Necroptosis in Esophageal Squamous Cell Carcinoma: An Independent Prognostic Factor and Its Correlation with Tumor-Infiltrating Lymphocytes
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Tissue Specimens
2.2. Neoadjuvant Chemotherapy and Surgery
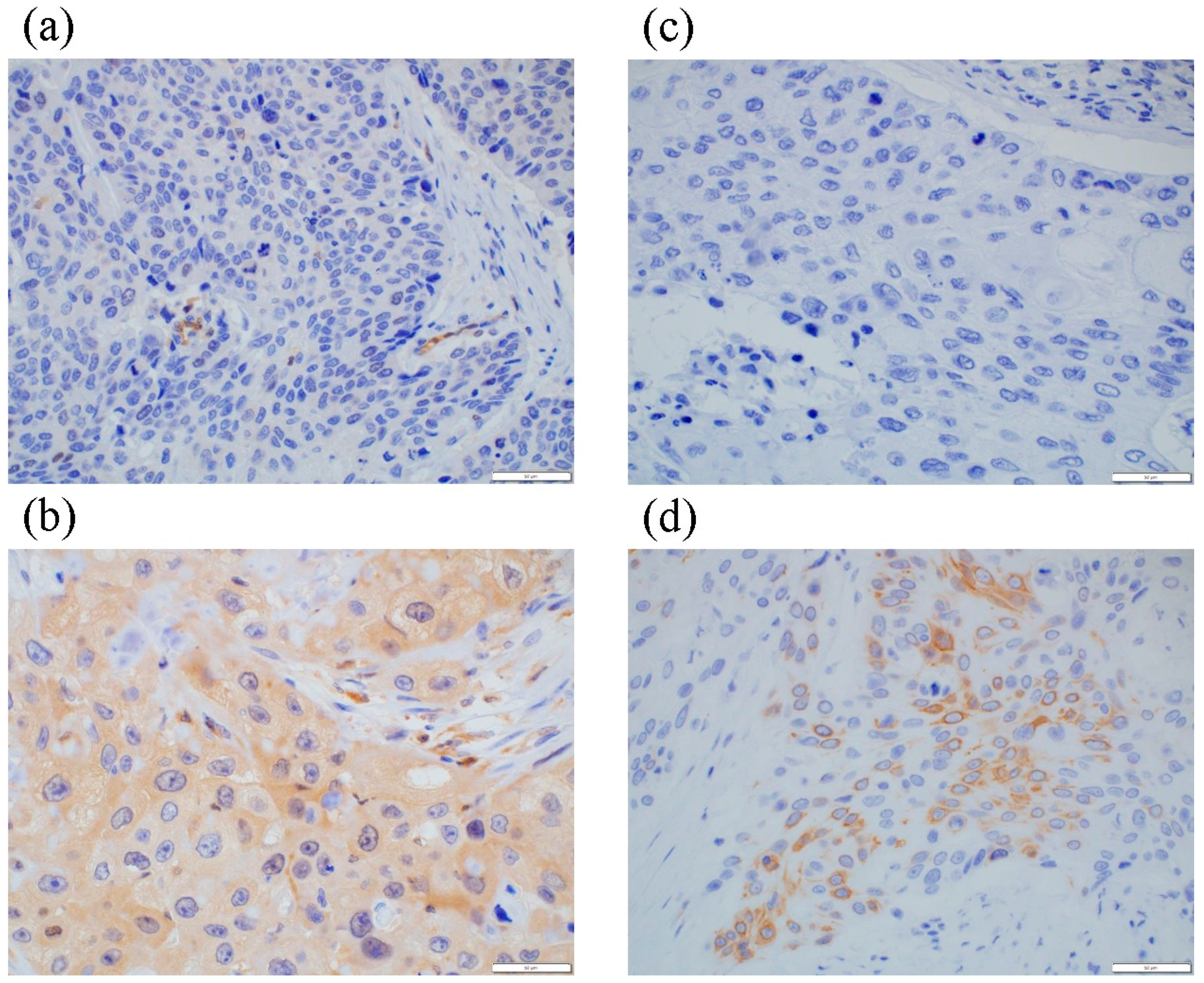
2.3. Immunohistochemical Staining
2.4. Statistical Analysis
3. Results
3.1. Post-NAC MLKL and pMLKL Status and Their Correlation with Clinicopathological Factors in ESCC Patients
3.2. Post-NAC MLKL and pMLKL Status and Their Correlation with the Clinical Outcome of ESCC Patients
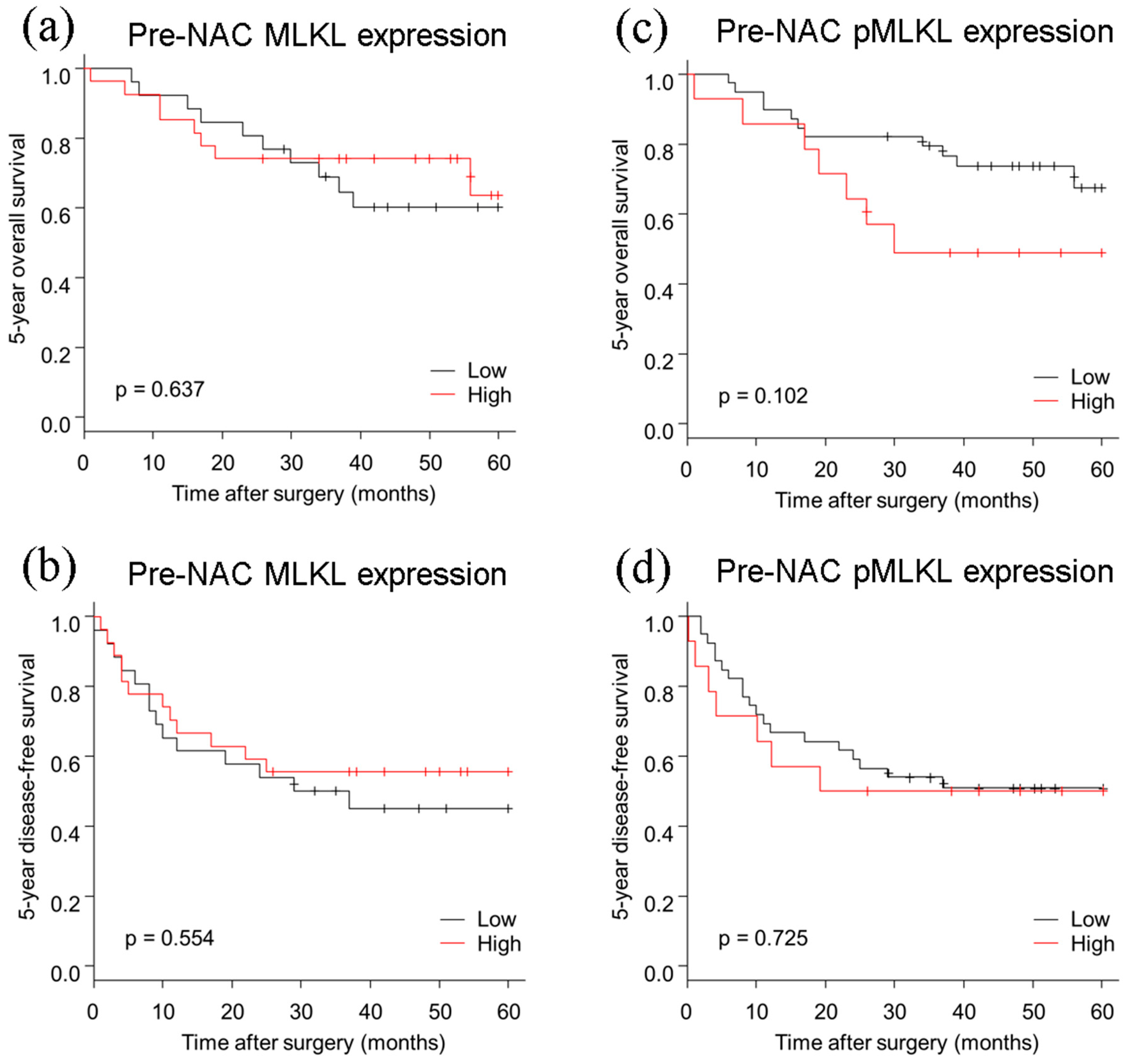
3.3. Pre-NAC MLKL and pMLKL and Their Correlation with Clinicopathological Factors of ESCC Patients
3.4. Pre-NAC MLKL and pMLKL Status and Their Correlation with Clinical Outcome of ESCC Patients
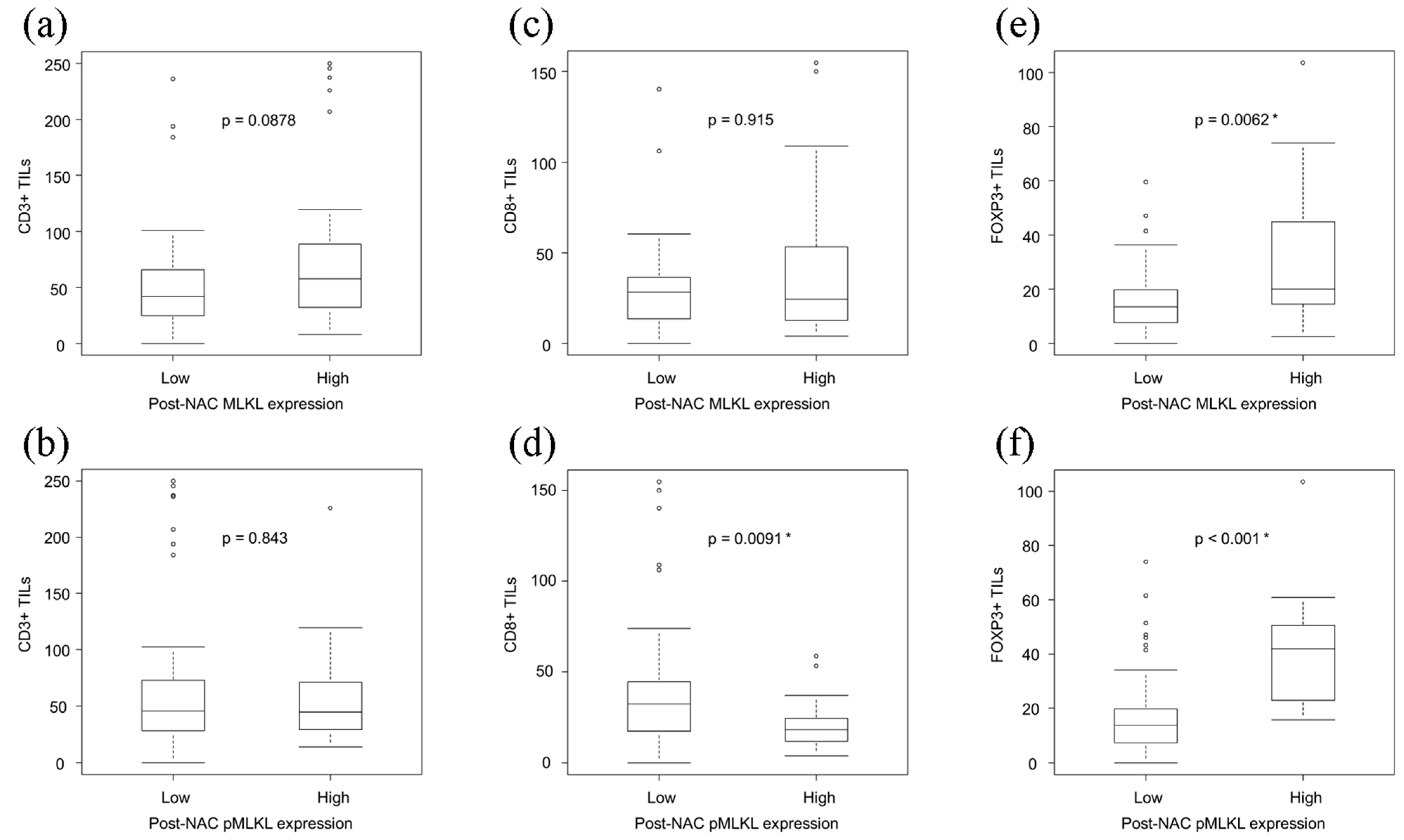
3.5. Correlations between the Necroptotic Biomarkers and TILs in ESCC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Kitagawa, Y.; Uno, T.; Oyama, T.; Kato, K.; Kato, H.; Kawakubo, H.; Kawamura, O.; Kusano, M.; Kuwano, H.; Takeuchi, H.; et al. Esophageal cancer practice guidelines 2017 edited by the Japan Esophageal Society: Part 1. Esophagus 2019, 16, 1–24. [Google Scholar] [CrossRef]
- Ando, N.; Kato, H.; Igaki, H.; Shinoda, M.; Ozawa, S.; Shimizu, H.; Nakamura, T.; Yabusaki, H.; Aoyama, N.; Kurita, A.; et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann. Surg. Oncol. 2012, 19, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Medical Research Council Oesophageal Cancer Working Group. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: A randomised controlled trial. Lancet 2002, 359, 1727–1733. [Google Scholar] [CrossRef]
- Linkermann, A.; Green, D.R. Necroptosis. N. Engl. J. Med. 2014, 370, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, A.; Vandenabeele, P.; Krysko, D.V. Necroptosis: The release of damage-associated molecular patterns and its physiological relevance. Immunity 2013, 38, 209–223. [Google Scholar] [CrossRef]
- Tonnus, W.; Meyer, C.; Paliege, A.; Belavgeni, A.; Von Mässenhausen, A.; Bornstein, S.R.; Hugo, C.; Becker, J.U.; Linkermann, A. The pathological features of regulated necrosis. J. Pathol. 2019, 247, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Seehawer, M.; Heinzmann, F.; D’Artista, L.; Harbig, J.; Roux, P.-F.; Hoenicke, L.; Dang, H.; Klotz, S.; Robinson, L.; Doré, G.; et al. Necroptosis microenvironment directs lineage commitment in liver cancer. Nature 2018, 562, 69–75. [Google Scholar] [CrossRef]
- Gong, Y.; Fan, Z.; Luo, G.; Yang, C.; Huang, Q.; Fan, K.; Cheng, H.; Jin, K.; Ni, Q.; Yu, X.; et al. The role of necroptosis in cancer biology and therapy. Mol. Cancer 2019, 18, 1–17. [Google Scholar] [CrossRef]
- Cho, Y.; Challa, S.; Moquin, D.; Genga, R.; Ray, T.D.; Guildford, M.; Chan, F.K.-M. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 2009, 137, 1112–1123. [Google Scholar] [CrossRef]
- He, S.; Wang, L.; Miao, L.; Wang, T.; Du, F.; Zhao, L.; Wang, X. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-α. Cell 2009, 137, 1100–1111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.-W.; Shao, J.; Lin, J.; Zhang, N.; Lu, B.-J.; Lin, S.-C.; Dong, M.-Q.; Han, J. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 2009, 325, 332–336. [Google Scholar] [CrossRef]
- Cai, Z.; Jitkaew, S.; Zhao, J.; Chiang, H.-C.; Choksi, S.; Liu, J.; Ward, Y.; Wu, L.-G.; Liu, Z.-G. Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat. Cell Biol. 2014, 16, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, W.; Ren, J.; Huang, D.; He, W.-T.; Song, Y.; Yang, C.; Li, W.; Zheng, X.; Chen, P.; et al. Translocation of mixed lineage kinase domain-like protein to plasma membrane leads to necrotic cell death. Cell Res. 2014, 24, 105–121. [Google Scholar] [CrossRef]
- Wang, H.; Sun, L.; Su, L.; Rizo, J.; Liu, L.; Wang, L.-F.; Wang, F.-S.; Wang, X. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol. Cell 2014, 54, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Pasparakis, M.; Vandenabeele, P. Necroptosis and its role in inflammation. Nature 2015, 517, 311–320. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhou, M.; Mei, L.; Ruan, J.; Hu, Q.; Peng, J.; Su, H.; Liao, H.; Liu, S.; Liu, W.; et al. Key roles of necroptotic factors in promoting tumor growth. Oncotarget 2016, 7, 22219–22233. [Google Scholar] [CrossRef]
- Seifert, L.; Werba, G.; Tiwari, S.; Giao Ly, N.N.; Alothman, S.; Alqunaibit, D.; Avanzi, A.; Barilla, R.; Daley, D.; Greco, S.H.; et al. The necrosome promotes pancreatic oncogenesis via CXCL1 and Mincle-induced immune suppression. Nature 2016, 532, 245–249. [Google Scholar] [CrossRef]
- Rosenbaum, S.R.; Wilski, N.A.; Aplin, A.E. Fueling the fire: Inflammatory forms of cell death and implications for cancer immunotherapy. Cancer Discov. 2021, 11, 266–281. [Google Scholar] [CrossRef]
- Mahmoud, S.M.A.; Paish, E.C.; Powe, D.G.; Macmillan, R.D.; Grainge, M.J.; Lee, A.H.S.; Ellis, I.O.; Green, A.R. Tumor-Infiltrating CD8+ Lymphocytes predict clinical outcome in breast cancer. J. Clin. Oncol. 2011, 29, 1949–1955. [Google Scholar] [CrossRef]
- Sato, E.; Olson, S.H.; Ahn, J.; Bundy, B.; Nishikawa, H.; Qian, F.; Jungbluth, A.A.; Frosina, D.; Gnjatic, S.; Ambrosone, C.; et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 18538–18543. [Google Scholar] [CrossRef]
- Farhood, B.; Najafi, M.; Mortezaee, K. CD8+cytotoxic T lymphocytes in cancer immunotherapy: A review. J. Cell Physiol. 2019, 234, 8509–8521. [Google Scholar] [CrossRef]
- Konno-Kumagai, T.; Fujishima, F.; Nakamura, Y.; Nakano, T.; Nagai, T.; Kamei, T.; Sasano, H. Programmed death-1 ligands and tumor infiltrating T lymphocytes in primary and lymph node metastasis of esophageal cancer patients. Dis. Esophagus 2019, 32, doy063. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, M.; Sasano, H.; Tamaki, K.; Chan, M.; Hirakawa, H.; Suzuki, A.; Tada, H.; Watanabe, G.; Nemoto, N.; Nakagawa, S.; et al. Tumor-infiltrating CD8+ and FOXP3+ lymphocytes in triple-negative breast cancer: Its correlation with pathological complete response to neoadjuvant chemotherapy. Breast Cancer Res. Treat. 2014, 148, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Baras, A.S.; Drake, C.; Liu, J.-J.; Gandhi, N.; Kates, M.; Hoque, M.O.; Meeker, A.; Hahn, N.; Taube, J.M.; Schoenberg, M.P.; et al. The ratio of CD8 to Treg tumor-infiltrating lymphocytes is associated with response to cisplatin-based neoadjuvant chemotherapy in patients with muscle invasive urothelial carcinoma of the bladder. Oncoimmunology 2016, 5, e1134412. [Google Scholar] [CrossRef] [PubMed]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. UICC TNM Classification of Malignant Tumours, 8th ed.; Wiley Blackwell: Oxford, UK, 2017. [Google Scholar]
- Japan Esophageal Society. Japanese Classification of Esophageal Cancer, 11th Edition: Part II and III. Esophagus 2017, 14, 37–65. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Fujishima, F.; Lomphithak, T.; Jitkaew, S.; Nio, M.; Sasano, H. Necroptosis in biliary atresia of the liver. Med. Mol. Morphol. 2021, 1–11. [Google Scholar] [CrossRef]
- Salgado, R.; Denkert, C.; Demaria, S.; Sirtaine, N.; Klauschen, F.; Pruneri, G.; Wienert, S.; Van Den Eynden, G.; Baehner, F.L.; Penault-Llorca, F.; et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: Recommendations by an International TILs Working Group 2014. Ann. Oncol. 2015, 26, 259–271. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Jiao, D.; Cai, Z.; Choksi, S.; Ma, D.; Choe, M.; Kwon, H.-J.; Baik, J.Y.; Rowan, B.G.; Liu, C.; Liu, Z.-G. Necroptosis of tumor cells leads to tumor necrosis and promotes tumor metastasis. Cell Res. 2018, 28, 868–870. [Google Scholar] [CrossRef]
- Li, J.; Huang, S.; Zeng, L.; Li, K.; Yang, L.; Gao, S.; Guan, C.; Zhang, S.; Lao, X.; Liao, G.; et al. Necroptosis in head and neck squamous cell carcinoma: Characterization of clinicopathological relevance and in vitro cell model. Cell Death Dis. 2020, 11, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lin, Z.; Zhao, N.; Zhou, L.; Liu, F.; Cichacz, Z.; Zhang, L.; Zhan, Q.; Zhao, X. Receptor interactive protein kinase 3 promotes cisplatin-triggered necrosis in apoptosis-resistant esophageal squamous cell carcinoma cells. PLoS ONE 2014, 9, e100127. [Google Scholar] [CrossRef][Green Version]
- Brown, M.F.; Leibowitz, B.J.; Chen, D.; He, K.; Zou, F.; Sobol, R.W.; Beer-Stolz, D.; Zhang, L.; Yu, J. Loss of Caspase-3 sensitizes colon cancer cells to genotoxic stress via RIP1-dependent necrosis. Cell Death Dis. 2015, 6, e1729. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Xia, L.; Klaiber, U.; Sachsenmaier, M.; Hinz, U.; Bergmann, F.; Strobel, O.; Büchler, M.W.; Neoptolemos, J.P.; Fortunato, F.; et al. Effects of neoadjuvant FOLFIRINOX and gemcitabine-based chemotherapy on cancer cell survival and death in patients with pancreatic ductal adenocarcinoma. Oncotarget 2019, 10, 7276–7287. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Galluzzi, L.; Kepp, O.; Zitvogel, L. Immunogenic cell death in cancer therapy. Annu Rev. Immunol 2013, 31, 51–72. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-G.; Jiao, D. Necroptosis, tumor necrosis and tumorigenesis. Cell Stress 2020, 4, 1–8. [Google Scholar] [CrossRef]
- Qin, X.; Ma, D.; Tan, Y.-X.; Wang, H.-Y.; Cai, Z. The role of necroptosis in cancer: A double-edged sword? Biochim. Et Biophys. Acta (BBA)-Rev. Cancer 2019, 1871, 259–266. [Google Scholar] [CrossRef]
- Togashi, Y.; Shitara, K.; Nishikawa, H. Regulatory T cells in cancer immunosuppression—Implications for anticancer therapy. Nat. Rev. Clin. Oncol. 2019, 16, 356–371. [Google Scholar] [CrossRef]
- Fassan, M.; Cavallin, F.; Guzzardo, V.; Kotsafti, A.; Scarpa, M.; Cagol, M.; Chiarion-Sileni, V.; Maria Saadeh, L.; Alfieri, R.; Castagliuolo, I.; et al. PD-L1 expression, CD8+ and CD4+ lymphocyte rate are predictive of pathological complete response after neoadjuvant chemoradiotherapy for squamous cell cancer of the thoracic esophagus. Cancer Med. 2019, 8, 6036–6048. [Google Scholar] [CrossRef] [PubMed]
- Vacchelli, E.; Semeraro, M.; Enot, D.P.; Chaba, K.; Colame, V.P.; Dartigues, P.; Perier, A.; Villa, I.; Rusakiewicz, S.; Gronnier, C.; et al. Negative prognostic impact of regulatory T cell infiltration in surgically resected esophageal cancer post-radiochemotherapy. Oncotarget 2015, 6, 20840–20850. [Google Scholar] [CrossRef] [PubMed]
- Eil, R.; Vodnala, S.K.; Clever, D.; Klebanoff, C.A.; Sukumar, M.; Pan, J.H.; Palmer, D.C.; Gros, A.; Yamamoto, T.N.; Patel, S.J.; et al. Ionic immune suppression within the tumour microenvironment limits T cell effector function. Nature 2016, 537, 539–543. [Google Scholar] [CrossRef] [PubMed]


| Variable | N | Post-NAC MLKL | p | Post-NAC pMLKL | p | ||
|---|---|---|---|---|---|---|---|
| High | Low | High | Low | ||||
| 88 | 44 | 44 | 19 | 69 | |||
| Age (years) | |||||||
| ≥65 | 57 | 25 | 32 | 0.180 | 8 | 49 | 0.029 * |
| <65 | 31 | 19 | 12 | 11 | 20 | ||
| Gender | |||||||
| Male | 74 | 37 | 37 | 1.000 | 13 | 61 | 0.069 |
| Female | 14 | 7 | 7 | 6 | 8 | ||
| pT | |||||||
| pT1-T2 | 37 | 17 | 20 | 0.666 | 3 | 34 | 0.009 * |
| pT3-T4 | 51 | 27 | 24 | 16 | 35 | ||
| pN | |||||||
| pN0 | 24 | 10 | 14 | 0.473 | 3 | 21 | 0.255 |
| pN1-N3 | 64 | 34 | 30 | 16 | 48 | ||
| Pathological stage | |||||||
| Stage I/II | 31 | 13 | 18 | 0.372 | 4 | 27 | 0.181 |
| Stage III/IV | 57 | 31 | 26 | 15 | 42 | ||
| Differentiation | |||||||
| Well, moderate | 76 | 37 | 39 | 0.200 | 18 | 58 | 0.661 |
| Poor | 10 | 7 | 3 | 1 | 9 | ||
| Unclassifiable | 2 | 0 | 2 | 0 | 2 | ||
| Resection margin | |||||||
| R0 | 80 | 39 | 41 | 0.713 | 18 | 62 | 1.000 |
| R1 | 8 | 5 | 3 | 1 | 7 | ||
| RECIST grade | |||||||
| CR/PR | 22 | 14 | 8 | 0.206 | 6 | 16 | 0.777 |
| SD/PD | 54 | 24 | 30 | 13 | 41 | ||
| Indeterminate | 12 | 6 | 6 | 0 | 12 | ||
| Histological NAC efficacy | |||||||
| Ineffective (Grade 0-1a) | 51 | 28 | 23 | 0.388 | 14 | 37 | 0.189 |
| Effective (Grade 1b-2) | 37 | 16 | 21 | 5 | 32 | ||
| Variable | Person-Year | Event | HR (95% CI) | p |
|---|---|---|---|---|
| Age (years) | ||||
| <65 | 101 | 11 | ref | |
| ≥65 | 194 | 22 | 1.08 (0.52–2.23) | 0.830 |
| Gender | ||||
| Female | 42 | 7 | ref | |
| Male | 253 | 26 | 0.63 (0.27–1.45) | 0.280 |
| pT | ||||
| pT1-2 | 138 | 9 | ref | |
| pT3-T4 | 157 | 24 | 2.26 (1.05–4.87) | 0.037 * |
| pN | ||||
| pN0 | 98 | 3 | ref | |
| pN1-N3 | 198 | 30 | 4.73 (1.44–15.53) | 0.010 * |
| Pathological stage | ||||
| Stage I/II | 129 | 5 | ref | |
| Stage III/IV | 167 | 28 | 4.08 (1.57–10.59) | 0.0039* |
| Differentiation | ||||
| Poor | 29 | 3 | ref | |
| Well, moderate | 258 | 30 | 1.24 (0.38–4.08) | 0.720 |
| Resection margin | ||||
| R0 | 282 | 26 | ref | |
| R1 | 14 | 7 | 5.03 (2.15–11.8) | <0.001 * |
| RECIST grade | ||||
| CR/PR | 77 | 5 | ref | |
| SD/PD | 175 | 24 | 2.13 (0.81–5.59) | 0.120 |
| Histological NAC efficacy | ||||
| Ineffective (Grade 0–1a) | 158 | 25 | ref | |
| Effective (Grade 1b–2) | 138 | 8 | 0.38 (0.17–0.84) | 0.017 * |
| post-NAC MLKL | ||||
| Low | 162 | 13 | ref | |
| High | 134 | 20 | 1.79 (0.89–3.60) | 0.100 |
| post-NAC pMLKL | ||||
| Low | 245 | 21 | ref | |
| High | 50 | 12 | 2.60 (1.27–5.31) | 0.0087 * |
| Variable | Person-Year | Event | HR (95% CI) | p |
|---|---|---|---|---|
| Age (years) | ||||
| <65 | 101 | 11 | ref | |
| ≥65 | 194 | 22 | 1.35 (0.63–2.92) | 0.440 |
| Gender | ||||
| Female | 42 | 7 | ref | |
| Male | 253 | 26 | 0.64 (0.25–1.61) | 0.344 |
| pT | ||||
| pT1-T2 | 138 | 9 | ref | |
| pT3-T4 | 157 | 24 | 1.30 (0.56–3.00) | 0.546 |
| pN | ||||
| pN0 | 98 | 3 | ref | |
| pN1-N3 | 198 | 30 | 4.42 (1.29–15.20) | 0.018 * |
| Resection margin | ||||
| R0 | 282 | 26 | ref | |
| R1 | 14 | 7 | 3.43 (1.30–9.05) | 0.013 * |
| Histological NAC efficacy | ||||
| Ineffective (Grade 0–1a) | 158 | 25 | ref | |
| Effective (Grade 1b–2) | 138 | 8 | 0.52 (0.22–1.21) | 0.127 |
| post-NAC pMLKL | ||||
| Low | 245 | 21 | ref | |
| High | 50 | 12 | 2.48 (1.11–5.57) | 0.027 * |
| Variable | N | Pre-NAC MLKL | p | Pre-NAC pMLKL | p | ||
|---|---|---|---|---|---|---|---|
| High | Low | High | Low | ||||
| 53 | 27 | 26 | 14 | 39 | |||
| Age (years) | |||||||
| ≥65 | 33 | 16 | 17 | 0.779 | 7 | 26 | 0.341 |
| <65 | 20 | 11 | 9 | 7 | 13 | ||
| Gender | |||||||
| Male | 45 | 25 | 20 | 0.142 | 11 | 34 | 0.422 |
| Female | 8 | 2 | 6 | 3 | 5 | ||
| pT | |||||||
| pT1-T2 | 28 | 13 | 15 | 0.586 | 6 | 22 | 0.534 |
| pT3-T4 | 25 | 14 | 11 | 8 | 17 | ||
| pN | |||||||
| pN0 | 17 | 5 | 12 | 0.042 * | 2 | 15 | 0.180 |
| pN1-N3 | 36 | 22 | 14 | 12 | 24 | ||
| Pathological stage | |||||||
| Stage I/II | 19 | 7 | 12 | 0.158 | 3 | 16 | 0.330 |
| Stage III/IV | 34 | 20 | 14 | 11 | 23 | ||
| Differentiation | |||||||
| Well, moderate | 45 | 20 | 25 | 0.010 * | 12 | 33 | 1.000 |
| Poor | 7 | 7 | 0 | 2 | 5 | ||
| Unclassifiable | 1 | 0 | 1 | 0 | 1 | ||
| Resection margin | |||||||
| R0 | 50 | 25 | 25 | 1.000 | 14 | 36 | 0.557 |
| R1 | 3 | 2 | 1 | 0 | 3 | ||
| RECIST grade | |||||||
| CR/PR | 16 | 8 | 8 | 0.540 | 3 | 13 | 0.195 |
| SD/PD | 28 | 17 | 11 | 11 | 17 | ||
| Indeterminate | 9 | 2 | 7 | 0 | 9 | ||
| Histological NAC efficacy | |||||||
| Ineffective (Grade 0–1a) | 30 | 17 | 13 | 0.412 | 12 | 18 | 0.013 * |
| Effective (Grade 1b–2) | 23 | 10 | 13 | 2 | 21 | ||
| Variable | Person-Year | Event | HR (95% CI) | p |
|---|---|---|---|---|
| pre-NAC MLKL | ||||
| Low | 86 | 10 | ref | |
| High | 88 | 8 | 0.80 (0.31–2.03) | 0.638 |
| pre-NAC pMLKL | ||||
| Low | 137 | 11 | ref | |
| High | 38 | 7 | 2.17 (0.84–5.64) | 0.111 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamauchi, T.; Fujishima, F.; Hashimoto, M.; Tsunokake, J.; Akaishi, R.; Gokon, Y.; Ueki, S.; Ozawa, Y.; Fukutomi, T.; Okamoto, H.; et al. Necroptosis in Esophageal Squamous Cell Carcinoma: An Independent Prognostic Factor and Its Correlation with Tumor-Infiltrating Lymphocytes. Cancers 2021, 13, 4473. https://doi.org/10.3390/cancers13174473
Yamauchi T, Fujishima F, Hashimoto M, Tsunokake J, Akaishi R, Gokon Y, Ueki S, Ozawa Y, Fukutomi T, Okamoto H, et al. Necroptosis in Esophageal Squamous Cell Carcinoma: An Independent Prognostic Factor and Its Correlation with Tumor-Infiltrating Lymphocytes. Cancers. 2021; 13(17):4473. https://doi.org/10.3390/cancers13174473
Chicago/Turabian StyleYamauchi, Takuro, Fumiyoshi Fujishima, Masatoshi Hashimoto, Junichi Tsunokake, Ryujiro Akaishi, Yusuke Gokon, Shunsuke Ueki, Yohei Ozawa, Toshiaki Fukutomi, Hiroshi Okamoto, and et al. 2021. "Necroptosis in Esophageal Squamous Cell Carcinoma: An Independent Prognostic Factor and Its Correlation with Tumor-Infiltrating Lymphocytes" Cancers 13, no. 17: 4473. https://doi.org/10.3390/cancers13174473
APA StyleYamauchi, T., Fujishima, F., Hashimoto, M., Tsunokake, J., Akaishi, R., Gokon, Y., Ueki, S., Ozawa, Y., Fukutomi, T., Okamoto, H., Sato, C., Taniyama, Y., Nakamura, T., Nakaya, N., Kamei, T., & Sasano, H. (2021). Necroptosis in Esophageal Squamous Cell Carcinoma: An Independent Prognostic Factor and Its Correlation with Tumor-Infiltrating Lymphocytes. Cancers, 13(17), 4473. https://doi.org/10.3390/cancers13174473






