The Landscape of Pediatric Precision Oncology: Program Design, Actionable Alterations, and Clinical Trial Development
Abstract
:Simple Summary
Abstract
1. Introduction
Methods
2. Molecularly Informed Personalized Medicine in Adult Oncology
3. The Differing Genomic Landscapes of Childhood and Adult Cancers
4. The Development of Precision Medicine Programs in Pediatric Oncology
5. Patient Accrual
6. Next-Generation Sequencing, Data Integration and Visualization
7. Translating Molecular Findings into Clinic: Identification and Prioritization of Targets
8. Germline Variants
9. Change or Refinement of Diagnosis
10. Targeted Therapy and Clinical Decision Making
11. Clinical Benefit
12. Clinical Trial Development: Innovative Global Collaboration
13. Ongoing and Future Perspectives in Pediatric Precision Oncology
13.1. Patient-Derived Models and Drug Sensitivity Profiling
13.2. Emerging Technologies: Liquid Biopsies
13.3. Novel Therapies: Immune Interventions
13.4. Clinical Trials: Incorporating Combination Strategies
13.5. Big Data
14. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| NGS | Next-Generation Sequencing |
| WGS | Whole-Genome Sequencing |
| lcWGS | Low-coverage Whole-Genome Sequencing |
| WES | Whole-Exome Sequencing |
| RNAseq | RNA sequencing |
| SNP array | Single Nucleotide Polymorphism array |
| aCGH | array Comparative Genomic Hybridization |
| FISH | Fluorescence in situ hybridization |
| IHC | Immunohistochemistry |
| BCR | Breakpoint Cluster Region |
| ABL | tyrosine-protein kinase ABL1 |
| HER2 | Human Epidermal growth factor Receptor 2 |
| EGFR | Epidermal Growth Factor Receptor |
| EML4 | EMAP Like 4 |
| ALK | Anaplastic Lymphoma Kinase |
| BRAF | v-raf murine sarcoma viral oncogene homolog B1 |
| MEK | Mitogen-Activated Protein Kinase Kinase |
| MAPK | Mitogen-Activated Protein Kinase |
| DNA | Deoxyribonucleic Acid |
| RNA | Ribonucleic Acid |
| MTB | Molecular Tumor Board |
| MSH6 | MutS Homolog 6 |
| PMS2 | PostMeiotic Segregation increased 2 |
| cfDNA | circulating free DNA |
| PDX | Patient-Derived Xenograft |
References
- Steliarova-Foucher, E.; Colombet, M.; Ries, L.A.G.; Moreno, F.; Dolya, A.; Bray, F.; Hesseling, P.; Shin, H.Y.; Stiller, C.A.; Bouzbid, S.; et al. International incidence of childhood cancer, 2001–10: A population-based registry study. Lancet Oncol. 2017, 18, 719–731. [Google Scholar] [CrossRef]
- Cunningham, R.M.; Walton, M.A.; Carter, P.M. The Major Causes of Death in Children and Adolescents in the United States. N. Engl. J. Med. 2018, 379, 2468–2475. [Google Scholar] [CrossRef] [PubMed]
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Niksic, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Esteve, J.; et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Galindo, C.; Friedrich, P.; Alcasabas, P.; Antillon, F.; Banavali, S.; Castillo, L.; Israels, T.; Jeha, S.; Harif, M.; Sullivan, M.J.; et al. Toward the Cure of All Children With Cancer Through Collaborative Efforts: Pediatric Oncology As a Global Challenge. J. Clin. Oncol. 2015, 33, 3065–3073. [Google Scholar] [CrossRef]
- Landier, W.; Skinner, R.; Wallace, W.H.; Hjorth, L.; Mulder, R.L.; Wong, F.L.; Yasui, Y.; Bhakta, N.; Constine, L.S.; Bhatia, S.; et al. Surveillance for Late Effects in Childhood Cancer Survivors. J. Clin. Oncol. 2018, 36, 2216–2222. [Google Scholar] [CrossRef] [PubMed]
- Kurzrock, R.; Giles, F.J. Precision oncology for patients with advanced cancer: The challenges of malignant snowflakes. Cell Cycle 2015, 14, 2219–2221. [Google Scholar] [CrossRef]
- Soverini, S.; Mancini, M.; Bavaro, L.; Cavo, M.; Martinelli, G. Chronic myeloid leukemia: The paradigm of targeting oncogenic tyrosine kinase signaling and counteracting resistance for successful cancer therapy. Mol. Cancer 2018, 17, 49. [Google Scholar] [CrossRef]
- Loibl, S.; Gianni, L. HER2-positive breast cancer. Lancet 2017, 389, 2415–2429. [Google Scholar] [CrossRef]
- Rosell, R.; Carcereny, E.; Gervais, R.; Vergnenegre, A.; Massuti, B.; Felip, E.; Palmero, R.; Garcia-Gomez, R.; Pallares, C.; Sanchez, J.M.; et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012, 13, 239–246. [Google Scholar] [CrossRef]
- Shaw, A.T.; Kim, D.W.; Nakagawa, K.; Seto, T.; Crinó, L.; Ahn, M.J.; De Pas, T.; Besse, B.; Solomon, B.J.; Blackhall, F.; et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N. Engl. J. Med. 2013, 368, 2385–2394. [Google Scholar] [CrossRef] [Green Version]
- Robert, C.; Grob, J.J.; Stroyakovskiy, D.; Karaszewska, B.; Hauschild, A.; Levchenko, E.; Chiarion Sileni, V.; Schachter, J.; Garbe, C.; Bondarenko, I.; et al. Five-Year Outcomes with Dabrafenib plus Trametinib in Metastatic Melanoma. N. Engl. J. Med. 2019, 381, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Von Hoff, D.D.; Stephenson, J.J., Jr.; Rosen, P.; Loesch, D.M.; Borad, M.J.; Anthony, S.; Jameson, G.; Brown, S.; Cantafio, N.; Richards, D.A.; et al. Pilot study using molecular profiling of patients’ tumors to find potential targets and select treatments for their refractory cancers. J. Clin. Oncol. 2010, 28, 4877–4883. [Google Scholar] [CrossRef]
- Tsimberidou, A.M.; Iskander, N.G.; Hong, D.S.; Wheler, J.J.; Falchook, G.S.; Fu, S.; Piha-Paul, S.; Naing, A.; Janku, F.; Luthra, R.; et al. Personalized medicine in a phase I clinical trials program: The MD Anderson Cancer Center initiative. Clin. Cancer Res. 2012, 18, 6373–6383. [Google Scholar] [CrossRef] [Green Version]
- Tsimberidou, A.M.; Wen, S.; Hong, D.S.; Wheler, J.J.; Falchook, G.S.; Fu, S.; Piha-Paul, S.; Naing, A.; Janku, F.; Aldape, K.; et al. Personalized medicine for patients with advanced cancer in the phase I program at MD Anderson: Validation and landmark analyses. Clin. Cancer Res. 2014, 20, 4827–4836. [Google Scholar] [CrossRef] [Green Version]
- Tsimberidou, A.M.; Hong, D.S.; Ye, Y.; Cartwright, C.; Wheler, J.J.; Falchook, G.S.; Naing, A.; Fu, S.; Piha-Paul, S.; Janku, F.; et al. Initiative for Molecular Profiling and Advanced Cancer Therapy (IMPACT): An MD Anderson Precision Medicine Study. JCO Precis. Oncol. 2017, 1, 1–18. [Google Scholar] [CrossRef]
- Massard, C.; Michiels, S.; Ferté, C.; Le Deley, M.-C.; Lacroix, L.; Hollebecque, A.; Verlingue, L.; Ileana, E.; Rosellini, S.; Ammari, S.; et al. High-Throughput Genomics and Clinical Outcome in Hard-to-Treat Advanced Cancers: Results of the MOSCATO 01 Trial. Cancer Discov. 2017, 7, 586–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murciano-Goroff, Y.R.; Drilon, A.; Stadler, Z.K. The NCI-MATCH: A National, Collaborative Precision Oncology Trial for Diverse Tumor Histologies. Cancer Cell 2021, 39, 22–24. [Google Scholar] [CrossRef]
- Flaherty, K.T.; Gray, R.J.; Chen, A.P.; Li, S.; McShane, L.M.; Patton, D.; Hamilton, S.R.; Williams, P.M.; Iafrate, A.J.; Sklar, J.; et al. Molecular Landscape and Actionable Alterations in a Genomically Guided Cancer Clinical Trial: National Cancer Institute Molecular Analysis for Therapy Choice (NCI-MATCH). J. Clin. Oncol. 2020, 38, 3883–3894. [Google Scholar] [CrossRef]
- Chen, A.P.; Kummar, S.; Moore, N.; Rubinstein, L.V.; Zhao, Y.; Williams, P.M.; Palmisano, A.; Sims, D.; O’Sullivan Coyne, G.; Rosenberger, C.L.; et al. Molecular Profiling-Based Assignment of Cancer Therapy (NCI-MPACT): A Randomized Multicenter Phase II Trial. JCO Precis. Oncol. 2021, 5, 133–144. [Google Scholar] [CrossRef]
- Coyne, G.O.; Takebe, N.; Chen, A.P. Defining precision: The precision medicine initiative trials NCI-MPACT and NCI-MATCH. Curr. Probl. Cancer 2017, 41, 182–193. [Google Scholar] [CrossRef]
- Schwaederle, M.; Parker, B.A.; Schwab, R.B.; Daniels, G.A.; Piccioni, D.E.; Kesari, S.; Helsten, T.L.; Bazhenova, L.A.; Romero, J.; Fanta, P.T.; et al. Precision Oncology: The UC San Diego Moores Cancer Center PREDICT Experience. Mol. Cancer Ther. 2016, 15, 743–752. [Google Scholar] [CrossRef] [Green Version]
- Kato, S.; Kim, K.H.; Lim, H.J.; Boichard, A.; Nikanjam, M.; Weihe, E.; Kuo, D.J.; Eskander, R.N.; Goodman, A.; Galanina, N.; et al. Real-world data from a molecular tumor board demonstrates improved outcomes with a precision N-of-One strategy. Nat. Commun. 2020, 11, 4965. [Google Scholar] [CrossRef]
- Sicklick, J.K.; Kato, S.; Okamura, R.; Schwaederle, M.; Hahn, M.E.; Williams, C.B.; De, P.; Krie, A.; Piccioni, D.E.; Miller, V.A.; et al. Molecular profiling of cancer patients enables personalized combination therapy: The I-PREDICT study. Nat. Med. 2019, 25, 744–750. [Google Scholar] [CrossRef]
- Hainsworth, J.D.; Meric-Bernstam, F.; Swanton, C.; Hurwitz, H.; Spigel, D.R.; Sweeney, C.; Burris, H.A.; Bose, R.; Yoo, B.; Stein, A.; et al. Targeted Therapy for Advanced Solid Tumors on the Basis of Molecular Profiles: Results From MyPathway, an Open-Label, Phase IIa Multiple Basket Study. J. Clin. Oncol. 2018, 36, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Trédan, O.; Wang, Q.; Pissaloux, D.; Cassier, P.; de la Fouchardière, A.; Fayette, J.; Desseigne, F.; Ray-Coquard, I.; de la Fouchardière, C.; Frappaz, D.; et al. Molecular screening program to select molecular-based recommended therapies for metastatic cancer patients: Analysis from the ProfiLER trial. Ann. Oncol. 2019, 30, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Rodon, J.; Soria, J.C.; Berger, R.; Miller, W.H.; Rubin, E.; Kugel, A.; Tsimberidou, A.; Saintigny, P.; Ackerstein, A.; Braña, I.; et al. Genomic and transcriptomic profiling expands precision cancer medicine: The WINTHER trial. Nat. Med. 2019, 25, 751–758. [Google Scholar] [CrossRef] [PubMed]
- van der Velden, D.L.; Hoes, L.R.; van der Wijngaart, H.; van Berge Henegouwen, J.M.; van Werkhoven, E.; Roepman, P.; Schilsky, R.L.; de Leng, W.W.J.; Huitema, A.D.R.; Nuijen, B.; et al. The Drug Rediscovery protocol facilitates the expanded use of existing anticancer drugs. Nature 2019, 574, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Fountzilas, E.; Tsimberidou, A.M. Overview of precision oncology trials: Challenges and opportunities. Expert Rev. Clin. Pharmacol. 2018, 11, 797–804. [Google Scholar] [CrossRef]
- Tsimberidou, A.M.; Fountzilas, E.; Nikanjam, M.; Kurzrock, R. Review of precision cancer medicine: Evolution of the treatment paradigm. Cancer Treat. Rev. 2020, 86, 102019. [Google Scholar] [CrossRef]
- Gambardella, V.; Tarazona, N.; Cejalvo, J.M.; Lombardi, P.; Huerta, M.; Rosello, S.; Fleitas, T.; Roda, D.; Cervantes, A. Personalized Medicine: Recent Progress in Cancer Therapy. Cancers 2020, 12, 1009. [Google Scholar] [CrossRef] [Green Version]
- Moreira, A.; Masliah-Planchon, J.; Callens, C.; Vacher, S.; Lecerf, C.; Frelaut, M.; Borcoman, E.; Torossian, N.; Ricci, F.; Hescot, S.; et al. Efficacy of molecularly targeted agents given in the randomised trial SHIVA01 according to the ESMO Scale for Clinical Actionability of molecular Targets. Eur. J. Cancer 2019, 121, 202–209. [Google Scholar] [CrossRef]
- Ma, X.; Liu, Y.; Liu, Y.; Alexandrov, L.B.; Edmonson, M.N.; Gawad, C.; Zhou, X.; Li, Y.; Rusch, M.C.; Easton, J.; et al. Pan-cancer genome and transcriptome analyses of 1,699 paediatric leukaemias and solid tumours. Nature 2018, 555, 371–376. [Google Scholar] [CrossRef]
- Gröbner, S.N.; Worst, B.C.; Weischenfeldt, J.; Buchhalter, I.; Kleinheinz, K.; Rudneva, V.A.; Johann, P.D.; Balasubramanian, G.P.; Segura-Wang, M.; Brabetz, S.; et al. The landscape of genomic alterations across childhood cancers. Nature 2018, 555, 321–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sweet-Cordero, E.A.; Biegel, J.A. The genomic landscape of pediatric cancers: Implications for diagnosis and treatment. Science 2019, 363, 1170–1175. [Google Scholar] [CrossRef]
- Pan-Cancer Analysis Of Whole Genomes. The ICGC/TCGA Pan-Cancer Analysis of Whole Genomes Consortium. Nature 2020, 578, 82–93. [Google Scholar] [CrossRef] [Green Version]
- Vogelstein, B.; Papadopoulos, N.; Velculescu, V.E.; Zhou, S.; Diaz, L.A., Jr.; Kinzler, K.W. Cancer genome landscapes. Science 2013, 339, 1546–1558. [Google Scholar] [CrossRef]
- Campbell, B.B.; Light, N.; Fabrizio, D.; Zatzman, M.; Fuligni, F.; de Borja, R.; Davidson, S.; Edwards, M.; Elvin, J.A.; Hodel, K.P.; et al. Comprehensive Analysis of Hypermutation in Human Cancer. Cell 2017, 171, 1042–1056.e1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, M.; Mayoh, C.; Lau, L.M.S.; Khuong-Quang, D.A.; Pinese, M.; Kumar, A.; Barahona, P.; Wilkie, E.E.; Sullivan, P.; Bowen-James, R.; et al. Whole genome, transcriptome and methylome profiling enhances actionable target discovery in high-risk pediatric cancer. Nat. Med. 2020, 26, 1742–1753. [Google Scholar] [CrossRef] [PubMed]
- Worst, B.C.; van Tilburg, C.M.; Balasubramanian, G.P.; Fiesel, P.; Witt, R.; Freitag, A.; Boudalil, M.; Previti, C.; Wolf, S.; Schmidt, S.; et al. Next-generation personalised medicine for high-risk paediatric cancer patients—The INFORM pilot study. Eur. J. Cancer 2016, 65, 91–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parsons, D.W.; Roy, A.; Yang, Y.; Wang, T.; Scollon, S.; Bergstrom, K.; Kerstein, R.A.; Gutierrez, S.; Petersen, A.K.; Bavle, A.; et al. Diagnostic Yield of Clinical Tumor and Germline Whole-Exome Sequencing for Children With Solid Tumors. JAMA Oncol. 2016, 2, 616–624. [Google Scholar] [CrossRef]
- Ortiz, M.V.; Kobos, R.; Walsh, M.; Slotkin, E.K.; Roberts, S.; Berger, M.F.; Hameed, M.; Solit, D.; Ladanyi, M.; Shukla, N.; et al. Integrating Genomics into Clinical Pediatric Oncology Using the Molecular Tumor Board at the Memorial Sloan Kettering Cancer Center. Pediatr. Blood Cancer 2016, 63, 1368–1374. [Google Scholar] [CrossRef] [Green Version]
- Marks, L.J.; Oberg, J.A.; Pendrick, D.; Sireci, A.N.; Glasser, C.; Coval, C.; Zylber, R.J.; Chung, W.K.; Pang, J.; Turk, A.T.; et al. Precision Medicine in Children and Young Adults with Hematologic Malignancies and Blood Disorders: The Columbia University Experience. Front. Pediatr. 2017, 5, 265. [Google Scholar] [CrossRef] [Green Version]
- Oberg, J.A.; Glade Bender, J.L.; Sulis, M.L.; Pendrick, D.; Sireci, A.N.; Hsiao, S.J.; Turk, A.T.; Dela Cruz, F.S.; Hibshoosh, H.; Remotti, H.; et al. Implementation of next generation sequencing into pediatric hematology-oncology practice: Moving beyond actionable alterations. Genome Med. 2016, 8, 133. [Google Scholar] [CrossRef] [Green Version]
- Mody, R.J.; Wu, Y.M.; Lonigro, R.J.; Cao, X.; Roychowdhury, S.; Vats, P.; Frank, K.M.; Prensner, J.R.; Asangani, I.; Palanisamy, N.; et al. Integrative Clinical Sequencing in the Management of Refractory or Relapsed Cancer in Youth. JAMA 2015, 314, 913–925. [Google Scholar] [CrossRef]
- Chang, W.; Brohl, A.S.; Patidar, R.; Sindiri, S.; Shern, J.F.; Wei, J.S.; Song, Y.K.; Yohe, M.E.; Gryder, B.; Zhang, S.; et al. MultiDimensional ClinOmics for Precision Therapy of Children and Adolescent Young Adults with Relapsed and Refractory Cancer: A Report from the Center for Cancer Research. Clin. Cancer Res. 2016, 22, 3810–3820. [Google Scholar] [CrossRef] [Green Version]
- Kline, C.N.; Joseph, N.M.; Grenert, J.P.; van Ziffle, J.; Talevich, E.; Onodera, C.; Aboian, M.; Cha, S.; Raleigh, D.R.; Braunstein, S.; et al. Targeted next-generation sequencing of pediatric neuro-oncology patients improves diagnosis, identifies pathogenic germline mutations, and directs targeted therapy. Neuro Oncol. 2017, 19, 699–709. [Google Scholar] [CrossRef]
- Harris, M.H.; DuBois, S.G.; Glade Bender, J.L.; Kim, A.; Crompton, B.D.; Parker, E.; Dumont, I.P.; Hong, A.L.; Guo, D.; Church, A.; et al. Multicenter Feasibility Study of Tumor Molecular Profiling to Inform Therapeutic Decisions in Advanced Pediatric Solid Tumors: The Individualized Cancer Therapy (iCat) Study. JAMA Oncol. 2016, 2, 608–615. [Google Scholar] [CrossRef] [Green Version]
- Parsons, D.W.; Janeway, K.A.; Patton, D.; Coffey, B.; Williams, P.M.; Hamilton, S.R.; Purkayastha, A.; Tsongalis, G.J.; Routbort, M.; Gastier-Foster, J.M.; et al. Identification of targetable molecular alterations in the NCI-COG Pediatric MATCH trial. J. Clin. Oncol. 2019, 37, 10011. [Google Scholar] [CrossRef]
- Grover, S.A.; Berman, J.N.; Chan, J.A.; Deyell, R.J.; Eisenstat, D.D.; Fernandez, C.V.; Grundy, P.E.; Hawkins, C.; Irwin, M.S.; Jabado, N.; et al. Abstract 5413: Terry Fox PRecision Oncology For Young peopLE (PROFYLE): A Canadian precision medicine program for children, adolescents and young adults with hard-to-treat cancer. Cancer Res. 2020, 80, 5413. [Google Scholar] [CrossRef]
- Khater, F.; Vairy, S.; Langlois, S.; Dumoucel, S.; Sontag, T.; St-Onge, P.; Bittencourt, H.; Dal Soglio, D.; Champagne, J.; Duval, M.; et al. Molecular Profiling of Hard-to-Treat Childhood and Adolescent Cancers. JAMA Netw. Open 2019, 2, e192906. [Google Scholar] [CrossRef] [PubMed]
- Villani, A.; Davidson, S.; Kanwar, N.; Lo, W.; Li, Y.; Cohen-Gogo, S.; Fuligni, F.; Waldman, L.; Harripaul, R.; Light, N.; et al. The clinical utility of genomics in childhood cancer extends beyond targetable mutations. In Proceedings of the Annual Meeting of The American Society of Human Genetics, Virtual, 27 October 2020. [Google Scholar]
- Pincez, T.; Clément, N.; Lapouble, E.; Pierron, G.; Kamal, M.; Bieche, I.; Bernard, V.; Fréneaux, P.; Michon, J.; Orbach, D.; et al. Feasibility and clinical integration of molecular profiling for target identification in pediatric solid tumors. Pediatr. Blood Cancer 2017, 64, e26365. [Google Scholar] [CrossRef]
- Harttrampf, A.C.; Lacroix, L.; Deloger, M.; Deschamps, F.; Puget, S.; Auger, N.; Vielh, P.; Varlet, P.; Balogh, Z.; Abbou, S.; et al. Molecular Screening for Cancer Treatment Optimization (MOSCATO-01) in Pediatric Patients: A Single-Institutional Prospective Molecular Stratification Trial. Clin. Cancer Res. 2017, 23, 6101–6112. [Google Scholar] [CrossRef] [Green Version]
- Benezech, S.; Saintigny, P.; Attignon, V.; Pissaloux, D.; Paindavoine, S.; Faure-Conter, C.; Corradini, N.; Marec-Berard, P.; Bergeron, C.; Cassier, P.; et al. Tumor Molecular Profiling: Pediatric Results of the ProfiLER Study. JCO Precis. Oncol. 2020, 785–795. [Google Scholar] [CrossRef]
- Berlanga, P.; Schleiermacher, G.; Lacroix, L.; Pierron, G.; Beaumais, T.A.d.; Chicard, M.; Iddir, Y.; Scoazec, J.Y.; Freneaux, P.; Bayar, M.A.; et al. Abstract CT081: Pediatric precision medicine program in recurrent tumors: Results of the first 500 patients included in the European MAPPYACTS molecular profiling trial. Cancer Res. 2019, 79, CT081. [Google Scholar] [CrossRef]
- Lau, L.; Byrne, J.; Ekert, P.G.; Failes, T.; Fellowes, A.; Fletcher, J.; Gifford, A.; Haber, M.; Kumar, A.; Lock, R.; et al. Pilot study of a comprehensive precision medicine platform for children with high-risk cancer. J. Clin. Oncol. 2017, 35, 10539. [Google Scholar] [CrossRef]
- van Tilburg, C.M.; Pfaff, E.; Pajtler, K.W.; Langenberg, K.P.S.; Fiesel, P.; Jones, B.C.; Balasubramanian, G.P.; Stark, S.; Johann, P.D.; Blattner-Johnson, M.; et al. The pediatric precision oncology INFORM registry: Clinical outcome and benefit for patients with very high-evidence targets. Cancer Discov. 2021. [Google Scholar] [CrossRef] [PubMed]
- Abstracts from the 51st Congress of the International Society of Paediatric Oncology (SIOP) Lyon, France, October 23-26, 2019. Pediatr Blood Cancer 2019, 66 (Suppl. S4), e27989. [CrossRef]
- Langenberg, K.; Dolman, E.; Molenaar, J. Abstract A40: Integration of high-throughput drug screening on patient-derived organoids into pediatric precision medicine programs: The future is now! Cancer Res. 2020, 80, A40. [Google Scholar] [CrossRef]
- George, S.L.; Izquierdo, E.; Campbell, J.; Koutroumanidou, E.; Proszek, P.; Jamal, S.; Hughes, D.; Yuan, L.; Marshall, L.V.; Carceller, F.; et al. A tailored molecular profiling programme for children with cancer to identify clinically actionable genetic alterations. Eur. J. Cancer 2019, 121, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Kim, N.K.D.; Lee, S.H.; Cho, H.W.; Ma, Y.; Ju, H.Y.; Yoo, K.H.; Sung, K.W.; Koo, H.H.; Park, W.Y. Discovery of actionable genetic alterations with targeted panel sequencing in children with relapsed or refractory solid tumors. PLoS ONE 2019, 14, e0224227. [Google Scholar] [CrossRef] [Green Version]
- Mueller, S.; Jain, P.; Liang, W.S.; Kilburn, L.; Kline, C.; Gupta, N.; Panditharatna, E.; Magge, S.N.; Zhang, B.; Zhu, Y.; et al. A pilot precision medicine trial for children with diffuse intrinsic pontine glioma-PNOC003: A report from the Pacific Pediatric Neuro-Oncology Consortium. Int. J. Cancer 2019, 145, 1889–1901. [Google Scholar] [CrossRef] [PubMed]
- Mody, R.J.; Prensner, J.R.; Everett, J.; Parsons, D.W.; Chinnaiyan, A.M. Precision medicine in pediatric oncology: Lessons learned and next steps. Pediatr. Blood Cancer 2017, 64, e26288. [Google Scholar] [CrossRef] [Green Version]
- Moreno, L.; Pearson, A.D.J.; Paoletti, X.; Jimenez, I.; Geoerger, B.; Kearns, P.R.; Zwaan, C.M.; Doz, F.; Baruchel, A.; Vormoor, J.; et al. Early phase clinical trials of anticancer agents in children and adolescents—An ITCC perspective. Nat. Rev. Clin. Oncol. 2017, 14, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Burdach, S.E.G.; Westhoff, M.A.; Steinhauser, M.F.; Debatin, K.M. Precision medicine in pediatric oncology. Mol. Cell Pediatr. 2018, 5, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vo, K.T.; Parsons, D.W.; Seibel, N.L. Precision Medicine in Pediatric Oncology. Surg. Oncol. Clin. N. Am. 2020, 29, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Forrest, S.J.; Geoerger, B.; Janeway, K.A. Precision medicine in pediatric oncology. Curr. Opin. Pediatr. 2018, 30, 17–24. [Google Scholar] [CrossRef]
- Hadjadj, D.; Deshmukh, S.; Jabado, N. Entering the era of precision medicine in pediatric oncology. Nat. Med. 2020, 26, 1684–1685. [Google Scholar] [CrossRef]
- Seibel, N.L.; Janeway, K.; Allen, C.E.; Chi, S.N.; Cho, Y.J.; Glade Bender, J.L.; Kim, A.; Laetsch, T.W.; Irwin, M.S.; Takebe, N.; et al. Pediatric oncology enters an era of precision medicine. Curr. Probl. Cancer 2017, 41, 194–200. [Google Scholar] [CrossRef]
- Zhang, Q.; Fu, Q.; Bai, X.; Liang, T. Molecular Profiling-Based Precision Medicine in Cancer: A Review of Current Evidence and Challenges. Front. Oncol. 2020, 10, 532403. [Google Scholar] [CrossRef] [PubMed]
- Capper, D.; Jones, D.T.W.; Sill, M.; Hovestadt, V.; Schrimpf, D.; Sturm, D.; Koelsche, C.; Sahm, F.; Chavez, L.; Reuss, D.E.; et al. DNA methylation-based classification of central nervous system tumours. Nature 2018, 555, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Koelsche, C.; Schrimpf, D.; Stichel, D.; Sill, M.; Sahm, F.; Reuss, D.E.; Blattner, M.; Worst, B.; Heilig, C.E.; Beck, K.; et al. Sarcoma classification by DNA methylation profiling. Nat. Commun. 2021, 12, 498. [Google Scholar] [CrossRef]
- Drilon, A.; Laetsch, T.W.; Kummar, S.; DuBois, S.G.; Lassen, U.N.; Demetri, G.D.; Nathenson, M.; Doebele, R.C.; Farago, A.F.; Pappo, A.S.; et al. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N. Engl. J. Med. 2018, 378, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Laetsch, T.W.; DuBois, S.G.; Mascarenhas, L.; Turpin, B.; Federman, N.; Albert, C.M.; Nagasubramanian, R.; Davis, J.L.; Rudzinski, E.; Feraco, A.M.; et al. Larotrectinib for paediatric solid tumours harbouring NTRK gene fusions: Phase 1 results from a multicentre, open-label, phase 1/2 study. Lancet Oncol. 2018, 19, 705–714. [Google Scholar] [CrossRef]
- Ravandi, F. How I treat Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood 2019, 133, 130–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hargrave, D.R.; Bouffet, E.; Tabori, U.; Broniscer, A.; Cohen, K.J.; Hansford, J.R.; Geoerger, B.; Hingorani, P.; Dunkel, I.J.; Russo, M.W.; et al. Efficacy and Safety of Dabrafenib in Pediatric Patients with BRAF V600 Mutation-Positive Relapsed or Refractory Low-Grade Glioma: Results from a Phase I/IIa Study. Clin. Cancer Res. 2019, 25, 7303–7311. [Google Scholar] [CrossRef]
- Andersson, N.; Bakker, B.; Karlsson, J.; Valind, A.; Holmquist Mengelbier, L.; Spierings, D.C.J.; Foijer, F.; Gisselsson, D. Extensive Clonal Branching Shapes the Evolutionary History of High-Risk Pediatric Cancers. Cancer Res. 2020, 80, 1512–1523. [Google Scholar] [CrossRef]
- Karlsson, J.; Valind, A.; Mengelbier, L.H.; Bredin, S.; Cornmark, L.; Jansson, C.; Wali, A.; Staaf, J.; Viklund, B.; Øra, I.; et al. Four evolutionary trajectories underlie genetic intratumoral variation in childhood cancer. Nat. Genet. 2018, 50, 944–950. [Google Scholar] [CrossRef]
- Eleveld, T.F.; Oldridge, D.A.; Bernard, V.; Koster, J.; Daage, L.C.; Diskin, S.J.; Schild, L.; Bentahar, N.B.; Bellini, A.; Chicard, M.; et al. Relapsed neuroblastomas show frequent RAS-MAPK pathway mutations. Nat. Genet. 2015, 47, 864–871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schramm, A.; Köster, J.; Assenov, Y.; Althoff, K.; Peifer, M.; Mahlow, E.; Odersky, A.; Beisser, D.; Ernst, C.; Henssen, A.G.; et al. Mutational dynamics between primary and relapse neuroblastomas. Nat. Genet. 2015, 47, 872–877. [Google Scholar] [CrossRef]
- Mullighan, C.G.; Phillips, L.A.; Su, X.; Ma, J.; Miller, C.B.; Shurtleff, S.A.; Downing, J.R. Genomic analysis of the clonal origins of relapsed acute lymphoblastic leukemia. Science 2008, 322, 1377–1380. [Google Scholar] [CrossRef] [Green Version]
- Oshima, K.; Zhao, J.; Pérez-Durán, P.; Brown, J.A.; Patiño-Galindo, J.A.; Chu, T.; Quinn, A.; Gunning, T.; Belver, L.; Ambesi-Impiombato, A.; et al. Mutational and functional genetics mapping of chemotherapy resistance mechanisms in relapsed acute lymphoblastic leukemia. Nat. Cancer 2020, 1, 1113–1127. [Google Scholar] [CrossRef]
- Schulte, M.; Köster, J.; Rahmann, S.; Schramm, A. Cancer evolution, mutations, and clonal selection in relapse neuroblastoma. Cell Tissue Res. 2018, 372, 263–268. [Google Scholar] [CrossRef]
- Morrissy, A.S.; Garzia, L.; Shih, D.J.; Zuyderduyn, S.; Huang, X.; Skowron, P.; Remke, M.; Cavalli, F.M.; Ramaswamy, V.; Lindsay, P.E.; et al. Divergent clonal selection dominates medulloblastoma at recurrence. Nature 2016, 529, 351–357. [Google Scholar] [CrossRef] [Green Version]
- McLeod, C.; Gout, A.M.; Zhou, X.; Thrasher, A.; Rahbarinia, D.; Brady, S.W.; Macias, M.; Birch, K.; Finkelstein, D.; Sunny, J.; et al. St. Jude Cloud: A Pediatric Cancer Genomic Data-Sharing Ecosystem. Cancer Discov. 2021, 11, 1082–1099. [Google Scholar] [CrossRef]
- R2: Genomics Analysis and Visualization Platform. Available online: http://r2.amc.nl (accessed on 11 May 2021).
- Zhang, J.; Walsh, M.F.; Wu, G.; Edmonson, M.N.; Gruber, T.A.; Easton, J.; Hedges, D.; Ma, X.; Zhou, X.; Yergeau, D.A.; et al. Germline Mutations in Predisposition Genes in Pediatric Cancer. N. Engl. J. Med. 2015, 373, 2336–2346. [Google Scholar] [CrossRef] [Green Version]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- MacFarland, S.P.; Zelley, K.; Surrey, L.F.; Gallo, D.; Luo, M.; Raman, P.; Wertheim, G.; Hunger, S.P.; Li, M.M.; Brodeur, G.M. Pediatric Somatic Tumor Sequencing Identifies Underlying Cancer Predisposition. JCO Precis. Oncol. 2019, 3, 1–26. [Google Scholar] [CrossRef]
- Villani, A.; Shore, A.; Wasserman, J.D.; Stephens, D.; Kim, R.H.; Druker, H.; Gallinger, B.; Naumer, A.; Kohlmann, W.; Novokmet, A.; et al. Biochemical and imaging surveillance in germline TP53 mutation carriers with Li-Fraumeni syndrome: 11 year follow-up of a prospective observational study. Lancet Oncol. 2016, 17, 1295–1305. [Google Scholar] [CrossRef]
- Conyers, R.; Devaraja, S.; Elliott, D. Systematic review of pharmacogenomics and adverse drug reactions in paediatric oncology patients. Pediatr. Blood Cancer 2018, 65, e26937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van de Haar, J.; Hoes, L.; Voest, E. Advancing molecular tumour boards: Highly needed to maximise the impact of precision medicine. ESMO Open 2019, 4, e000516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishiwaki, S.; Ando, Y. Gap between pediatric and adult approvals of molecular targeted drugs. Sci. Rep. 2020, 10, 17145. [Google Scholar] [CrossRef] [PubMed]
- Nader, J.H.; Neel, D.V.; Shulman, D.S.; Ma, C.; Bourgeois, F.; DuBois, S.G. Landscape of phase 1 clinical trials for minors with cancer in the United States. Pediatr. Blood Cancer 2020, 67, e28694. [Google Scholar] [CrossRef]
- Pearson, A.D.; Stegmaier, K.; Bourdeaut, F.; Reaman, G.; Heenen, D.; Meyers, M.L.; Armstrong, S.A.; Brown, P.; De Carvalho, D.; Jabado, N.; et al. Paediatric Strategy Forum for medicinal product development of epigenetic modifiers for children: ACCELERATE in collaboration with the European Medicines Agency with participation of the Food and Drug Administration. Eur. J. Cancer 2020, 139, 135–148. [Google Scholar] [CrossRef]
- Pearson, A.D.J.; Karres, D.; Reaman, G.; DuBois, S.G.; Knox, L.; Scobie, N.; Vassal, G. The RACE to accelerate drug development for children with cancer. Lancet Child Adolesc. Health 2020, 4, 714–716. [Google Scholar] [CrossRef]
- DuBois, S.G.; Corson, L.B.; Stegmaier, K.; Janeway, K.A. Ushering in the next generation of precision trials for pediatric cancer. Science 2019, 363, 1175–1181. [Google Scholar] [CrossRef]
- Pearson, A.D.; Herold, R.; Rousseau, R.; Copland, C.; Bradley-Garelik, B.; Binner, D.; Capdeville, R.; Caron, H.; Carleer, J.; Chesler, L.; et al. Implementation of mechanism of action biology-driven early drug development for children with cancer. Eur. J. Cancer 2016, 62, 124–131. [Google Scholar] [CrossRef]
- Pasqualini, C.; Rubino, J.; Brard, C.; Cassard, L.; Andre, N.; Rondof, W.; Scoazec, J.Y.; Marchais, A.; Nebchi, S.; Boselli, L.; et al. Phase II and biomarker study of programmed cell death protein 1 inhibitor nivolumab and metronomic cyclophosphamide in paediatric relapsed/refractory solid tumours: Arm G of AcSe-ESMART, a trial of the European Innovative Therapies for Children With Cancer Consortium. Eur. J. Cancer 2021, 150, 53–62. [Google Scholar] [CrossRef]
- van Tilburg, C.M.; Witt, R.; Heiss, M.; Pajtler, K.W.; Plass, C.; Poschke, I.; Platten, M.; Harting, I.; Sedlaczek, O.; Freitag, A.; et al. INFORM2 NivEnt: The first trial of the INFORM2 biomarker driven phase I/II trial series: The combination of nivolumab and entinostat in children and adolescents with refractory high-risk malignancies. BMC Cancer 2020, 20, 523. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.E.; Laetsch, T.W.; Mody, R.; Irwin, M.S.; Lim, M.S.; Adamson, P.C.; Seibel, N.L.; Parsons, D.W.; Cho, Y.J.; Janeway, K.; et al. Target and Agent Prioritization for the Children’s Oncology Group-National Cancer Institute Pediatric MATCH Trial. J. Natl. Cancer Inst. 2017, 109, djw274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, E.; Federico, S.M.; Chen, X.; Shelat, A.A.; Bradley, C.; Gordon, B.; Karlstrom, A.; Twarog, N.R.; Clay, M.R.; Bahrami, A.; et al. Orthotopic patient-derived xenografts of paediatric solid tumours. Nature 2017, 549, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Bleijs, M.; van de Wetering, M.; Clevers, H.; Drost, J. Xenograft and organoid model systems in cancer research. Embo J. 2019, 38, e101654. [Google Scholar] [CrossRef]
- Tsoli, M.; Wadham, C.; Pinese, M.; Failes, T.; Joshi, S.; Mould, E.; Yin, J.X.; Gayevskiy, V.; Kumar, A.; Kaplan, W.; et al. Integration of genomics, high throughput drug screening, and personalized xenograft models as a novel precision medicine paradigm for high risk pediatric cancer. Cancer Biol. Ther. 2018, 19, 1078–1087. [Google Scholar] [CrossRef]
- Clevers, H. Modeling Development and Disease with Organoids. Cell 2016, 165, 1586–1597. [Google Scholar] [CrossRef] [Green Version]
- Drost, J.; Clevers, H. Translational applications of adult stem cell-derived organoids. Development 2017, 144, 968–975. [Google Scholar] [CrossRef] [Green Version]
- Calandrini, C.; Schutgens, F.; Oka, R.; Margaritis, T.; Candelli, T.; Mathijsen, L.; Ammerlaan, C.; van Ineveld, R.L.; Derakhshan, S.; de Haan, S.; et al. An organoid biobank for childhood kidney cancers that captures disease and tissue heterogeneity. Nat. Commun. 2020, 11, 1310. [Google Scholar] [CrossRef]
- Ponsioen, B.; Post, J.B.; des Amorie, J.R.B.; Laskaris, D.; van Ineveld, R.L.; Kersten, S.; Bertotti, A.; Sassi, F.; Sipieter, F.; Cappe, B.; et al. Quantifying single-cell ERK dynamics in colorectal cancer organoids reveals EGFR as an amplifier of oncogenic MAPK pathway signalling. Nat. Cell Biol. 2021, 23, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Vlachogiannis, G.; Hedayat, S.; Vatsiou, A.; Jamin, Y.; Fernández-Mateos, J.; Khan, K.; Lampis, A.; Eason, K.; Huntingford, I.; Burke, R.; et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science 2018, 359, 920–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van de Wetering, M.; Francies, H.E.; Francis, J.M.; Bounova, G.; Iorio, F.; Pronk, A.; van Houdt, W.; van Gorp, J.; Taylor-Weiner, A.; Kester, L.; et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell 2015, 161, 933–945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tucker, E.R.; George, S.; Angelini, P.; Bruna, A.; Chesler, L. The Promise of Patient-Derived Preclinical Models to Accelerate the Implementation of Personalised Medicine for Children with Neuroblastoma. J. Pers. Med. 2021, 11, 248. [Google Scholar] [CrossRef] [PubMed]
- Diaz, L.A., Jr.; Bardelli, A. Liquid biopsies: Genotyping circulating tumor DNA. J. Clin. Oncol. 2014, 32, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Van Paemel, R.; Vlug, R.; De Preter, K.; Van Roy, N.; Speleman, F.; Willems, L.; Lammens, T.; Laureys, G.; Schleiermacher, G.; Tytgat, G.A.M.; et al. The pitfalls and promise of liquid biopsies for diagnosing and treating solid tumors in children: A review. Eur. J. Pediatr. 2020, 179, 191–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rothwell, D.G.; Ayub, M.; Cook, N.; Thistlethwaite, F.; Carter, L.; Dean, E.; Smith, N.; Villa, S.; Dransfield, J.; Clipson, A.; et al. Utility of ctDNA to support patient selection for early phase clinical trials: The TARGET study. Nat. Med. 2019, 25, 738–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inaba, H.; Pui, C.H. Immunotherapy in pediatric acute lymphoblastic leukemia. Cancer Metastasis Rev. 2019, 38, 595–610. [Google Scholar] [CrossRef]
- Yu, A.L.; Gilman, A.L.; Ozkaynak, M.F.; London, W.B.; Kreissman, S.G.; Chen, H.X.; Smith, M.; Anderson, B.; Villablanca, J.G.; Matthay, K.K.; et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N. Engl. J. Med. 2010, 363, 1324–1334. [Google Scholar] [CrossRef] [Green Version]
- Bouffet, E.; Larouche, V.; Campbell, B.B.; Merico, D.; de Borja, R.; Aronson, M.; Durno, C.; Krueger, J.; Cabric, V.; Ramaswamy, V.; et al. Immune Checkpoint Inhibition for Hypermutant Glioblastoma Multiforme Resulting From Germline Biallelic Mismatch Repair Deficiency. J. Clin. Oncol. 2016, 34, 2206–2211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutzen, B.; Paudel, S.N.; Naeimi Kararoudi, M.; Cassady, K.A.; Lee, D.A.; Cripe, T.P. Immunotherapies for pediatric cancer: Current landscape and future perspectives. Cancer Metastasis Rev. 2019, 38, 573–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitt, M.W.; Loeb, L.A.; Salk, J.J. The influence of subclonal resistance mutations on targeted cancer therapy. Nat. Rev. Clin. Oncol. 2016, 13, 335–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dentro, S.C.; Leshchiner, I.; Haase, K.; Tarabichi, M.; Wintersinger, J.; Deshwar, A.G.; Yu, K.; Rubanova, Y.; Macintyre, G.; Demeulemeester, J.; et al. Characterizing genetic intra-tumor heterogeneity across 2,658 human cancer genomes. Cell 2021, 184, 2239–2254.e2239. [Google Scholar] [CrossRef]
- Moreno, L.; Barone, G.; DuBois, S.G.; Molenaar, J.; Fischer, M.; Schulte, J.; Eggert, A.; Schleiermacher, G.; Speleman, F.; Chesler, L.; et al. Accelerating drug development for neuroblastoma: Summary of the Second Neuroblastoma Drug Development Strategy forum from Innovative Therapies for Children with Cancer and International Society of Paediatric Oncology Europe Neuroblastoma. Eur. J. Cancer 2020, 136, 52–68. [Google Scholar] [CrossRef]
- Kieran, M.W.; Caron, H.; Winther, J.F.; Henderson, T.O.; Haupt, R.; Hjorth, L.; Hudson, M.M.; Kremer, L.C.M.; van der Pal, H.J.; Pearson, A.D.J.; et al. A global approach to long-term follow-up of targeted and immune-based therapy in childhood and adolescence. Pediatr. Blood Cancer 2021, 68, e29047. [Google Scholar] [CrossRef]
- Hulsen, T.; Jamuar, S.S.; Moody, A.R.; Karnes, J.H.; Varga, O.; Hedensted, S.; Spreafico, R.; Hafler, D.A.; McKinney, E.F. From Big Data to Precision Medicine. Front. Med. 2019, 6, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buechner, P.; Hinderer, M.; Unberath, P.; Metzger, P.; Boeker, M.; Acker, T.; Haller, F.; Mack, E.; Nowak, D.; Paret, C.; et al. Requirements Analysis and Specification for a Molecular Tumor Board Platform Based on cBioPortal. Diagnostics 2020, 10, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
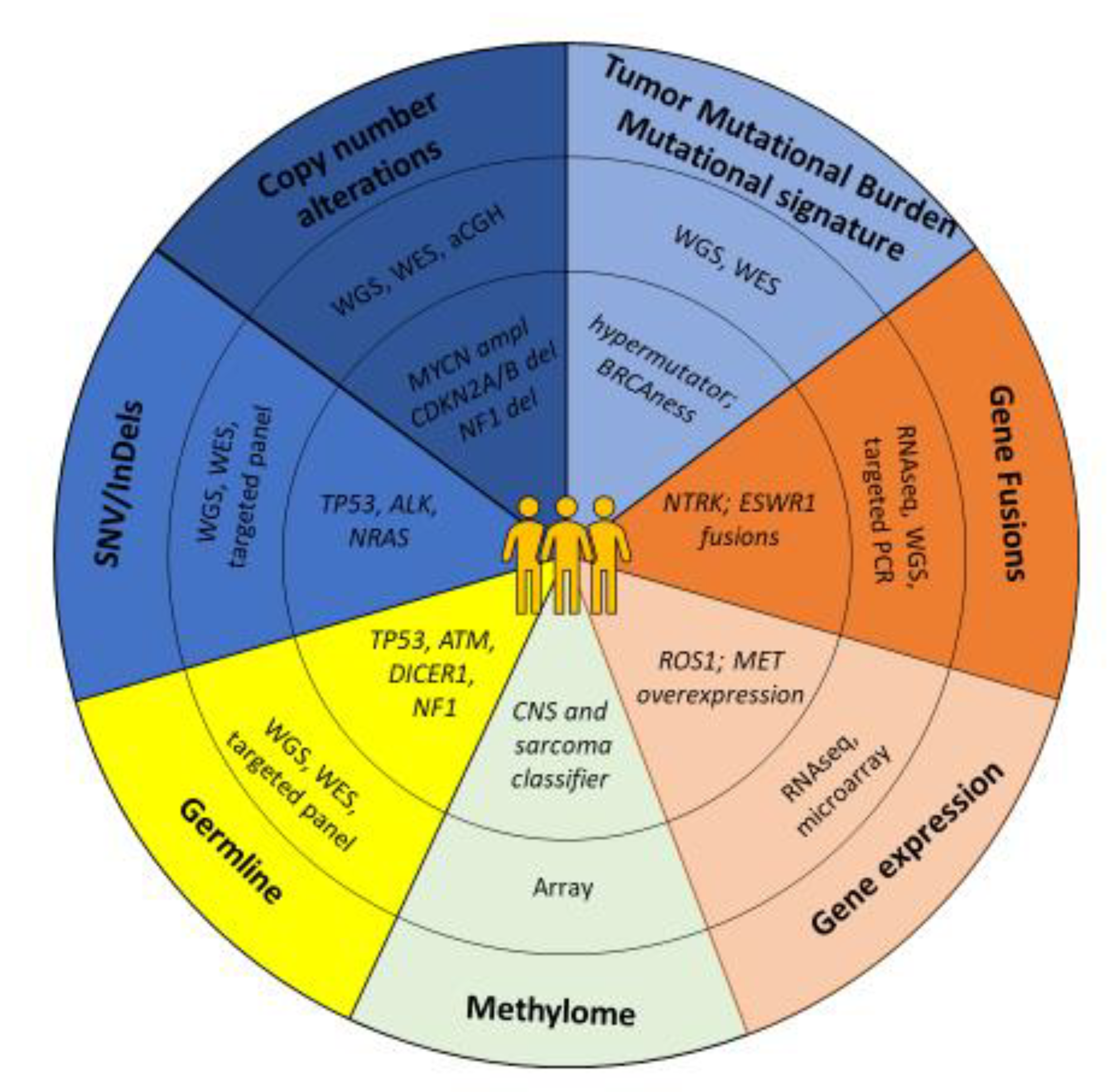
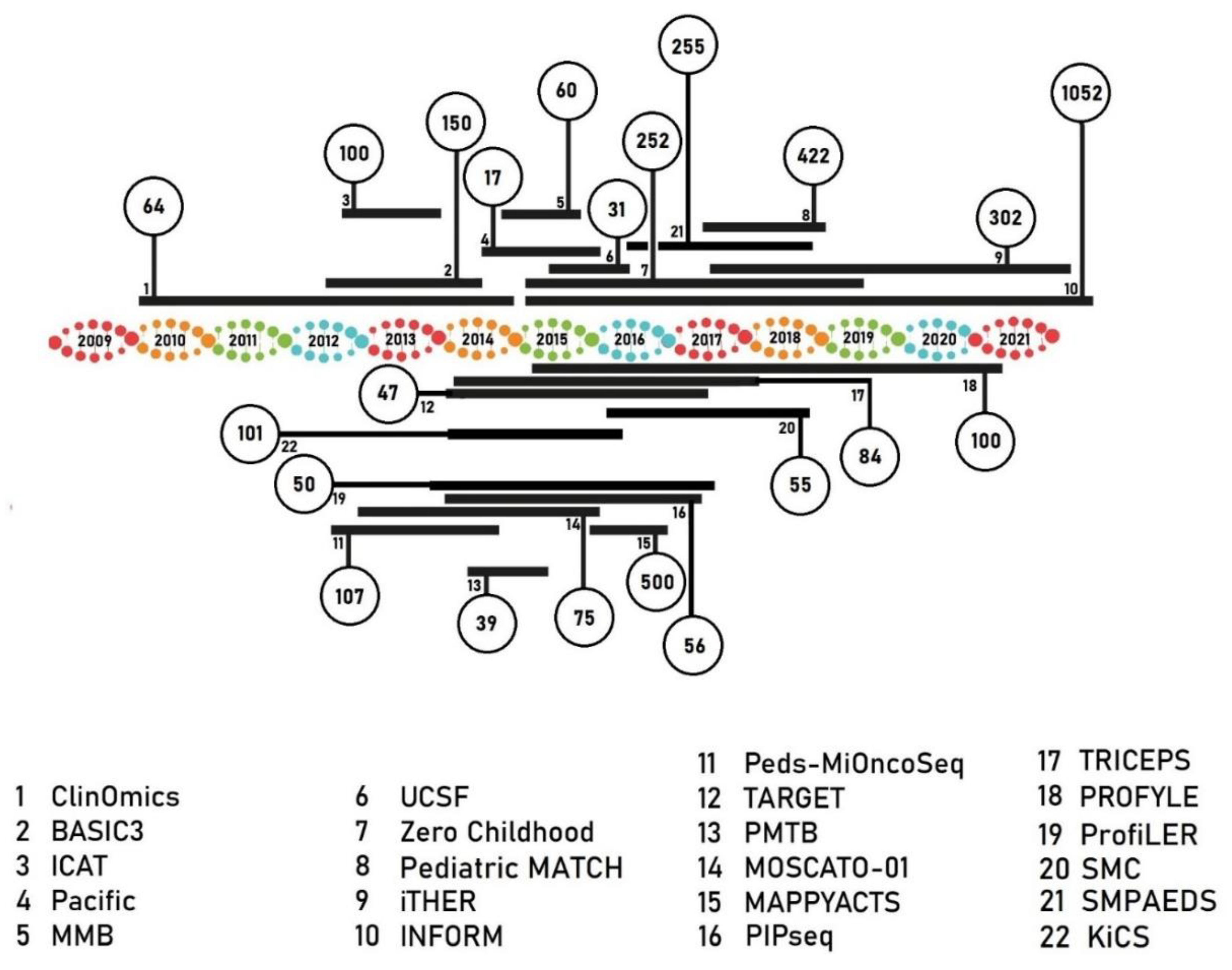
| Program Name/Sponsor | No. of Samples Included | No of Samples Analyzed | Inclusion Criteria | NGS Technique | Tumor Subtypes | Data Reported | Time to Results (Days) | % Actionable Alterations | % Patients Receiving Targeted Therapy (of All Samples Sequenced Successfully) | % Change or Refinement of Diagnosis | % Germline Aberrations |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ClinOmics [45] USA, NCI Center for Cancer Research | 64 | 59 | Relapse/refractory | WES; RNAseq; SNP array | Solid tumors | Somatic & germline | NR | 51 | NR | 7 | 12 |
| Peds-MiOncoSeq [44] USA, University of Michigan | 107 | 101 | Primary high-risk; relapse/refractory; rare cancers | WES; RNAseq | Solid tumors; hematological malignancies | Somatic & germline | 54 (average) | 46 | 15 | 2 | 10 |
| BASIC3 [40] USA, Baylor College of Medicine | 150 | 121 | Primary high-risk (newly diagnosed and untreated) | WES | Solid & CNS tumors | Somatic & germline | NR | 27 | NR | NR | 10 |
| iCAT [47] USA, Dana Farber Cancer Institute | 100 | 89 | Primary high-risk; relapse/refractory | NGS panel; aCGH | Solid tumors | Somatic & germline | NR | 39 | 3 | 3 | 12 |
| MOSCATO-01 [53] ** France, Gustave Roussy Cancer Center | 75 | 69 | Relapse/refractory | WES; NGS panel; RNAseq; aCGH | Solid & CNS tumors | Somatic & germline | 19–41 (26 average) | 61 | 19 | 4 | 10 |
| ProfiLER [54] France, Centre Léon Bérard | 50 | 43 | Primary high-risk; relapse | 69 gene panel; aCGH | Solid & CNS tumors; hematological malignancies | Somatic | NR | 23 | 9 | NR | NA |
| PIPseq [42,43] USA, Columbia University | - | 56 | Relapse/refractory; unusual presentation for age; rare cancers | WES; NGS panel; RNAseq | Hematological malignancies | Somatic & germline | 40 (median) | 80 | 13 | 11 | 24 |
| TRICEPS [50] *** Canada, CHU Sainte-Justine | 84 | 62 | Relapse/refractory | WES or NGS panel; RNAseq | Solid & CNS tumors; hematological malignancies | Somatic & germline | 32–120 (61 median) | 87 | 41 | 22 | 13 |
| PMTB [41] USA, Memorial Sloan Kettering Cancer Center | - | 39 | Primary high-risk; relapse/refractory; remission | WES; Hybrid-capture based DNA and RNA sequencing assay; RNAseq; FISH | Solid & CNS tumors; hematological malignancies | Somatic & germline | NR | 73 | 54 | NR | NR |
| PNOC003 [62] Transnational, Pacific Pediatric Neuro-oncology consortium | 17 | 17 | Primary high-risk | WES; WGS (60x); RNAseq; | CNS tumors | Somatic & germline | 6–22 (13 median) | 100 | 47 | NR | NR |
| MMB [52] France, Institut Curie | 60 | 58 | Primary high-risk; relapse/refractory | NGS panel; aCGH | Solid & CNS tumors | Somatic | 26–58 (42 median) | 40 | 10 | NR | NA |
| INFORM [39,57] Germany, German Cancer Research Center | 1052 | 928 | Primary high-risk; relapse/refractory | WES; lcWGS; RNAseq; 850K methylation | Solid & CNS tumors; hematological malignancies | Somatic & germline | 25 (average) | 85 | 28 | 7 | 8 |
| TARGET [56] * Australia, Manchester Cancer Research Centre | - | 47 | Primary high-risk | NGS panel; RNAseq | Solid & CNS tumors; hematological malignancies | Somatic & germline | NR | 61 | NR | NR | NR |
| Zero Childhood Cancer [38] * Australia, CCI | 252 | 252 | Primary high-risk; relapse/refractory | WGS, RNAseq, 850K methylation | Solid & CNS tumors; hematological malignancies | Somatic & germline | 53 (average) | 71 | 17 | 5 | 16 |
| PROFYLE [49] *** Canada, The Terry Fox Research Institute | - | 100 | Relapse; hard-to-treat cancer | NGS panel; WGS; RNAseq; | Solid & CNS tumors; hematological malignancies | Somatic & germline | NR | 82 | 58 | NR | 14 |
| UCSF [46] USA, UCSF Medical Center | 31 | 31 | Relapse/refractory; no standard therapy available | NGS panel | CNS tumors | Somatic & germline | 14–21 | 61 | NR | 19 | 35 |
| MAPPYACTS [55] ** France, Gustave Roussy Cancer Center | 500 | 390 | Relapse/refractory | WES; RNAseq | Solid & CNS tumors; hematological malignancies | Somatic & germline | NR | 70 | 28 | NR | 6 |
| SMC [61] Republic of Korea, Samsung Medical Center | 55 | 53 | Relapse/refractory | 381 gene panel; 22 intron panel | Solid tumors | Somatic | NR | 36 | 2 | NR | NA |
| SMPAEDS [60] UK, Royal Marsden Hospital | 255 | 209 | Relapse/refractory | 78 or 91 gene panel | Solid tumors | Somatic | NR | 51 | 2 | NR | NA |
| iTHER [58,59] The Netherlands, Princess Máxima Center | 302 | 226 | Primary high-risk; relapse/refractory cancers | WES; lcWGS; RNAseq; 850K methylation | Solid & CNS tumors; hematological malignancies | Somatic & germline | 35 (average) | 89 | 12 | 4 | 10 |
| Pediatric MATCH [48] USA, National Cancer Institute–Children’s Oncology Group | 422 | 357 | Relapse/refractory | NGS gene panel; IHC | Solid & CNS tumors; hematological malignancies | Somatic | 15 (average) | 29 | 24 | NR | NA |
| KiCS [51] *** Canada, The Hospital for Sick Children (SickKids) | - | 200 | Poor prognosis; rare tumors; cancer predisposition | 864 gene panel; RNAseq; WGS | Solid & CNS tumors; hematological malignancies | Somatic & germline | NR | 53 | NR | NR | 12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Langenberg, K.P.S.; Looze, E.J.; Molenaar, J.J. The Landscape of Pediatric Precision Oncology: Program Design, Actionable Alterations, and Clinical Trial Development. Cancers 2021, 13, 4324. https://doi.org/10.3390/cancers13174324
Langenberg KPS, Looze EJ, Molenaar JJ. The Landscape of Pediatric Precision Oncology: Program Design, Actionable Alterations, and Clinical Trial Development. Cancers. 2021; 13(17):4324. https://doi.org/10.3390/cancers13174324
Chicago/Turabian StyleLangenberg, Karin P. S., Eleonora J. Looze, and Jan J. Molenaar. 2021. "The Landscape of Pediatric Precision Oncology: Program Design, Actionable Alterations, and Clinical Trial Development" Cancers 13, no. 17: 4324. https://doi.org/10.3390/cancers13174324
APA StyleLangenberg, K. P. S., Looze, E. J., & Molenaar, J. J. (2021). The Landscape of Pediatric Precision Oncology: Program Design, Actionable Alterations, and Clinical Trial Development. Cancers, 13(17), 4324. https://doi.org/10.3390/cancers13174324





