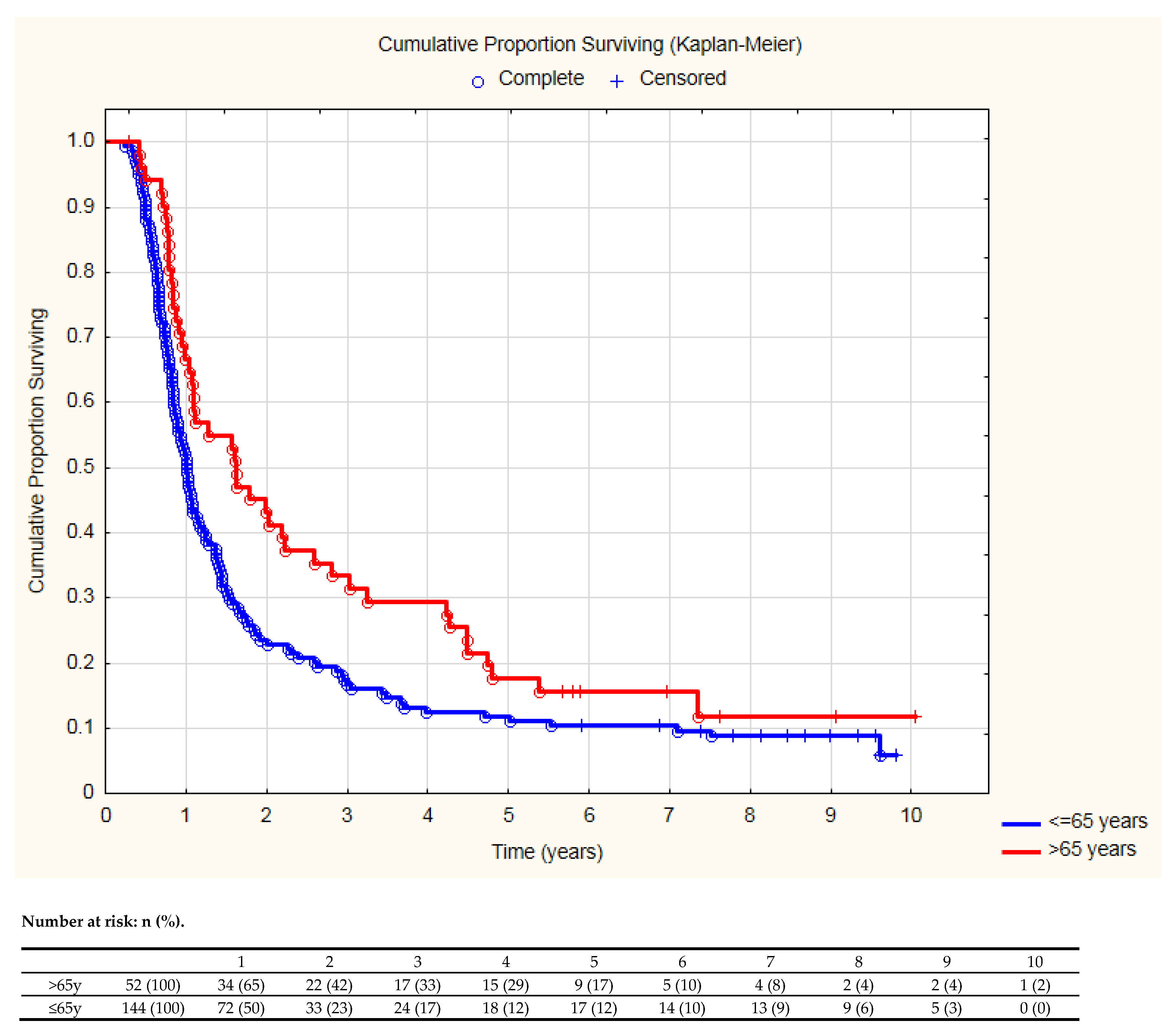
| Age > 65 years | 0.66 | 0.46–0.94 | 0.02 | 0.54 | 0.36–0.83 | <0.01 |
| Obesity (BMI ≥ 30kg/m2) | 0.92 | 0.65–1.31 | 0.64 | 0.95 | 0.65–1.4 | 0.8 |
| Squamous–cell carcinoma | 0.99 | 0.73–1.34 | 0.94 | 0.95 | 0.64–1.40 | 0.78 |
| Adenocarcinoma | 0.81 | 0.56–1.16 | 0. 24 | 0.84 | 0.54–1.32 | 0.45 |
| Stage IIIA vs. IIIB | 0.68 | 0.50–0.92 | 0.01 | 0.7 | 0.47–1.06 | 0.09 |
| T4 | 1.12 | 0.81–1.54 | 0.5 | 0.96 | 0.63–1.46 | 0.85 |
| N3 | 1.10 | 0.68–1.80 | 0.69 | 1.02 | 0.57–1.85 | 0.94 |
| Smoking ≥50 pack years | 0.90 | 0.62–1.30 | 0.56 | 0.85 | 0.58–1. 25 | 0.41 |
| Weight loss ≥10% | 1.61 | 1.03–2.53 | 0.04 | 1.50 | 0.91–2.48 | 0.11 |
| Performance status KPS = 100 | 0.96 | 0.69–1.32 | 0.79 | 0.89 | 0.62–1. 26 | 0.5 |
| Charlson Comorbidity Index (CCI) >4 | 1.12 | 0.83–1.52 | 0.46 | 1.45 | 0.97–2.15 | 0.07 |
| Simplified Charlson Comorbidity Index (SCCI) >8 | 1.0 | 0.72–1.39 | 0.99 | 0.9 | 0.62–1.32 | 0.59 |
| ≤65 Years (144 Patients) | >65 Years (52 Patients) | p-Value | ||
|---|---|---|---|---|
| Demographic | Female | 52 (36.1%) | 17 (32.7%) | 0.7 |
| Obesity (BMI ≥30 kg/m2) | 32 (22.2%) | 19 (36.5%) | 0.04 | |
| Pathology | Squamous–cell carcinoma | 64 (44.4%) | 27 (51.9%) | 0.35 |
| Adenocarcinoma | 35 (24.3%) | 12 (23.1%) | 0.86 | |
| Other types | 45 (31.3%) | 13 (25%) | 0.4 | |
| Primary tumor and mediastinal nodes stage | Clinical stage IIIA | 67 (46.5%) | 27 (51.9%) | 0.5 |
| T4 | 43 (29.9%) | 21 (40.4%) | 0.17 | |
| N3 | 14 (9.7%) | 6 (11.5%) | 0.7 | |
| Smoking | ≥20 pack years | 104 (72.2%) | 35 (67.3%) | 0.5 |
| ≥50 pack years | 33 (22.9%) | 12 (23.1%) | 0.98 | |
| Weight loss | Not observed | 79 (54.9%) | 28 (53.9%) | 0.9 |
| ≥10% | 20 (13.9%) | 4 (7.7%) | 0.36 | |
| Performance status KPS = 100 | 59 (40.1%) | 8 (15.4%) | <0.01 | |
| Comorbidities | Charlson Comorbidity Index (CCI) >4 | 42 (29.2%) | 43 (82.7%) | <0.01 |
| Simplified Charlson Comorbidity Index (SCCI) >8 | 37 (25.7%) | 22 (42.3%) | 0.03 | |
| ≤65 Years (144 Patients) | >65 Years (52 Patients) | p-Value | ||
|---|---|---|---|---|
| Chemotherapy | Cisplatin + vinorelbine | 121 (84%) | 37 (71. 2%) | 0.04 |
| Regimen without cisplatin | 9 (6.3%) | 12 (23.1%) | <0.01 | |
| Longer time between the end of chemotherapy and start of radiotherapy (defined as > 42 days/6 weeks) | 31 (21.5%) | 14 (26.9%) | 0.4 | |
| Deterioration of performance status at least by 10 points (KPS) | 45 (31.3%) | 8 (15.4%) | 0.03 | |
| Occurrence of complications grade 3/4 according to CTCAE | 45 (31.3%) | 22 (42.3%) | 0.15 | |
| Response according to RECIST evaluated 6 weeks after the end of radiotherapy | CR | 7 (4.9%) | 8 (15.4%) | 0.01 |
| PR | 95 (66%) | 28 (53.9%) | 0.12 | |
| SD | 30 (20.8%) | 14 (26.9%) | 0.37 | |
| PD | 12 (8.3%) | 2 (3.9%) | 0.45 | |
| Type of cancer disease progression | Local progression | 59 (40.1%) | 28 (53.9%) | 0.11 |
| Distant metastases | 66 (45.8%) | 15 (28.9%) | 0.03 | |
| Secondary cancer disease | 7 (4.9%) | 2 (3.9%) | 0.93 | |
| Subsequent Systemic Therapies | ≤65 Years (144 Patients) | >65 Years (52 Patients) | p-Value |
|---|---|---|---|
| Chemotherapy | 69 (47.9%) | 24 (46. 2%) | 0.83 |
| Number of lines of chemotherapy | |||
| 1. | 43 (29.9%) | 20 (38.5%) | 0.31 |
| 2. | 17 (11.8%) | 4 (7.7%) | |
| 3. | 7 (4.9%) | 0 | |
| 4. | 2 (1.4%) | 0 | |
| Immunotherapy | 4 (2.8%) | 2 (3.8%) | 0.93 |
| Targeted therapy | 2 (1.4%) | 1 (1.9%) | |
| Anti–EGFR | 1 | 1 | |
| Anti–ALK | 1 | 0 | 0.7 |
| First Author of the Study | Design of the Study | Main Result or Conclusion |
|---|---|---|
| Atagi S. [9] | Patients: 71 years of age or older. Randomization: radiotherapy alone vs. chemoradiotherapy (concurrent use of carboplatin) | Terminated due to treatment-related deaths. |
| Stinchcombe T.E. [10] | 16 phase II or III trials of concurrent chemoradiotherapy | Elderly patients under concurrent chemoradiotherapy had unbeneficial OS, higher rate of toxicity (including death). |
| Miller E.D. [11] | Patients: elderly (≥70 years old). Comparative effectiveness study of radiation therapy versus chemoradiation | Sequential chemotherapy and radiation resulted in a 9% mortality reduction in comparison to concurrent treatment. |
| Lee J.H. [22] | Patients: aged 70 years or more. Treatment: radical radiotherapy with or without chemotherapy | Simplified comorbidity score (SCS) was the independent prognostic factor for OS. Chemoradiotherapy was superior to radiotherapy in the fit elderly with SCS < 10. |
| Atagi S. [32] | Patients older than 70 years. Randomized, controlled, phase 3 trial: chemoradiotherapy (concurrent low–dose carboplatin) or radiotherapy alone, | Some elderly should be considered for chemoradiotherapy due to benefit of decreased mortality (HR = 0.68, p = 0.0179). Chemoradiotherapy was associated with more rate of grade 3–4 hematological toxicity. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaborowska-Szmit, M.; Olszyna-Serementa, M.; Kowalski, D.M.; Szmit, S.; Krzakowski, M. Elderly Patients with Locally Advanced and Unresectable Non-Small-Cell Lung Cancer May Benefit from Sequential Chemoradiotherapy. Cancers 2021, 13, 4534. https://doi.org/10.3390/cancers13184534
Zaborowska-Szmit M, Olszyna-Serementa M, Kowalski DM, Szmit S, Krzakowski M. Elderly Patients with Locally Advanced and Unresectable Non-Small-Cell Lung Cancer May Benefit from Sequential Chemoradiotherapy. Cancers. 2021; 13(18):4534. https://doi.org/10.3390/cancers13184534
Chicago/Turabian StyleZaborowska-Szmit, Magdalena, Marta Olszyna-Serementa, Dariusz M. Kowalski, Sebastian Szmit, and Maciej Krzakowski. 2021. "Elderly Patients with Locally Advanced and Unresectable Non-Small-Cell Lung Cancer May Benefit from Sequential Chemoradiotherapy" Cancers 13, no. 18: 4534. https://doi.org/10.3390/cancers13184534
APA StyleZaborowska-Szmit, M., Olszyna-Serementa, M., Kowalski, D. M., Szmit, S., & Krzakowski, M. (2021). Elderly Patients with Locally Advanced and Unresectable Non-Small-Cell Lung Cancer May Benefit from Sequential Chemoradiotherapy. Cancers, 13(18), 4534. https://doi.org/10.3390/cancers13184534






