The Day after Mass COVID-19 Vaccination: Higher Hypermetabolic Lymphadenopathy Detection on PET/CT and Impact on Oncologic Patients Management
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
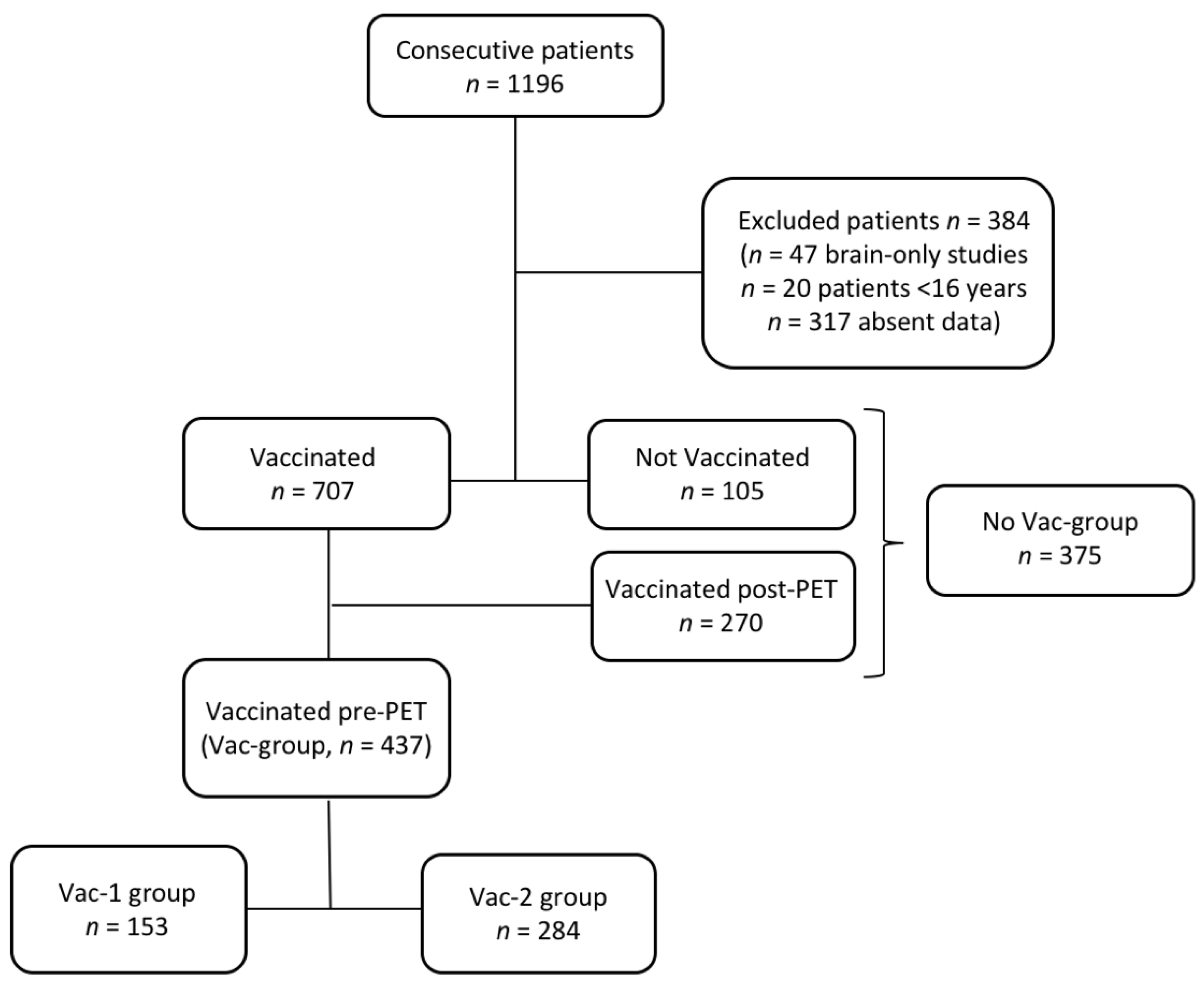
2.1. Study Design and Population
2.2. PET/CT Acquisition
2.3. Image Interpretation and Data Analysis
2.4. Statistical Analysis
3. Results
3.1. Patients Characteristics
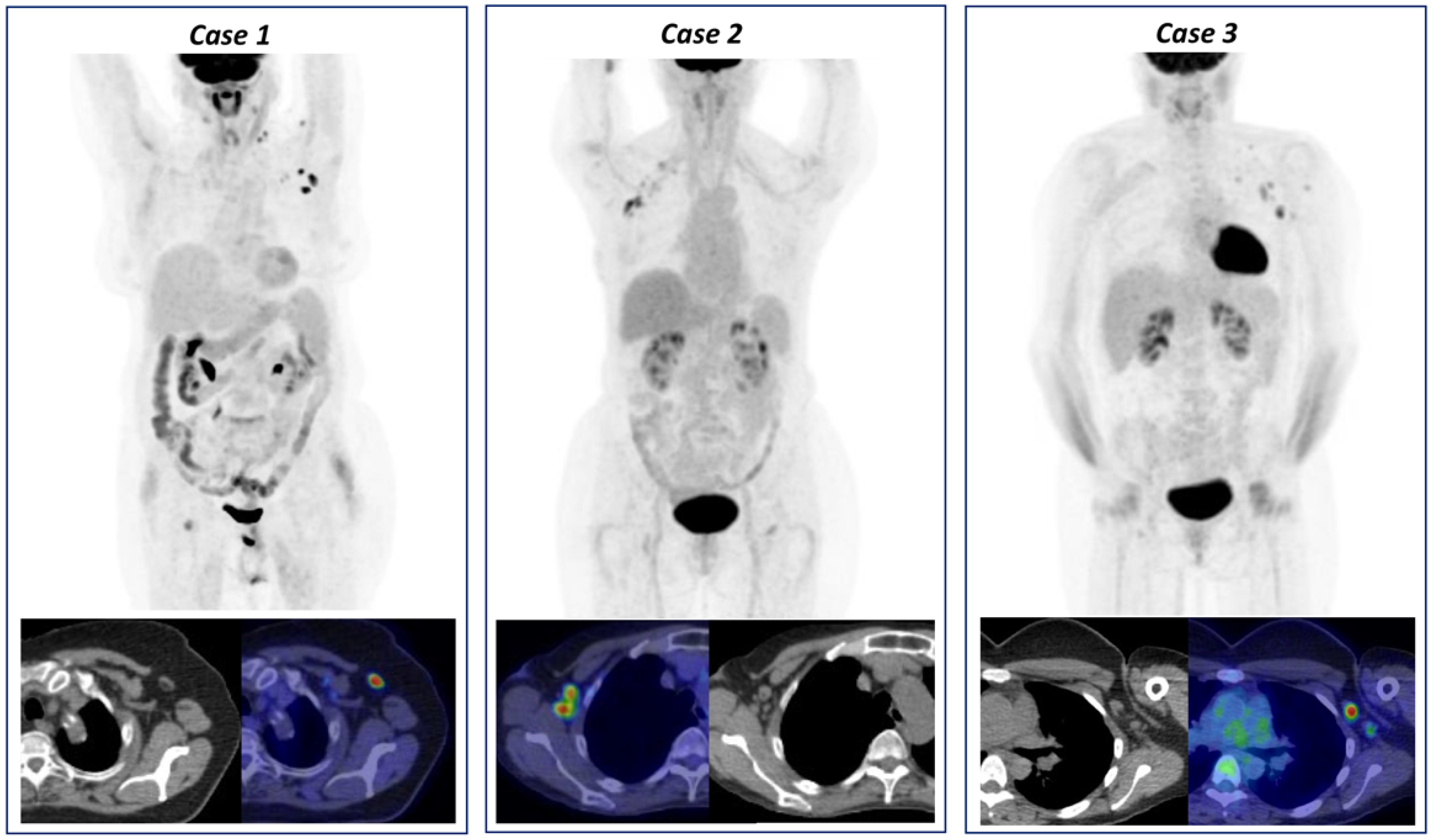
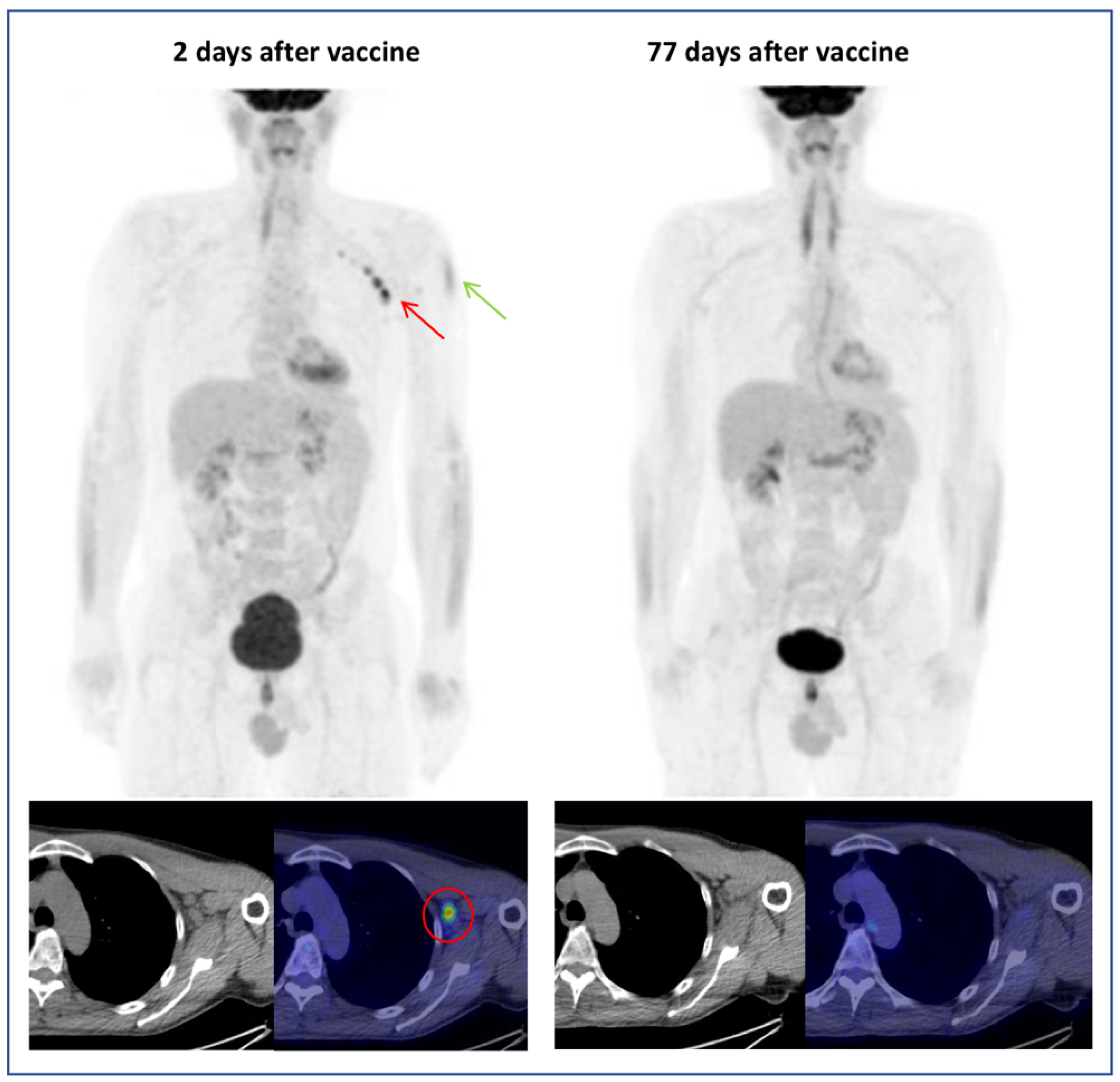
3.2. Hypermetabolic Lymph Nodes Detection and Categorization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Treglia, G.; Cuzzocrea, M.; Muoio, B.; Elzi, L. PET findings after COVID-19 vaccination: “Keep Calm and Carry On”. Clin. Transl. Imaging 2021, 9, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Burger, I.A.; Husmann, L.; Hany, T.F.; Schmid, D.T.; Schaefer, N. Incidence and Intensity of F-18 FDG Uptake After Vaccination With H1N1 Vaccine. Clin. Nucl. Med. 2011, 36, 848–853. [Google Scholar] [CrossRef] [PubMed]
- Coates, E.E.; Costner, P.J.; Nason, M.C.; Herrin, D.M.; Conant, S.; Herscovitch, P.; Sarwar, U.N.; Holman, L.; Mitchell, J.; Yamshchikov, G.; et al. Lymph Node Activation by PET/CT Following Vaccination With Licensed Vaccines for Human Papillomaviruses. Clin. Nucl. Med. 2017, 42, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Panagiotidis, E.; Exarhos, D.; Housianakou, I.; Bournazos, A.; Datseris, I. FDG uptake in axillary lymph nodes after vaccination against pandemic (H1N1). Eur. Radiol. 2010, 20, 1251–1253. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.; Krauthammer, S.H.; Wolf, I.; Even-Sapir, E. Hypermetabolic lymphadenopathy following administration of BNT162b2 mRNA Covid-19 vaccine: Incidence assessed by [18F]FDG PET-CT and relevance to study interpretation. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1854–1863. [Google Scholar] [CrossRef] [PubMed]
- Eifer, M.; Eshet, Y. Imaging of COVID-19 Vaccination at FDG PET/CT. Radiology 2021, 299, E248. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Kaur, P.; Kumar, V.; Maroules, M. COVID-19 vaccine induced Axillary and Pectoral Lymphadenopathy on PET scan. Radiol. Case Rep. 2021, 16, 1819–1821. [Google Scholar] [CrossRef] [PubMed]
- Seban, R.-D.; Champion, L.; Deleval, N.; Richard, C.; Provost, C. Immune Response Visualized In Vivo by [18F]-FDG PET/CT after COVID-19 Vaccine. Diagnostics 2021, 11, 676. [Google Scholar] [CrossRef]
- Adin, M.E.; Isufi, E.; Kulon, M.; Pucar, D. Association of COVID-19 mRNA Vaccine With Ipsilateral Axillary Lymph Node Reactivity on Imaging. JAMA Oncol. 2021, 7, 1241. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.J.; Van Abel, K.; Ma, D.; Johnson, D.R. FDG Avid Axillary Lymph Nodes After COVID-19 Vaccination. J. Nucl. Med. 2021, 121. [Google Scholar] [CrossRef]
- Minamimoto, R.; Kiyomatsu, T. Effects of COVID-19 vaccination on FDG-PET/CT imaging: A literature review. Glob. Heal. Med. 2021, 3, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- COVID-19 Resources. Available online: https://www.nccn.org/covid-19 (accessed on 12 August 2021).
- Eshet, Y.; Tau, N.; Alhoubani, Y.; Kanana, N.; Domachevsky, L.; Eifer, M. Prevalence of Increased FDG PET/CT Axillary Lymph Node Uptake Beyond 6 Weeks after mRNA COVID-19 Vaccination. Radiology 2021, 210886. [Google Scholar] [CrossRef]
- Seban, R.-D.; Champion, L.; Yeh, R.; Schwartz, L.H.; Dercle, L. Assessing immune response upon systemic RNA vaccination on [18F]-FDG PET/CT for COVID-19 vaccine and then for immuno-oncology? Eur. J. Nucl. Med. Mol. Imaging 2021, 1–2. [Google Scholar] [CrossRef]


| Patients Characteristics | All-Vac Group (n = 437) | No Vac-Group (n = 375) |
|---|---|---|
| Mean age ± SD, years (range) | 64 ± 14 (21–88) | 61 ± 14.5 (16–87) |
| Female, n (%) | 205 (47%) | 178 (48%) |
| PET/CT indication, n (%) | ||
| Hematological malignancy | 107 (25%) | 93 (25%) |
| Breast malignancy | 53 (12%) | 47 (13%) |
| Lung malignancy | 76 (17%) | 53 (14%) |
| Gastrointestinal malignancy | 56 (13%) | 45 (12%) |
| Gynecological malignancy | 21 (5%) | 15 (4%) |
| Genitourinary malignancy | 61 (14%) | 50 (13%) |
| Head and neck malignancy | 23 (5%) | 26 (7%) |
| Sarcoma | 5 (1%) | 4 (1%) |
| Melanoma | 17 (4%) | 17 (5%) |
| Inflammation/infection | 10 (2%) | 12 (3%) |
| Other malignancy | 8 (2%) | 13 (3%) |
| Extension of disease, n (%) | ||
| Localized disease | 162 (37%) | 157 (42%) |
| Metastatic disease | 275 (63%) | 218 (58%) |
| Status of Disease, n (%) | ||
| Diagnosis/staging | 129 (30%) | 96 (26%) |
| Interim/post-therapy | 159 (36%) | 146 (39%) |
| Follow-up | 149 (34%) | 133 (35%) |
| Treatment, n (%) | ||
| No current treatment | 306 (70%) | 283 (75%) |
| Chemotherapy | 55 (13%) | 35 (9%) |
| Immunosuppressive/ Immunotherapy | 27 (6%) | 21 (6%) |
| Other | 49 (11%) | 36 (10%) |
| Vaccination data, n (%) | ||
| Vac-1 group | 153 (35%) | |
| Vac-2 group | 284 (65%) | |
| Type of vaccination, n (%) | ||
| BioNTech/Pfizer | 380 (87%) | |
| Moderna | 26 (6%) | |
| AstraZeneca | 31 (7%) | |
| Median time vaccination-PET/CT, days | 23 |
| Lymphadenopathy Characteristics | No Vac-Group (n = 375) | All Vac Group (n = 437) | p |
|---|---|---|---|
| HLN | 55 (15%) | 120 (27%) | <0.001 |
| MHL | 28 (23%) | ||
| VAHL | 65 (54%) | ||
| EqHL | 27 (23%) | ||
| Median SUVmax | 5.3 (1.5–33.4) | 4.1 (1.4–24.5) | p = 0.141 |
| Grading uptake | |||
| Grade 1 | 6 (11%) | 13 (11%) | |
| Grade 2 | 7 (13%) | 45 (37%) | |
| Grade 3 | 42 (76%) | 62 (52%) |
| Variables | Detection Rate | p Value |
|---|---|---|
| Sex, n (%) | <0.001 | |
| Female | 46/230 (20%) | |
| Male | 74/207 (36%) | |
| Type of disease, n (%) | 0.599 | |
| Hematological disease | 32/107 (30%) | |
| Solid tumor | 83/316 (26%) | |
| Other | 5/14 (36%) | |
| Type of treatment, n (%) | 0.662 | |
| No treatment | 85/306 (28%) | |
| Chemotherapy | 12/55 (22%) | |
| Immunosuppressive/immunotherapy | 7/27 (26%) | |
| Other | 16/49 (33%) | |
| Type of vaccination, n (%) | 0.141 | |
| BioNTech/Pfizer | 103/380 (27%) | |
| Moderna | 11/26 (42%) | |
| AstraZeneca/Vaxzevria | 6/31 (19%) | |
| Number of vaccination dose pre-PET, n (%) | 0.456 | |
| Vac-1 | 41/153 (27%) | |
| Vac-2 | 79/284 (28%) | |
| Number of days from vaccine to PET, n (%) | 0.01 | |
| 0–6 | 21/60 (35%) | |
| 7–19 | 45/129 (35%) | |
| ≥20 | 54/248 (22%) | |
| PET Radiopharmaceutical, n (%) | 0.002 | |
| [18F]Fluorodeoxyglucose | 117/389 (30%) | |
| [18F]Fluorocholine | 3/45 (7%) | |
| [18F]Fluciclovine | 0/3 (0%) |
| Variables | SUVmax Grade | p Value | ||
|---|---|---|---|---|
| Grade 1 (<2.2) | Grade 2 (2.2–4) | Grade 3 (>4) | ||
| Sex, n (%) | 0.405 | |||
| Female | 5/43 (12%) | 15/43 (35%) | 23/43 (53%) | |
| Male | 1/22 (5%) | 11/22 (50%) | 10/22 (45%) | |
| Type of malignancy, n (%) | 0.109 | |||
| Hematological disease | 0/13 (0%) | 3/13 (23%) | 10/13 (77%) | |
| Solid tumor | 6/50 (12%) | 21/50 (42%) | 23/50 (46%) | |
| Other | 0/2 (0%) | 2/2 (100%) | 0/2 (0%) | |
| Type of treatment, n (%) | 0.129 | |||
| No treatment | 3/46 (7%) | 19/46 (41%) | 24/46 (52%) | |
| Chemotherapy | 0/5 (0%) | 1/5 (20%) | 4/5 (80%) | |
| Immunosuppressive/ immunotherapy | 0/5 (0%) | 2/5 (40%) | 3/5 (60%) | |
| Other | 3/9 (33%) | 4/9 (45%) | 2/9 (22%) | |
| Type of vaccination, n (%) | 0.117 | |||
| BioNTech/Pfizer | 4/51 (8%) | 22/51 (43%) | 25/51 (47%) | |
| Moderna | 0/9 (0%) | 3/9 (33%) | 6/9 (67%) | |
| AstraZeneca/Vaxzevria | 2/5 (40%) | 1/5 (20%) | 2/5 (40%) | |
| Number of vaccination dose pre-PET, n (%) | 0.334 | |||
| Vac-1 | 1/24 (4%) | 12/24 (50%) | 11/24 (46%) | |
| Vac-2 | 5/41 (12%) | 14/41 (34%) | 22/41 (54%) | |
| Median time vaccine to PET, days (range) | 37.5 (11–133) | 19 (2-71) | 12 (3-50) | 0.004 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrari, C.; Nappi, A.G.; Santo, G.; Mammucci, P.; Rubini, D.; Tucci, M.; Pisani, A.R. The Day after Mass COVID-19 Vaccination: Higher Hypermetabolic Lymphadenopathy Detection on PET/CT and Impact on Oncologic Patients Management. Cancers 2021, 13, 4340. https://doi.org/10.3390/cancers13174340
Ferrari C, Nappi AG, Santo G, Mammucci P, Rubini D, Tucci M, Pisani AR. The Day after Mass COVID-19 Vaccination: Higher Hypermetabolic Lymphadenopathy Detection on PET/CT and Impact on Oncologic Patients Management. Cancers. 2021; 13(17):4340. https://doi.org/10.3390/cancers13174340
Chicago/Turabian StyleFerrari, Cristina, Anna Giulia Nappi, Giulia Santo, Paolo Mammucci, Dino Rubini, Marco Tucci, and Antonio Rosario Pisani. 2021. "The Day after Mass COVID-19 Vaccination: Higher Hypermetabolic Lymphadenopathy Detection on PET/CT and Impact on Oncologic Patients Management" Cancers 13, no. 17: 4340. https://doi.org/10.3390/cancers13174340
APA StyleFerrari, C., Nappi, A. G., Santo, G., Mammucci, P., Rubini, D., Tucci, M., & Pisani, A. R. (2021). The Day after Mass COVID-19 Vaccination: Higher Hypermetabolic Lymphadenopathy Detection on PET/CT and Impact on Oncologic Patients Management. Cancers, 13(17), 4340. https://doi.org/10.3390/cancers13174340






