Efficacy of Endoscopic Ultrasound-Guided Ablation with the HybridTherm Probe in Locally Advanced or Borderline Resectable Pancreatic Cancer: A Phase II Randomized Controlled Trial
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Design
2.2. Study Methods
2.3. Study Outcomes
2.4. Sample Size Calculation and Statistical Analysis
3. Results
3.1. Patients and Therapeutic Features
3.2. Six-Months Progression-Free Survival and Radiological Response to Therapy
3.3. Biological Response to Therapy
3.4. Tumour Volume Changes
3.5. Surgical Outcomes
3.6. Adverse Events
3.7. Progression-Free and Overall Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology; Pancreatic Adenocarcinoma; Version 2; National Comprehensive Cancer Network: Fort Washington, PA, USA, 2016. [Google Scholar]
- Tempero, M.A.; Malafa, M.P.; Chiorean, E.G.; Czito, B.; Scaife, C.; Narang, A.K.; Fountzilas, C.; Wolpin, B.M.; Al-Hawary, M.; Asbun, H.; et al. Pancreatic Adenocarcinoma, Version 1.2019. J. Natl. Compr. Cancer Netw. 2019, 17, 202–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muranaka, T.; Kuwatani, M.; Komatsu, Y.; Sawada, K.; Nakatsumi, H.; Kawamoto, Y.; Yuki, S.; Kubota, Y.; Kubo, K.; Kawahata, S.; et al. Comparison of efficacy and toxicity of FOLFIRINOX and gemcitabine with nab-paclitaxel in unresectable pancreatic cancer. J. Gastrointest. Oncol. 2017, 8, 566–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammel, P.; Huguet, F.; van Laethem, J.L.; Goldstein, D.; Glimelius, B.; Artru, P.; Borbath, I.; Bouché, O.; Shannon, J.; André, T.; et al. Effect of Chemoradiotherapy vs Chemotherapy on Survival in Patients with Locally Advanced Pancreatic Cancer Controlled After 4 Months of Gemcitabine with or without Erlotinib: The LAP07 Randomized Clinical Trial. JAMA 2016, 315, 1844–1853. [Google Scholar] [CrossRef] [PubMed]
- Assifi, M.M.; Lu, X.; Eibl, G.; Reber, H.A.; Li, G.; Hines, O.J. Neoadjuvant therapy in pancreatic adenocarcinoma: A meta-analysis of phase II trials. Surgery 2011, 150, 466–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larghi, A.; Rimbas, M.; Rizzatti, G.; Carbone, C.; Gasbarrini, A.; Costamagna, G.; Alfieri, S.; Tortora, G. Endoscopic ultrasound-guided therapies for pancreatic solid tumors: An overview. Semin. Oncol. 2021, 7, 1–11. [Google Scholar]
- Ware, M.J.; Curtis, L.T.; Wu, M.; Ho, J.C.; Corr, S.J.; Curley, S.A.; Godin, B.; Frieboes, H.B. Pancreatic adenocarcinoma response to chemotherapy enhanced with non-invasive radiofrequency evaluated via an integrated experimental/computational approach. Sci. Rep. 2017, 7, 3437. [Google Scholar] [CrossRef]
- Testoni, S.G.G.; Healey, A.J.; Dietrich, C.F.; Arcidiacono, P.G. Systematic review of endoscopy ultrasound-guided thermal ablation treatment for pancreatic cancer. Endosc. Ultrasound. 2020, 9, 83–100. [Google Scholar] [PubMed]
- Arcidiacono, P.G.; Carrara, S.; Reni, M.; Cappio, S.; Balzano, G.; Boemo, C.; Cereda, S.; Nicoletti, R.; Enderle, M.D.; Neugebauer, A.; et al. Feasibility and safety of EUS-guided cryothermal ablation in patients with locally advanced pancreatic cancer. Gastrointest. Endosc. 2012, 76, 1142–1151. [Google Scholar] [CrossRef]
- Testoni, S.G.G.; Capurso, G.; Petrone, M.C.; Barbera, M.; Linzenbold, W.; Enderle, M.; Gusmini, S.; Nicoletti, R.; Della Torre, E.; Mariani, A.; et al. Necrosis volume and Choi criteria predict the response to endoscopic ultrasonography-guided HybridTherm ablation of locally advanced pancreatic cancer. Endosc. Int. Open 2020, 8, E1511–E1519. [Google Scholar] [CrossRef]
- Cong, L.; Liu, Q.; Zhang, R.; Cui, M.; Zhang, X.; Gao, X.; Guo, J.; Dai, M.; Zhang, T.; Liao, Q.; et al. Tumor Size Classification of the 8 th Edition of TNM Staging System Is Superior to That of the 7 th Edition in Predicting the Survival Outcome of Pancreatic Cancer Patients After Radical Resection and Adjuvant Chemotherapy. Sci. Rep. 2018, 8, 10383. [Google Scholar] [CrossRef]
- Cotton, P.B.; Eisen, G.M.; Aabakken, L.; Baron, T.H.; Hutter, M.H.; Jacobson, B.C.; Mergener, K.; Nemcek, A., Jr.; Petersen, B.T.; Petrini, J.L.; et al. A lexicon for endoscopic adverse events: Report of an ASGE workshop. Gastrointest. Endosc. 2010, 71, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Reni, M.; Cereda, S.; Balzano, G.; Passoni, P.; Rognone, A.; Fugazza, C.; Mazza, E.; Zerbi, A.; Di Carlo, V.; Villa, E. Carbohydrate Antigen 19-9 change during chemotherapy for advanced pancreatic adenocarcinoma. Cancer 2009, 115, 2630–2639. [Google Scholar] [CrossRef] [PubMed]
- Ronot, M.; Bouattour, M.; Wassermann, J.; Bruno, O.; Dreyer, C.; Larroque, B.; Castera, L.; Vilgrain, V.; Belghiti, J.; Raymond, E.; et al. Alternative Response Criteria (Choi, European association for the study of the liver, and modified Response Evaluation Criteria in Solid Tumors [RECIST]) Versus RECIST 1.1 in patients with advanced hepatocellular carcinoma treated with sorafenib. Oncologist 2014, 19, 394–402. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.; Charnsangavej, C.; Faria, S.C.; Macapinlac, H.A.; Burgess, M.A.; Patel, S.R.; Chen, L.L.; Podoloff, D.A.; Benjamin, R.S. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: Proposal of new computed tomography response criteria. J. Clin. Oncol. 2007, 25, 1753–1759. [Google Scholar] [CrossRef]
- Ebner, L.; Roos, J.E.; Christensen, J.D.; Dobrocky, T.; Leidolt, L.; Brela, B.; Obmann, V.C.; Joy, S.; Huber, A.; Christe, A. Maximum-Intensity-Projection and Computer-Aided-Detection Algorithms as Stand-Alone Reader Devices in Lung Cancer Screening Using Different Dose Levels and Reconstruction Kernels. AJR Am. J. Roentgenol. 2016, 207, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Tan, Y.; Li, W.; Gong, J.; Zhou, Z.; Huang, Y.; Zheng, J.; Deng, Y.; Wang, L.; Peng, J.; et al. Tumor volume reduction rate is superior to RECIST for predicting the pathological response of rectal cancer treated with neoadjuvant chemoradiation: Results from a prospective study. Oncol. Lett. 2015, 9, 2680–2686. [Google Scholar] [CrossRef]
- Nougaret, S.; Rouanet, P.; Molinari, N.; Pierredon, M.A.; Bibeau, F.; Azria, D.; Lemanski, C.; Assenat, E.; Duffour, J.; Ychou, M.; et al. MR Volumetric measurement of low rectal cancer helps predict tumor response and outcome after combined chemotherapy and radiation therapy. Radiology 2012, 263, 409–418. [Google Scholar] [CrossRef]
- Loehrer, P.J.; Feng, Y.; Cardenes, H.; Wagner, L.; Brell, J.M.; Cella, D.; Flynn, P.; Ramanathan, R.K.; Crane, C.H.; Alberts, S.R.; et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: An Eastern Cooperative Oncology Group trial. J. Clin. Oncol. 2011, 29, 4105–4112. [Google Scholar] [CrossRef]
- Chauffert, B.; Mornex, F.; Bonnetain, F.; Rougier, P.; Mariette, C.; Bouché, O.; Bosset, J.F.; Aparicio, T.; Mineur, L.; Azzedine, A.; et al. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000-01 FFCD/SFRO study. Ann. Oncol. 2008, 19, 1592–1599. [Google Scholar]
- Brookmeyer, R.; Crowley, J.A. A confidence interval for the median survival time. Biometrics 1982, 38, 29–41. [Google Scholar] [CrossRef]
- Altman, D.G. Diagnostic tests. In Statistics with Confidence, 2nd ed.; Altman, D.G., Machin, D., Bryant, T.N., Gardner, M.J., Eds.; BMJ Books: London, UK, 2000; pp. 105–119. [Google Scholar]
- Hartman, D.J.; Krasinskas, A.M. Assessing treatment effect in pancreatic cancer. Arch. Pathol. Lab. Med. 2012, 136, 100–109. [Google Scholar] [CrossRef] [Green Version]
- NCI. CTCAE. Available online: http://evs.nci.nih.gov/ftp1/CTCAE/About.html (accessed on 1 December 2014).
- Saccomandi, P.; Lapergola, A.; Longo, F.; Schena, E.; Quero, G. Thermal ablation of pancreatic cancer: A systematic review of clinical practice and pre-clinical studies. Int. J. Hyperth. 2018, 35, 398–418. [Google Scholar] [CrossRef]
- Reni, M.; Zanon, S.; Peretti, U.; Chiaravalli, M.; Barone, D.; Pircher, C.; Balzano, G.; Macchini, M.; Romi, S.; Gritti, E.; et al. Nab-paclitaxel plus gemcitabine with or without capecitabine and cisplatin in metastatic pancreatic adenocarcinoma (PACT-19): A randomized phase 2 trial. Lancet Gastroenterol. Hepatol. 2018, 3, 691–697. [Google Scholar] [CrossRef]
- Yoo, C.; Hwang, I.; Song, T.J.; Lee, S.S.; Jeong, J.H.; Park, D.H.; Seo, D.W.; Lee, S.K.; Kim, M.H.; Byun, J.H.; et al. FOLFIRINOX in borderline resectable and locally advanced unresectable pancreatic adenocarcinoma. Ther. Adv. Med. Oncol. 2020, 12, 1758835920953294. [Google Scholar] [CrossRef] [PubMed]
- Song, T.J.; Seo, D.W.; Lakhtakia, S.; Reddy, N.; Oh, D.W.; Park, D.H.; Lee, S.S.; Lee, S.K.; Kim, M.H. Initial experience of EUS-guided radiofrequency ablation of unresectable pancreatic cancer. Gastrointest. Endosc. 2016, 83, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Crinò, S.F.; D’Onofrio, M.; Bernardoni, L.; Frulloni, L.; Iannelli, M.; Malleo, G.; Paiella, S.; Larghi, A.; Gabbrielli, A. EUS-guided Radiofrequency Ablation (EUS-RFA) of Solid Pancreatic Neoplasm Using an 18-gauge Needle Electrode: Feasibility, Safety, and Technical Success. J. Gastrointestin Liver Dis. 2018, 27, 67–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scopelliti, F.; Pea, A.; Conigliaro, R.; Butturini, G.; Frigerio, I.; Regi, P.; Giardino, A.; Bertani, H.; Paini, M.; Pederzoli, P.; et al. Technique, safety, and feasibility of EUS-guided radiofrequency ablation in unresectable pancreatic cancer. Surg. Endosc. 2018, 32, 4022–4028. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Y.; Zhao, Y.; Wu, X.; Zhang, M.; Hou, W.; Chen, Q.; Cheng, B. Endoscopic ultrasound-guided radiofrequency ablation of unresectable pancreatic cancer with low ablation power and multiple applications: A preliminary study of 11 patients. Ann. Palliat. Med. 2021, 10, 1842–1850. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Lou, Q.; Yang, J.; Zhang, X. Feasibility and safety of EUS-guided radiofrequency ablation in treatment of locally advanced, unresectable pancreatic cancer. Endosc. Ultrasound. 2021. online ahead of print. [Google Scholar]
- Chu, K.F.; Dupuy, D.E. Thermal ablation of tumours: Biological mechanisms and advances in therapy. Nat. Rev. Cancer 2014, 14, 199–208. [Google Scholar] [CrossRef]
- Ansari, D.; Bauden, M.; Bergström, S.; Rylance, R.; Marko-Varga, G.; Andersson, R. Relationship between tumour size and outcome in pancreatic ductal adenocarcinoma. Br. J. Surg. 2017, 104, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Paiella, S.; Malleo, G.; Cataldo, I.; Gasparini, C.; De Pastena, M.; De Marchi, G.; Marchegiani, G.; Rusev, B.; Scarpa, A.; Girelli, R.; et al. Radiofrequency ablation for locally advanced pancreatic cancer: SMAD4 analysis segregates a responsive subgroup of patients. Langenbecks Arch. Surg. 2018, 403, 213–220. [Google Scholar] [CrossRef]
- Rombouts, S.J.E.; Vogel, J.A.; Van Santvoort, H.C.; van Lienden, K.P.; van Hillegersberg, R.; Busch, O.R.C.; Besselink, M.G.H.; Molenaar, I.Q. Systematic review of innovative ablative therapies for the treatment of locally advanced pancreatic cancer. Br. J. Surg. 2015, 102, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Maiettini, D.; Mauri, G.; Varano, G.; Bonomo, G.; Della Vigna, P.; Rebonato, A.; Orsi, F. Pancreatic ablation: Minimally invasive treatment options. Int. J. Hyperth. 2019, 36, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Takaki, H.; Cornelis, F.; Kako, Y.; Kobayashi, K.; Kamikonya, N.; Yamakado, K. Thermal ablation and immunomodulation: From preclinical experiments to clinical trials. Diagn. Interv. Imaging 2017, 98, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Chen, J.; He, L.; Liao, M.; Yuan, Y.; Zeng, J.; Li, J.; Zuo, J.; Xu, K. Combination treatment with comprehensive cryoablation and immunotherapy in metastatic pancreatic cancer. Pancreas 2013, 42, 1143–1149. [Google Scholar] [CrossRef]

| Variables | HTP-CT Arm | CT Arm | p-Value |
|---|---|---|---|
| Patients enrolled, N. | 17 | 20 | |
| Sex, males/females, N. (%) | 9 (52.9)/8 (47.1) | 9 (45)/11 (55) | 0.74 |
| Age (years), mean ± StDe | 63.5 ± 11.4 | 65.1 ± 5.03 | 0.58 |
| Tumour site, head/body-tail, N. (%) | 10 (58.8)/7(41.2) | 12 (60)/8 (40) | 1.00 |
| Tumour size (mm) at MDCT scan, mean (± StDe) | |||
| short axis | 33.3 ± 9 | 29.8 ± 10.7 | 0.51 |
| long axis | 47.1 ± 11.6 | 43 ± 16.2 | 0.30 |
| Tumour volume (cc) at MDCT scan, median (IQR) | 32.1 (22.8–47.5) | 20.2 (11–28.4) | 0.23 |
| Tumour staging, N. (%) | |||
| borderline resectable | 5 (29.4) | 5 (25) | 1.00 |
| locally advanced | 12 (70.6) | 15 (75) | |
| 7th TNM classification, N. (%) [11] | |||
| T3 | 6 (35.3) | 9 (45) | 0.55 |
| T4 | 7 (41.2) | 6 (30) | 0.48 |
| 8th TNM classification, N. (%) [11] | |||
| T2 | 1 (5.9) | 1 (5) | 0.91 |
| T3 | 0 | 2 (10) | 0.19 |
| T4 | 3 (17.6) | 2 (10) | 0.51 |
| CA19.9 serum levels (U/mL), median (IQR) | 733.2 (54.4–4049.9) | 835 (75.2–5411.2) | 0.61 |
| >upper laboratory normal limit, N. (%) | 16 (94.1) | 14 (70) | |
| Metal biliary stent, N. (%) | 8 (47.05) | 10 (50) | 0.88 |
| Vessel invasion in locally advanced, N. (%) * | |||
| superior mesenteric/portal vein | 10 (83.3) | 13 (86.7) | |
| hepatic artery | 2 (16.7) | 3 (20) | |
| superior mesenteric artery | 7 (58.3) | 5 (33.3) | |
| celiac axis | 2 (16.7) | 3 (20) | |
| splenic vein | 3 (25) | 5 (33.3) | |
| splenic artery | 5 (41.7) | 4 (26.7) | |
| gastroduodenal artery | 0 | 3 (20) | |
| Vessel invasion in borderline resectable, N. (%) * | |||
| superior mesenteric/portal vein | 4 (80) | 5 (100) | |
| splenic vein | 1 (20) | 0 | |
| splenic artery | 1 (20) | 0 |
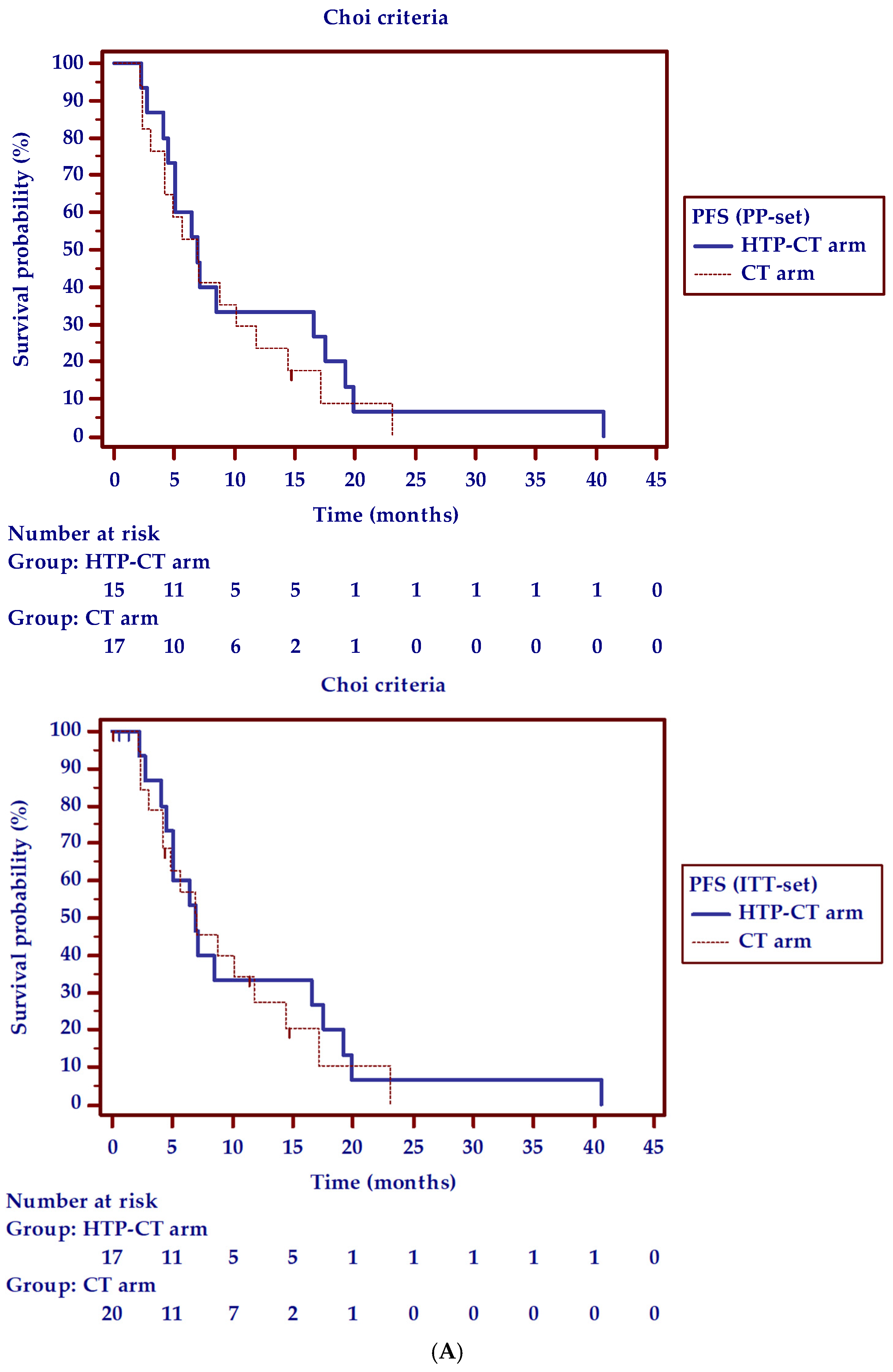
| 6-Months Progression-Free Survival and Radiological Response to Therapy | HTP-CT Arm | CT Arm | p-Value | |||
|---|---|---|---|---|---|---|
| PP-Set | ITT-Set | PP-Set | ITT-Set | PP-Set | ITT-Set | |
| Radiological disease control rate (Choi criteria) | ||||||
| 2 months, % (N.) | 80 (12/15) | 71 (12/17) | 72.2 (13/18 *) | 65 (13/20) | 0.61 | 0.70 |
| N. 8 PR, N. 4 SD | N. 7 PR, N. 6 SD | |||||
| 4 months, % (N.) | 66.7 (8/12) | 47.1 (8/17) | 69.2 (9/13 §) | 45 (9/20) | 0.90 | 0.90 |
| N. 3 PR, N. 5 SD | N. 5 PR, N. 4 SD | |||||
| 6 months, % (N.) | 87.5 (7/8) | 41.2 (7/17) | 66.7 (6/9) | 30 (6/20) | 0.33 | 0.48 |
| N. 4 PR, N. 3 SD | N. 3 PR, N. 3 SD | |||||
| Overall response to therapy, % (N.) | 80 (12/15) | 71 (12/17) | 72.2 (13/18 *) | 65 (13/20) | 0.88 | 0.95 |
| N. 9 PR, N. 3 SD | N. 11 PR, N. 3 SD | |||||
| 6-PFS, % (N.) | 46.7 (7/15) | 41.2 (7/17) | 35.3 (6/17) | 30 (6/20) | 0.52 | 0.48 |
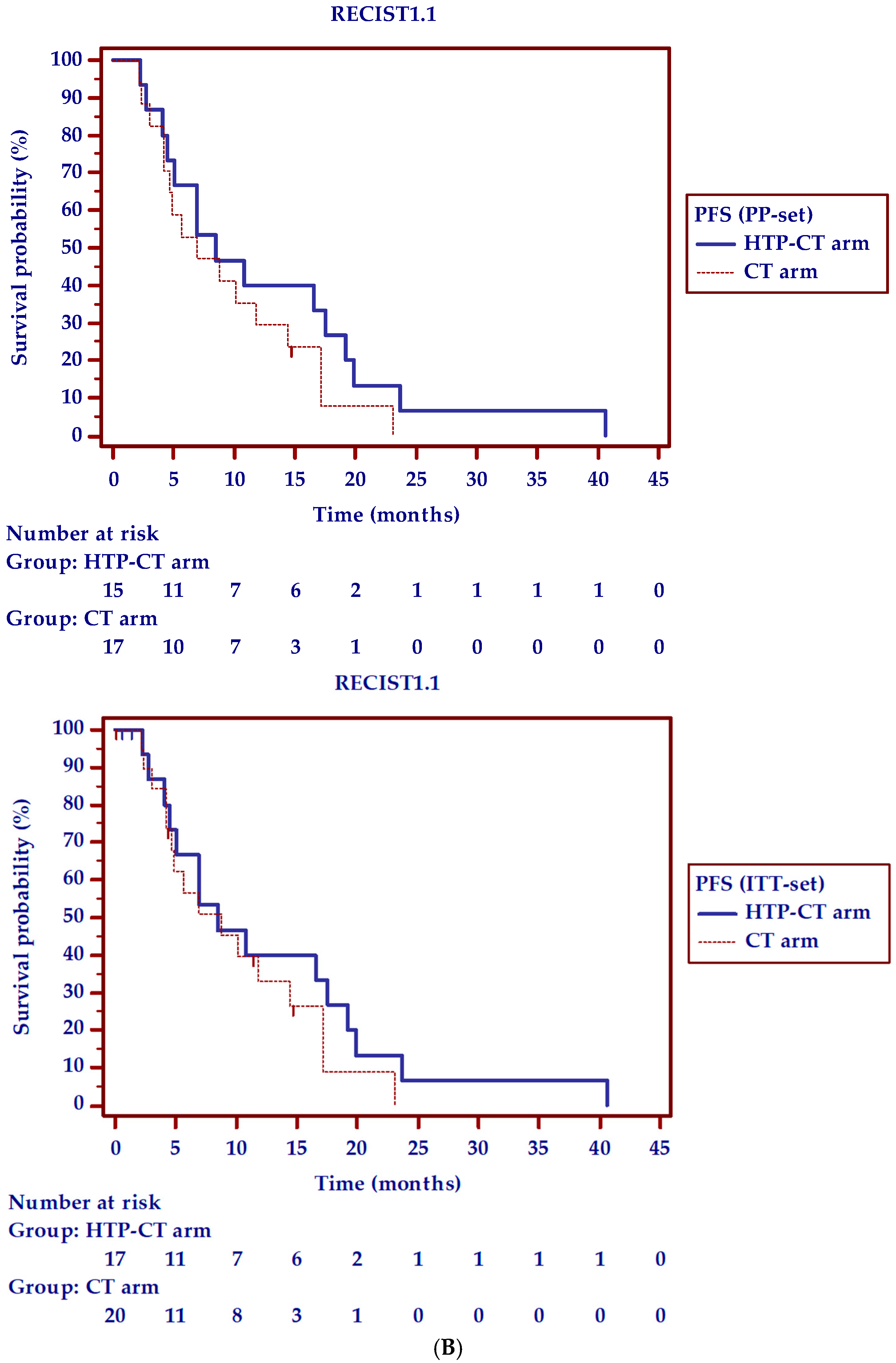
| Radiological disease control rate (RECIST1.1) | ||||||
| 2 months, % (N.) | 80 (12/15) | 71 (12/17) | 83.3 (15/18) | 75 (15/20) | 0.81 | 0.79 |
| N. 12 SD | N. 4 PR, N. 11 SD | |||||
| 4 months, % (N.) | 75 (9/12) | 52.9 (9/17) | 71.4 (10/14 §) | 50 (10/20) | 0.84 | 0.86 |
| N. 9 SD | N. 1 PR, N. 9 SD | |||||
| 6 months, % (N.) | 100 (9/9) | 52.9 (9/17) | 70 (7/10) | 35 (7/20) | 0.08 | 0.28 |
| N. 2 PR, N. 7 SD | N. 1 PR, N. 6 SD | |||||
| Overall response to therapy, % (N.) | 80 (12/15) | 71 (12/17) | 83.3 (15/18) | 75 (15/20) | 0.81 | 0.79 |
| N. 2 PR, N. 10 SD | N. 6 PR, N. 9 SD | |||||
| 6-PFS, % (N.) | 60 (9/15) | 52.9 (9/17) | 41.2 (7/17) | 35 (7/20) | 0.30 | 0.28 |
| CA19.9 Percentage Change versus Basal Value | HTP-CT Arm | CT Arm | p-Value | |||
|---|---|---|---|---|---|---|
| PP-Set | ITT-Set | PP-Set | ITT-Set | PP-Set | ITT-Set | |
| CA19.9 decrease < 50%, % (N.) | ||||||
| 2 months | 0 | 0 | 21.4 (3/14 *) | 21.4 (3/14) | 0.07 | 0.06 |
| 4 months | 0 | 0 | 15.4 (2/13) | 14.3 (2/14) | 0.15 | 0.12 |
| 6 months | 0 | 0 | 18.2 (2/11) | 14.3 (2/14) | 0.15 | 0.12 |
| CA19.9 decrease 50–89%, % (N.) | ||||||
| 2 months | 71.4 (10/14) | 62.5 (10/16) | 50 (7/14 *) | 50 (7/14) | 0.26 | 0.50 |
| 4 months | 61.5 (8/13) | 50 (8/16) | 38.5 (5/13) | 35.7 (5/14) | 0.25 | 0.44 |
| 6 months | 54.5 (6/11) | 37.5 (6/16) | 18.2 (2/11) | 14.3 (2/14) | 0.08 | 0.16 |
| CA19.9 decrease ≥ 90%, % (N.) | ||||||
| 2 months | 14.3 (2/14) | 12.5 (2/16) | 21.4 (3/14 *) | 21.4 (3/14) | 0.63 | 0.52 |
| 4 months | 23.1 (3/13) | 18.8 (3/16) | 30.8 (4/13) | 28.6 (4/14) | 0.66 | 0.53 |
| 6 months | 27.3 (3/11) | 18.8 (3/16) | 36.4 (4/11) | 28.6 (4/14) | 0.65 | 0.53 |
| Overall CA19.9 decrease ≥ 50%, % (N.) | 85.7 (12/14) | 75 (12/16) | 64.3 (9/14) | 64.3 (9/14) | 0.20 | 0.53 |
| Vital Tumour Volume (cc) | HTP-CT Arm | CT Arm | p-Value | Reduction Rate vs. Basal Tumour Volume (Median, IQR) | p-Value vs. Basal Tumour Volume | ||
|---|---|---|---|---|---|---|---|
| Basal, median, (IQR) | 31.3 | 20.2 | 0.06 | HTP-CT Arm | CT Arm | HTP-CT Arm | CT Arm |
| (22.6–49.1) | (11–28.4) | ||||||
| 2 months, median (IQR) | 30.4 | 18.1 | 0.02 | −16.7 | −5.9% | 0.82 | 0.85 |
| (18.5–37) | (15.5–25.8) | (−38.5–45.1%) | (−21.6–3%) | ||||
| 4 months, median (IQR) | 31.4 | 16.7 | 0.17 | −14.3 | −20.5% | 0.60 | 0.77 |
| (15.6–39.4) | (13.8–23) | (−64.6–51.1%) | (−28.3–9.6) | ||||
| 6 months, median (IQR) | 20.1 | 18.2 | 0.39 | −43.7% | −22.1% | 0.31 | 0.89 |
| (16.5–34.7) | (14.3–28.7) | (−64.8–27.7%) | (−31–43.9%) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Testoni, S.G.G.; Petrone, M.C.; Reni, M.; Rossi, G.; Barbera, M.; Nicoletti, V.; Gusmini, S.; Balzano, G.; Linzenbold, W.; Enderle, M.; et al. Efficacy of Endoscopic Ultrasound-Guided Ablation with the HybridTherm Probe in Locally Advanced or Borderline Resectable Pancreatic Cancer: A Phase II Randomized Controlled Trial. Cancers 2021, 13, 4512. https://doi.org/10.3390/cancers13184512
Testoni SGG, Petrone MC, Reni M, Rossi G, Barbera M, Nicoletti V, Gusmini S, Balzano G, Linzenbold W, Enderle M, et al. Efficacy of Endoscopic Ultrasound-Guided Ablation with the HybridTherm Probe in Locally Advanced or Borderline Resectable Pancreatic Cancer: A Phase II Randomized Controlled Trial. Cancers. 2021; 13(18):4512. https://doi.org/10.3390/cancers13184512
Chicago/Turabian StyleTestoni, Sabrina Gloria Giulia, Maria Chiara Petrone, Michele Reni, Gemma Rossi, Maurizio Barbera, Valeria Nicoletti, Simone Gusmini, Gianpaolo Balzano, Walter Linzenbold, Markus Enderle, and et al. 2021. "Efficacy of Endoscopic Ultrasound-Guided Ablation with the HybridTherm Probe in Locally Advanced or Borderline Resectable Pancreatic Cancer: A Phase II Randomized Controlled Trial" Cancers 13, no. 18: 4512. https://doi.org/10.3390/cancers13184512
APA StyleTestoni, S. G. G., Petrone, M. C., Reni, M., Rossi, G., Barbera, M., Nicoletti, V., Gusmini, S., Balzano, G., Linzenbold, W., Enderle, M., Della-Torre, E., De Cobelli, F., Doglioni, C., Falconi, M., Capurso, G., & Arcidiacono, P. G. (2021). Efficacy of Endoscopic Ultrasound-Guided Ablation with the HybridTherm Probe in Locally Advanced or Borderline Resectable Pancreatic Cancer: A Phase II Randomized Controlled Trial. Cancers, 13(18), 4512. https://doi.org/10.3390/cancers13184512










