Elevated Flt3L Predicts Long-Term Survival in Patients with High-Grade Gastroenteropancreatic Neuroendocrine Neoplasms
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Serum Samples
2.2. NEN Transcriptome Data
2.3. Determination of Circulating Flt3L
2.4. Software and Statistical Analyses
3. Results
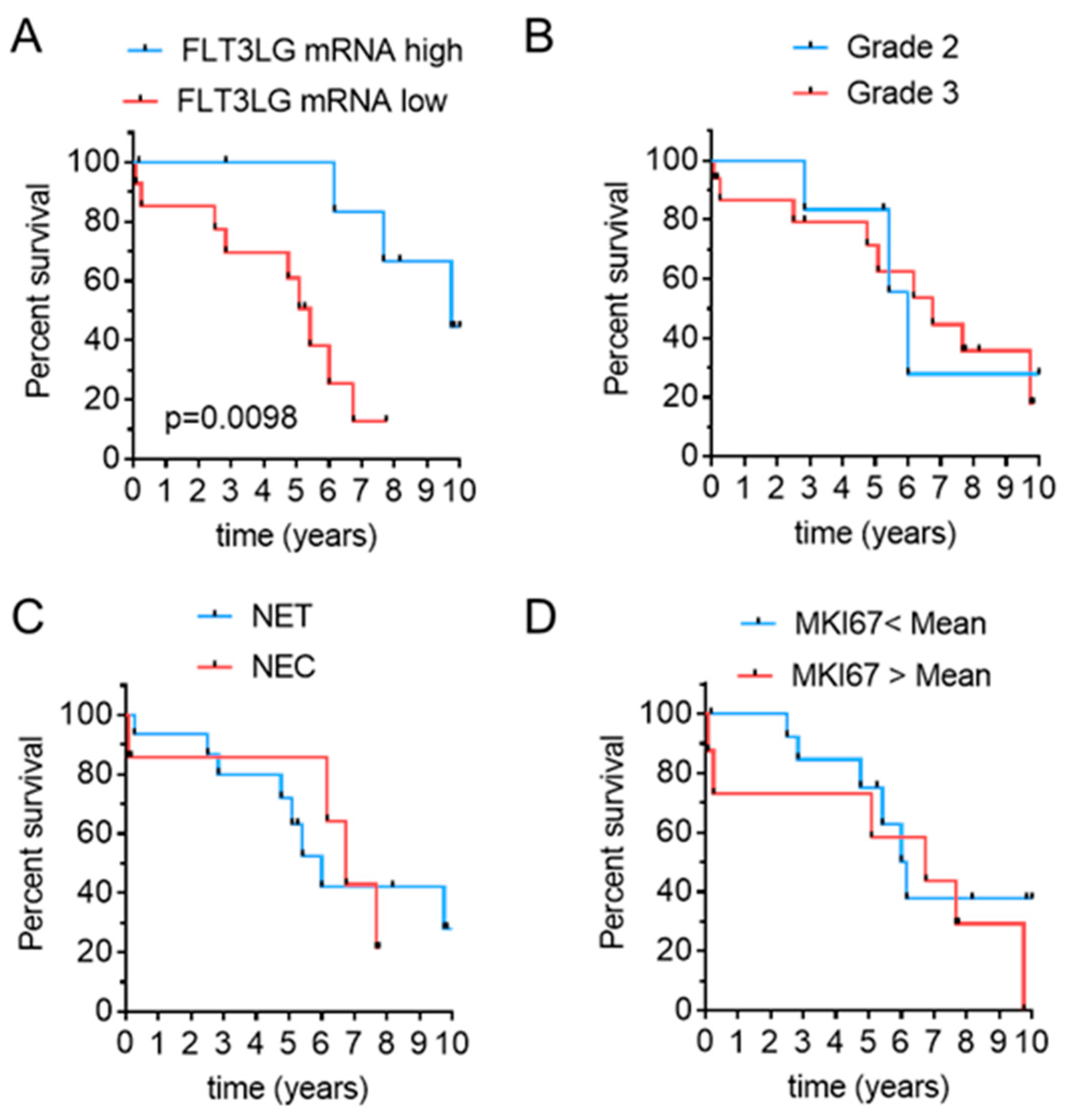
3.1. Flt3L mRNA Expression Is Increased in a Subset of G3 NEN
3.2. Flt3L mRNA Expression Predicts Disease-Related Survival in Pancreatic and Gastric High Grade NEN
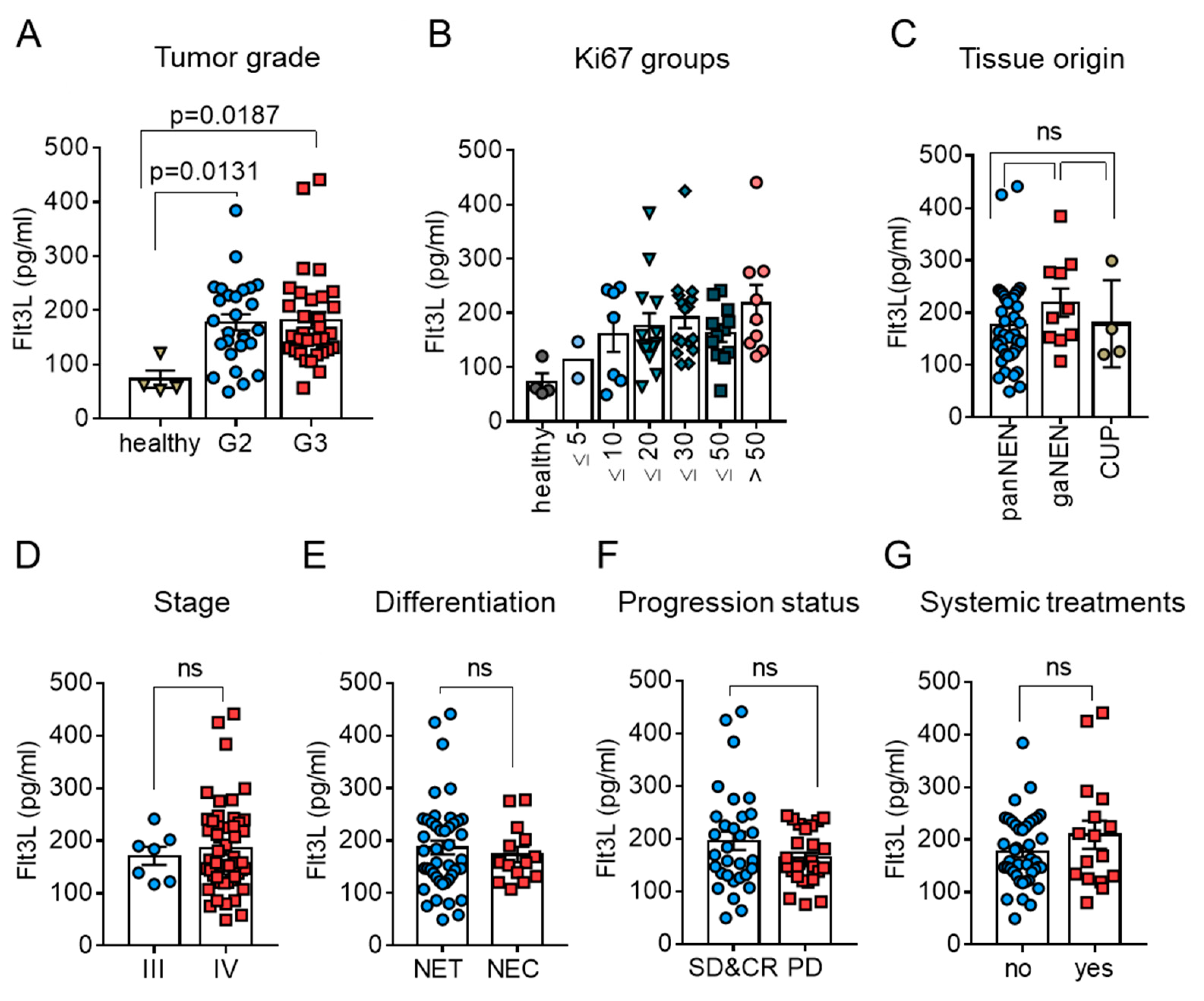
3.3. Circulating Levels of Flt3L Are Elevated in Highly Proliferative NEN
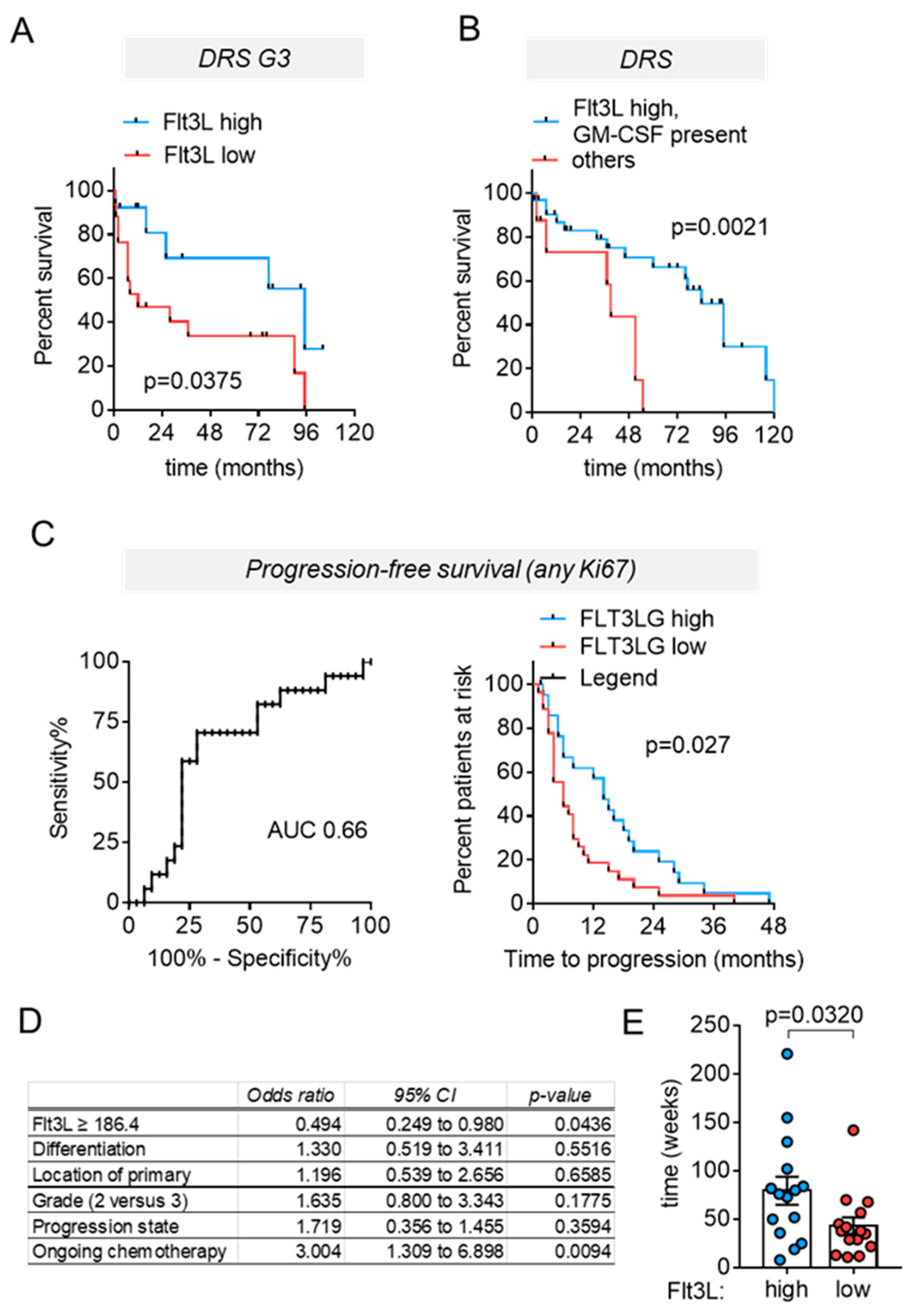
3.4. High Circulating Flt3L Predicts Longer Disease-Related Survival in NEN Patients with Highly Proliferative Tumors
3.5. High Circulating Flt3L Predicts Longer Progression–Free-Survival in NEN Patients with Highly Proliferative Tumors
3.6. High Circulating Flt3L Correlates to Extended Treatment-Induced Disease Stabilization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hofland, J.; Kaltsas, G.; de Herder, W.W. Advances in the Diagnosis and Management of Well-Differentiated Neuroendocrine Neoplasms. Endocr. Rev. 2020, 41, 371–403. [Google Scholar] [CrossRef] [Green Version]
- Sorbye, H.; Welin, S.; Langer, S.W.; Vestermark, L.W.; Holt, N.; Osterlund, P.; Dueland, S.; Hofsli, E.; Guren, M.G.; Ohrling, K.; et al. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): The NORDIC NEC study. Ann. Oncol. 2013, 24, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Milione, M.; Maisonneuve, P.; Spada, F.; Pellegrinelli, A.; Spaggiari, P.; Albarello, L.; Pisa, E.; Barberis, M.; Vanoli, A.; Buzzoni, R.; et al. The Clinicopathologic Heterogeneity of Grade 3 Gastroenteropancreatic Neuroendocrine Neoplasms: Morphological Differentiation and Proliferation Identify Different Prognostic Categories. Neuroendocrinology 2017, 104, 85–93. [Google Scholar] [CrossRef]
- Marion-Audibert, A.M.; Barel, C.; Gouysse, G.; Dumortier, J.; Pilleul, F.; Pourreyron, C.; Hervieu, V.; Poncet, G.; Lombard-Bohas, C.; Chayvialle, J.A.; et al. Low microvessel density is an unfavorable histoprognostic factor in pancreatic endocrine tumors. Gastroenterology 2003, 125, 1094–1104. [Google Scholar] [CrossRef]
- Scoazec, J.Y. Angiogenesis in neuroendocrine tumors: Therapeutic applications. Neuroendocrinology 2013, 97, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Motz, G.T.; Coukos, G. The parallel lives of angiogenesis and immunosuppression: Cancer and other tales. Nat. Rev. Immunol. 2011, 11, 702–711. [Google Scholar] [CrossRef] [PubMed]
- De Palma, M.; Biziato, D.; Petrova, T.V. Microenvironmental regulation of tumour angiogenesis. Nat. Rev. Cancer 2017, 17, 457–474. [Google Scholar] [CrossRef] [PubMed]
- Scarpa, A.; Chang, D.K.; Nones, K.; Corbo, V.; Patch, A.M.; Bailey, P.; Lawlor, R.T.; Johns, A.L.; Miller, D.K.; Mafficini, A.; et al. Whole-genome landscape of pancreatic neuroendocrine tumours. Nature 2017, 543, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.J.; Subramaniam, P.S.; Tang, L.H.; Grunn, A.; Aburi, M.; Rieckhof, G.; Komissarova, E.V.; Hagan, E.A.; Bodei, L.; Clemons, P.A.; et al. A precision oncology approach to the pharmacological targeting of mechanistic dependencies in neuroendocrine tumors. Nat. Genet. 2018, 50, 979–989. [Google Scholar] [CrossRef]
- Young, K.; Lawlor, R.T.; Ragulan, C.; Patil, Y.; Mafficini, A.; Bersani, S.; Antonello, D.; Mansfield, D.; Cingarlini, S.; Landoni, L.; et al. Immune landscape, evolution, hypoxia-mediated viral mimicry pathways and therapeutic potential in molecular subtypes of pancreatic neuroendocrine tumours. Gut 2020, 1–10. [Google Scholar] [CrossRef]
- Busse, A.; Mochmann, L.H.; Spenke, C.; Arsenic, R.; Briest, F.; Johrens, K.; Lammert, H.; Sipos, B.; Kuhl, A.A.; Wirtz, R.; et al. Immunoprofiling in Neuroendocrine Neoplasms Unveil Immunosuppressive Microenvironment. Cancers 2020, 12, 3448. [Google Scholar] [CrossRef]
- Katz, S.C.; Donkor, C.; Glasgow, K.; Pillarisetty, V.G.; Gonen, M.; Espat, N.J.; Klimstra, D.S.; D’Angelica, M.I.; Allen, P.J.; Jarnagin, W.; et al. T cell infiltrate and outcome following resection of intermediate-grade primary neuroendocrine tumours and liver metastases. HPB (Oxf.) 2010, 12, 674–683. [Google Scholar] [CrossRef] [Green Version]
- Cai, L.; Michelakos, T.; Deshpande, V.; Arora, K.S.; Yamada, T.; Ting, D.T.; Taylor, M.S.; Castillo, C.F.; Warshaw, A.L.; Lillemoe, K.D.; et al. Role of Tumor-Associated Macrophages in the Clinical Course of Pancreatic Neuroendocrine Tumors (PanNETs). Clin. Cancer Res. 2019, 25, 2644–2655. [Google Scholar] [CrossRef]
- Imam, R.; Chang, Q.; Black, M.; Yu, C.; Cao, W. CD47 expression and CD163(+) macrophages correlated with prognosis of pancreatic neuroendocrine tumor. BMC Cancer 2021, 21, 320. [Google Scholar] [CrossRef]
- Milione, M.; Miceli, R.; Barretta, F.; Pellegrinelli, A.; Spaggiari, P.; Tagliabue, G.; Centonze, G.; Paolino, C.; Mangogna, A.; Kankava, K.; et al. Microenvironment and tumor inflammatory features improve prognostic prediction in gastro-entero-pancreatic neuroendocrine neoplasms. J. Pathol. Clin. Res. 2019, 5, 217–226. [Google Scholar] [CrossRef] [Green Version]
- Centonze, G.; Lagano, V.; Sabella, G.; Mangogna, A.; Garzone, G.; Filugelli, M.; Belmonte, B.; Cattaneo, L.; Crisafulli, V.; Pellegrinelli, A.; et al. Myeloid and T-Cell Microenvironment Immune Features Identify Two Prognostic Sub-Groups in High-Grade Gastroenteropancreatic Neuroendocrine Neoplasms. J. Clin. Med. 2021, 10, 1741. [Google Scholar] [CrossRef]
- Yao, J.C.; Strosberg, J.; Fazio, N.; Pavel, M.E.; Bergsland, E.; Ruszniewski, P.; Halperin, D.M.; Li, D.; Tafuto, S.; Raj, N.; et al. Spartalizumab in metastatic, well/poorly-differentiated neuroendocrine neoplasms. Endocr.-Relat. Cancer 2021, 28, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.P.; Othus, M.; Chae, Y.K.; Giles, F.J.; Hansel, D.E.; Singh, P.P.; Fontaine, A.; Shah, M.H.; Kasi, A.; Baghdadi, T.A.; et al. A Phase II Basket Trial of Dual Anti-CTLA-4 and Anti-PD-1 Blockade in Rare Tumors (DART SWOG 1609) in Patients with Nonpancreatic Neuroendocrine Tumors. Clin. Cancer Res. 2020, 26, 2290–2296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, S.P.; Mayerson, E.; Chae, Y.K.; Strosberg, J.; Wang, J.; Konda, B.; Hayward, J.; McLeod, C.M.; Chen, H.X.; Sharon, E.; et al. A phase II basket trial of Dual Anti-CTLA-4 and Anti-PD-1 Blockade in Rare Tumors (DART) SWOG S1609: High-Grade Neuroendocrine Neoplasm Cohort. Cancer 2021, 127, 3194–3201. [Google Scholar] [CrossRef] [PubMed]
- Raufi, A.G.; May, M.; Greendyk, R.A.; Iuga, A.; Ahmed, F.; Mansukhani, M.; Manji, G.A. Spontaneous Regression and Complete Response to Immune Checkpoint Blockade in a Case of High-Grade Neuroendocrine Carcinoma. JCO Precis. Oncol. 2020, 4, 1006–1011. [Google Scholar] [CrossRef]
- Ozdirik, B.; Jann, H.; Bischoff, P.; Fehrenbach, U.; Tacke, F.; Roderburg, C.; Wiedenmann, B. PD-L1—Inhibitors in neuroendocrine neoplasia: Results from a real-life study. Medicine (Baltim.) 2021, 100, e23835. [Google Scholar] [CrossRef] [PubMed]
- Stuven, A.K.; Wiedenmann, B. Sustained partial remission of a metastatic NEN using off-label immunotherapy with pembrolizumab. Oncotarget 2019, 10, 3302–3311. [Google Scholar] [CrossRef]
- Sorbye, H.; Baudin, E.; Borbath, I.; Caplin, M.; Chen, J.; Cwikla, J.B.; Frilling, A.; Grossman, A.; Kaltsas, G.; Scarpa, A.; et al. Unmet Needs in High-Grade Gastroenteropancreatic Neuroendocrine Neoplasms (WHO G3). Neuroendocrinology 2019, 108, 54–62. [Google Scholar] [CrossRef]
- Anandasabapathy, N.; Breton, G.; Hurley, A.; Caskey, M.; Trumpfheller, C.; Sarma, P.; Pring, J.; Pack, M.; Buckley, N.; Matei, I.; et al. Efficacy and safety of CDX-301, recombinant human Flt3L, at expanding dendritic cells and hematopoietic stem cells in healthy human volunteers. Bone Marrow Transplant. 2015, 50, 924–930. [Google Scholar] [CrossRef]
- Waskow, C.; Liu, K.; Darrasse-Jeze, G.; Guermonprez, P.; Ginhoux, F.; Merad, M.; Shengelia, T.; Yao, K.; Nussenzweig, M. The receptor tyrosine kinase Flt3 is required for dendritic cell development in peripheral lymphoid tissues. Nat. Immunol. 2008, 9, 676–683. [Google Scholar] [CrossRef]
- Gasparetto, C.; Gasparetto, M.; Morse, M.; Rooney, B.; Vredenburgh, J.J.; Long, G.D.; Rizzieri, D.A.; Loftis, J.; Chao, N.J.; Smith, C. Mobilization of dendritic cells from patients with breast cancer into peripheral blood stem cell leukapheresis samples using Flt-3-Ligand and G-CSF or GM-CSF. Cytokine 2002, 18, 8–19. [Google Scholar] [CrossRef]
- Fong, L.; Hou, Y.; Rivas, A.; Benike, C.; Yuen, A.; Fisher, G.A.; Davis, M.M.; Engleman, E.G. Altered peptide ligand vaccination with Flt3 ligand expanded dendritic cells for tumor immunotherapy. Proc. Natl. Acad. Sci. USA 2001, 98, 8809–8814. [Google Scholar] [CrossRef] [Green Version]
- Broz, M.L.; Binnewies, M.; Boldajipour, B.; Nelson, A.E.; Pollack, J.L.; Erle, D.J.; Barczak, A.; Rosenblum, M.D.; Daud, A.; Barber, D.L.; et al. Dissecting the Tumor Myeloid Compartment Reveals Rare Activating Antigen-Presenting Cells Critical for T Cell Immunity. Cancer Cell 2014, 26, 938. [Google Scholar] [CrossRef] [PubMed]
- Ger, M.; Kaupinis, A.; Petrulionis, M.; Kurlinkus, B.; Cicenas, J.; Sileikis, A.; Valius, M.; Strupas, K. Proteomic Identification of FLT3 and PCBP3 as Potential Prognostic Biomarkers for Pancreatic Cancer. Anticancer Res. 2018, 38, 5759–5765. [Google Scholar] [CrossRef] [PubMed]
- Hammerich, L.; Marron, T.U.; Upadhyay, R.; Svensson-Arvelund, J.; Dhainaut, M.; Hussein, S.; Zhan, Y.; Ostrowski, D.; Yellin, M.; Marsh, H.; et al. Systemic clinical tumor regressions and potentiation of PD1 blockade with in situ vaccination. Nat. Med. 2019, 25, 814–824. [Google Scholar] [CrossRef] [PubMed]
- Barry, K.C.; Hsu, J.; Broz, M.L.; Cueto, F.J.; Binnewies, M.; Combes, A.J.; Nelson, A.E.; Loo, K.; Kumar, R.; Rosenblum, M.D.; et al. A natural killer-dendritic cell axis defines checkpoint therapy-responsive tumor microenvironments. Nat. Med. 2018, 24, 1178–1191. [Google Scholar] [CrossRef]
- Bottcher, J.P.; Reis e Sousa, C. The Role of Type 1 Conventional Dendritic Cells in Cancer Immunity. Trends Cancer 2018, 4, 784–792. [Google Scholar] [CrossRef] [Green Version]
- Salmon, H.; Idoyaga, J.; Rahman, A.; Leboeuf, M.; Remark, R.; Jordan, S.; Casanova-Acebes, M.; Khudoynazarova, M.; Agudo, J.; Tung, N.; et al. Expansion and Activation of CD103(+) Dendritic Cell Progenitors at the Tumor Site Enhances Tumor Responses to Therapeutic PD-L1 and BRAF Inhibition. Immunity 2016, 44, 924–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garg, A.D.; De Ruysscher, D.; Agostinis, P. Immunological metagene signatures derived from immunogenic cancer cell death associate with improved survival of patients with lung, breast or ovarian malignancies: A large-scale meta-analysis. Oncoimmunology 2016, 5, e1069938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gentles, A.J.; Newman, A.M.; Liu, C.L.; Bratman, S.V.; Feng, W.; Kim, D.; Nair, V.S.; Xu, Y.; Khuong, A.; Hoang, C.D.; et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat. Med. 2015, 21, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.M.; Liu, C.L.; Green, M.R.; Gentles, A.J.; Feng, W.; Xu, Y.; Hoang, C.D.; Diehn, M.; Alizadeh, A.A. Robust enumeration of cell subsets from tissue expression profiles. Nat. Methods 2015, 12, 453–457. [Google Scholar] [CrossRef] [Green Version]
- Melaiu, O.; Chierici, M.; Lucarini, V.; Jurman, G.; Conti, L.A.; De Vito, R.; Boldrini, R.; Cifaldi, L.; Castellano, A.; Furlanello, C.; et al. Cellular and gene signatures of tumor-infiltrating dendritic cells and natural-killer cells predict prognosis of neuroblastoma. Nat. Commun. 2020, 11, 5992. [Google Scholar] [CrossRef]
- Lyman, S.D.; Seaberg, M.; Hanna, R.; Zappone, J.; Brasel, K.; Abkowitz, J.L.; Prchal, J.T.; Schultz, J.C.; Shahidi, N.T. Plasma/serum levels of flt3 ligand are low in normal individuals and highly elevated in patients with Fanconi anemia and acquired aplastic anemia. Blood 1995, 86, 4091–4096. [Google Scholar] [CrossRef] [Green Version]
- Balan, S.; Arnold-Schrauf, C.; Abbas, A.; Couespel, N.; Savoret, J.; Imperatore, F.; Villani, A.C.; Vu Manh, T.P.; Bhardwaj, N.; Dalod, M. Large-Scale Human Dendritic Cell Differentiation Revealing Notch-Dependent Lineage Bifurcation and Heterogeneity. Cell Rep. 2018, 24, 1902–1915.e6. [Google Scholar] [CrossRef] [Green Version]
- Villani, A.C.; Satija, R.; Reynolds, G.; Sarkizova, S.; Shekhar, K.; Fletcher, J.; Griesbeck, M.; Butler, A.; Zheng, S.; Lazo, S.; et al. Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science 2017, 356, eaah4573. [Google Scholar] [CrossRef] [Green Version]
- Bota, D.A.; Chung, J.; Dandekar, M.; Carrillo, J.A.; Kong, X.T.; Fu, B.D.; Hsu, F.P.; Schonthal, A.H.; Hofman, F.M.; Chen, T.C.; et al. Phase II study of ERC1671 plus bevacizumab versus bevacizumab plus placebo in recurrent glioblastoma: Interim results and correlations with CD4(+) T-lymphocyte counts. CNS Oncol. 2018, 7, CNS22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bottcher, J.P.; Bonavita, E.; Chakravarty, P.; Blees, H.; Cabeza-Cabrerizo, M.; Sammicheli, S.; Rogers, N.C.; Sahai, E.; Zelenay, S.; Reis e Sousa, C. NK Cells Stimulate Recruitment of cDC1 into the Tumor Microenvironment Promoting Cancer Immune Control. Cell 2018, 172, 1022–1037.e14. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Huang, M.J.; Zhang, D.; Zhang, L.; Zhao, Y.; Yu, L.; Chang, Z.; Pei, H.Z.; Chen, M.C.; Xue, M.H.; et al. Tumor Associated Macrophages Express High-Levels of FLT3 Ligand, Which Induces Activation of FLT3 Signaling That Promotes Survival of Neoplastic Cells in B-Cell Acute Lymphoblastic Leukemia. Blood 2020, 136, 36–37. [Google Scholar] [CrossRef]
- Spranger, S.; Dai, D.; Horton, B.; Gajewski, T.F. Tumor-Residing Batf3 Dendritic Cells Are Required for Effector T Cell Trafficking and Adoptive T Cell Therapy. Cancer Cell 2017, 31, 711–723.e4. [Google Scholar] [CrossRef] [Green Version]
- Theisen, D.J.; Davidson, J.T.; Briseno, C.G.; Gargaro, M.; Lauron, E.J.; Wang, Q.; Desai, P.; Durai, V.; Bagadia, P.; Brickner, J.R.; et al. WDFY4 is required for cross-presentation in response to viral and tumor antigens. Science 2018, 362, 694–699. [Google Scholar] [CrossRef] [Green Version]
- Hildner, K.; Edelson, B.T.; Purtha, W.E.; Diamond, M.; Matsushita, H.; Kohyama, M.; Calderon, B.; Schraml, B.U.; Unanue, E.R.; Diamond, M.S.; et al. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science 2008, 322, 1097–1100. [Google Scholar] [CrossRef] [Green Version]
- Galluzzi, L.; Humeau, J.; Buque, A.; Zitvogel, L.; Kroemer, G. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2020, 17, 725–741. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Galluzzi, L.; Kepp, O.; Zitvogel, L. Immunogenic cell death in cancer therapy. Annu. Rev. Immunol. 2013, 31, 51–72. [Google Scholar] [CrossRef] [PubMed]
- Voorwerk, L.; Slagter, M.; Horlings, H.M.; Sikorska, K.; van de Vijver, K.K.; de Maaker, M.; Nederlof, I.; Kluin, R.J.C.; Warren, S.; Ong, S.; et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: The TONIC trial. Nat. Med. 2019, 25, 920–928. [Google Scholar] [CrossRef]
- Pfirschke, C.; Engblom, C.; Rickelt, S.; Cortez-Retamozo, V.; Garris, C.; Pucci, F.; Yamazaki, T.; Poirier-Colame, V.; Newton, A.; Redouane, Y.; et al. Immunogenic Chemotherapy Sensitizes Tumors to Checkpoint Blockade Therapy. Immunity 2016, 44, 343–354. [Google Scholar] [CrossRef] [Green Version]
- Kalanxhi, E.; Meltzer, S.; Schou, J.V.; Larsen, F.O.; Dueland, S.; Flatmark, K.; Jensen, B.V.; Hole, K.H.; Seierstad, T.; Redalen, K.R.; et al. Systemic immune response induced by oxaliplatin-based neoadjuvant therapy favours survival without metastatic progression in high-risk rectal cancer. Br. J. Cancer 2018, 118, 1322–1328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abrahamsson, H.; Jensen, B.V.; Berven, L.L.; Nielsen, D.L.; Saltyte Benth, J.; Johansen, J.S.; Larsen, F.O.; Johansen, J.S.; Ree, A.H. Antitumour immunity invoked by hepatic arterial infusion of first-line oxaliplatin predicts durable colorectal cancer control after liver metastasis ablation: 8–12 years of follow-up. Int. J. Cancer 2020, 146, 2019–2026. [Google Scholar] [CrossRef] [PubMed]


| n = 59 | % | |||
|---|---|---|---|---|
| Age | Median: 59 Years (Range 29 to 75) | |||
| Gender | Female | 22 | 37 | |
| Male | 37 | 63 | ||
| Tumor Site | Pancreas | 45 | 76 | |
| Stomach | 10 | 17 | ||
| CUP | 4 | 7 | ||
| Tumor Grade | Grade 2 | 27 | 46 | |
| Grade 3 NET | 18 | 30 | ||
| Grade 3 NEC | 14 | 24 | ||
| Ki67 Index | <15% | 14 | 24 | |
| ≥15% | 45 | 76 | ||
| Tumor Stage | IV | 52 | 88 | |
| I to III | 7 | 12 | ||
| Progression State at Blood Sampling | Complete Remission | 7 | 12 | |
| Stable Disease/Partial Remission | 24 | 41 | ||
| Progressive Disease | 26 | 44 | ||
| Treatment | Chemotherapy | 11 | 19 | |
| (At the Time of Blood Sampling) | Targeted Therapy | 2 | 3 | |
| PRRT | 1 | 2 | ||
| None | 45 | 76 | ||
| Prior Treatments | Grade 2 | Naïve | 8 | 14 |
| Pretreated | 21 | 35 | ||
| Grade 3 | Naïve | 8 | 14 | |
| Pretreated | 21 | 35 | ||
| Missing | 1 | 2 | ||
| Number of Treatment Modalities Before Blood Sampling | Grade 2 | Median: 1 (Range 1 to 7) | ||
| Grade 3 | Median: 1 (Range 1 to 5) | |||
| Type of Treatment | Streptozotocin/5-FU | 11 | 19 | |
| Temozolomide/Capecitabine | 9 | 15 | ||
| FOLFOX | 6 | 10 | ||
| Grade 2 | Somatostatin Analogs (SSA) | 5 | 8 | |
| Everolimus | 3 | 5 | ||
| PRRT | 2 | 3 | ||
| SIRT, Brachytherapy, TAE | 2 | 3 | ||
| Sunitinib/Bevacizumab | 1 | 2 | ||
| Irinotecan | 1 | 2 | ||
| Grade 3 | FOLFOX | 9 | 15 | |
| Temozolomide/Capecitabine | 7 | 12 | ||
| Cisplatin/Etoposide | 6 | 10 | ||
| Streptozotocin/5-FU | 5 | 8 | ||
| SSA | 3 | 5 | ||
| PRRT | 3 | 5 | ||
| FOLFIRI | 3 | 5 | ||
| Carboplatin/Etoposide | 2 | 3 | ||
| SIRT, Brachytherapy, TAE | 1 | 2 | ||
| Everolimus | 1 | 2 | ||
| Sunitinib/Bevacizumab | 1 | 2 | ||
| Capecitabine/Oxaliplatin | 1 | 2 | ||
| Carboplatin/Irinotecan | 1 | 2 | ||
| Carboplatin | 1 | 2 | ||
| Cisplatin | 1 | 2 | ||
| Dacarbacin | 1 | 2 | ||
| Checkpoint Inhibitor | 1 | 2 | ||
| Cisplatin/5-FU/Docetaxel | 1 | 2 |
| Combined Cohort | % | Charité Cohort | % | |||
|---|---|---|---|---|---|---|
| Number of Samples | n = 54 | 100 | n = 25 | 100 | ||
| Age | Median: 64 Years | 64 Years | 61 Years | |||
| Range: 32–79 Years | 32–79 Years | 32–74 Years | ||||
| Sex | Male | 38 | 70 | 17 | 68 | |
| Female | 16 | 30 | 8 | 32 | ||
| Tumor Site of Origin | Pancreas | 49 | 91 | 20 | 80 | |
| Stomach (All Grade 3) | 5 | 9 | 5 | 20 | ||
| Tissue Type | Pimary | 38 | 70 | 9 | 36 | |
| Local Recurrence | 1 | 2 | 1 | 4 | ||
| Metastasis | 15 | 28 | 15 | 60 | ||
| Tumor Stage (AJCC) | IA | 5 | 9 | 0 | 0 | |
| IB | 11 | 20 | 1 | 4 | ||
| IIA | 5 | 9 | 1 | 4 | ||
| IIB | 11 | 20 | 4 | 16 | ||
| III | 1 | 2 | 1 | 4 | ||
| IV | 21 | 39 | 18 | 72 | ||
| Tumor Grade | G1 | 14 | 26 | 0 | 0 | |
| G2 | 19 | 35 | 6 | 24 | ||
| G3 NET | 12 | 22 | 10 | 40 | ||
| G3 NEC | 9 | 17 | 9 | 36 | ||
| Prior Treatments | Naïve | N/A | 15 | 60 | ||
| Pretreated | N/A | 9 | 36 | |||
| Missing | N/A | 1 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Detjen, K.M.; Otto, R.; Giesecke, Y.; Geisler, L.; Riemer, P.; Jann, H.; Grötzinger, C.; Sers, C.; Pascher, A.; Lüdde, T.; et al. Elevated Flt3L Predicts Long-Term Survival in Patients with High-Grade Gastroenteropancreatic Neuroendocrine Neoplasms. Cancers 2021, 13, 4463. https://doi.org/10.3390/cancers13174463
Detjen KM, Otto R, Giesecke Y, Geisler L, Riemer P, Jann H, Grötzinger C, Sers C, Pascher A, Lüdde T, et al. Elevated Flt3L Predicts Long-Term Survival in Patients with High-Grade Gastroenteropancreatic Neuroendocrine Neoplasms. Cancers. 2021; 13(17):4463. https://doi.org/10.3390/cancers13174463
Chicago/Turabian StyleDetjen, Katharina M., Raik Otto, Yvonne Giesecke, Lukas Geisler, Pamela Riemer, Henning Jann, Carsten Grötzinger, Christine Sers, Andreas Pascher, Tom Lüdde, and et al. 2021. "Elevated Flt3L Predicts Long-Term Survival in Patients with High-Grade Gastroenteropancreatic Neuroendocrine Neoplasms" Cancers 13, no. 17: 4463. https://doi.org/10.3390/cancers13174463






