How to Differentiate Benign from Malignant Adrenocortical Tumors?
Abstract
Simple Summary
Abstract
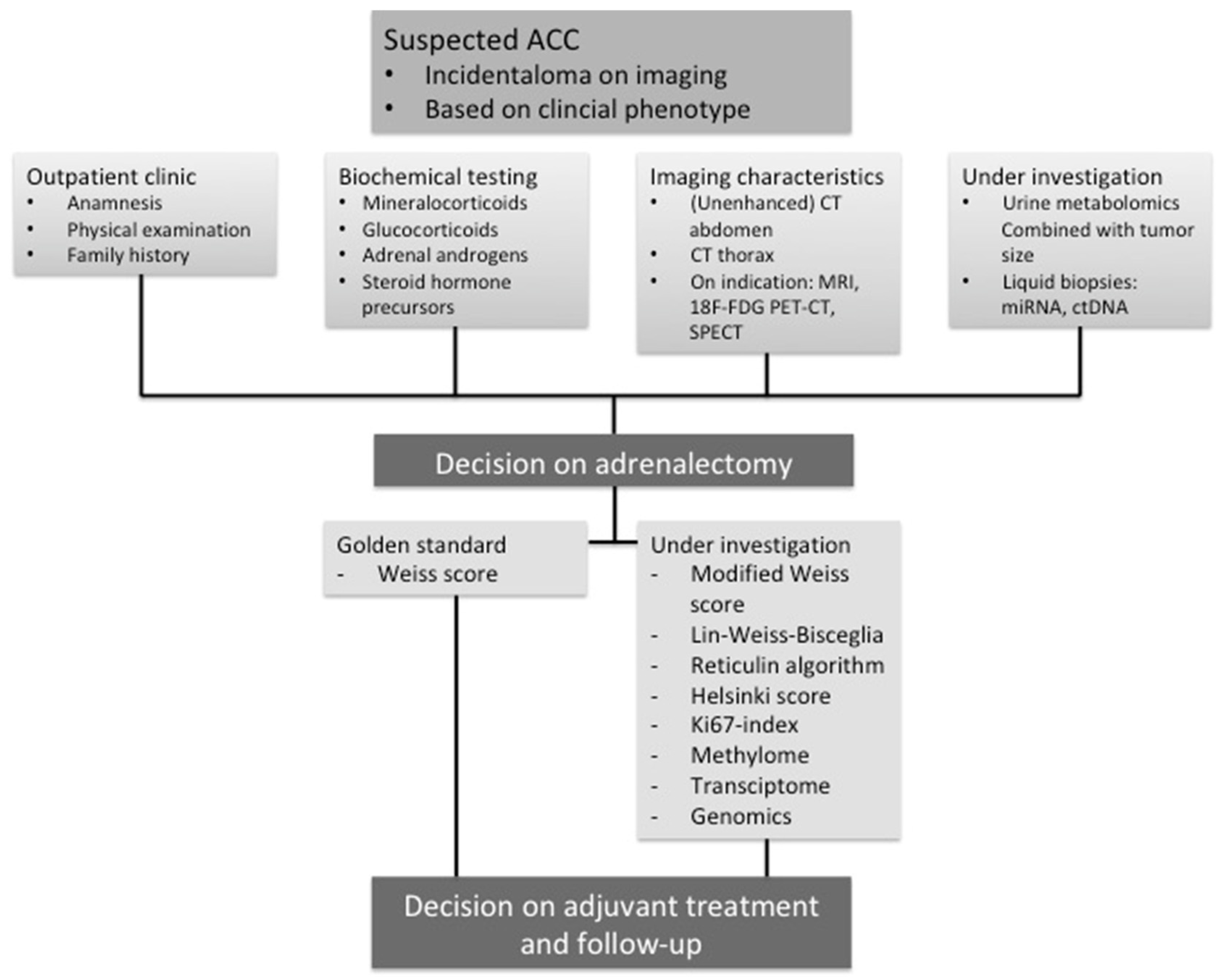
1. Introduction
2. Imaging Strategies to Differentiate Benign from Malignant Adrenocortical Tumors
3. Hormonal Evaluation
3.1. Biochemical Diagnostic Procedures
3.2. Urine Steroid Metabolomics
4. Histopathology
4.1. Weiss Score
4.2. Revised Weiss Score
4.3. Lin-Weiss-Bisceglia System
4.4. Reticulin Algorithm
4.5. Helsinki Score
4.6. Ki67-Index
4.7. Other Immunohistochemical Assessments
5. Diagnostic Molecular Biomarkers
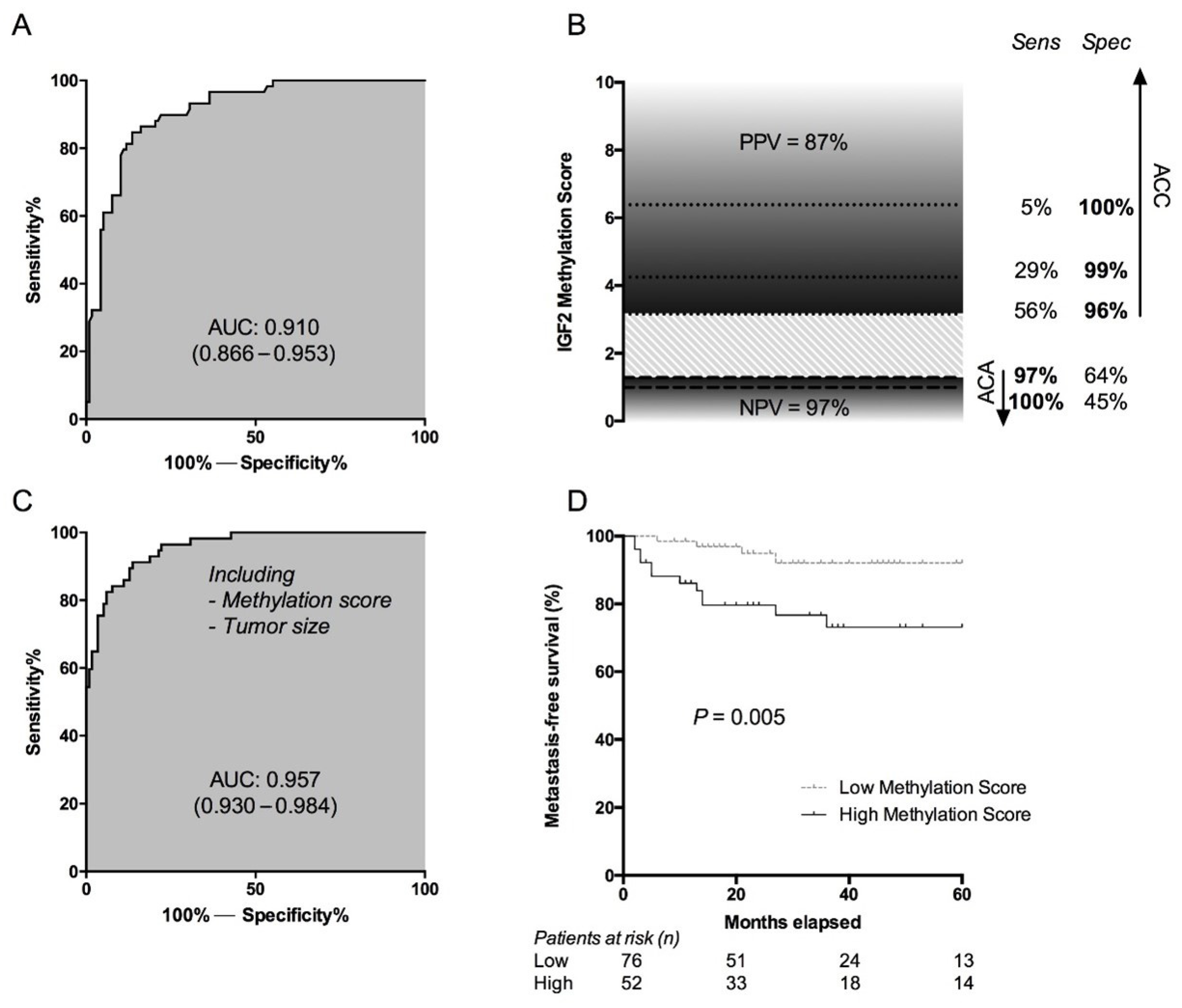
5.1. Methylome
5.2. Transcriptome
5.3. MiRNAome
5.4. Chromosomal Aberrations
5.5. DNA Mutations
5.6. Liquid Biopsies
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kloos, R.T.; Gross, M.D.; Francis, I.R.; Korobkin, M.; Shapiro, B. Incidentally discovered adrenal masses. Endocr. Rev. 1995, 16, 460–484. [Google Scholar] [PubMed]
- Mantero, F.; Terzolo, M.; Arnaldi, G.; Osella, G.; Masini, A.M.; Ali, A.; Giovagnetti, M.; Opocher, G.; Angeli, A. A survey on adrenal incidentaloma in italy. Study group on adrenal tumors of the italian society of endocrinology. J. Clin. Endocrinol. Metab. 2000, 85, 637–644. [Google Scholar] [PubMed]
- Bancos, I.; Taylor, A.E.; Chortis, V.; Sitch, A.J.; Jenkinson, C.; Davidge-Pitts, C.J.; Lang, K.; Tsagarakis, S.; Macech, M.; Riester, A.; et al. Urine steroid metabolomics for the differential diagnosis of adrenal incidentalomas in the eurine-act study: A prospective test validation study. Lancet Diabetes Endocrinol. 2020, 8, 773–781. [Google Scholar] [CrossRef]
- Bovio, S.; Cataldi, A.; Reimondo, G.; Sperone, P.; Novello, S.; Berruti, A.; Borasio, P.; Fava, C.; Dogliotti, L.; Scagliotti, G.V.; et al. Prevalence of adrenal incidentaloma in a contemporary computerized tomography series. J. Endocrinol. Investig. 2006, 29, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Young, W.F.J. Clinical practice. The incidentally discovered adrenal mass. N. Engl. J. Med. 2007, 356, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Fassnacht, M.; Kroiss, M.; Allolio, B. Update in adrenocortical carcinoma. J. Clin. Endocrinol. Metab. 2013, 98, 4551–4564. [Google Scholar] [CrossRef] [PubMed]
- Kerkhofs, T.M.; Verhoeven, R.H.; Van der Zwan, J.M.; Dieleman, J.; Kerstens, M.N.; Links, T.P.; Van de Poll-Franse, L.V.; Haak, H.R. Adrenocortical carcinoma: A population-based study on incidence and survival in the netherlands since 1993. Eur. J. Cancer 2013, 49, 2579–2586. [Google Scholar] [CrossRef] [PubMed]
- Lughezzani, G.; Sun, M.; Perrotte, P.; Jeldres, C.; Alasker, A.; Isbarn, H.; Budaus, L.; Shariat, S.F.; Guazzoni, G.; Montorsi, F.; et al. The european network for the study of adrenal tumors staging system is prognostically superior to the international union against cancer-staging system: A north american validation. Eur. J. Cancer 2010, 46, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Sharma, E.; Dahal, S.; Sharma, P.; Bhandari, A.; Gupta, V.; Amgai, B.; Dahal, S. The characteristics and trends in adrenocortical carcinoma: A united states population based study. J. Clin. Med. Res. 2018, 10, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Wanis, K.N.; Kanthan, R. Diagnostic and prognostic features in adrenocortical carcinoma: A single institution case series and review of the literature. World J. Surg. Oncol. 2015, 13, 117. [Google Scholar] [CrossRef]
- Fassnacht, M.; Dekkers, O.M.; Else, T.; Baudin, E.; Berruti, A.; de Krijger, R.; Haak, H.R.; Mihai, R.; Assie, G.; Terzolo, M. European society of endocrinology clinical practice guidelines on the management of adrenocortical carcinoma in adults, in collaboration with the european network for the study of adrenal tumors. Eur. J. Endocrinol. 2018, 179, G1–G46. [Google Scholar] [CrossRef]
- Lau, S.K.; Weiss, L.M. The weiss system for evaluating adrenocortical neoplasms: 25 years later. Hum. Pathol. 2009, 40, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Fassnacht, M.; Johanssen, S.; Quinkler, M.; Bucsky, P.; Willenberg, H.S.; Beuschlein, F.; Terzolo, M.; Mueller, H.H.; Hahner, S.; Allolio, B.; et al. Limited prognostic value of the 2004 international union against cancer staging classification for adrenocortical carcinoma: Proposal for a revised tnm classification. Cancer 2009, 115, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Dinnes, J.; Bancos, I.; Ferrante di Ruffano, L.; Chortis, V.; Davenport, C.; Bayliss, S.; Sahdev, A.; Guest, P.; Fassnacht, M.; Deeks, J.J.; et al. Management of endocrine disease: Imaging for the diagnosis of malignancy in incidentally discovered adrenal masses: A systematic review and meta-analysis. Eur. J. Endocrinol. 2016, 175, R51–R64. [Google Scholar] [CrossRef] [PubMed]
- Hamrahian, A.H.; Ioachimescu, A.G.; Remer, E.M.; Motta-Ramirez, G.; Bogabathina, H.; Levin, H.S.; Reddy, S.; Gill, I.S.; Siperstein, A.; Bravo, E.L. Clinical utility of noncontrast computed tomography attenuation value (hounsfield units) to differentiate adrenal adenomas/hyperplasias from nonadenomas: Cleveland clinic experience. J. Clin. Endocrinol. Metab. 2005, 90, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Marty, M.; Gaye, D.; Perez, P.; Auder, C.; Nunes, M.L.; Ferriere, A.; Haissaguerre, M.; Tabarin, A. Diagnostic accuracy of computed tomography to identify adenomas among adrenal incidentalomas in an endocrinological population. Eur. J. Endocrinol. 2018, 178, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Caoili, E.M.; Davenport, M.S.; Poznanski, A.; Francis, I.R.; Giordano, T.; Dunnick, N.R. Ct imaging characteristics of oncocytic adrenal neoplasms (oans): Comparison with adrenocortical carcinomas. Abdom. Imaging 2014, 39, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Choyke, P.L. ACR appropriateness criteria on incidentally discovered adrenal mass. J. Am. Coll. Radiol. 2006, 3, 498–504. [Google Scholar] [CrossRef]
- Korobkin, M.; Brodeur, F.J.; Francis, I.R.; Quint, L.E.; Dunnick, N.R.; Londy, F. CT time-attenuation washout curves of adrenal adenomas and nonadenomas. Am. J. Roentgenol. 1998, 170, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Szolar, D.H.; Kammerhuber, F.H. Adrenal adenomas and nonadenomas: Assessment of washout at delayed contrast-enhanced CT. Radiology 1998, 207, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Caoili, E.M.; Korobkin, M.; Francis, I.R.; Cohan, R.H.; Dunnick, N.R. Delayed enhanced ct of lipid-poor adrenal adenomas. Am. J. Roentgenol. 2000, 175, 1411–1415. [Google Scholar] [CrossRef] [PubMed]
- Sturgeon, C.; Shen, W.T.; Clark, O.H.; Duh, Q.Y.; Kebebew, E. Risk assessment in 457 adrenal cortical carcinomas: How much does tumor size predict the likelihood of malignancy? J. Am. Coll. Surg. 2006, 202, 423–430. [Google Scholar] [CrossRef]
- Ilias, I.; Sahdev, A.; Reznek, R.H.; Grossman, A.B.; Pacak, K. The optimal imaging of adrenal tumours: A comparison of different methods. Endocr. Relat. Cancer 2007, 14, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, S.; Balachandran, A.; Habra, M.A.; Phan, A.T.; Bassett, R.L.J.; Macapinlac, H.A.; Chuang, H.H. Impact of (1)(8)f-fdg pet/ct on the management of adrenocortical carcinoma: Analysis of 106 patients. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 2066–2073. [Google Scholar] [CrossRef]
- Ansquer, C.; Scigliano, S.; Mirallie, E.; Taieb, D.; Brunaud, L.; Sebag, F.; Leux, C.; Drui, D.; Dupas, B.; Renaudin, K.; et al. 18f-fdg pet/ct in the characterization and surgical decision concerning adrenal masses: A prospective multicentre evaluation. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1669–1678. [Google Scholar] [CrossRef] [PubMed]
- Han, S.J.; Kim, T.S.; Jeon, S.W.; Jeong, S.J.; Yun, M.; Rhee, Y.; Kang, E.S.; Cha, B.S.; Lee, E.J.; Lee, H.C.; et al. Analysis of adrenal masses by 18f-fdg positron emission tomography scanning. Int. J. Clin. Pract. 2007, 61, 802–809. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Caoili, E.M.; Avram, A.M.; Miller, B.S.; Else, T. 18f-fdg-pet/ct evaluation of indeterminate adrenal masses in non-cancer patients. J. Clin. Endocrinol. Metab. 2021, 106, 1448–1449. [Google Scholar] [CrossRef]
- Hahner, S.; Caoili, E.; Else, T. 5th international ACC symposium: Imaging for diagnosis and surveillance of adrenal tumors—New advances and reviews of old concepts. Horm. Cancer 2016, 7, 40–43. [Google Scholar] [CrossRef]
- Boland, G.W.; Dwamena, B.A.; Sangwaiya, M.J.; Goehler, A.G.; Blake, M.A.; Hahn, P.F.; Scott, J.A.; Kalra, M.K. Characterization of adrenal masses by using fdg pet: A systematic review and meta-analysis of diagnostic test performance. Radiology 2011, 259, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Fassnacht, M.; Arlt, W.; Bancos, I.; Dralle, H.; Newell-Price, J.; Sahdev, A.; Tabarin, A.; Terzolo, M.; Tsagarakis, S.; Dekkers, O.M. Management of adrenal incidentalomas: European society of endocrinology clinical practice guideline in collaboration with the european network for the study of adrenal tumors. Eur. J. Endocrinol. 2016, 175, G1–G34. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, T.M.; Lirov, R.; Caoili, E.M.; Lerario, A.M.; Miller, B.S.; Fragoso, M.C.; Dunnick, N.R.; Hammer, G.D.; Else, T. Radiographic characteristics of adrenal masses preceding the diagnosis of adrenocortical cancer. Horm. Cancer 2015, 6, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Bergstrom, M.; Juhlin, C.; Bonasera, T.A.; Sundin, A.; Rastad, J.; Akerstrom, G.; Langstrom, B. Pet imaging of adrenal cortical tumors with the 11beta-hydroxylase tracer 11c-metomidate. J. Nucl. Med. 2000, 41, 275–282. [Google Scholar] [PubMed]
- Minn, H.; Salonen, A.; Friberg, J.; Roivainen, A.; Viljanen, T.; Langsjo, J.; Salmi, J.; Valimaki, M.; Nagren, K.; Nuutila, P. Imaging of adrenal incidentalomas with pet using (11)c-metomidate and (18)f-fdg. J. Nucl. Med. 2004, 45, 972–979. [Google Scholar] [PubMed]
- Zettinig, G.; Mitterhauser, M.; Wadsak, W.; Becherer, A.; Pirich, C.; Vierhapper, H.; Niederle, B.; Dudczak, R.; Kletter, K. Positron emission tomography imaging of adrenal masses: (18)f-fluorodeoxyglucose and the 11beta-hydroxylase tracer (11)c-metomidate. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 1224–1230. [Google Scholar] [CrossRef]
- Hennings, J.; Lindhe, O.; Bergstrom, M.; Langstrom, B.; Sundin, A.; Hellman, P. [11c]metomidate positron emission tomography of adrenocortical tumors in correlation with histopathological findings. J. Clin. Endocrinol. Metab. 2006, 91, 1410–1414. [Google Scholar] [CrossRef]
- Hahner, S.; Sundin, A. Metomidate-based imaging of adrenal masses. Horm. Cancer 2011, 2, 348–353. [Google Scholar] [CrossRef]
- Hahner, S.; Kreissl, M.C.; Fassnacht, M.; Haenscheid, H.; Bock, S.; Verburg, F.A.; Knoedler, P.; Lang, K.; Reiners, C.; Buck, A.K.; et al. Functional characterization of adrenal lesions using [123i]imto-spect/ct. J. Clin. Endocrinol. Metab. 2013, 98, 1508–1518. [Google Scholar] [CrossRef] [PubMed]
- Kreissl, M.C.; Schirbel, A.; Fassnacht, M.; Haenscheid, H.; Verburg, F.A.; Bock, S.; Saeger, W.; Knoedler, P.; Reiners, C.; Buck, A.K.; et al. [(1)(2)(3)i]iodometomidate imaging in adrenocortical carcinoma. J. Clin. Endocrinol. Metab. 2013, 98, 2755–2764. [Google Scholar] [CrossRef] [PubMed]
- Fassnacht, M.; Allolio, B. Clinical management of adrenocortical carcinoma. Best Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 273–289. [Google Scholar] [CrossRef] [PubMed]
- Nieman, L.K. Approach to the patient with an adrenal incidentaloma. J. Clin. Endocrinol. Metab. 2010, 95, 4106–4113. [Google Scholar] [CrossRef]
- Schwarte, S.; Brabant, E.G.; Bastian, L.; Bruns, F. Cortisol as a possible marker of metastatic adrenocortical carcinoma: A case report with 3-year follow-up. Anticancer Res. 2007, 27, 1917–1920. [Google Scholar]
- Funder, J.W.; Carey, R.M.; Mantero, F.; Murad, M.H.; Reincke, M.; Shibata, H.; Stowasser, M.; Young, W.F.J. The management of primary aldosteronism: Case detection, diagnosis, and treatment: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2016, 101, 1889–1916. [Google Scholar] [CrossRef]
- Luton, J.P.; Cerdas, S.; Billaud, L.; Thomas, G.; Guilhaume, B.; Bertagna, X.; Laudat, M.H.; Louvel, A.; Chapuis, Y.; Blondeau, P.; et al. Clinical features of adrenocortical carcinoma, prognostic factors, and the effect of mitotane therapy. N. Engl. J. Med. 1990, 322, 1195–1201. [Google Scholar] [CrossRef]
- Malunowicz, E.M.; Ginalska-Malinowska, M.; Romer, T.E.; Ruszczynska-Wolska, A.; Dura, M. Heterogeneity of urinary steroid profiles in children with adrenocortical tumors. Horm. Res. 1995, 44, 182–188. [Google Scholar]
- Minowada, S.; Kinoshita, K.; Hara, M.; Isurugi, K.; Uchikawa, T.; Niijima, T. Measurement of urinary steroid profile in patients with adrenal tumor as a screening method for carcinoma. Endocrinol. Jpn. 1985, 32, 29–37. [Google Scholar] [CrossRef]
- Doerr, H.G.; Sippell, W.G.; Drop, S.L.; Bidlingmaier, F.; Knorr, D. Evidence of 11 beta-hydroxylase deficiency in childhood adrenocortical tumors. The plasma corticosterone/11-deoxycorticosterone ratio as a possible marker for malignancy. Cancer 1987, 60, 1625–1629. [Google Scholar] [CrossRef]
- Arlt, W.; Biehl, M.; Taylor, A.E.; Hahner, S.; Libe, R.; Hughes, B.A.; Schneider, P.; Smith, D.J.; Stiekema, H.; Krone, N.; et al. Urine steroid metabolomics as a biomarker tool for detecting malignancy in adrenal tumors. J. Clin. Endocrinol. Metab. 2011, 96, 3775–3784. [Google Scholar] [CrossRef] [PubMed]
- Kerkhofs, T.M.; Kerstens, M.N.; Kema, I.P.; Willems, T.P.; Haak, H.R. Diagnostic value of urinary steroid profiling in the evaluation of adrenal tumors. Horm. Cancer 2015, 6, 168–175. [Google Scholar] [CrossRef]
- Velikanova, L.I.; Shafigullina, Z.R.; Lisitsin, A.A.; Vorokhobina, N.V.; Grigoryan, K.; Kukhianidze, E.A.; Strelnikova, E.G.; Krivokhizhina, N.S.; Krasnov, L.M.; Fedorov, E.A.; et al. Different types of urinary steroid profiling obtained by high-performance liquid chromatography and gas chromatography-mass spectrometry in patients with adrenocortical carcinoma. Horm. Cancer 2016, 7, 327–335. [Google Scholar] [CrossRef]
- Bancos, I.; Tamhane, S.; Shah, M.; Delivanis, D.A.; Alahdab, F.; Arlt, W.; Fassnacht, M.; Murad, M.H. Diagnosis of endocrine disease: The diagnostic performance of adrenal biopsy: A systematic review and meta-analysis. Eur. J. Endocrinol. 2016, 175, R65–R80. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.; LeBlanc, J.; Al-Haddad, M.; Sherman, S.; DeWitt, J. Role of endoscopic ultrasound fine-needle aspiration evaluating adrenal gland enlargement or mass. World J. Nephrol. 2014, 3, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Delivanis, D.A.; Erickson, D.; Atwell, T.D.; Natt, N.; Maraka, S.; Schmit, G.D.; Eiken, P.W.; Nathan, M.A.; Young, W.F.J.; Bancos, I. Procedural and clinical outcomes of percutaneous adrenal biopsy in a high-risk population for adrenal malignancy. Clin. Endocrinol. 2016, 85, 710–716. [Google Scholar] [CrossRef]
- Point du Jour, K.S.; Alwelaie, Y.; Coleman, A.; Tadros, T.; Aneja, R.; Reid, M.D. Adrenal gland fine needle aspiration: A multi-institutional analysis of 139 cases. J. Am. Soc. Cytopathol. 2021, 10, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Uemura, S.; Yasuda, I.; Kato, T.; Doi, S.; Kawaguchi, J.; Yamauchi, T.; Kaneko, Y.; Ohnishi, R.; Suzuki, T.; Yasuda, S.; et al. Preoperative routine evaluation of bilateral adrenal glands by endoscopic ultrasound and fine-needle aspiration in patients with potentially resectable lung cancer. Endoscopy 2013, 45, 195–201. [Google Scholar] [CrossRef]
- Duregon, E.; Volante, M.; Bollito, E.; Goia, M.; Buttigliero, C.; Zaggia, B.; Berruti, A.; Scagliotti, G.V.; Papotti, M. Pitfalls in the diagnosis of adrenocortical tumors: A lesson from 300 consultation cases. Hum. Pathol. 2015, 46, 1799–1807. [Google Scholar] [CrossRef]
- Mete, O.; Gucer, H.; Kefeli, M.; Asa, S.L. Diagnostic and prognostic biomarkers of adrenal cortical carcinoma. Am. J. Surg. Pathol. 2018, 42, 201–213. [Google Scholar] [CrossRef]
- Sbiera, S.; Schmull, S.; Assie, G.; Voelker, H.-U.; Kraus, L.; Beyer, M.; Ragazzon, B.; Beuschlein, F.; Willenberg, H.S.; Hahner, S.; et al. High diagnostic and prognostic value of steroidogenic factor-1 expression in adrenal tumors. J. Clin. Endocrinol. Metab. 2010, 95, E161–E171. [Google Scholar] [CrossRef]
- Hodgson, A.; Pakbaz, S.; Mete, O. A diagnostic approach to adrenocortical tumors. Surg. Pathol. Clin. 2019, 12, 967–995. [Google Scholar] [CrossRef]
- Lam, A. Adrenocortical carcinoma: Updates of clinical and pathological features after renewed world health organisation classification and pathology staging. Biomedicines 2021, 9, 175. [Google Scholar] [CrossRef]
- Soon, P.; Gill, A.; Benn, D.; Clarkson, A.; Robinson, B.; McDonald, K.; Sidhu, S. Microarray gene expression and immunohistochemistry analyses of adrenocortical tumors identify IGF2 and KI-67 as useful in differentiating carcinomas from adenomas. Endocr.-Relat. Cancer 2009, 16, 573–583. [Google Scholar] [CrossRef]
- Kanitra, J.J.; Hardaway, J.C.; Soleimani, T.; Koehler, T.J.; McLeod, M.K.; Kavuturu, S. Adrenocortical oncocytic neoplasm: A systematic review. Surgery 2018, 164, 1351–1359. [Google Scholar] [CrossRef]
- Papotti, M.; Volante, M.; Duregon, E.; Delsedime, L.; Terzolo, M.; Berruti, A.; Rosai, J. Adrenocortical tumors with myxoid features: A distinct morphologic and phenotypical variant exhibiting malignant behavior. Am. J. Surg. Pathol. 2010, 34, 973–983. [Google Scholar] [CrossRef]
- Papathomas, T.G.; Duregon, E.; Korpershoek, E.; Restuccia, D.F.; van Marion, R.; Cappellesso, R.; Sturm, N.; Rossi, G.; Coli, A.; Zucchini, N.; et al. Sarcomatoid adrenocortical carcinoma: A comprehensive pathological, immunohistochemical, and targeted next-generation sequencing analysis. Hum. Pathol. 2016, 58, 113–122. [Google Scholar] [CrossRef]
- Hough, A.J.; Hollifield, J.W.; Page, D.L.; Hartmann, W.H. Prognostic factors in adrenal cortical tumors: A mathematical analysis of clinical and morphologic data. Am. J. Clin. Pathol. 1979, 72, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Weiss, L.M. Comparative histologic study of 43 metastasizing and nonmetastasizing adrenocortical tumors. Am. J. Surg. Pathol. 1984, 8, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Van Slooten, H.; Schaberg, A.; Smeenk, D.; Moolenaar, A.J. Morphologic characteristics of benign and malignant adrenocortical tumors. Cancer 1985, 55, 766–773. [Google Scholar] [CrossRef]
- Weiss, L.M.; Medeiros, L.J.; Vickery, A.L.J. Pathologic features of prognostic significance in adrenocortical carcinoma. Am. J. Surg. Pathol. 1989, 13, 202–206. [Google Scholar] [CrossRef]
- Aubert, S.; Wacrenier, A.; Leroy, X.; Devos, P.; Carnaille, B.; Proye, C.; Wemeau, J.L.; Lecomte-Houcke, M.; Leteurtre, E. Weiss system revisited: A clinicopathologic and immunohistochemical study of 49 adrenocortical tumors. Am. J. Surg. Pathol. 2002, 26, 1612–1619. [Google Scholar] [CrossRef]
- Wieneke, J.A.; Thompson, L.D.; Heffess, C.S. Adrenal cortical neoplasms in the pediatric population: A clinicopathologic and immunophenotypic analysis of 83 patients. Am. J. Surg. Pathol. 2003, 27, 867–881. [Google Scholar] [CrossRef]
- Bisceglia, M.; Ludovico, O.; Di Mattia, A.; Ben-Dor, D.; Sandbank, J.; Pasquinelli, G.; Lau, S.K.; Weiss, L.M. Adrenocortical oncocytic tumors: Report of 10 cases and review of the literature. Int. J. Surg. Pathol. 2004, 12, 231–243. [Google Scholar] [CrossRef]
- Volante, M.; Bollito, E.; Sperone, P.; Tavaglione, V.; Daffara, F.; Porpiglia, F.; Terzolo, M.; Berruti, A.; Papotti, M. Clinicopathological study of a series of 92 adrenocortical carcinomas: From a proposal of simplified diagnostic algorithm to prognostic stratification. Histopathology 2009, 55, 535–543. [Google Scholar] [CrossRef]
- Pennanen, M.; Heiskanen, I.; Sane, T.; Remes, S.; Mustonen, H.; Haglund, C.; Arola, J. Helsinki score—A novel model for prediction of metastases in adrenocortical carcinomas. Hum. Pathol. 2015, 46, 404–410. [Google Scholar] [CrossRef]
- Johanssen, S.; Hahner, S.; Saeger, W.; Quinkler, M.; Beuschlein, F.; Dralle, H.; Haaf, M.; Kroiss, M.; Jurowich, C.; Langer, P.; et al. Deficits in the management of patients with adrenocortical carcinoma in germany. Dtsch. Ärzteblatt Int. 2010, 107, 885. [Google Scholar] [CrossRef]
- Tissier, F.; Aubert, S.; Leteurtre, E.; Al Ghuzlan, A.; Patey, M.; Decaussin, M.; Doucet, L.; Gobet, F.; Hoang, C.; Mazerolles, C. Adrenocortical tumors: Improving the practice of the weiss system through virtual microscopy: A national program of the french network inca-comete. Am. J. Surg. Pathol. 2012, 36, 1194–1201. [Google Scholar] [CrossRef]
- Wong, D.D.; Spagnolo, D.V.; Bisceglia, M.; Havlat, M.; McCallum, D.; Platten, M.A. Oncocytic adrenocortical neoplasms—A clinicopathologic study of 13 new cases emphasizing the importance of their recognition. Hum. Pathol. 2011, 42, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Renaudin, K.; Smati, S.; Wargny, M.; Al Ghuzlan, A.; Aubert, S.; Leteurtre, E.; Patey, M.; Sibony, M.; Sturm, N.; Tissier, F. Clinicopathological description of 43 oncocytic adrenocortical tumors: Importance of Ki-67 in histoprognostic evaluation. Mod. Pathol. 2018, 31, 1708–1716. [Google Scholar] [CrossRef] [PubMed]
- Duregon, E.; Fassina, A.; Volante, M.; Nesi, G.; Santi, R.; Gatti, G.; Cappellesso, R.; Ciaramella, P.D.; Ventura, L.; Gambacorta, M. The reticulin algorithm for adrenocortical tumor diagnosis: A multicentric validation study on 245 unpublished cases. Am. J. Surg. Pathol. 2013, 37, 1433–1440. [Google Scholar] [CrossRef]
- Duregon, E.; Volante, M.; Cappia, S.; Cuccurullo, A.; Bisceglia, M.; Wong, D.D.; Spagnolo, D.V.; Szpak-Ulczok, S.; Bollito, E.; Daffara, F. Oncocytic adrenocortical tumors: Diagnostic algorithm and mitochondrial DNA profile in 27 cases. Am. J. Surg. Pathol. 2011, 35, 1882–1893. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, D.; Murthy, S.S.; Tagore, K.R.; Rao, B.V.; Thamminedi, S.R.; Raju, K.; Sharma, R.; Challa, S. Diagnosis of adrenocortical tumors by reticulin algorithm. Indian J. Endocrinol. Metab. 2017, 21, 734. [Google Scholar] [CrossRef] [PubMed]
- Duregon, E.; Cappellesso, R.; Maffeis, V.; Zaggia, B.; Ventura, L.; Berruti, A.; Terzolo, M.; Fassina, A.; Volante, M.; Papotti, M. Validation of the prognostic role of theHelsinki Score in 225 cases of adrenocortical carcinoma. Hum. Pathol. 2017, 62, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Terzolo, M.; Boccuzzi, A.; Bovio, S.; Cappia, S.; De Giuli, P.; Alì, A.; Paccotti, P.; Porpiglia, F.; Fontana, D.; Angeli, A. Immunohistochemical assessment of Ki-67 in the differential diagnosis of adrenocortical tumors. Urology 2001, 57, 176–182. [Google Scholar] [CrossRef]
- Arola, J.; Salmenkivi, K.; Liu, J.; Kahri, A.; Heikkila, P. P53 and ki67 in adrenocortical tumors. Endocr. Res. 2000, 26, 861–865. [Google Scholar] [CrossRef]
- Stojadinovic, A.; Brennan, M.F.; Hoos, A.; Omeroglu, A.; Leung, D.H.; Dudas, M.E.; Nissan, A.; Cordon-Cardo, C.; Ghossein, R.A. Adrenocortical adenoma and carcinoma: Histopathological and molecular comparative analysis. Mod. Pathol. 2003, 16, 742–751. [Google Scholar] [CrossRef]
- Schmitt, A.; Saremaslani, P.; Schmid, S.; Rousson, V.; Montani, M.; Schmid, D.; Heitz, P.U.; Komminoth, P.; Perren, A. IGFII and MIB1 immunohistochemistry is helpful for the differentiation of benign from malignant adrenocortical tumours. Histopathology 2006, 49, 298–307. [Google Scholar] [CrossRef]
- Beuschlein, F.; Weigel, J.; Saeger, W.; Kroiss, M.; Wild, V.; Daffara, F.; Libé, R.; Ardito, A.; Al Ghuzlan, A.; Quinkler, M.; et al. Major prognostic role of Ki67 in localized adrenocortical carcinoma after complete resection. J. Clin. Endocrinol. Metab. 2015, 100, 841–849. [Google Scholar] [CrossRef]
- Duregon, E.; Molinaro, L.; Volante, M.; Ventura, L.; Righi, L.; Bolla, S.; Terzolo, M.; Sapino, A.; Papotti, M.G. Comparative diagnostic and prognostic performances of the hematoxylin-eosin and phospho-histone H3 mitotic count and Ki-67 index in adrenocortical carcinoma. Mod. Pathol. 2014, 27, 1246–1254. [Google Scholar] [CrossRef]
- Tissier, F. Classification of adrenal cortical tumors: What limits for the pathological approach? Best Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 877–885. [Google Scholar] [CrossRef]
- McNicol, A.M. A diagnostic approach to adrenal cortical lesions. Endocr. Pathol. 2008, 19, 241–251. [Google Scholar] [CrossRef]
- Mukherjee, G.; Datta, C.; Chatterjee, U.; Sengupta, M.; Chatterjee, G.; Bera, M.; Chowdhury, S. Histopathological study of adrenocortical masses with special references to Weiss score, Ki-67 index and P53 status. Indian J. Pathol. Microbiol. 2015, 58, 175–180. [Google Scholar] [PubMed]
- Papathomas, T.G.; Pucci, E.; Giordano, T.J.; Lu, H.; Duregon, E.; Volante, M.; Papotti, M.; Lloyd, R.V.; Tischler, A.S.; Van Nederveen, F.H.; et al. An international Ki67 reproducibility study in adrenal cortical carcinoma. Am. J. Surg. Pathol. 2016, 40, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Papathomas, T.G.; van Zessen, D.; Palli, I.; de Krijger, R.R.; van der Spek, P.J.; Dinjens, W.N.; Stubbs, A.P. Automated selection of hotspots (Ash): Enhanced automated segmentation and adaptive step finding for Ki67 hotspot detection in adrenal cortical cancer. Diagn. Pathol. 2014, 9, 216. [Google Scholar] [CrossRef]
- Boulle, N.; Gicquel, C.; Logié, A.; Christol, R.; Feige, J.J.; Le Bouc, Y. Fibroblast growth factor-2 inhibits the maturation of pro-insulin-like growth factor-ii (pro-IGF-II) and the expression of insulin-like growth factor binding protein-2 (IGFBP-2) in the human adrenocortical tumor cell line NCI-H295R. Endocrinology 2000, 141, 3127–3136. [Google Scholar] [CrossRef][Green Version]
- Boulle, N.; Logié, A.; Gicquel, C.; Perin, L.; Le Bouc, Y. Increased levels of insulin-like growth factor ii (Igf-II) and igf-binding protein-2 are associated with malignancy in sporadic adrenocortical tumors. J. Clin. Endocrinol. Metab. 1998, 83, 1713–1720. [Google Scholar]
- Reincke, M.; Karl, M.; Travis, W.H.; Mastorakos, G.; Allolio, B.; Linehan, H.M.; Chrousos, G.P. P53 mutations in human adrenocortical neoplasms: Immunohistochemical and molecular studies. J. Clin. Endocrinol. Metab. 1994, 78, 790–794. [Google Scholar]
- Tissier, F.; Cavard, C.; Groussin, L.; Perlemoine, K.; Fumey, G.; Hagneré, A.-M.; René-Corail, F.; Jullian, E.; Gicquel, C.; Bertagna, X.; et al. Mutations of β-catenin in adrenocortical tumors: Activation of the wnt signaling pathway is a frequent event in both benign and malignant adrenocortical tumors. Cancer Res. 2005, 65, 7622–7627. [Google Scholar] [CrossRef]
- Kulis, M.; Esteller, M. DNA methylation and cancer. Adv. Genet. 2010, 70, 27–56. [Google Scholar]
- Fonseca, A.L.; Kugelberg, J.; Starker, L.F.; Scholl, U.; Choi, M.; Hellman, P.; Åkerström, G.; Westin, G.; Lifton, R.P.; Björklund, P.; et al. Comprehensive DNA methylation analysis of benign and malignant adrenocortical tumors. Genes Chromosomes Cancer 2012, 51, 949–960. [Google Scholar] [CrossRef]
- Barreau, O.; Assié, G.; Wilmot-Roussel, H.; Ragazzon, B.; Baudry, C.; Perlemoine, K.; Rene-Corail, F.; Bertagna, X.; Dousset, B.; Hamzaoui, N.; et al. Identification of a cpg island methylator phenotype in adrenocortical carcinomas. J. Clin. Endocrinol. Metab. 2013, 98, E174–E184. [Google Scholar] [CrossRef]
- Rechache, N.S.; Wang, Y.; Stevenson, H.S.; Killian, J.K.; Edelman, D.C.; Merino, M.; Zhang, L.; Nilubol, N.; Stratakis, C.A.; Meltzer, P.S.; et al. DNA methylation profiling identifies global methylation differences and markers of adrenocortical tumors. J. Clin. Endocrinol. Metab. 2012, 97, E1004–E1013. [Google Scholar] [CrossRef]
- Gao, Z.-H.; Suppola, S.; Liu, J.; Heikkilä, P.i.; Jänne, J.; Voutilainen, R. Association of H19 promoter methylation with the expression of H19 and igf-ii genes in adrenocortical tumors. J. Clin. Endocrinol. Metab. 2002, 87, 1170–1176. [Google Scholar] [CrossRef]
- Creemers, S.; Van Koetsveld, P.; van Kemenade, F.; Papathomas, T.; Franssen, G.; Dogan, F.; Eekhoff, E.; van der Valk, P.; de Herder, W.; Janssen, J.; et al. Methylation of IGF2 regulatory regions to diagnose adrenocortical carcinomas. Endocr.-Relat. Cancer 2016, 23, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Creemers, S.; Feelders, R.; Valdes, N.; Ronchi, C.; Volante, M.; van Hemel, B.; Luconi, M.; Ettaieb, M.; Mannelli, M.; Chiara, M.; et al. The IGF2 methylation score for adrenocortical cancer: An ensat validation study. Endocr.-Relat. Cancer 2020, 27, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Assié, G.; Guillaud-Bataille, M.; Ragazzon, B.; Bertagna, X.; Bertherat, J.; Clauser, E. The pathophysiology, diagnosis and prognosis of adrenocortical tumors revisited by transcriptome analyses. Trends Endocrinol. Metab. 2010, 21, 325–334. [Google Scholar] [CrossRef]
- Ragazzon, B.; Assie, G.; Bertherat, J. Transcriptome analysis of adrenocortical cancers: From molecular classification to the identification of new treatments. Endocr.-Relat. Cancer 2011, 18, R15–R17. [Google Scholar] [CrossRef] [PubMed]
- Armignacco, R.; Cantini, G.; Canu, L.; Poli, G.; Ercolino, T.; Mannelli, M.; Luconi, M. Adrenocortical carcinoma: The dawn of a new era of genomic and molecular biology analysis. J. Endocrinol. Investig. 2018, 41, 499–507. [Google Scholar] [CrossRef]
- Giordano, T.J.; Kuick, R.; Else, T.; Gauger, P.G.; Vinco, M.; Bauersfeld, J.; Sanders, D.; Thomas, D.G.; Doherty, G.; Hammer, G. Molecular classification and prognostication of adrenocortical tumors by transcriptome profiling. Clin. Cancer Res. 2009, 15, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Giordano, T.J.; Thomas, D.G.; Kuick, R.; Lizyness, M.; Misek, D.E.; Smith, A.L.; Sanders, D.; Aljundi, R.T.; Gauger, P.G.; Thompson, N.W.; et al. Distinct transcriptional profiles of adrenocortical tumors uncovered by DNA microarray analysis. Am. J. Pathol. 2003, 162, 521–531. [Google Scholar] [CrossRef]
- De Fraipont, F.; El Atifi, M.; Cherradi, N.; Le Moigne, G.; Defaye, G.; Houlgatte, R.; Bertherat, J.; Bertagna, X.; Plouin, P.-F.; Baudin, E.; et al. Gene expression profiling of human adrenocortical tumors using complementary deoxyribonucleic acid microarrays identifies several candidate genes as markers of malignancy. J. Clin. Endocrinol. Metab. 2005, 90, 1819–1829. [Google Scholar] [CrossRef]
- Velázquez-Fernández, D.; Laurell, C.; Geli, J.; Höög, A.; Odeberg, J.; Kjellman, M.; Lundeberg, J.; Hamberger, B.; Nilsson, P.; Bäckdahl, M. Expression profiling of adrenocortical neoplasms suggests a molecular signature of malignancy. Surgery 2005, 138, 1087–1094. [Google Scholar] [CrossRef]
- Szabo, P.; Tamasi, V.; Molnar, V.; Andrasfalvy, M.; Tömböl, Z.; Farkas, R.; Kövesdi, K.; Patocs, A.; Toth, M.; Szalai, C.; et al. Meta-analysis of adrenocortical tumour genomics data: Novel pathogenic pathways revealed. Oncogene 2010, 29, 3163–3172. [Google Scholar] [CrossRef]
- Tömböl, Z.; Szabo, P.M.; Molnar, V.; Wiener, Z.; Tölgyesi, G.; Horanyi, J.; Riesz, P.; Reismann, P.; Patocs, A.; Liko, I.; et al. Integrative molecular bioinformatics study of human adrenocortical tumors: Microrna, tissue-specific target prediction, and pathway analysis. Endocr.-Relat. Cancer 2009, 16, 895–906. [Google Scholar] [CrossRef]
- Laurell, C.; Velázquez-Fernández, D.; Lindsten, K.; Juhlin, C.; Enberg, U.; Geli, J.; Höög, A.; Kjellman, M.; Lundeberg, J.; Hamberger, B.; et al. Transcriptional profiling enables molecular classification of adrenocortical tumours. Eur. J. Endocrinol. 2009, 161, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Ranvier, G.G.; Weng, J.; Yeh, R.-F.; Khanafshar, E.; Suh, I.; Barker, C.; Duh, Q.Y.; Clark, O.H.; Kebebew, E. Identification of biomarkers of adrenocortical carcinoma using genomewide gene expression profiling. Arch. Surg. 2008, 143, 841–846. [Google Scholar] [CrossRef]
- Bonnet-Serrano, F.; Bertherat, J. Genetics of tumors of the adrenal cortex. Endocr. -Relat. Cancer 2018, 25, R131–R152. [Google Scholar] [CrossRef] [PubMed]
- Slater, E.; Diehl, S.; Langer, P.; Samans, B.; Ramaswamy, A.; Zielke, A.; Bartsch, D. Analysis by cdna microarrays of gene expression patterns of human adrenocortical tumors. Eur. J. Endocrinol. 2006, 154, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Sun, Y.; Wu, H.; Zhao, D.; Chen, J. Distinguishing adrenal cortical carcinomas and adenomas: A study of clinicopathological features and biomarkers. Histopathology 2014, 64, 567–576. [Google Scholar] [CrossRef]
- de Reyniès, A.; Assié, G.; Rickman, D.S.; Tissier, F.; Groussin, L.; René-Corail, F.; Dousset, B.; Bertagna, X.; Clauser, E.; Bertherat, J. Gene expression profiling reveals a new classification of adrenocortical tumors and identifies molecular predictors of malignancy and survival. J. Clin. Oncol. 2009, 27, 1108–1115. [Google Scholar] [CrossRef]
- Phan, L.M.; Fuentes-Mattei, E.; Wu, W.; Velazquez-Torres, G.; Sircar, K.; Wood, C.G.; Hai, T.; Jimenez, C.; Cote, G.J.; Ozsari, L.; et al. Hepatocyte growth factor/cmet pathway activation enhances cancer hallmarks in adrenocortical carcinoma. Cancer Res. 2015, 75, 4131–4142. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Qian, G.; Chen, L.; Wu, C.-L.; Dan, H.C.; Xiao, Y.; Wang, X. Co-expression network analysis of biomarkers for adrenocortical carcinoma. Front. Genet. 2018, 9, 328. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Fullwood, M.J. Roles, functions, and mechanisms of long non-coding RNAS in cancer. Genom. Proteom. Bioinform. 2016, 14, 42–54. [Google Scholar] [CrossRef]
- Buishand, F.O.; Liu-Chittenden, Y.; Fan, Y.; Tirosh, A.; Gara, S.K.; Patel, D.; Meerzaman, D.; Kebebew, E. Adrenocortical tumors have a distinct, long, non-coding rna expression profile and linc00271 is downregulated in malignancy. Surgery 2020, 167, 224–232. [Google Scholar] [CrossRef]
- Malumbres, M. Mirnas and cancer: An epigenetics view. Mol. Asp. Med. 2013, 34, 863–874. [Google Scholar] [CrossRef]
- Igaz, P.; Igaz, I.; Nagy, Z.; Nyírő, G.; Szabó, P.M.; Falus, A.; Patócs, A.; Rácz, K. Micrornas in adrenal tumors: Relevance for pathogenesis, diagnosis, and therapy. Cell. Mol. Life Sci. 2015, 72, 417–428. [Google Scholar] [CrossRef]
- Soon, P.S.H.; Tacon, L.J.; Gill, A.J.; Bambach, C.P.; Sywak, M.S.; Campbell, P.R.; Yeh, M.W.; Wong, S.G.; Clifton-Bligh, R.J.; Robinson, B.G.; et al. MiR-195 and miR-483-5p identified as predictors of poor prognosis in adrenocortical cancer. Clin. Cancer Res. 2009, 15, 7684–7692. [Google Scholar] [CrossRef] [PubMed]
- Özata, D.M.; Caramuta, S.; Velázquez-Fernández, D.; Akçakaya, P.; Xie, H.; Höög, A.; Zedenius, J.; Bäckdahl, M.; Larsson, C.; Lui, W.-O. The role of microRNA deregulation in the pathogenesis of adrenocortical carcinoma. Endocr.-Relat. Cancer 2011, 18, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Patterson, E.E.; Holloway, A.K.; Weng, J.; Fojo, T.; Kebebew, E. Microrna profiling of adrenocortical tumors reveals miR-483 as a marker of malignancy. Cancer 2011, 117, 1630–1639. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Boufraqech, M.; Jain, M.; Zhang, L.; He, M.; Gesuwan, K.; Gulati, N.; Nilubol, N.; Fojo, T.; Kebebew, E. MiR-34a and mir-483-5p are candidate serum biomarkers for adrenocortical tumors. Surgery 2013, 154, 1224–1229. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, K.; Helwig, J.; Bertram, S.; Sheu, S.; Suttorp, A.; Seggewiss, J.; Willscher, E.; Walz, M.; Worm, K.; Schmid, K. Differential expression of microRNA-675, microRNA-139-3p and microRNA-335 in benign and malignant adrenocortical tumours. J. Clin. Pathol. 2011, 64, 529–535. [Google Scholar] [CrossRef]
- Chehade, M.; Bullock, M.; Glover, A.; Hutvagner, G.; Sidhu, S. Key microrna’s and their targetome in adrenocortical cancer. Cancers 2020, 12, 2198. [Google Scholar] [CrossRef]
- Chabre, O.; Libé, R.; Assie, G.; Barreau, O.; Bertherat, J.; Bertagna, X.; Feige, J.J.; Cherradi, N. Serum mir-483-5p and mir-195 are predictive of recurrence risk in adrenocortical cancer patients. Endocr.-Relat. Cancer 2013, 20, 579–594. [Google Scholar] [CrossRef]
- Singh, P.; Soon, P.S.; Feige, J.-J.; Chabre, O.; Zhao, J.T.; Cherradi, N.; Lalli, E.; Sidhu, S.B. Dysregulation of micrornas in adrenocortical tumors. Mol. Cell. Endocrinol. 2012, 351, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Agosta, C.; Laugier, J.; Guyon, L.; Denis, J.; Bertherat, J.; Libé, R.; Boisson, B.; Sturm, N.; Feige, J.J.; Chabre, O.; et al. MiR-483-5p and miR-139-5p promote aggressiveness by targeting n-myc downstream-regulated gene family members in adrenocortical cancer. Int. J. Cancer 2018, 143, 944–957. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.; Wang, P.; Pan, B.; Nie, J.; Wang, S.; He, B. The diagnostic and prognostic values of microRNA-196a in cancer. Biosci. Rep. 2021, 41, BSR20203559. [Google Scholar] [CrossRef] [PubMed]
- Igaz, P. Circulating micrornas in adrenal tumors. Curr. Opin. Endocrinol. Diabetes Obes. 2019, 26, 155–159. [Google Scholar] [CrossRef]
- Perge, P.; Butz, H.; Pezzani, R.; Bancos, I.; Nagy, Z.; Pálóczi, K.; Nyírő, G.; Decmann, Á.; Pap, E.; Luconi, M.; et al. Evaluation and diagnostic potential of circulating extracellular vesicle-associated micrornas in adrenocortical tumors. Sci. Rep. 2017, 7, 5474. [Google Scholar] [CrossRef]
- Koperski, Ł.; Kotlarek, M.; Świerniak, M.; Kolanowska, M.; Kubiak, A.; Górnicka, B.; Jażdżewski, K.; Wójcicka, A. Next-generation sequencing reveals microrna markers of adrenocortical tumors malignancy. Oncotarget 2017, 8, 49191. [Google Scholar] [CrossRef]
- Ronchi, C.L.; Sbiera, S.; Leich, E.; Henzel, K.; Rosenwald, A.; Allolio, B.; Fassnacht, M. Single nucleotide polymorphism array profiling of adrenocortical tumors-evidence for an adenoma carcinoma sequence? PLoS ONE 2013, 8, e73959. [Google Scholar] [CrossRef]
- Else, T.; Kim, A.C.; Sabolch, A.; Raymond, V.M.; Kandathil, A.; Caoili, E.M.; Jolly, S.; Miller, B.S.; Giordano, T.J.; Hammer, G.D. Adrenocortical carcinoma. Endocr. Rev. 2014, 35, 282–326. [Google Scholar] [CrossRef]
- Zheng, S.; Cherniack, A.D.; Dewal, N.; Moffitt, R.A.; Danilova, L.; Murray, B.A.; Lerario, A.M.; Else, T.; Knijnenburg, T.A.; Ciriello, G.; et al. Comprehensive pan-genomic characterization of adrenocortical carcinoma. Cancer Cell 2016, 29, 723–736. [Google Scholar] [CrossRef]
- Barreau, O.; de Reynies, A.; Wilmot-Roussel, H.; Guillaud-Bataille, M.; Auzan, C.; René-Corail, F.; Tissier, F.; Dousset, B.; Bertagna, X.; Bertherat, J.; et al. Clinical and pathophysiological implications of chromosomal alterations in adrenocortical tumors: An integrated genomic approach. J. Clin. Endocrinol. Metab. 2012, 97, E301–E311. [Google Scholar] [CrossRef]
- Kjellman, M.; Kallioniemi, O.-P.; Karhu, R.; Höög, A.; Farnebo, L.-O.; Auer, G.; Larsson, C.; Bäckdahl, M. Genetic aberrations in adrenocortical tumors detected using comparative genomic hybridization correlate with tumor size and malignancy. Cancer Res. 1996, 56, 4219–4223. [Google Scholar] [PubMed]
- Zhao, J.; Speel, E.J.; Muletta-Feurer, S.; Rütimann, K.; Saremaslani, P.; Roth, J.; Heitz, P.U.; Komminoth, P. Analysis of genomic alterations in sporadic adrenocortical lesions: Gain of chromosome 17 is an early event in adrenocortical tumorigenesis. Am. J. Pathol. 1999, 155, 1039–1045. [Google Scholar] [CrossRef]
- Assié, G.; Letouzé, E.; Fassnacht, M.; Jouinot, A.; Luscap, W.; Barreau, O.; Omeiri, H.; Rodriguez, S.; Perlemoine, K.; René-Corail, F.; et al. Integrated genomic characterization of adrenocortical carcinoma. Nat. Genet. 2014, 46, 607–612. [Google Scholar] [CrossRef]
- Juhlin, C.C.; Goh, G.; Healy, J.M.; Fonseca, A.L.; Scholl, U.I.; Stenman, A.; Kunstman, J.W.; Brown, T.C.; Overton, J.D.; Mane, S.M.; et al. Whole-exome sequencing characterizes the landscape of somatic mutations and copy number alterations in adrenocortical carcinoma. J. Clin. Endocrinol. Metab. 2015, 100, E493–E502. [Google Scholar] [CrossRef]
- Fassnacht, M.; Libé, R.; Kroiss, M.; Allolio, B. Adrenocortical carcinoma: A clinician’s update. Nat. Rev. Endocrinol. 2011, 7, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Gicquel, C.; Bertagna, X.; Gaston, V.; Coste, J.; Louvel, A.; Baudin, E.; Bertherat, J.; Chapuis, Y.; Duclos, J.-M.; Schlumberger, M.; et al. Molecular markers and long-term recurrences in a large cohort of patients with sporadic adrenocortical tumors. Cancer Res. 2001, 61, 6762–6767. [Google Scholar]
- Kjellman, M.; Roshani, L.; Teh, B.T.; Kallioniemi, O.P.; Höög, A.; Gray, S.; Farnebo, L.O.; Holst, M.; Bäckdahl, M.; Larsson, C. Genotyping of adrenocortical tumors: Very frequent deletions of the men1 locus in 11q13 and of a 1-centimorgan region in 2p16. J. Clin. Endocrinol. Metab. 1999, 84, 730–735. [Google Scholar] [CrossRef]
- Libé, R.; Bertherat, J. Molecular genetics of adrenocortical tumours, from familial to sporadic diseases. Eur. J. Endocrinol. 2005, 153, 477–487. [Google Scholar] [CrossRef]
- Darabi, S.; Braxton, D.R.; Eisenberg, B.L.; Demeure, M.J. Molecular genomic profiling of adrenocortical cancers in clinical practice. Surgery 2021, 169, 138–144. [Google Scholar] [CrossRef]
- De Martino, M.C.; Al Ghuzlan, A.; Aubert, S.; Assié, G.; Scoazec, J.-Y.; Leboulleux, S.; Do Cao, C.; Libè, R.; Nozières, C.; Lombès, M. Molecular screening for a personalized treatment approach in advanced adrenocortical cancer. J. Clin. Endocrinol. Metab. 2013, 98, 4080–4088. [Google Scholar] [CrossRef]
- Ragazzon, B.; Libé, R.; Assié, G.; Tissier, F.; Barreau, O.; Houdayer, C.; Perlemoine, K.; Audebourg, A.; Clauser, E.; René-Corail, F.; et al. Mass-array screening of frequent mutations in cancers reveals rb1 alterations in aggressive adrenocortical carcinomas. Eur. J. Endocrinol. 2014, 170, 385–391. [Google Scholar] [CrossRef]
- Ross, J.S.; Wang, K.; Rand, J.V.; Gay, L.; Presta, M.J.; Sheehan, C.E.; Ali, S.M.; Elvin, J.A.; Labrecque, E.; Hiemstra, C.; et al. Next-generation sequencing of adrenocortical carcinoma reveals new routes to targeted therapies. J. Clin. Pathol. 2014, 67, 968–973. [Google Scholar] [CrossRef]
- Wasserman, J.D.; Novokmet, A.; Eichler-Jonsson, C.; Ribeiro, R.C.; Rodriguez-Galindo, C.; Zambetti, G.P.; Malkin, D. Prevalence and functional consequence of tp53 mutations in pediatric adrenocortical carcinoma: A children’s oncology group study. J. Clin. Oncol. 2015, 33, 602. [Google Scholar] [CrossRef]
- Libè, R.; Groussin, L.; Tissier, F.; Elie, C.; René-Corail, F.; Fratticci, A.; Jullian, E.; Beck-Peccoz, P.; Bertagna, X.; Gicquel, C.; et al. Somatic TP53 mutations are relatively rare among adrenocortical cancers with the frequent 17p13 loss of heterozygosity. Clin. Cancer Res. 2007, 13, 844–850. [Google Scholar] [CrossRef]
- Bond, C.E.; McKeone, D.M.; Kalimutho, M.; Bettington, M.L.; Pearson, S.A.; Dumenil, T.D.; Wockner, L.F.; Burge, M.; Leggett, B.A.; Whitehall, V.L. Rnf43 and znrf3 are commonly altered in serrated pathway colorectal tumorigenesis. Oncotarget 2016, 7, 70589–70600. [Google Scholar] [CrossRef]
- Qiu, W.; Yang, Z.; Fan, Y.; Zheng, Q. ZNRF3 is downregulated in papillary thyroid carcinoma and suppresses the proliferation and invasion of papillary thyroid cancer cells. Tumor Biol. 2016, 37, 12665–12672. [Google Scholar] [CrossRef]
- Zheng, G.-Y.; Zhang, X.-B.; Li, H.-Z.; Zhang, Y.-S.; Deng, J.-H.; Wu, X.-C. Sum of high-risk gene mutation (shgm): A novel attempt to assist differential diagnosis for adrenocortical carcinoma with benign adenoma, based on detection of mutations of nine target genes. Biochem. Genet. 2021, 59, 902–918. [Google Scholar] [CrossRef]
- Alix-Panabières, C.; Pantel, K. Clinical applications of circulating tumor cells and circulating tumor DNA as liquid biopsy. Cancer Discov. 2016, 6, 479–491. [Google Scholar] [CrossRef]
- Batth, I.S.; Mitra, A.; Manier, S.; Ghobrial, I.M.; Menter, D.; Kopetz, S.; Li, S. Circulating tumor markers: Harmonizing the yin and yang of ctcs and ctdna for precision medicine. Ann. Oncol. 2017, 28, 468–477. [Google Scholar] [CrossRef]
- Perakis, S.; Speicher, M.R. Emerging concepts in liquid biopsies. BMC Med. 2017, 15, 75. [Google Scholar] [CrossRef]
- Cantini, G.; Canu, L.; Armignacco, R.; Salvianti, F.; De Filpo, G.; Ercolino, T.; Nesi, G.; Maggi, M.; Mannelli, M.; Pinzani, P.; et al. Prognostic and monitoring value of circulating tumor cells in adrenocortical carcinoma: A preliminary monocentric study. Cancers 2020, 12, 3176. [Google Scholar] [CrossRef] [PubMed]
- Pinzani, P.; Scatena, C.; Salvianti, F.; Corsini, E.; Canu, L.; Poli, G.; Paglierani, M.; Piccini, V.; Pazzagli, M.; Nesi, G.; et al. Detection of circulating tumor cells in patients with adrenocortical carcinoma: A monocentric preliminary study. J. Clin. Endocrinol. Metab. 2013, 98, 3731–3738. [Google Scholar] [CrossRef] [PubMed]
- Salvianti, F.; Canu, L.; Poli, G.; Armignacco, R.; Scatena, C.; Cantini, G.; Di Franco, A.; Gelmini, S.; Ercolino, T.; Pazzagli, M.; et al. New insights in the clinical and translational relevance of miR483-5p in adrenocortical cancer. Oncotarget 2017, 8, 65525. [Google Scholar] [CrossRef] [PubMed]
- Szabó, D.R.; Luconi, M.; Szabó, P.M.; Tóth, M.; Szücs, N.; Horányi, J.; Nagy, Z.; Mannelli, M.; Patócs, A.; Rácz, K.; et al. Analysis of circulating microRNAS in adrenocortical tumors. Lab. Investig. 2014, 94, 331–339. [Google Scholar] [CrossRef]
- Decmann, A.; Bancos, I.; Khanna, A.; Thomas, M.A.; Turai, P.; Perge, P.; Pintér, J.Z.; Tóth, M.; Patócs, A.; Igaz, P. Comparison of plasma and urinary microRNA-483-5p for the diagnosis of adrenocortical malignancy. J. Biotechnol. 2019, 297, 49–53. [Google Scholar] [CrossRef]
- Creemers, S.G.; Korpershoek, E.; Atmodimedjo, P.N.; Dinjens, W.N.; van Koetsveld, P.M.; Feelders, R.A.; Hofland, L.J. Identification of mutations in cell-free circulating tumor DNA in adrenocortical carcinoma: A case series. J. Clin. Endocrinol. Metab. 2017, 102, 3611–3615. [Google Scholar] [CrossRef]
- Garinet, S.; Nectoux, J.; Neou, M.; Pasmant, E.; Jouinot, A.; Sibony, M.; Orhant, L.; da Fonseca, J.P.; Perlemoine, K.; Bricaire, L.; et al. Detection and monitoring of circulating tumor DNA in adrenocortical carcinoma. Endocr.-Relat. Cancer 2018, 25, L13–L17. [Google Scholar] [CrossRef]
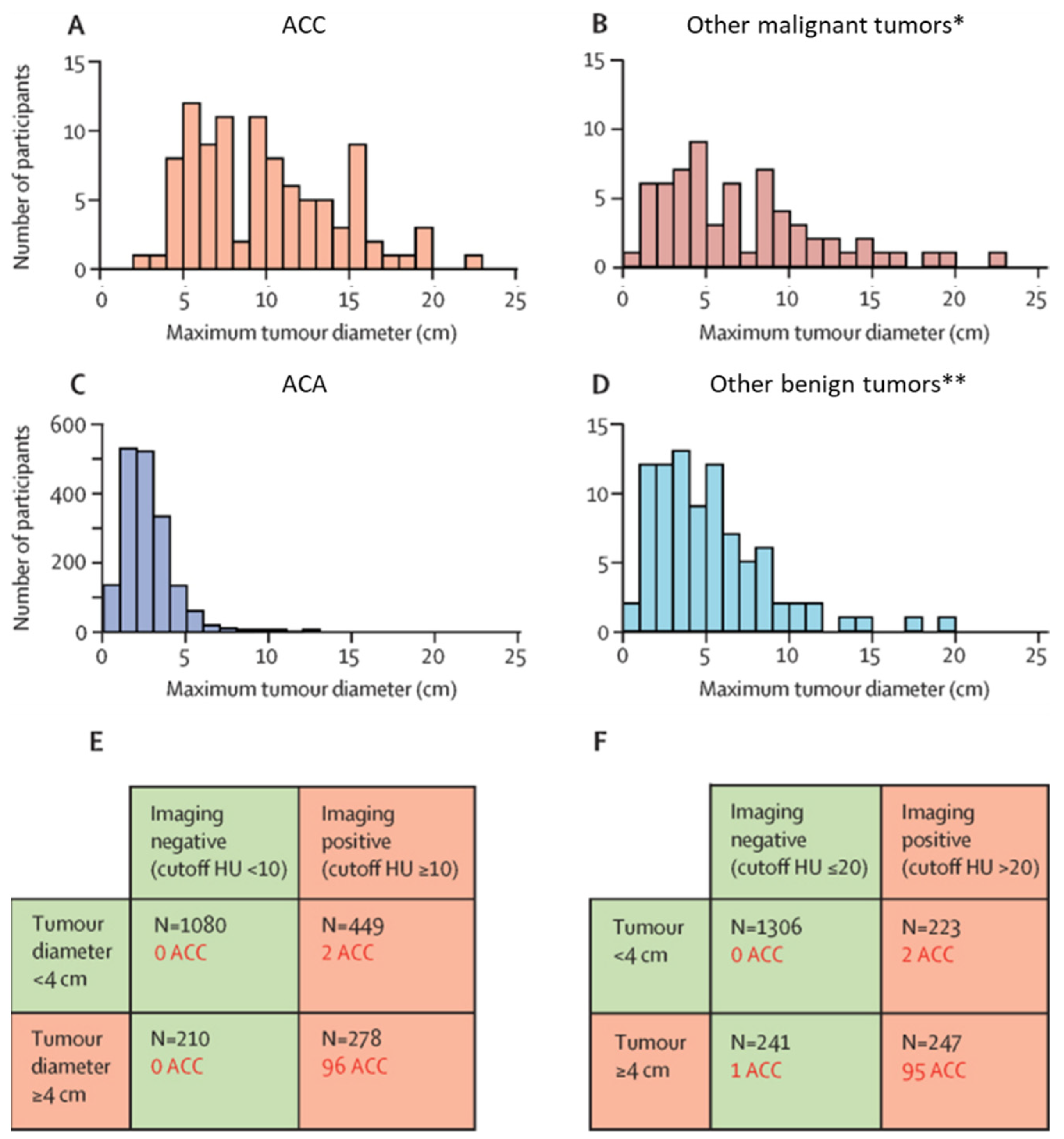
| ENSAT Stage | T | N | M |
|---|---|---|---|
| I | 1 | 0 | 0 |
| II | 2 | 0 | 0 |
| III | 1, 2 | 1 | 0 |
| 3, 4 | 0, 1 | 0 | |
| IV | 1–4 | 0, 1 | 1 |
| Parameter | Weiss (1989) [67] | Modified Weiss (2002) [68] | Lin-Weiss-Bisceglia (2004) [70] | Reticulin Algorithm (2009) [71] | Helsinki Score (2015) [72] |
|---|---|---|---|---|---|
| Tumor type(s): | Conventional | Conventional | Oncocytic | Conventional Oncocytic Myxoid | Conventional Oncocytic Myxoid |
| Criteria: | |||||
| Mitosis | >5/50HPF | >5/50HPF | >5/50HPF | >5/50HPF | >5/50HPF (x3) |
| Atypical mitosis | x | x | x | ||
| Necrosis | x | x | x | x | x (x5) |
| Clear cells ≤25% | x | x (x2) | |||
| Venous invasion | x | x | x | ||
| Capsular invasion | x | x | x | ||
| Sinusoidal invasion | x | x | |||
| Diffuse architecture >30% | x | ||||
| FNG 3 or 4 | x | ||||
| >10 cm and/or >200 g | x | ||||
| Altered reticulin network | x | ||||
| Numeric value of Ki67% | x | ||||
| Cutoff for malignancy: | ≥3 points | ≥3 points | 1 major * | Reticulin + 1 | >8.5 points |
| Micro-RNA | Number of Patients | AUC | Sensitivity (%) | Specificity (%) | Reference |
|---|---|---|---|---|---|
| miR-34a | 17 ACC, 22 ACA | 0.81 | - | - | [127] |
| miR-483-5p | 17 ACC, 22 ACA | 0.74 | - | - | [127] |
| miR-195 | 14 aACC, 9 naACC, 14 ACA | 0.948 | 90.9 | 100 | [130] |
| miR-139-5p | 14 aACC, 9 naACC, 14 ACA | 0.714 | 87.5 | 65 | [130] |
| miR-335 | 14 aACC, 9 naACC, 14 ACA | 0.837 | 95.2 | 71.4 | [130] |
| miR-376a | 14 aACC, 9 naACC, 14 ACA | 0.811 | 71.4 | 85.7 | [130] |
| miR-483-5p | 14 aACC, 9 naACC, 14 ACA | 0.929 * | 85.7 * | 100 * | [130] |
| miR-210/181b | 9 ACC, 8 ACA | 0.87 | 88 | 75 | [164] |
| miR-100/181b | 9 ACC, 8 ACA | 0.85 | 77.8 | 100 | [164] |
| miR-483-5p | 16 ACC, 18 ACA | 0.965 | 87.5 | 94.4 | [135] |
| miR-483-5p | 23 ACC, 23 ACA | 0.88 | 87 | 78.3 | [165] |
| Marker | Reference | Number of Patients | Sensitivity (%) | Specificity (%) | Other Accuracy Measures |
|---|---|---|---|---|---|
| Radiological Markers | |||||
| Diameter ≤4 cm + HU ≤20 1 | [16] | 233 | 76.4 | 96.9 | PPV 98.6%, NPV 59.0% |
| Diameter ≥4 cm | [22] | 504 | 96 | 52 | LR malignancy 4.4 |
| Diameter ≥6 cm | [22] | 504 | 90 | 80 | LR malignancy 16.9 |
| Diameter ≥6.5 cm | [68] | 49 | 100 | 91.7 | |
| HU ≥ 10 | [3] | 2017 | 100 | 64.0 | - |
| HU ≥ 20 | [3] | 2017 | 100 | 80.0 | - |
| FDG-PET | [29] | 1217 | 97 2 | 91 2 | PLR 11.1, NLR 0.04, OR 294 2 |
| MTO-PET | [35] | 173 | 89 3 | 96 3 | - |
| I123IMTO | [37,38] | 51–58 | 38–83 3 | 86–100 3 | - |
| Biochemical Markers | |||||
| Urine steroid metabolomics | [47] | 147 | 90 | 90 | AUC 0.97 |
| Imaging + urine | [3] | 2017 | - | - | PPV 76.4%, NPV 99.7% |
| Histopathological and Immunohistochemical Markers | |||||
| Weiss | [72,74] | 50–177 | 86–100 | 90.2 | Misclassification in 9–13%, AUC 0.624 |
| Revised Weiss | [68,72] | 49–177 | 100 | 96.9 | - |
| Reticulin algorithm | [71,77] | 139–245 | 97–100 | 100 | - |
| Helsinki score | [72] | 177 | 100 4 | 99.4 4 | AUC 0.729 4 |
| Ki67/MIB-1 labeling index | [60,68,81,84,116] | 37–64 | 64–100 | 91.7–100 | - |
| IGF2 expression | [60,84,116] | 39–64 | 64–76.5 | 72–100 | - |
| IGF2 + Ki67 | [60] | 34 | 96 | 100 | - |
| IGF2 + MAD2L1 | [60] | 34 | 100 | 95 | - |
| IGF2 + CNNb1 | [60] | 34 | 91 | 100 | - |
| IGF2 + MIB-1 | [84] | 39 | 100 | 95.5 | - |
| P53 | [84] | 39 | 17.6 | 100 | - |
| Molecular Markers | |||||
| IGF2 methylation score | [101,102] | 22–194 | 89–96 | 92–100 | AUC 0.910–0.997 |
| IGF2 methylation score + tumor size | [102] | 194 | - | - | AUC 0.957 |
| miR-483-5p | [126,130,135] | 31–34 | 80–87.5 | 94.4–100 | PPV 100%, NPV 92%, AUC 0.90–0.96 |
| miR-195 | [130] | 31 | - | - | AUC 0.83 |
| miR-335 | [130] | 31 | - | - | AUC 0.87 |
| Combi 6 miR’s 5 | [136] | 28 | - | - | 95% accuracy |
| miR-511 + miR-503 | [111] | 36 | 100 | 93 | - |
| miR-511 + miR184 | [111] | 36 | 100 | 80 | - |
| miR-483-3p + Smad4 | [116] | 50 | - | 92.8 | - |
| Alterations 6 loci 6 | [140] | 138 | 100 | 83 | - |
| >60% amplification chrom5 | [137] | 46 | 77.3 | 100 | - |
| Combi >50 CNV’s + >10 LOH events | [137] | 46 | 82 | 100 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viëtor, C.L.; Creemers, S.G.; van Kemenade, F.J.; van Ginhoven, T.M.; Hofland, L.J.; Feelders, R.A. How to Differentiate Benign from Malignant Adrenocortical Tumors? Cancers 2021, 13, 4383. https://doi.org/10.3390/cancers13174383
Viëtor CL, Creemers SG, van Kemenade FJ, van Ginhoven TM, Hofland LJ, Feelders RA. How to Differentiate Benign from Malignant Adrenocortical Tumors? Cancers. 2021; 13(17):4383. https://doi.org/10.3390/cancers13174383
Chicago/Turabian StyleViëtor, Charlotte L., Sara G. Creemers, Folkert J. van Kemenade, Tessa M. van Ginhoven, Leo J. Hofland, and Richard A. Feelders. 2021. "How to Differentiate Benign from Malignant Adrenocortical Tumors?" Cancers 13, no. 17: 4383. https://doi.org/10.3390/cancers13174383
APA StyleViëtor, C. L., Creemers, S. G., van Kemenade, F. J., van Ginhoven, T. M., Hofland, L. J., & Feelders, R. A. (2021). How to Differentiate Benign from Malignant Adrenocortical Tumors? Cancers, 13(17), 4383. https://doi.org/10.3390/cancers13174383






