Photodynamic Therapy: A Compendium of Latest Reviews
Abstract
Simple Summary
Abstract
1. Introduction
2. Latest Reviews of PDT
2.1. Overviews
2.2. Specific Cancers and Meta-Analyses
2.2.1. Gastrointestinal Cancer
2.2.2. Skin Cancer
2.2.3. Lung Cancer
2.2.4. Prostate Cancer
2.2.5. Head and Neck Cancer
2.2.6. Breast Cancer
2.2.7. Brain Cancer
2.2.8. Bladder Cancer
2.3. Photosensitizers
2.4. PDT Mechanisms
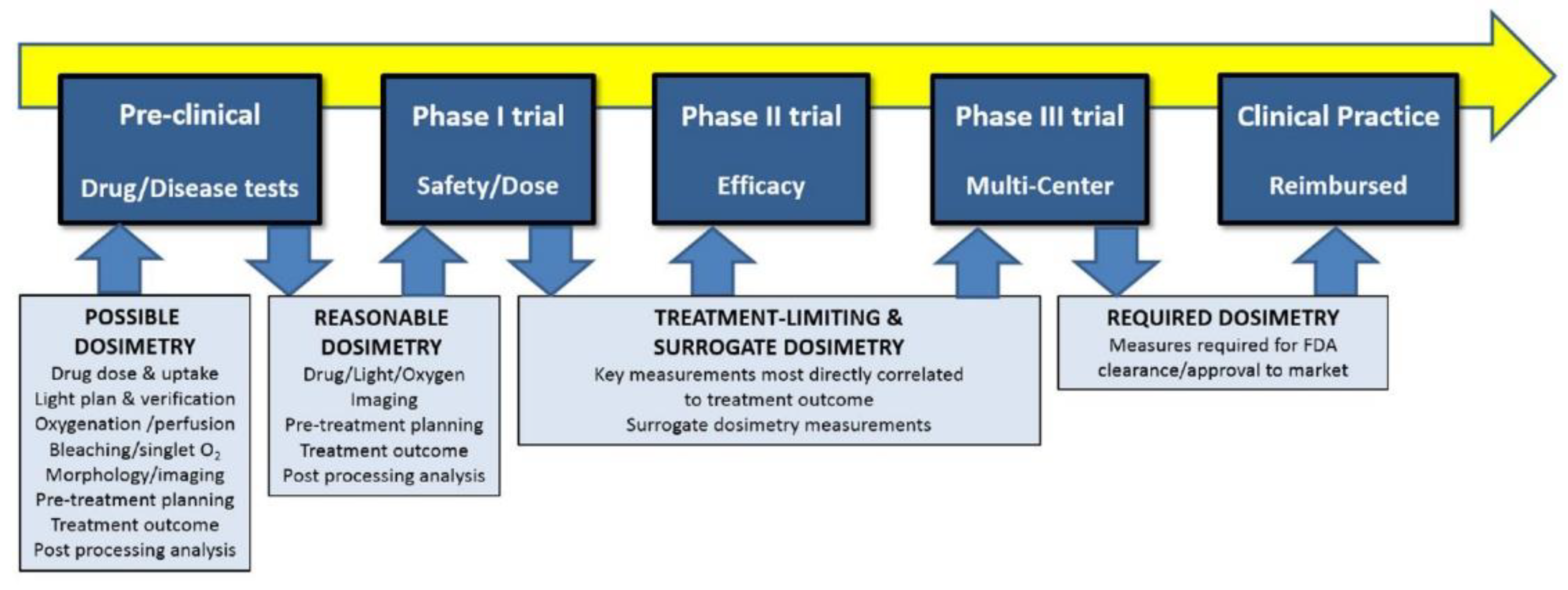
2.5. Dosimetry and Sources
3. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2018; Available online: https://gco.iarc.fr/today (accessed on 2 June 2021).
- Hamblin, M.R. Photodynamic Therapy for Cancer: What’s Past Is Prologue. Photochem. Photobiol. 2020, 96, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Ackroyd, R.; Kelty, C.; Brown, N.; Reed, M. The History of Photodetection and Photodynamic Therapy. Photochem. Photobiol. 2001, 74, 656–669. [Google Scholar] [CrossRef]
- Prime, J. Les Accidentes Toxiques Par L’eosinate de Sodium; Jouve and Boyer: Paris, France, 1900. [Google Scholar]
- Tappeiner, H.; Jesionek, A. Therapeutische Versuche Mit Fluoreszierenden Stoffen. Muench. Med. Wochenschr. 1903, 1, 2042–2044. [Google Scholar]
- Tappeiner, H.; Jodlbauer, A. Die Sensibilisierende Wirkung Fluorieszierender Substanzer. Gesammte Untersuchungen Uber Die Photodynamische Erscheinung; FCW Vogel: Leipzig, Germany, 1907. [Google Scholar]
- Diamond, I.; Mcdonagh, A.F.; Wilson, C.B.; Granelli, S.G.; Nielsen, S.; Jaenicke, R. Photodynamic Therapy of Malignant Tumours. Lancet 1972, 300, 1175–1177. [Google Scholar] [CrossRef]
- Dougherty, T.J.; Grindey, G.B.; Fiel, R.; Weishaupt, K.R.; Boyle, D.G. Photoradiation Therapy. II. Cure of Animal Tumors with Hematoporphyrin and Light. J. Natl. Cancer Inst. 1975, 55, 115–121. [Google Scholar] [CrossRef]
- Kelly, J.F.; Snell, M.E. Hematoporphyrin Derivative: A Possible Aid in the Diagnosis and Therapy of Carcinoma of the Bladder. J. Urol. 1976, 115, 150–151. [Google Scholar] [CrossRef]
- Dougherty, T.J.; Kaufman, J.E.; Goldfarb, A.; Weishaupt, K.R.; Boyle, D.; Mittleman, A. Photoradiation Therapy for the Treatment of Malignant Tumors. Cancer Res. 1978, 38, 2628–2635. [Google Scholar]
- U.S. National Library of Medicine. Available online: https://clinicaltrials.gov/ (accessed on 14 April 2021).
- Moan, J.; Berg, K. Photochemotherapy of Cancer: Experimental Research. Photochem. Photobiol. 1992, 55, 931–948. [Google Scholar] [CrossRef]
- Moan, J.; Peng, Q. An Outline of the Hundred-Year History of PDT. Anticancer Res. 2003, 23, 3591–3600. [Google Scholar]
- Barr, H.; Tralau, C.J.; Boulos, P.B.; MacRobert, A.J.; Tilly, R.; Bown, S.G. The Contrasting Mechanisms of Colonic Collagen Damage between Photodynamic Therapy and Thermal Injury. Photochem. Photobiol. 1987, 46, 795–800. [Google Scholar] [CrossRef]
- Aniogo, E.C.; Plackal Adimuriyil George, B.; Abrahamse, H. The Role of Photodynamic Therapy on Multidrug Resistant Breast Cancer. Cancer Cell Int. 2019, 19, 91. [Google Scholar] [CrossRef] [PubMed]
- Spring, B.Q.; Rizvi, I.; Xu, N.; Hasan, T. The Role of Photodynamic Therapy in Overcoming Cancer Drug Resistance. Photochem. Photobiol. Sci. 2015, 14, 1476–1491. [Google Scholar] [CrossRef]
- Kleinovink, J.W.; Van Driel, P.B.; Snoeks, T.J.; Prokopi, N.; Fransen, M.F.; Cruz, L.J.; Mezzanotte, L.; Chan, A.; Löwik, C.W.; Ossendorp, F. Combination of Photodynamic Therapy and Specific Immunotherapy Efficiently Eradicates Established Tumors. Clin. Cancer Res. 2016, 22, 1459–1468. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, I.J.; Dougherty, T.J. Basic Principles of Photodynamic Therapy. J. Porphyr. Phthalocyanines 2001, 5, 105–129. [Google Scholar] [CrossRef]
- Wilson, B.C. Photodynamic Therapy for Cancer: Principles. Can. J. Gastroenterol. 2002, 16, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, D.E.J.G.J.; Fukumura, D.; Jain, R.K. Photodynamic Therapy for Cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef]
- Brown, S.B.; Brown, E.A.; Walker, I. The Present and Future Role of Photodynamic Therapy in Cancer Treatment. Lancet Oncol. 2004, 5, 497–508. [Google Scholar] [CrossRef]
- Wilson, B.C.; Patterson, M.S. The Physics, Biophysics and Technology of Photodynamic Therapy. Phys. Med. Biol. 2008, 53, R61. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic Therapy of Cancer: An Update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- van Straten, D.; Mashayekhi, V.; de Bruijn, H.S.; Oliveira, S.; Robinson, D.J. Oncologic Photodynamic Therapy: Basic Principles, Current Clinical Status and Future Directions. Cancers 2017, 9, 19. [Google Scholar] [CrossRef]
- Dos Santos, A.F.; De Almeida, D.R.Q.; Terra, L.F.; Baptista, M.S.; Labriola, L. Photodynamic Therapy in Cancer Treatment–an Update Review. J. Cancer Metastasis Treat. 2019, 5, 1–20. [Google Scholar] [CrossRef]
- Yang, M.; Yang, T.; Mao, C. Enhancement of Photodynamic Cancer Therapy by Physical and Chemical Factors. Angew. Chem. Int. Ed. 2019, 58, 14066–14080. [Google Scholar] [CrossRef]
- RL, Y.; DW, B.; GS, R.; SJ, I.; ST, C. Photodynamic Therapy for Solid Tumors: A Review of the Literature. Photodermatol. Photoimmunol. Photomed. 2019, 35, 295–303. [Google Scholar] [CrossRef]
- Kessel, D. Photodynamic Therapy: A Brief History. J. Clin. Med. 2019, 8, 1581. [Google Scholar] [CrossRef]
- Li, X.; Lovell, J.F.; Yoon, J.; Chen, X. Clinical Development and Potential of Photothermal and Photodynamic Therapies for Cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar] [CrossRef]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic Therapy for the Treatment and Diagnosis of Cancer–A Review of the Current Clinical Status. Front. Chem. 2021, 9, 686303. [Google Scholar] [CrossRef]
- Ortner, M.-A. Photodynamic Therapy for Cholangiocarcinoma. Lasers Surg. Med. 2011, 43, 776–780. [Google Scholar] [CrossRef]
- Tomizawa, Y.; Tian, J. Photodynamic Therapy for Unresectable Cholangiocarcinoma. Dig. Dis. Sci. 2012, 57, 274–283. [Google Scholar] [CrossRef]
- Shishkova, N.; Kuznetsova, O.; Berezov, T. Photodynamic Therapy in Gastroenterology. J. Gastrointest. Cancer 2013, 44, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Rizk, N.; Kahaleh, M. Role of Photodynamic Therapy and Intraductal Radiofrequency Ablation in Cholangiocarcinoma. Best Pract. Res. Clin. Gastroenterol. 2015, 29, 309–318. [Google Scholar] [CrossRef]
- Kawczyk-Krupka, A.; Bugaj, A.M.; Latos, W.; Zaremba, K.; Wawrzyniec, K.; Sieroń, A. Photodynamic Therapy in Colorectal Cancer Treatment: The State of the Art in Clinical Trials; Elsevier: Amsterdam, The Netherlands, 2015; Volume 12, pp. 545–553. [Google Scholar]
- Bown, S.G. Photodynamic Therapy for Cancer of the Pancreas-The Story so Far. Photonics Lasers Med. 2016, 5, 91–100. [Google Scholar] [CrossRef]
- Kawczyk-Krupka, A.; Bugaj, A.M.; Latos, W.; Zaremba, K.; Wawrzyniec, K.; Kucharzewski, M.; Sieroń, A. Photodynamic Therapy in Colorectal Cancer Treatment-The State of the Art in Preclinical Research. Photodiagnosis Photodyn. Ther. 2016, 13, 158–174. [Google Scholar] [CrossRef]
- Moole, H.; Tathireddy, H.; Dharmapuri, S.; Moole, V.; Boddireddy, R.; Yedama, P.; Dharmapuri, S.; Uppu, A.; Bondalapati, N.; Duvvuri, A. Success of Photodynamic Therapy in Palliating Patients with Nonresectable Cholangiocarcinoma: A Systematic Review and Meta-Analysis. World J. Gastroenterol. 2017, 23, 1278–1288. [Google Scholar] [CrossRef]
- Hodgkinson, N.; Kruger, C.A.; Abrahamse, H. Targeted Photodynamic Therapy as Potential Treatment Modality for the Eradication of Colon Cancer and Colon Cancer Stem Cells. Tumor Biol. 2017, 39, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kaleta-Richter, M.; Kawczyk-Krupka, A.; Aebisher, D.; Bartusik-Aebisher, D.; Czuba, Z.; Cieślar, G. The Capability and Potential of New Forms of Personalized Colon Cancer Treatment: Immunotherapy and Photodynamic Therapy. Photodiagnosis Photodyn. Ther. 2019, 25, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Wang, F.; Zhou, J.-J.; Liu, X.; He, Q.; Wang, C.; Zheng, Y.-W.; Wen, Y.; Xiong, L. Application of Photodynamic Therapy for Liver Malignancies. J. Gastrointest. Oncol. 2020, 11, 431–442. [Google Scholar] [CrossRef]
- Nkune, N.W.; Kruger, C.A.; Abrahamse, H. Possible Enhancement of Photodynamic Therapy (PDT) Colorectal Cancer Treatment When Combined with Cannabidiol. Anticancer. Agents Med. Chem. 2021, 21, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liu, R.; Xu, Y.; Qian, L.; Dai, Z. Photosensitizer Nanoparticles Boost Photodynamic Therapy for Pancreatic Cancer Treatment. Nano-Micro Lett. 2021, 13, 1–16. [Google Scholar] [CrossRef]
- Fayter, D.; Corbett, M.; Heirs, M.; Fox, D.; Eastwood, A. A Systematic Review of Photodynamic Therapy in the Treatment of Precancerous Skin Conditions, Barrett’s Oesophagus and Cancers of the Biliary Tract, Brain, Head and Neck, Lung, Oesophagus and Skin. Health Technol. Assess. 2010, 14, 3–129. [Google Scholar] [CrossRef]
- Zhao, B.; He, Y.-Y. Recent Advances in the Prevention and Treatment of Skin Cancer Using Photodynamic Therapy. Expert Rev. Anticancer Ther. 2010, 10, 1797–1809. [Google Scholar] [CrossRef] [PubMed]
- Horlings, R.K.; Terra, J.B.; Witjes, M.J.H. MTHPC Mediated, Systemic Photodynamic Therapy (PDT) for Nonmelanoma Skin Cancers: Case and Literature Review. Lasers Surg. Med. 2015, 47, 779–787. [Google Scholar] [CrossRef]
- Lucena, S.R.; Salazar, N.; Gracia-Cazaña, T.; Zamarrón, A.; González, S.; Juarranz, Á.; Gilaberte, Y. Combined Treatments with Photodynamic Therapy for Non-Melanoma Skin Cancer. Int. J. Mol. Sci. 2015, 16, 25912–25933. [Google Scholar] [CrossRef]
- Griffin, L.L.; Lear, J.T. Photodynamic Therapy and Non-Melanoma Skin Cancer. Cancers 2016, 8, 98. [Google Scholar] [CrossRef]
- Cohen, D.K.; Lee, P.K. Photodynamic Therapy for Non-Melanoma Skin Cancers. Cancers 2016, 8, 90. [Google Scholar] [CrossRef]
- Erkiert-Polguj, A.; Halbina, A.; Polak-Pacholczyk, I.; Rotsztejn, H. Light-Emitting Diodes in Photodynamic Therapy in Non-Melanoma Skin Cancers–Own Observations and Literature Review. J. Cosmet. Laser Ther. 2016, 18, 105–110. [Google Scholar] [CrossRef]
- Yu, X.; Zheng, H.; Chan, M.T.V.; Wu, W.K.K. Immune Consequences Induced by Photodynamic Therapy in Non-Melanoma Skin Cancers: A Review. Environ. Sci. Pollut. Res. 2018, 25, 20569–20574. [Google Scholar] [CrossRef]
- Morton, C.A. A Synthesis of the World’s Guidelines on Photodynamic Therapy for Non-Melanoma Skin Cancer. Ital. J. Dermatol. Venereol. 2018, 153, 783–792. [Google Scholar] [CrossRef]
- Tampa, M.; Sarbu, M.I.; Matei, C.; Mitran, C.I.; Mitran, M.I.; Caruntu, C.; Constantin, C.; Neagu, M.; Georgescu, S.R. Photodynamic Therapy: A Hot Topic in Dermato-Oncology (Review). Oncol. Lett. 2019, 17, 4085–4093. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.C.; Fu, C.; Qin, L.; Zeng, X.Y.; Liu, Q. Photodynamic Therapy with Methyl-5-Aminolevulinate for Basal Cell Carcinoma: A Systematic Review and Meta-Analysis. Photodiagnosis Photodyn. Ther. 2020, 29, 101667. [Google Scholar] [CrossRef]
- Allegra, A.; Pioggia, G.; Tonacci, A.; Musolino, C.; Gangemi, S. Oxidative Stress and Photodynamic Therapy of Skin Cancers: Mechanisms, Challenges and Promising Developments. Antioxidants 2020, 9, 448. [Google Scholar] [CrossRef]
- Allison, R.; Moghissi, K.; Downie, G.; Dixon, K. Photodynamic Therapy (PDT) for Lung Cancer. Photodiagnosis Photodyn. Ther. 2011, 8, 231–239. [Google Scholar] [CrossRef]
- Ikeda, N.; Usuda, J.; Kato, H.; Ishizumi, T.; Ichinose, S.; Otani, K.; Honda, H.; Furukawa, K.; Okunaka, T.; Tsutsui, H. New Aspects of Photodynamic Therapy for Central Type Early Stage Lung Cancer. Lasers Surg. Med. 2011, 43, 749–754. [Google Scholar] [CrossRef]
- Chiaviello, A.; Postiglione, I.; Palumbo, G. Targets and Mechanisms of Photodynamic Therapy in Lung Cancer Cells: A Brief Overview. Cancers 2011, 3, 1014–1041. [Google Scholar] [CrossRef]
- Simone, C.B.; Friedberg, J.S.; Glatstein, E.; Stevenson, J.P.; Sterman, D.H.; Hahn, S.M.; Cengel, K.A. Photodynamic Therapy for the Treatment of Non-Small Cell Lung Cancer. J. Thorac. Dis. 2012, 4, 63–75. [Google Scholar] [PubMed]
- Kato, H. Our Experience with Photodynamic Diagnosis and Photodynamic Therapy for Lung Cancer. JNCCN J. Natl. Compr. Cancer Netw. 2012, 10, S3–S8. [Google Scholar] [CrossRef][Green Version]
- Simone, C.B.; Cengel, K.A. Photodynamic Therapy for Lung Cancer and Malignant Pleural Mesothelioma. Semin. Oncol. 2014, 41, 820–830. [Google Scholar] [CrossRef] [PubMed]
- Shafirstein, G.; Battoo, A.; Harris, K.; Baumann, H.; Gollnick, S.O.; Lindenmann, J.; Nwogu, C.E. Photodynamic Therapy of Non-Small Cell Lung Cancer Narrative Review and Future Directions. Ann. Am. Thorac. Soc. 2016, 13, 265–275. [Google Scholar] [CrossRef]
- Mokwena, M.G.; Kruger, C.A.; Ivan, M.T.; Heidi, A. A Review of Nanoparticle Photosensitizer Drug Delivery Uptake Systems for Photodynamic Treatment of Lung Cancer. Photodiagnosis Photodyn. Ther. 2018, 22, 147–154. [Google Scholar] [CrossRef]
- Wang, K.; Yu, B.; Pathak, J.L. An Update in Clinical Utilization of Photodynamic Therapy for Lung Cancer. J. Cancer 2021, 12, 1154–1160. [Google Scholar] [CrossRef]
- El-Hussein, A.; Manoto, S.L.; Ombinda-Lemboumba, S.; Alrowaili, Z.A.; Mthunzi-Kufa, P. A Review of Chemotherapy and Photodynamic Therapy for Lung Cancer Treatment. Anti-cancer Agents Med. Chem. 2021, 21, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Arumainayagam, N.; Moore, C.M.; Ahmed, H.U.; Emberton, M. Photodynamic Therapy for Focal Ablation of the Prostate. World J. Urol. 2010, 28, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.M.; Emberton, M.; Bown, S.G. Photodynamic Therapy for Prostate Cancer-an Emerging Approach for Organ-Confined Disease. Lasers Surg. Med. 2011, 43, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Kawczyk-Krupka, A.; Wawrzyniec, K.; Musiol, S.K.; Potempa, M.; Bugaj, A.M.; Sieroń, A. Treatment of Localized Prostate Cancer Using WST-09 and WST-11 Mediated Vascular Targeted Photodynamic Therapy-A Review. Photodiagnosis Photodyn. Ther. 2015, 12, 567–574. [Google Scholar] [CrossRef]
- Gheewala, T.; Skwor, T.; Munirathinam, G. Photosensitizers in Prostate Cancer Therapy. Oncotarget 2017, 8, 30524–30538. [Google Scholar] [CrossRef]
- Kleinclauss, F.; Frontczak, A.; Balssa, L.; Lebdai, S.; Azzouzi, R. Vascular Targeted Photodynamic Therapy in Low-Risk Prostate Cancer. A Literature Review. Prog. Urol. 2019, 29, 393–401. [Google Scholar] [CrossRef]
- Ferroni, C.; Del Rio, A.; Martini, C.; Manoni, E.; Varchi, G. Light-Induced Therapies for Prostate Cancer Treatment. Front. Chem. 2019, 7, 719. [Google Scholar] [CrossRef]
- Wang, L.; Yang, H.; Li, B. Photodynamic Therapy for Prostate Cancer: A Systematic Review and Meta-Analysis. Prostate Int. 2019, 7, 83–90. [Google Scholar] [CrossRef]
- Osuchowski, M.; Bartusik-Aebisher, D.; Osuchowski, F.; Aebisher, D. Photodynamic Therapy for Prostate Cancer–A Narrative Review. Photodiagnosis Photodyn. Ther. 2021, 33, 102158. [Google Scholar] [CrossRef]
- Guo, R.-Q.; Guo, X.-X.; Li, Y.-M.; Bie, Z.-X.; Li, B.; Li, X.-G. Cryoablation, High-Intensity Focused Ultrasound, Irreversible Electroporation, and Vascular-Targeted Photodynamic Therapy for Prostate Cancer: A Systemic Review and Meta-Analysis. Int. J. Clin. Oncol. 2021, 26, 461–484. [Google Scholar] [CrossRef]
- Quon, H.; Grossman, C.E.; Finlay, J.C.; Zhu, T.C.; Clemmens, C.S.; Malloy, K.M.; Busch, T.M. Photodynamic Therapy in the Management of Pre-Malignant Head and Neck Mucosal Dysplasia and Microinvasive Carcinoma. Photodiagnosis Photodyn. Ther. 2011, 8, 75–85. [Google Scholar] [CrossRef]
- Chen, H.-M.; Yu, C.-H.; Lin, H.-P.; Cheng, S.-J.; Chiang, C.-P. 5-Aminolevulinic Acid-Mediated Photodynamic Therapy for Oral Cancers and Precancers. J. Dent. Sci. 2012, 7, 307–315. [Google Scholar] [CrossRef]
- De Visscher, S.A.H.J.; Dijkstra, P.U.; Tan, I.B.; Roodenburg, J.L.N.; Witjes, M.J.H. MTHPC Mediated Photodynamic Therapy (PDT) of Squamous Cell Carcinoma in the Head and Neck: A Systematic Review. Oral Oncol. 2013, 49, 192–210. [Google Scholar] [CrossRef]
- Vohra, F.; Al-Kheraif, A.A.; Qadri, T.; Hassan, M.I.A.; Ahmed, A.; Warnakulasuriya, S.; Javed, F. Efficacy of Photodynamic Therapy in the Management of Oral Premalignant Lesions. A Systematic Review. Photodiagnosis Photodyn. Ther. 2015, 12, 150–159. [Google Scholar] [CrossRef]
- Saini, R.; Lee, N.V.; Liu, K.Y.P.; Poh, C.F. Prospects in the Application of Photodynamic Therapy in Oral Cancer and Premalignant Lesions. Cancers 2016, 8, 83. [Google Scholar] [CrossRef] [PubMed]
- Nelke, K.H.; Pawlak, W.; Leszczyszyn, J.; Gerber, H. Photodynamic Therapy in Head and Neck Cancer. Postepy Hig. Med. Dosw. 2014, 68, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Civantos, F.J.; Karakullukcu, B.; Biel, M.; Silver, C.E.; Rinaldo, A.; Saba, N.F.; Takes, R.P.; Vander Poorten, V.; Ferlito, A. A Review of Photodynamic Therapy for Neoplasms of the Head and Neck. Adv. Ther. 2018, 35, 324–340. [Google Scholar] [CrossRef] [PubMed]
- Meulemans, J.; Delaere, P.; Vander Poorten, V. Photodynamic Therapy in Head and Neck Cancer: Indications, Outcomes, and Future Prospects. Curr. Opin. Otolaryngol. Head Neck Surg. 2019, 27, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Adimuriyil George, B.P.; Abrahamse, H. A Review on Novel Breast Cancer Therapies: Photodynamic Therapy and Plant Derived Agent Induced Cell Death Mechanisms. Anti-Cancer Agents Med. Chem. 2016, 16, 793–801. [Google Scholar] [CrossRef]
- Banerjee, S.M.; MacRobert, A.J.; Mosse, C.A.; Periera, B.; Bown, S.G.; Keshtgar, M.R.S. Photodynamic Therapy: Inception to Application in Breast Cancer. Breast 2017, 31, 105–113. [Google Scholar] [CrossRef]
- Rakhimzhanova, R.I.; Shanazarov, N.A.; Turzhanova, D.E. Photodynamic Therapy of Intradermal Metastatic Breast Cancer (Literature Review). Biomed. Photonics 2019, 8, 36–42. [Google Scholar] [CrossRef]
- Montaseri, H.; Kruger, C.A.; Abrahamse, H. Review: Organic Nanoparticle Based Active Targeting for Photodynamic Therapy Treatment of Breast Cancer Cells. Oncotarget 2020, 11, 2120–2136. [Google Scholar] [CrossRef] [PubMed]
- Ostańska, E.; Aebisher, D.; Bartusik-Aebisher, D. The Potential of Photodynamic Therapy in Current Breast Cancer Treatment Methodologies. Biomed. Pharmacother. 2021, 137, 111302. [Google Scholar] [CrossRef] [PubMed]
- Bechet, D.; Mordon, S.R.; Guillemin, F.; Barberi-Heyob, M.A. Photodynamic Therapy of Malignant Brain Tumours: A Complementary Approach to Conventional Therapies. Cancer Treat. Rev. 2014, 40, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Quirk, B.J.; Brandal, G.; Donlon, S.; Vera, J.C.; Mang, T.S.; Foy, A.B.; Lew, S.M.; Girotti, A.W.; Jogal, S.; LaViolette, P.S.; et al. Photodynamic Therapy (PDT) for Malignant Brain Tumors—Where Do We Stand? Photodiagnosis Photodyn. Ther. 2015, 12, 530–544. [Google Scholar] [CrossRef]
- Akimoto, J. Photodynamic Therapy for Malignant Brain Tumors. Neurol. Med. Chir. 2016, 56, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Tzerkovsky, D.A.; Maslakov, E.A.; Bagrintsev, D.A.; Semak, I.A.; Protopovich, Y.L.; Chizh, A.G.; Tatur, A.A.; Fomenkov, I.S.; Stupak, D.S. The Role of Photodynamic Therapy in the Treatment of Primary, Recurrent and Metastatic Malignant Brain Tumors. Biomed. Photonics 2018, 7, 37–49. [Google Scholar] [CrossRef]
- Abdurashitov, A.; Tuchin, V.; Semyachkina-Glushkovskaya, O. Photodynamic Therapy of Brain Tumors and Novel Optical Coherence Tomography Strategies for in Vivo Monitoring of Cerebral Fluid Dynamics. J. Innov. Opt. Health Sci. 2020, 13, 2030004. [Google Scholar] [CrossRef]
- Yavari, N.; Andersson-Engels, S.; Segersten, U.; Malmstrom, P.-U. An Overview on Preclinical and Clinical Experiences with Photodynamic Therapy for Bladder Cancer. Can. J. Urol. 2011, 18, 5778–5786. [Google Scholar] [PubMed]
- Inoue, K. 5-Aminolevulinic Acid-Mediated Photodynamic Therapy for Bladder Cancer. Int. J. Urol. 2017, 24, 97–101. [Google Scholar] [CrossRef]
- Railkar, R.; Agarwal, P.K. Photodynamic Therapy in the Treatment of Bladder Cancer: Past Challenges and Current Innovations. Eur. Urol. Focus 2018, 4, 509–511. [Google Scholar] [CrossRef]
- Wen, X.; Li, Y.; Hamblin, M.R. Photodynamic Therapy in Dermatology beyond Non-Melanoma Cancer: An Update. Photodiagnosis Photodyn. Ther. 2017, 19, 140–152. [Google Scholar] [CrossRef]
- National Cancer Institute, Head and Neck Cancers. Available online: https://www.cancer.gov/types/head-and-neck/head-neck-fact-sheet (accessed on 10 July 2021).
- Lamberti, M.J.; Rumie Vittar, N.B.; Rivarola, V.A. Breast Cancer as Photodynamic Therapy Target: Enhanced Therapeutic Efficiency by Overview of Tumor Complexity. World J. Clin. Oncol. 2014, 5, 901–907. [Google Scholar] [CrossRef]
- Banerjee, S.M.; El-Sheikh, S.; Malhotra, A.; Mosse, C.A.; Parker, S.; Williams, N.R.; MacRobert, A.J.; Hamoudi, R.; Bown, S.G.; Keshtgar, M.R.S. Photodynamic Therapy in Primary Breast Cancer. J. Clin. Med. 2020, 9, 483. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Diaz, R.R.; Cho, K.S.; Lim, M.S.; Chung, J.S.; Kim, W.T.; Ham, W.S.; Choi, Y.D. Efficacy and Safety of Photodynamic Therapy for Recurrent, High Grade Nonmuscle Invasive Bladder Cancer Refractory or Intolerant to Bacille Calmette-Guérin Immunotherapy. J. Urol. 2013, 190, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in Photodynamic Therapy: Part One–Photosensitizers, Photochemistry and Cellular Localization. Photodiagnosis Photodyn. Ther. 2004, 1, 279–293. [Google Scholar] [CrossRef]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in Photodynamic Therapy: Part Three—Photosensitizer Pharmacokinetics, Biodistribution, Tumor Localization and Modes of Tumor Destruction. Photodiagnosis Photodyn. Ther. 2005, 2, 91–106. [Google Scholar] [CrossRef]
- Palumbo, G. Photodynamic Therapy and Cancer: A Brief Sightseeing Tour. Expert Opin. Drug Deliv. 2007, 4, 131–148. [Google Scholar] [CrossRef]
- Kudinova, N.V.; Berezov, T.T. Photodynamic Therapy of Cancer: Search for Ideal Photosensitizer. Biochem. Suppl. Ser. B Biomed. Chem. 2010, 4, 95–103. [Google Scholar] [CrossRef]
- Anand, S.; Ortel, B.J.; Pereira, S.P.; Hasan, T.; Maytin, E.V. Biomodulatory Approaches to Photodynamic Therapy for Solid Tumors. Cancer Lett. 2012, 326, 8–16. [Google Scholar] [CrossRef]
- Stockert, J.C.; Cañete, M.; Juarranz, A.; Villanueva, A.; Horobin, R.W.; Borrell, J.I.; Teixidó, J.; Nonell, S. Porphycenes: Facts and Prospects in Photodynamic Therapy of Cancer. Curr. Med. Chem. 2007, 14, 997–1026. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, P. Methyl Aminolaevulinate-Photodynamic Therapy: A Review of Clinical Trials in the Treatment of Actinic Keratoses and Nonmelanoma Skin Cancer. Br. J. Dermatol. 2007, 156, 793–801. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, A.E.; Gallagher, W.M.; Byrne, A.T. Porphyrin and Nonporphyrin Photosensitizers in Oncology: Preclinical and Clinical Advances in Photodynamic Therapy. Photochem. Photobiol. 2009, 85, 1053–1074. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.R.; Sibata, C.H. Oncologic Photodynamic Therapy Photosensitizers: A Clinical Review. Photodiagnosis Photodyn. Ther. 2010, 7, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Wachowska, M.; Muchowicz, A.; Firczuk, M.; Gabrysiak, M.; Winiarska, M.; Wańczyk, M.; Bojarczuk, K.; Golab, J. Aminolevulinic Acid (Ala) as a Prodrug in Photodynamic Therapy of Cancer. Molecules 2011, 16, 4140–4164. [Google Scholar] [CrossRef]
- Zeitouni, N.C.; Paquette, A.D.; Housel, J.P.; Shi, Y.; Wilding, G.E.; Foster, T.H.; Henderson, B.W. A Retrospective Review of Pain Control by a Two-Step Irradiance Schedule during Topical ALA-Photodynamic Therapy of Non-Melanoma Skin Cancer. Lasers Surg. Med. 2013, 45, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, R.; Lee, J.; Yang, S.-G. Clinical Development of Photodynamic Agents and Therapeutic Applications. Biomater. Res. 2018, 22, 25. [Google Scholar] [CrossRef] [PubMed]
- Díez Valle, R.; Hadjipanayis, C.G.; Stummer, W. Established and Emerging Uses of 5-ALA in the Brain: An Overview. J. Neurooncol. 2019, 141, 487–494. [Google Scholar] [CrossRef]
- Santos, K.L.M.; Barros, R.M.; da Silva Lima, D.P.; Nunes, A.M.A.; Sato, M.R.; Faccio, R.; de Lima Damasceno, B.P.G.; Oshiro-Junior, J.A. Prospective Application of Phthalocyanines in the Photodynamic Therapy against Microorganisms and Tumor Cells: A Mini-Review. Photodiagnosis Photodyn. Ther. 2020, 32, 102032. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; De Zheng, B.; Peng, X.H.; Li, S.Z.; Ying, J.W.; Zhao, Y.; Huang, J.D.; Yoon, J. Phthalocyanines as Medicinal Photosensitizers: Developments in the Last Five Years. Coord. Chem. Rev. 2019, 379, 147–160. [Google Scholar] [CrossRef]
- Dąbrowski, J.M.; Pucelik, B.; Regiel-Futyra, A.; Brindell, M.; Mazuryk, O.; Kyzioł, A.; Stochel, G.; Macyk, W.; Arnaut, L.G. Engineering of Relevant Photodynamic Processes through Structural Modifications of Metallotetrapyrrolic Photosensitizers. Coord. Chem. Rev. 2016, 325, 67–101. [Google Scholar] [CrossRef]
- Yuan, A.; Wu, J.; Tang, X.; Zhao, L.; Xu, F.; Hu, Y. Application of Near-Infrared Dyes for Tumor Imaging, Photothermal, and Photodynamic Therapies. J. Pharm. Sci. 2013, 102, 6–28. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Wu, J.B.; Pan, D. Review on Near-Infrared Heptamethine Cyanine Dyes as Theranostic Agents for Tumor Imaging, Targeting, and Photodynamic Therapy. J. Biomed. Opt. 2016, 21, 050901. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, C.; Rees, T.W.; Ke, L.; Ji, L.; Chao, H. Harnessing Ruthenium(II) as Photodynamic Agents: Encouraging Advances in Cancer Therapy. Coord. Chem. Rev. 2018, 363, 17–28. [Google Scholar] [CrossRef]
- Monro, S.; Colón, K.L.; Yin, H.; Roque, J.; Konda, P.; Gujar, S.; Thummel, R.P.; Lilge, L.; Cameron, C.G.; McFarland, S.A. Transition Metal Complexes and Photodynamic Therapy from a Tumor-Centered Approach: Challenges, Opportunities, and Highlights from the Development of TLD1433. Chem. Rev. 2019, 119, 797–828. [Google Scholar] [CrossRef]
- Chinna Ayya Swamy, P.; Sivaraman, G.; Priyanka, R.N.; Raja, S.O.; Ponnuvel, K.; Shanmugpriya, J.; Gulyani, A. Near Infrared (NIR) Absorbing Dyes as Promising Photosensitizer for Photodynamic Therapy. Coord. Chem. Rev. 2020, 411, 213233. [Google Scholar] [CrossRef]
- Lo, P.-C.; Rodríguez-Morgade, M.S.; Pandey, R.K.; Ng, D.K.P.; Torres, T.; Dumoulin, F. The Unique Features and Promises of Phthalocyanines as Advanced Photosensitisers for Photodynamic Therapy of Cancer. Chem. Soc. Rev. 2020, 49, 1041–1056. [Google Scholar] [CrossRef]
- Pucelik, B.; Sułek, A.; Dąbrowski, J.M. Bacteriochlorins and Their Metal Complexes as NIR-Absorbing Photosensitizers: Properties, Mechanisms, and Applications. Coord. Chem. Rev. 2020, 416, 213340. [Google Scholar] [CrossRef]
- Mfouo-Tynga, I.S.; Dias, L.D.; Inada, N.M.; Kurachi, C. Features of Third Generation Photosensitizers Used in Anticancer Photodynamic Therapy: Review. Photodiagnosis Photodyn. Ther. 2021, 34, 102091. [Google Scholar] [CrossRef] [PubMed]
- Gomez, S.; Tsung, A.; Hu, Z. Current Targets and Bioconjugation Strategies in Photodynamic Diagnosis and Therapy of Cancer. Molecules 2020, 25, 4964. [Google Scholar] [CrossRef]
- Pereira, P.M.R.; Korsak, B.; Sarmento, B.; Schneider, R.J.; Fernandes, R.; Tomé, J.P.C. Antibodies Armed with Photosensitizers: From Chemical Synthesis to Photobiological Applications. Org. Biomol. Chem. 2015, 13, 2518–2529. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.R.G.; Fernandes, R.; Sarmento, B.; Pereira, P.M.R.; Tomé, J.P.C. Photoimmunoconjugates: Novel Synthetic Strategies to Target and Treat Cancer by Photodynamic Therapy. Org. Biomol. Chem. 2019, 17, 2579–2593. [Google Scholar] [CrossRef]
- Sandland, J.; Boyle, R.W. Photosensitizer Antibody-Drug Conjugates: Past, Present, and Future. Bioconjug. Chem. 2019, 30, 975–993. [Google Scholar] [CrossRef]
- Mesquita, M.Q.; Dias, C.J.; Gamelas, S.; Fardilha, M.; Neves, M.G.P.M.S.; Faustino, M.A.F. An Insight on the Role of Photosensitizer Nanocarriers for Photodynamic Therapy. An. Acad. Bras. Cienc. 2018, 90, 1101–1130. [Google Scholar] [CrossRef]
- Oba, T. Photosensitizer Nanoparticles for Photodynamic Therapy. Curr. Bioact. Compd. 2007, 3, 239–251. [Google Scholar] [CrossRef]
- Juzenas, P.; Chen, W.; Sun, Y.-P.; Coelho, M.A.N.; Generalov, R.; Generalova, N.; Christensen, I.L. Quantum Dots and Nanoparticles for Photodynamic and Radiation Therapies of Cancer. Adv. Drug Deliv. Rev. 2008, 60, 1600–1614. [Google Scholar] [CrossRef]
- Chen, W. Nanoparticle Self-Lighting Photodynamic Therapy for Cancer Treatment. J. Biomed. Nanotechnol. 2008, 4, 369–376. [Google Scholar] [CrossRef]
- Li, W.-T. Nanotechology-Based Strategies to Enhance the Efficacy of Photodynamic Therapy for Cancers. Curr. Drug Metab. 2010, 10, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wei, S.; Ge, X.; Zhou, J.; Yu, B.; Shen, J. External Heavy-Atomic Construction of Photosensitizer Nanoparticles for Enhanced in Vitro Photodynamic Therapy of Cancer. J. Phys. Chem. B 2012, 116, 12744–12749. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.K.; Heo, J.; Shin, S.; Jeong, K.; Seo, Y.H.; Jang, W.D.; Park, C.R.; Park, S.Y.; Kim, S.; Kwon, I.C. Nanophotosensitizers toward Advanced Photodynamic Therapy of Cancer. Cancer Lett. 2013, 334, 176–187. [Google Scholar] [CrossRef]
- Debele, T.A.; Peng, S.; Tsai, H.C. Drug Carrier for Photodynamic Cancer Therapy. Int. J. Mol. Sci. 2015, 16, 22094–22136. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Shuhendler, A.J.; Ye, D.; Xu, J.J.; Chen, H.Y. Two-Photon Excitation Nanoparticles for Photodynamic Therapy. Chem. Soc. Rev. 2016, 45, 6725–6741. [Google Scholar] [CrossRef]
- Calixto, G.M.F.; Bernegossi, J.; De Freitas, L.M.; Fontana, C.R.; Chorilli, M.; Grumezescu, A.M. Nanotechnology-Based Drug Delivery Systems for Photodynamic Therapy of Cancer: A Review. Molecules 2016, 21, 342. [Google Scholar] [CrossRef]
- Hong, E.J.; Choi, D.G.; Shim, M.S. Targeted and Effective Photodynamic Therapy for Cancer Using Functionalized Nanomaterials. Acta Pharm. Sin. B 2016, 6, 297–307. [Google Scholar] [CrossRef]
- Li, X.; Kolemen, S.; Yoon, J.; Akkaya, E.U. Activatable Photosensitizers: Agents for Selective Photodynamic Therapy. Adv. Funct. Mater. 2017, 27, 1604053. [Google Scholar] [CrossRef]
- Abrahamse, H.; Kruger, C.A.; Kadanyo, S.; Mishra, A. Nanoparticles for Advanced Photodynamic Therapy of Cancer. Photomed. Laser Surg. 2017, 35, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Chizenga, E.P.; Abrahamse, H. Nanotechnology in Modern Photodynamic Therapy of Cancer: A Review of Cellular Resistance Patterns Affecting the Therapeutic Response. Pharmaceutics 2020, 12, 632. [Google Scholar] [CrossRef]
- Molaei, M.J. Two-Dimensional (2D) Materials beyond Graphene in Cancer Drug Delivery, Photothermal and Photodynamic Therapy, Recent Advances and Challenges Ahead: A Review. J. Drug Deliv. Sci. Technol. 2020, 61, 101830. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, W.; Zhang, T.; Jiang, X.; Hu, Y. Hybrid Nanoparticle Composites Applied to Photodynamic Therapy: Strategies and Applications. J. Mater. Chem. B 2020, 8, 4726–4737. [Google Scholar] [CrossRef] [PubMed]
- Sztandera, K.; Gorzkiewicz, M.; Klajnert-Maculewicz, B. Nanocarriers in Photodynamic Therapy—In Vitro and In Vivo Studies. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1509. [Google Scholar] [CrossRef]
- Shibu, E.S.; Hamada, M.; Murase, N.; Biju, V. Nanomaterials Formulations for Photothermal and Photodynamic Therapy of Cancer. J. Photochem. Photobiol. C Photochem. Rev. 2013, 15, 53–72. [Google Scholar] [CrossRef]
- Bugaj, A.M. Targeted Photodynamic Therapy–A Promising Strategy of Tumor Treatment. Photochem. Photobiol. Sci. 2011, 10, 1097–1109. [Google Scholar] [CrossRef]
- Lin, L.; Xiong, L.; Wen, Y.; Lei, S.; Deng, X.; Liu, Z.; Chen, W.; Miao, X. Active Targeting of Nano-Photosensitizer Delivery Systems for Photodynamic Therapy of Cancer Stem Cells. J. Biomed. Nanotechnol. 2015, 11, 531–554. [Google Scholar] [CrossRef]
- Weijer, R.; Broekgaarden, M.; Kos, M.; van Vught, R.; Rauws, E.A.J.; Breukink, E.; van Gulik, T.M.; Storm, G.; Heger, M. Enhancing Photodynamic Therapy of Refractory Solid Cancers: Combining Second-Generation Photosensitizers with Multi-Targeted Liposomal Delivery. J. Photochem. Photobiol. C Photochem. Rev. 2015, 23, 103–131. [Google Scholar] [CrossRef]
- Šošić, L.; Selbo, P.K.; Kotkowska, Z.K.; Kündig, T.M.; Høgset, A.; Johansen, P. Photochemical Internalization: Light Paves Way for New Cancer Chemotherapies and Vaccines. Cancers 2020, 12, 165. [Google Scholar] [CrossRef]
- Zhou, Z.; Song, J.; Nie, L.; Chen, X. Reactive Oxygen Species Generating Systems Meeting Challenges of Photodynamic Cancer Therapy. Chem. Soc. Rev. 2016, 45, 6597–6626. [Google Scholar] [CrossRef]
- Kessel, D. Apoptosis, Paraptosis and Autophagy: Death and Survival Pathways Associated with Photodynamic Therapy. Photochem. Photobiol. 2019, 95, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Nowis, D.; Makowski, M.; Stokłosa, T.; Legat, M.; Issat, T.; Goła̧b, J. Direct Tumor Damage Mechanisms of Photodynamic Therapy. Acta Biochim. Pol. 2005, 52, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Plaetzer, K.; Kiesslich, T.; Oberdanner, C.B.; Krammer, B. Apoptosis Following Photodynamic Tumor Therapy: Induction, Mechanisms and Detection. Curr. Pharm. Des. 2005, 11, 1151–1165. [Google Scholar] [CrossRef] [PubMed]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in Photodynamic Therapy: Part Two–Cellular Signaling, Cell Metabolism and Modes of Cell Death. Photodiagnosis Photodyn. Ther. 2005, 2, 1–23. [Google Scholar] [CrossRef]
- Robertson, C.A.; Evans, D.H.; Abrahamse, H. Photodynamic Therapy (PDT): A Short Review on Cellular Mechanisms and Cancer Research Applications for PDT. J. Photochem. Photobiol. B Biol. 2009, 96, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mroz, P.; Yaroslavsky, A.; Kharkwal, G.B.; Hamblin, M.R. Cell Death Pathways in Photodynamic Therapy of Cancer. Cancers 2011, 3, 2516–2539. [Google Scholar] [CrossRef]
- Kessel, D.; Oleinick, N.L. Cell Death Pathways Associated with Photodynamic Therapy: An Update. Photochem. Photobiol. 2018, 94, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Kessel, D. Photodynamic Therapy: Apoptosis, Paraptosis and Beyond. Apoptosis 2020, 25, 611–615. [Google Scholar] [CrossRef]
- Yoo, J.O.; Ha, K.S. New Insights into the Mechanisms for Photodynamic Therapy-Induced Cancer Cell Death. Int. Rev. Cell Mol. Biol. 2012, 295, 139–174. [Google Scholar] [PubMed]
- Kushibiki, T.; Hirasawa, T.; Okawa, S.; Ishihara, M. Responses of Cancer Cells Induced by Photodynamic Therapy. J. Healthc. Eng. 2013, 4, 87–108. [Google Scholar] [CrossRef]
- Allison, R.R.; Moghissi, K. Photodynamic Therapy (PDT): PDT Mechanisms. Clin. Endosc. 2013, 46, 24–29. [Google Scholar] [CrossRef]
- Milla Sanabria, L.; Rodríguez, M.E.; Cogno, I.S.; Rumie Vittar, N.B.; Pansa, M.F.; Lamberti, M.J.; Rivarola, V.A. Direct and Indirect Photodynamic Therapy Effects on the Cellular and Molecular Components of the Tumor Microenvironment. Biochim. Biophys. Acta-Rev. Cancer 2013, 1835, 36–45. [Google Scholar] [CrossRef]
- Hwang, H.S.; Shin, H.; Han, J.; Na, K. Combination of Photodynamic Therapy (PDT) and Anti-Tumor Immunity in Cancer Therapy. J. Pharm. Investig. 2018, 48, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.D.; Agostinis, P. ER Stress, Autophagy and Immunogenic Cell Death in Photodynamic Therapy-Induced Anti-Cancer Immune Responses. Photochem. Photobiol. Sci. 2014, 13, 474–487. [Google Scholar] [CrossRef]
- Zawacka-Pankau, J.; Krachulec, J.; Grulkowski, I.; Bielawski, K.P.; Selivanova, G. The P53-Mediated Cytotoxicity of Photodynamic Therapy of Cancer: Recent Advances. Toxicol. Appl. Pharmacol. 2008, 232, 487–497. [Google Scholar] [CrossRef]
- Bhuvaneswari, R.; Gan, Y.Y.; Soo, K.C.; Olivo, M. The Effect of Photodynamic Therapy on Tumor Angiogenesis. Cell. Mol. Life Sci. 2009, 66, 2275–2283. [Google Scholar] [CrossRef]
- Broekgaarden, M.; Weijer, R.; van Gulik, T.M.; Hamblin, M.R.; Heger, M. Tumor Cell Survival Pathways Activated by Photodynamic Therapy: A Molecular Basis for Pharmacological Inhibition Strategies. Cancer Metastasis Rev. 2015, 34, 643–690. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Gao, J.; Wei, Q. Combination of Photodynamic Therapy with Radiotherapy for Cancer Treatment. J. Nanomater. 2016, 2016, 8507924. [Google Scholar] [CrossRef]
- Castano, A.P.; Mroz, P.; Hamblin, M.R. Photodynamic Therapy and Anti-Tumour Immunity. Nat. Rev. Cancer 2006, 6, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.D.; Nowis, D.; Golab, J.; Agostinis, P. Photodynamic Therapy: Illuminating the Road from Cell Death towards Anti-Tumour Immunity. Apoptosis 2010, 15, 1050–1071. [Google Scholar] [CrossRef]
- Mroz, P.; Hashmi, J.T.; Huang, Y.-Y.; Lange, N.; Hamblin, M.R. Stimulation of Anti-Tumor Immunity by Photodynamic Therapy. Expert Rev. Clin. Immunol. 2011, 7, 75–91. [Google Scholar] [CrossRef]
- Garg, A.D.; Krysko, D.V.; Vandenabeele, P.; Agostinis, P. DAMPs and PDT-Mediated Photo-Oxidative Stress: Exploring the Unknown. Photochem. Photobiol. Sci. 2011, 10, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.E.; Cogno, I.S.; Milla Sanabria, L.S.; Morán, Y.S.; Rivarola, V.A. Heat Shock Proteins in the Context of Photodynamic Therapy: Autophagy, Apoptosis and Immunogenic Cell Death. Photochem. Photobiol. Sci. 2016, 15, 1090–1102. [Google Scholar] [CrossRef]
- Nath, S.; Obaid, G.; Hasan, T. The Course of Immune Stimulation by Photodynamic Therapy: Bridging Fundamentals of Photochemically Induced Immunogenic Cell Death to the Enrichment of T-Cell Repertoire. Photochem. Photobiol. 2019, 95, 1288–1305. [Google Scholar] [CrossRef]
- Asadzadeh, Z.; Safarzadeh, E.; Safaei, S.; Baradaran, A.; Mohammadi, A.; Hajiasgharzadeh, K.; Derakhshani, A.; Argentiero, A.; Silvestris, N.; Baradaran, B. Current Approaches for Combination Therapy of Cancer: The Role of Immunogenic Cell Death. Cancers 2020, 12, 1047. [Google Scholar] [CrossRef]
- Beltrán Hernández, I.; Yu, Y.; Ossendorp, F.; Korbelik, M.; Oliveira, S. Preclinical and Clinical Evidence of Immune Responses Triggered in Oncologic Photodynamic Therapy: Clinical Recommendations. J. Clin. Med. 2020, 9, 333. [Google Scholar] [CrossRef]
- Brackett, C.M.; Gollnick, S.O. Photodynamic Therapy Enhancement of Anti-Tumor Immunity. Photochem. Photobiol. Sci. 2011, 10, 649–652. [Google Scholar] [CrossRef] [PubMed]
- Panzarini, E.; Inguscio, V.; Dini, L. Immunogenic Cell Death: Can It Be Exploited in Photodynamic Therapy for Cancer? Biomed. Res. Int. 2013, 2013, 482160. [Google Scholar] [CrossRef] [PubMed]
- Anzengruber, F.; Avci, P.; De Freitas, L.F.; Hamblin, M.R. T-Cell Mediated Anti-Tumor Immunity after Photodynamic Therapy: Why Does It Not Always Work and How Can We Improve It? Photochem. Photobiol. Sci. 2015, 14, 1492–1509. [Google Scholar] [CrossRef] [PubMed]
- Alzeibak, R.; Mishchenko, T.A.; Shilyagina, N.Y.; Balalaeva, I.V.; Vedunova, M.V.; Krysko, D.V. Targeting Immunogenic Cancer Cell Death by Photodynamic Therapy: Past, Present and Future. J. Immunother. Cancer 2021, 9, e001926. [Google Scholar] [CrossRef]
- Dang, J.; He, H.; Chen, D.; Yin, L. Manipulating Tumor Hypoxia toward Enhanced Photodynamic Therapy (PDT). Biomater. Sci. 2017, 5, 1500–1511. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kwon, N.; Guo, T.; Liu, Z.; Yoon, J. Innovative Strategies for Hypoxic-Tumor Photodynamic Therapy. Angew. Chem. Int. Ed. 2018, 57, 11522–11531. [Google Scholar] [CrossRef]
- Pucelik, B.; Sułek, A.; Barzowska, A.; Dąbrowski, J.M. Recent Advances in Strategies for Overcoming Hypoxia in Photodynamic Therapy of Cancer. Cancer Lett. 2020, 492, 116–135. [Google Scholar] [CrossRef]
- Wei, F.; Rees, T.W.; Liao, X.; Ji, L.; Chao, H. Oxygen Self-Sufficient Photodynamic Therapy. Coord. Chem. Rev. 2021, 432, 213714. [Google Scholar] [CrossRef]
- Hu, T.; Wang, Z.; Shen, W.; Liang, R.; Yan, D.; Wei, M. Recent Advances in Innovative Strategies for Enhanced Cancer Photodynamic Therapy. Theranostics 2021, 11, 3278–3300. [Google Scholar] [CrossRef]
- De Vijlder, H.C.; Sterenborg, H.J.C.M.; Martino Neumann, H.A.; Robinson, D.J.; De Haas, E.R.M. Light Fractionation Significantly Improves the Response of Superficial Basal Cell Carcinoma to Aminolaevulinic Acid Photodynamic Therapy: Five-Year Follow-up of a Randomized, Prospective Trial. Acta Derm. Venereol. 2012, 92, 641–647. [Google Scholar] [CrossRef]
- Shams, M.; Owczarczak, B.; Manderscheid-Kern, P.; Bellnier, D.A.; Gollnick, S.O. Development of Photodynamic Therapy Regimens That Control Primary Tumor Growth and Inhibit Secondary Disease. Cancer Immunol. Immunother. 2015, 64, 287–297. [Google Scholar] [CrossRef]
- Zuluaga, M.-F.; Lange, N. Combination of Photodynamic Therapy with Anti-Cancer Agents. Curr. Med. Chem. 2008, 15, 1655–1673. [Google Scholar] [CrossRef]
- Dąbrowski, J.M.; Arnaut, L.G. Photodynamic Therapy (PDT) of Cancer: From Local to Systemic Treatment. Photochem. Photobiol. Sci. 2015, 14, 1765–1780. [Google Scholar] [CrossRef]
- Wilson, B.C.; Patterson, M.S.; Lilge, L. Implicit and Explicit Dosimetry in Photodynamic Therapy: A New Paradigm. Lasers Med. Sci. 1997, 12, 182–199. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.C.; Finlay, J.C.; Wilson, B. TH-A-T-6C-01: Photodynamic Therapy: Fundamentals and Dosimetry. Med. Phys. 2005, 32, 2150. [Google Scholar] [CrossRef]
- Kim, M.M.; Darafsheh, A. Light Sources and Dosimetry Techniques for Photodynamic Therapy. Photochem. Photobiol. 2020, 96, 280–294. [Google Scholar] [CrossRef] [PubMed]
- Pogue, B.W.; Elliott, J.T.; Kanick, S.C.; Davis, S.C.; Samkoe, K.S.; Maytin, E.V.; Pereira, S.P.; Hasan, T. Revisiting Photodynamic Therapy Dosimetry: Reductionist & Surrogate Approaches to Facilitate Clinical Success. Phys. Med. Biol. 2016, 61, R57–R89. [Google Scholar]
- Jacques, S.L. How Tissue Optics Affect Dosimetry of Photodynamic Therapy. J. Biomed. Opt. 2010, 15, 051608. [Google Scholar] [CrossRef]
- Swartling, J.; Axelsson, J.; Ahlgren, G.; Kälkner, K.M.; Nilsson, S.; Svanberg, S.; Svanberg, K.; Andersson-Engels, S. System for Interstitial Photodynamic Therapy with Online Dosimetry: First Clinical Experiences of Prostate Cancer. J. Biomed. Opt. 2010, 15, 058003. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lin, L.; Lin, H.; Wilson, B.C. Photosensitized Singlet Oxygen Generation and Detection: Recent Advances and Future Perspectives in Cancer Photodynamic Therapy. J. Biophotonics 2016, 9, 1314–1325. [Google Scholar] [CrossRef]
- Kim, M.M.; Ghogare, A.A.; Greer, A.; Zhu, T.C. On the in Vivo Photochemical Rate Parameters for PDT Reactive Oxygen Species Modeling. Phys. Med. Biol. 2017, 62, R1–R48. [Google Scholar] [CrossRef]
- Mallidi, S.; Spring, B.Q.; Chang, S.; Vakoc, B.; Hasan, T. Optical Imaging, Photodynamic Therapy and Optically Triggered Combination Treatments. Cancer J. 2015, 21, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Celli, J.P.; Spring, B.Q.; Rizvi, I.; Evans, C.L.; Samkoe, K.S.; Verma, S.; Pogue, B.W.; Hasan, T. Imaging and Photodynamic Therapy: Mechanisms, Monitoring, and Optimization. Chem. Rev. 2010, 110, 2795–2838. [Google Scholar] [CrossRef] [PubMed]
- De Silva, P.; Saad, M.A.; Thomsen, H.C.; Bano, S.; Ashraf, S.; Hasan, T. Photodynamic Therapy, Priming and Optical Imaging: Potential Co-Conspirators in Treatment Design and Optimization–A Thomas Dougherty Award for Excellence in PDT Paper. J. Porphyr. Phthalocyanines 2020, 24, 1320–1360. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Huang, Y. Imaging in Photodynamic Therapy; Taylor & Francis: Oxfordshire, UK, 2017. [Google Scholar]
- Hester, S.C.; Kuriakose, M.; Nguyen, C.D.; Mallidi, S. Role of Ultrasound and Photoacoustic Imaging in Photodynamic Therapy for Cancer. Photochem. Photobiol. 2020, 96, 260–279. [Google Scholar] [CrossRef] [PubMed]
- Kachynski, A.V.; Pliss, A.; Kuzmin, A.N.; Ohulchanskyy, T.Y.; Baev, A.; Qu, J.; Prasad, P.N. Photodynamic Therapy by in Situ Nonlinear Photon Conversion. Nat. Photonics 2014, 8, 455–461. [Google Scholar] [CrossRef]
- Parodi, V.; Jacchetti, E.; Osellame, R.; Cerullo, G.; Polli, D.; Raimondi, M.T. Nonlinear Optical Microscopy: From Fundamentals to Applications in Live Bioimaging. Front. Bioeng. Biotechnol. 2020, 8, 1174. [Google Scholar] [CrossRef]
- Brancaleon, L.; Moseley, H. Laser and Non-Laser Light Sources for Photodynamic Therapy. Lasers Med. Sci. 2002, 17, 173–186. [Google Scholar] [CrossRef]
- Mang, T.S. Lasers and Light Sources for PDT: Past, Present and Future. Photodiagnosis Photodyn. Ther. 2004, 1, 43–48. [Google Scholar] [CrossRef]
- Huang, Z.; Xu, H.; Meyers, A.D.; Musani, A.I.; Wang, L.; Tagg, R.; Barqawi, A.B.; Chen, Y.K. Photodynamic Therapy for Treatment of Solid Tumors–Potential and Technical Challenges. Technol. Cancer Res. Treat. 2008, 7, 309–320. [Google Scholar] [CrossRef]
- Finlay, J.C.; Darafsheh, A. Light sources, drugs, and dosimetry. In Biomedical Optics in Otorhinolaryngology: Head and Neck Surgery; Wong, B.J.-F., Ilgner, J., Eds.; Springer: New York, NY, USA, 2016; pp. 311–336. ISBN 9781493917587. [Google Scholar]
- Mallidi, S.; Anbil, S.; Bulin, A.L.; Obaid, G.; Ichikawa, M.; Hasan, T. Beyond the Barriers of Light Penetration: Strategies, Perspectives and Possibilities for Photodynamic Therapy. Theranostics 2016, 6, 2458–2487. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Tang, Y.; Elmenoufy, A.H.; Xu, H.; Cheng, Z.; Yang, X. Nanocomposite-Based Photodynamic Therapy Strategies for Deep Tumor Treatment. Small 2015, 11, 5860–5887. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, C.M.; Esteves da Silva, J.C.G.; Pinto da Silva, L. Chemiluminescence and Bioluminescence as an Excitation Source in the Photodynamic Therapy of Cancer: A Critical Review. ChemPhysChem 2016, 17, 2286–2294. [Google Scholar] [CrossRef]
- Fan, W.; Huang, P.; Chen, X. Overcoming the Achilles’ Heel of Photodynamic Therapy. Chem. Soc. Rev. 2016, 45, 6488–6519. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cheng, L.; Liu, Z. Upconversion Nanoparticles for Photodynamic Therapy and Other Cancer Therapeutics. Theranostics 2013, 3, 317–330. [Google Scholar] [CrossRef]
- Hamblin, M.R. Upconversion in Photodynamic Therapy: Plumbing the Depths. Dalt. Trans. 2018, 47, 8571–8580. [Google Scholar] [CrossRef]
- Liu, Y.; Meng, X.; Bu, W. Upconversion-Based Photodynamic Cancer Therapy; Elsevier B.V.: Amsterdam, The Netherlands, 2019; Volume 379, pp. 82–98. [Google Scholar]
- Cline, B.; Delahunty, I.; Xie, J. Nanoparticles to Mediate X-Ray-Induced Photodynamic Therapy and Cherenkov Radiation Photodynamic Therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1541. [Google Scholar] [CrossRef]
- Chen, X.; Song, J.; Chen, X.; Yang, H. X-Ray-Activated Nanosystems for Theranostic Applications. Chem. Soc. Rev. 2019, 48, 3073–3101. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Zhou, Z.; Pratx, G.; Chen, X.; Chen, H. Nanoscintillator-Mediated X-Ray Induced Photodynamic Therapy for Deep-Seated Tumors: From Concept to Biomedical Applications. Theranostics 2020, 10, 1296–1318. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.D.; Hao, X.Y.; Li, H.C.; Ke, M.R.; Zheng, B.Y.; Huang, J.D. Progress in the Development of Nanosensitizers for X-Ray-Induced Photodynamic Therapy. Drug Discov. Today 2018, 23, 1791–1800. [Google Scholar] [CrossRef] [PubMed]
- Larue, L.; Ben Mihoub, A.; Youssef, Z.; Colombeau, L.; Acherar, S.; André, J.C.; Arnoux, P.; Baros, F.; Vermandel, M.; Frochot, C. Using X-Rays in Photodynamic Therapy: An Overview. Photochem. Photobiol. Sci. 2018, 17, 1612–1650. [Google Scholar] [CrossRef] [PubMed]
- Daouk, J.; Dhaini, B.; Petit, J.; Frochot, C.; Barberi-Heyob, M.; Schohn, H. Can Cerenkov Light Really Induce an Effective Photodynamic Therapy? Radiation 2020, 1, 2. [Google Scholar] [CrossRef]
- Huang, Y.; Qiu, F.; Chen, R.; Yan, D.; Zhu, X. Fluorescence Resonance Energy Transfer-Based Drug Delivery Systems for Enhanced Photodynamic Therapy. J. Mater. Chem. B 2020, 8, 3772–3788. [Google Scholar] [CrossRef]
- Fan, W.; Lu, N.; Xu, C.; Liu, Y.; Lin, J.; Wang, S.; Shen, Z.; Yang, Z.; Qu, J.; Wang, T.; et al. Enhanced Afterglow Performance of Persistent Luminescence Implants for Efficient Repeatable Photodynamic Therapy. ACS Nano 2017, 11, 5864–5872. [Google Scholar] [CrossRef]
- Hu, L.; Wang, P.; Zhao, M.; Liu, L.; Zhou, L.; Li, B.; Albaqami, F.H.; El-Toni, A.M.; Li, X.; Xie, Y.; et al. Near-Infrared Rechargeable “Optical Battery” Implant for Irradiation-Free Photodynamic Therapy. Biomaterials 2018, 163, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Teh, D.B.L.; Bansal, A.; Chai, C.; Toh, T.B.; Tucker, R.A.J.; Gammad, G.G.L.; Yeo, Y.; Lei, Z.; Zheng, X.; Yang, F.; et al. A Flexi-PEGDA Upconversion Implant for Wireless Brain Photodynamic Therapy. Adv. Mater. 2020, 32, 2001459. [Google Scholar] [CrossRef]
- Bansal, A.; Yang, F.; Xi, T.; Zhang, Y.; Ho, J.S. In Vivo Wireless Photonic Photodynamic Therapy. Proc. Natl. Acad. Sci. USA 2018, 115, 1469–1474. [Google Scholar] [CrossRef]
- Yamagishi, K.; Kirino, I.; Takahashi, I.; Amano, H.; Takeoka, S.; Morimoto, Y.; Fujie, T. Tissue-Adhesive Wirelessly Powered Optoelectronic Device for Metronomic Photodynamic Cancer Therapy. Nat. Biomed. Eng. 2019, 3, 27–36. [Google Scholar] [CrossRef]
- Kim, A.; Zhou, J.; Samaddar, S.; Song, S.H.; Elzey, B.D.; Thompson, D.H.; Ziaie, B. An Implantable Ultrasonically-Powered Micro-Light-Source (ΜLight) for Photodynamic Therapy. Sci. Rep. 2019, 9, 1395. [Google Scholar] [CrossRef]
- Algorri, J.F.; Ochoa, M.; Roldán-Varona, P.; Rodríguez-Cobo, L.; López-Higuera, J.M. Light Technology for Efficient and Effective Photodynamic Therapy: A Critical Review. Cancers 2021, 13, 3484. [Google Scholar] [CrossRef] [PubMed]
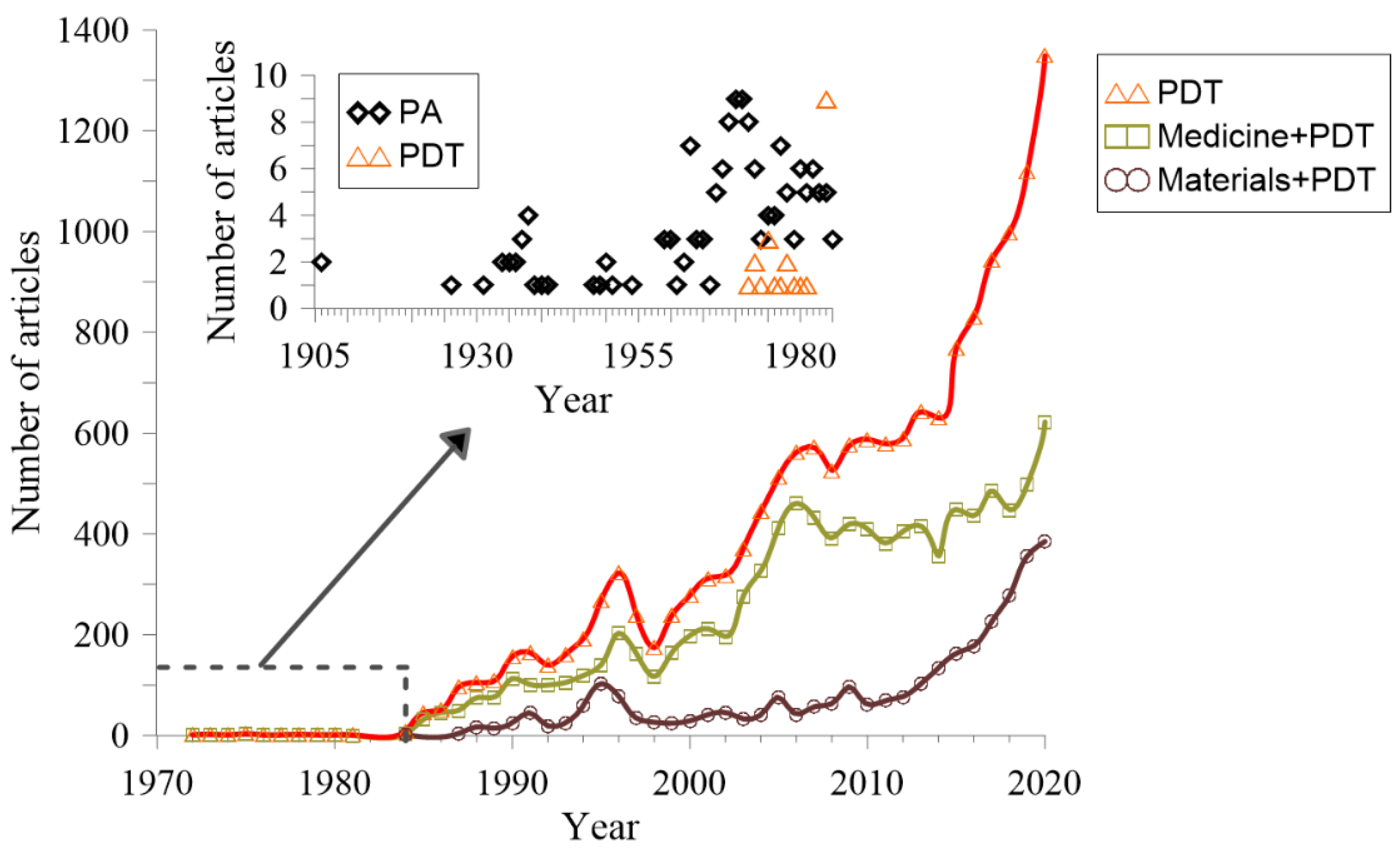
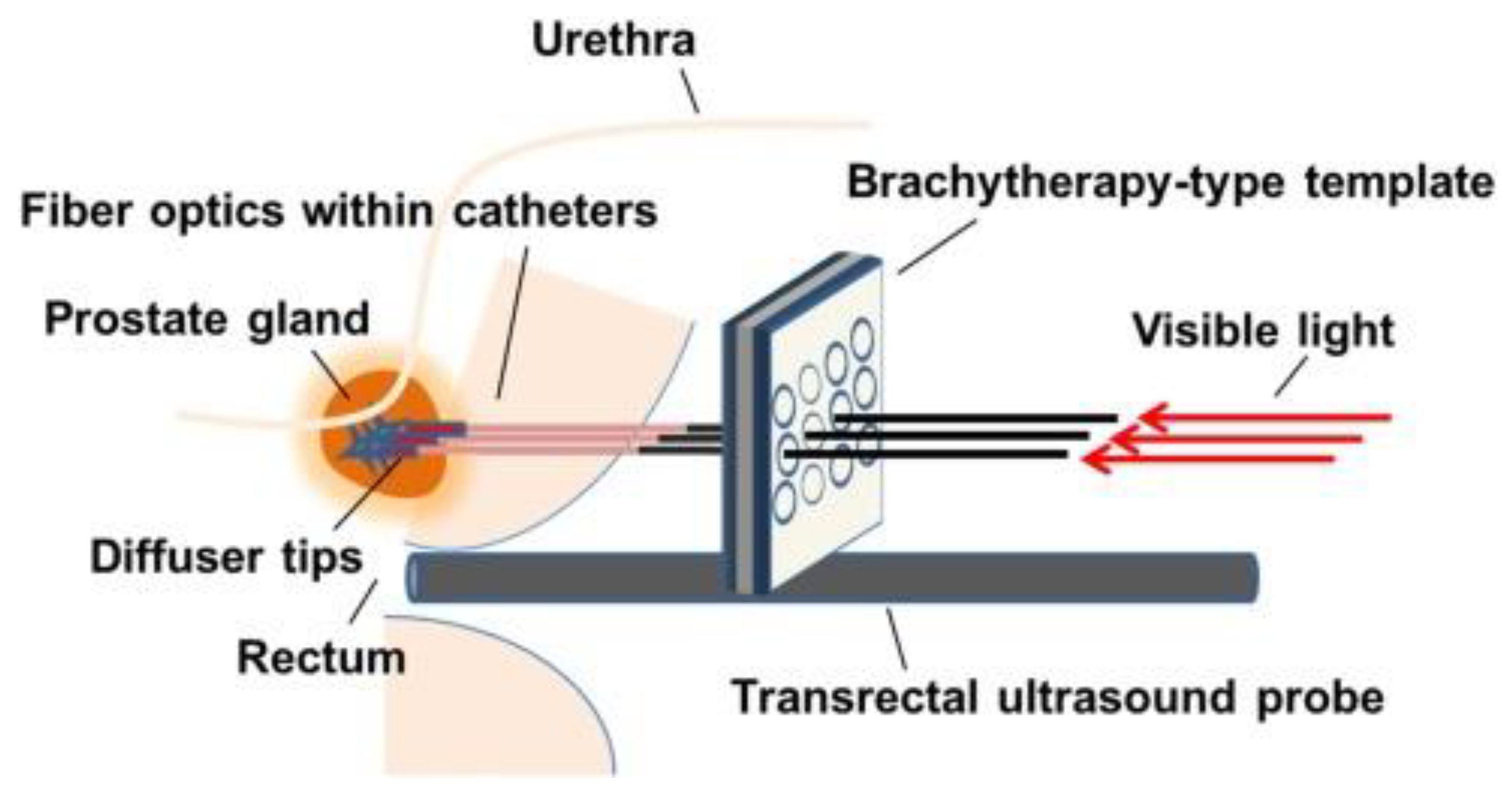


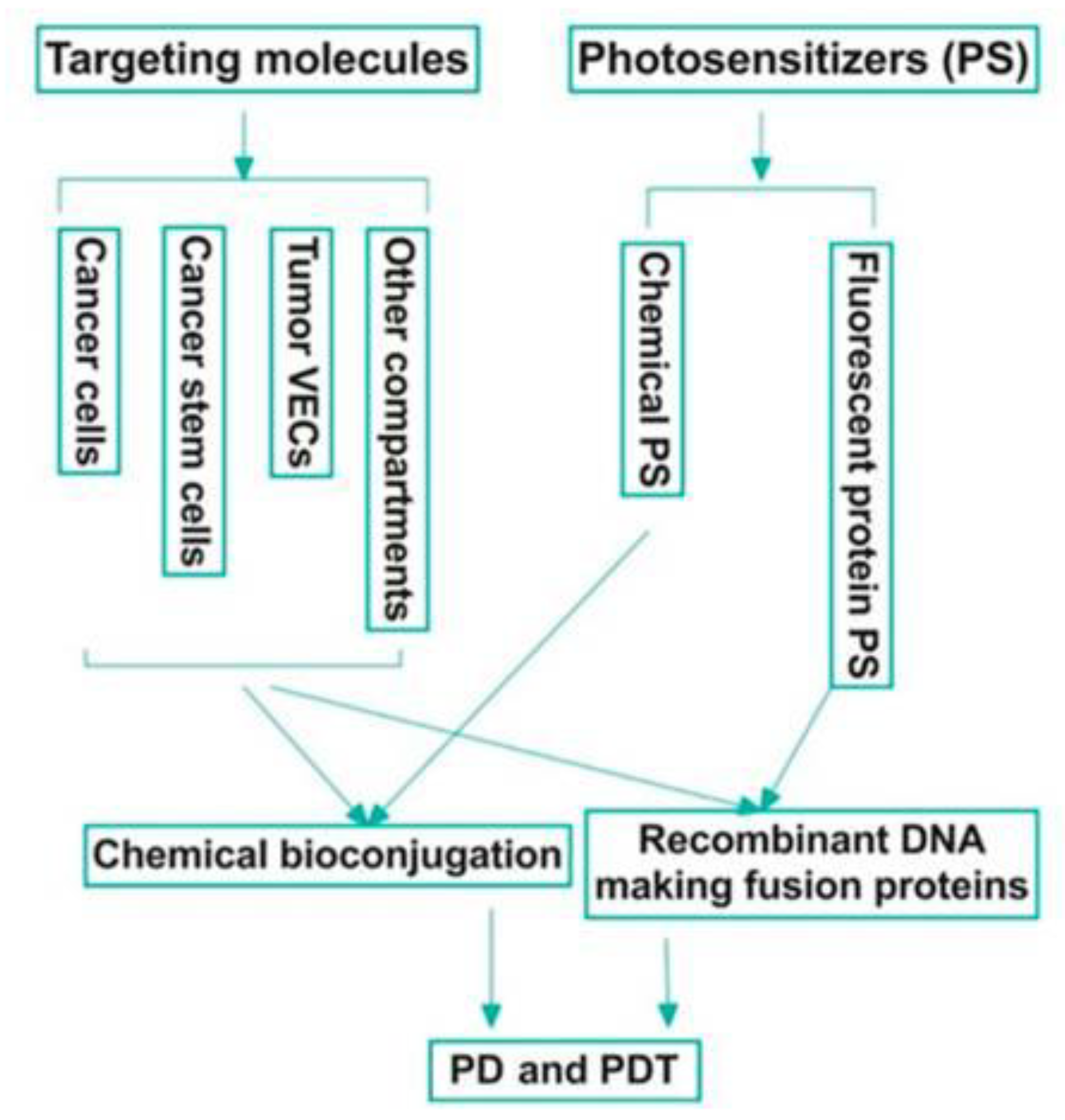
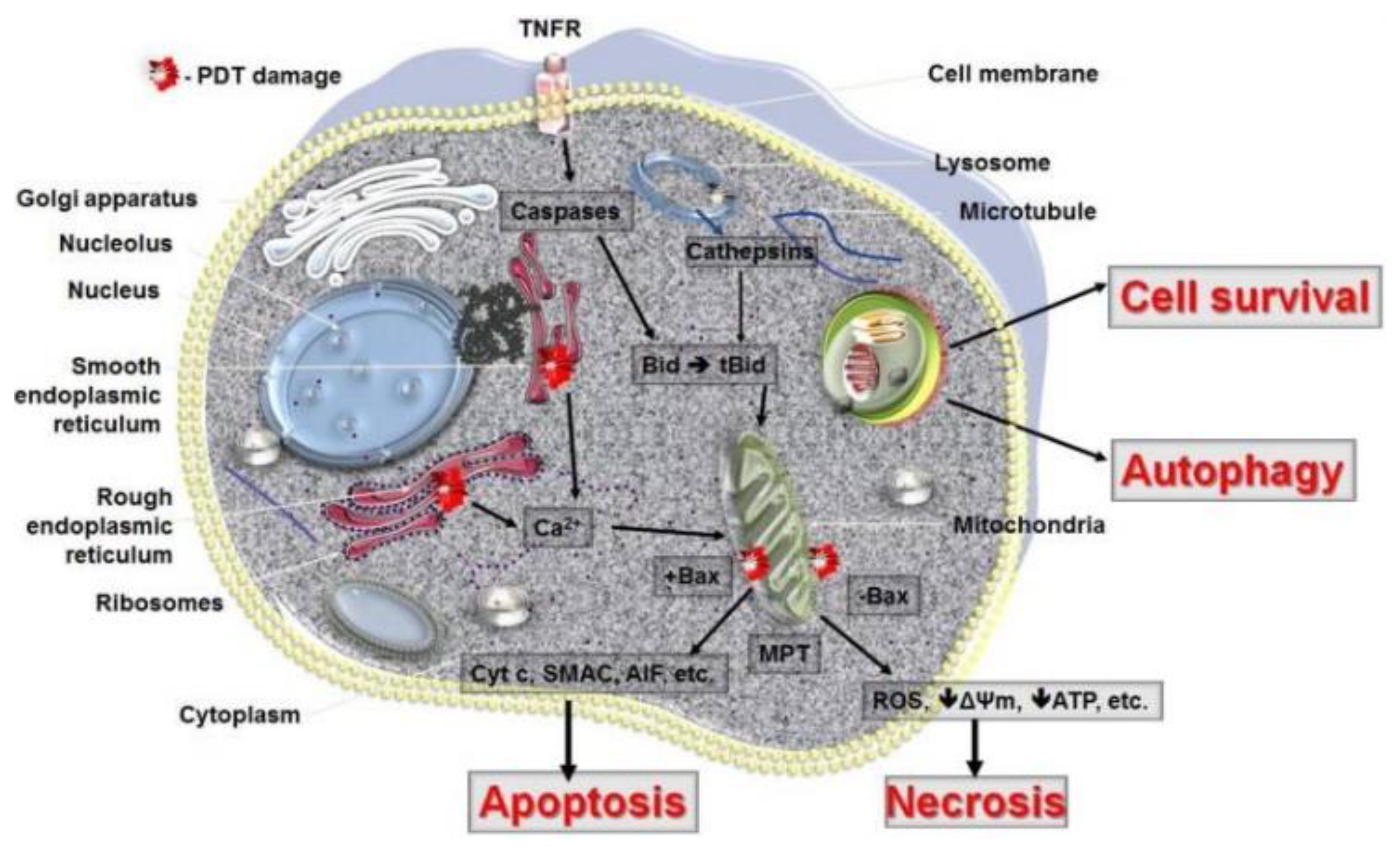
| Type of Cancer | Year | Ref. | Content |
|---|---|---|---|
| Gastrointestinal (liver and bile ducts, pancreas, small intestine, colon, and rectum) | 2011 | [31] | State-of-the-art on cholangiocarcinoma. |
| 2012 | [32] | Unresectable cholangiocarcinoma. | |
| 2013 | [33] | PDT in gastroenterology. | |
| 2015 | [34,35] | Cholangiocarcinoma; colorectal clinical trials. | |
| 2016 | [36,37] | Pancreas PDT; colorectal preclinical research. | |
| 2017 | [38,39] | Cholangiocarcinoma; colon cancer and stem cells. | |
| 2019 | [40] | Colon (immunotherapy and PDT). | |
| 2020 | [41] | Liver malignancies. | |
| 2021 | [42,43] | Colorectal (PDT and cannabidiol); pancreas (PS nanoparticles). | |
| Skin | 2010 | [44,45] | Skin and other types of cancer reviews. |
| 2015 | [46,47] | MTHPC for non-melanoma skin cancers; combined strategy. | |
| 2016 | [48,49,50] | Non-melanoma skin cancer. | |
| 2018 | [51,52] | Immune consequences induced by PDT; non-melanoma. | |
| 2019 | [53] | Dermato-oncology. | |
| 2020 | [54,55] | Methyl-5-aminolevulinate for basal cell carcinoma; mechanisms, challenges, and promising developments | |
| Lung | 2011 | [56,57,58] | General review; early-stage; targets and mechanisms. |
| 2012 | [59,60] | Non-small-cell lung cancer, Kato’s 30-year experience. | |
| 2014 | [61] | Lung cancer and malignant pleural mesothelioma. | |
| 2016 | [62] | Non-small-cell lung cancer, review and future directions. | |
| 2018 | [63] | Nanoparticle PS drug delivery uptake systems. | |
| 2021 | [64,65] | Update; chemotherapy and PDT in lung cancer. | |
| Prostate | 2010 | [66] | PDT for focal ablation of the prostate. |
| 2011 | [67] | Perspective. | |
| 2015 | [68] | WST-09 and WST-11 mediated vascular-targeted PDT. | |
| 2017 | [69] | Photosensitizers in prostate cancer therapy. | |
| 2019 | [70,71,72] | Low-risk prostate cancer, review and meta-analysis. | |
| 2021 | [73,74] | Narrative review; comparison with minimally invasive techniques. | |
| Head and neck | 2011 | [75] | Mucosal dysplasia and microinvasive carcinoma. |
| 2012 | [76] | 5-Aminolevulinic acid-mediated PDT. | |
| 2013 | [77] | MTHPC mediated squamous cell carcinoma. | |
| 2015 | [78] | Oral premalignant lesions. | |
| 2016 | [79] | Oral cancer and premalignant lesions. | |
| 2014 | [80] | Detailed review about PS. | |
| 2018 | [81] | Neoplasms of the head and neck. | |
| 2019 | [82] | Indications, outcomes, and prospects. | |
| Breast | 2016 | [83] | The plant-derived agent-induced cell death mechanisms. |
| 2017 | [84] | PDT: inception to application in breast cancer. | |
| 2019 | [15,85] | Multidrug-resistant; intradermal metastatic breast cancer. | |
| 2020 | [86] | Organic nanoparticle-based active targeting for PDT. | |
| 2021 | [87] | Perspective. | |
| Brain | 2014 | [88] | General review (sources, dosimetry, PS, etc). |
| 2015 | [89] | Perspective. | |
| 2016 | [90] | Current status and prospects of PDT in Japan. | |
| 2018 | [91] | A systematic review of clinical trials. | |
| 2020 | [92] | Complete review of PDT and novel OCT strategies. | |
| Bladder | 2011 | [93] | Overview of preclinical and clinical experiences. |
| 2017 | [94] | 5-Aminolevulinic acid-mediated PDT. | |
| 2018 | [95] | Past challenges and current innovations. |
| Name | λexc (nm) | Manufacturer | Application |
|---|---|---|---|
| FIRST-GENERATION PHOTOSENSITIZERS | |||
| Porfimer sodium | 630 | Axcan Pharma | PDT of esophageal cancer, lung adenocarcinoma, and endobronchial cancer |
| SECOND-GENERATION PHOTOSENSITIZERS/Prodrugs | |||
| 5-aminolaevulinic acid | 635 | DUSA Stabiopharma | PDT of mild to moderate actinic keratosis, with fluorescence-guided resection of glioma |
| Methyl-aminolevulinic acid | 579–670 | Galderma | PDT of non-hyperkeratotic actinic keratosis and basal cell carcinoma |
| Temoporfin | 652 | Biolitec | PDT of advanced head and neck cancer |
| Talaporfin | 664 | Meiji SeikaNovartis | PDT of early centrally located lung cancer |
| Verteporfin | 690 | Novartis | PDT of age-related macular degeneration |
| Redaporfin | 749 | Luzitin | PDT of biliary tract cancer |
| PHOTOSENSITIZERS UNDER CLINICAL INVESTIGATIONS | |||
| Fotolon | 665 | Apocare Pharma | PDT of nasopharyngeal sarcoma |
| Hexylaminolevulinate | 635 | Photocure | PDT of HPV-induced cervical precancerous lesions and non-muscle invasive bladder cancer |
| Radachlorin | 662 | Rada-pharma | PDT of skin cancer |
| Photochlor (HTTP) | 664 | Rosewell Park | PDT of head and neck cancer |
| Padeliporfin | 762 | Negma-Lerads | PDT of prostate cancer |
| Motexafin lutetium | 732 | Pharmacyclics | PDT of coronary artery disease |
| Rostaprofin | 664 | Miravant | PDT of age-related macular degeneration |
| Talaporfin | 664 | Meiji Seika | PDT of colorectal neoplasms, liver metastasis |
| Fimaporfin | 435 | PCI Biotech | PCI of cutaneous or sub-cutaneous malignancies, cholangiocarcinoma, and PCI of vaccine antigens |
| Anti-Tumour PDT Mechanisms | ||
|---|---|---|
| Organelles | Processes | |
| Direct cell damage | Mitochondria: | Apoptosis |
| ||
Cytoplasm:
| ||
Endoplasmatic reticulum
| Autophagy | |
| Cell membrane disintegration | Necrosis | |
| Vascular shutdown | Local depletion of oxygen and nutrients | Apoptosis Necrosis Autophagy |
| Activation of immune response | Cytotoxic T cells | Granzyme mediated apoptosis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Algorri, J.F.; Ochoa, M.; Roldán-Varona, P.; Rodríguez-Cobo, L.; López-Higuera, J.M. Photodynamic Therapy: A Compendium of Latest Reviews. Cancers 2021, 13, 4447. https://doi.org/10.3390/cancers13174447
Algorri JF, Ochoa M, Roldán-Varona P, Rodríguez-Cobo L, López-Higuera JM. Photodynamic Therapy: A Compendium of Latest Reviews. Cancers. 2021; 13(17):4447. https://doi.org/10.3390/cancers13174447
Chicago/Turabian StyleAlgorri, José Francisco, Mario Ochoa, Pablo Roldán-Varona, Luís Rodríguez-Cobo, and José Miguel López-Higuera. 2021. "Photodynamic Therapy: A Compendium of Latest Reviews" Cancers 13, no. 17: 4447. https://doi.org/10.3390/cancers13174447
APA StyleAlgorri, J. F., Ochoa, M., Roldán-Varona, P., Rodríguez-Cobo, L., & López-Higuera, J. M. (2021). Photodynamic Therapy: A Compendium of Latest Reviews. Cancers, 13(17), 4447. https://doi.org/10.3390/cancers13174447







