The “K-Sign”—A Novel CT Finding Suggestive before the Appearance of Pancreatic Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
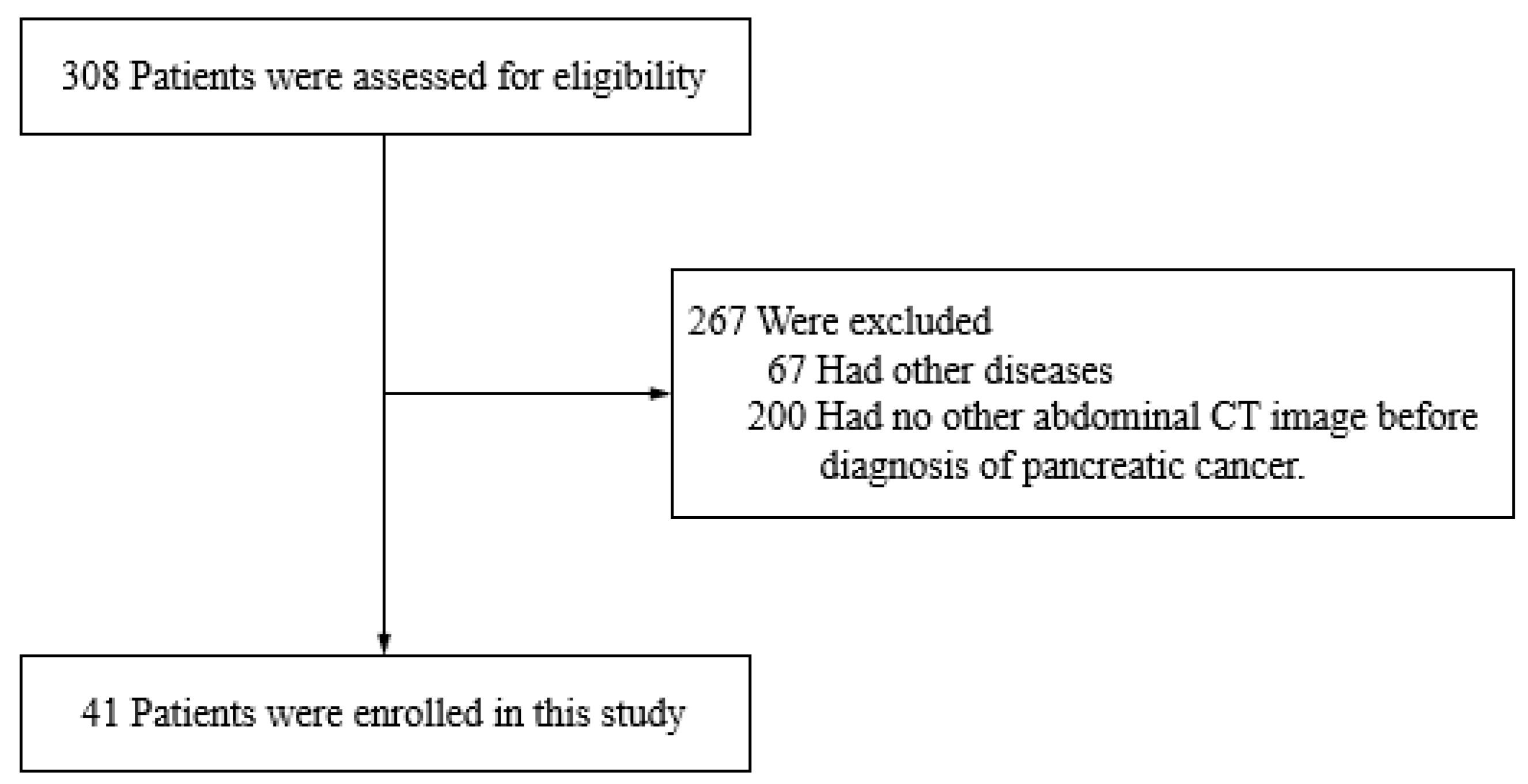
2.1. Study Design and Patient Population
2.2. Study Variables
2.3. Diagnosis Management and Imaging Modality
2.4. Ethical Approval
3. Results
3.1. Clinical Characteristics
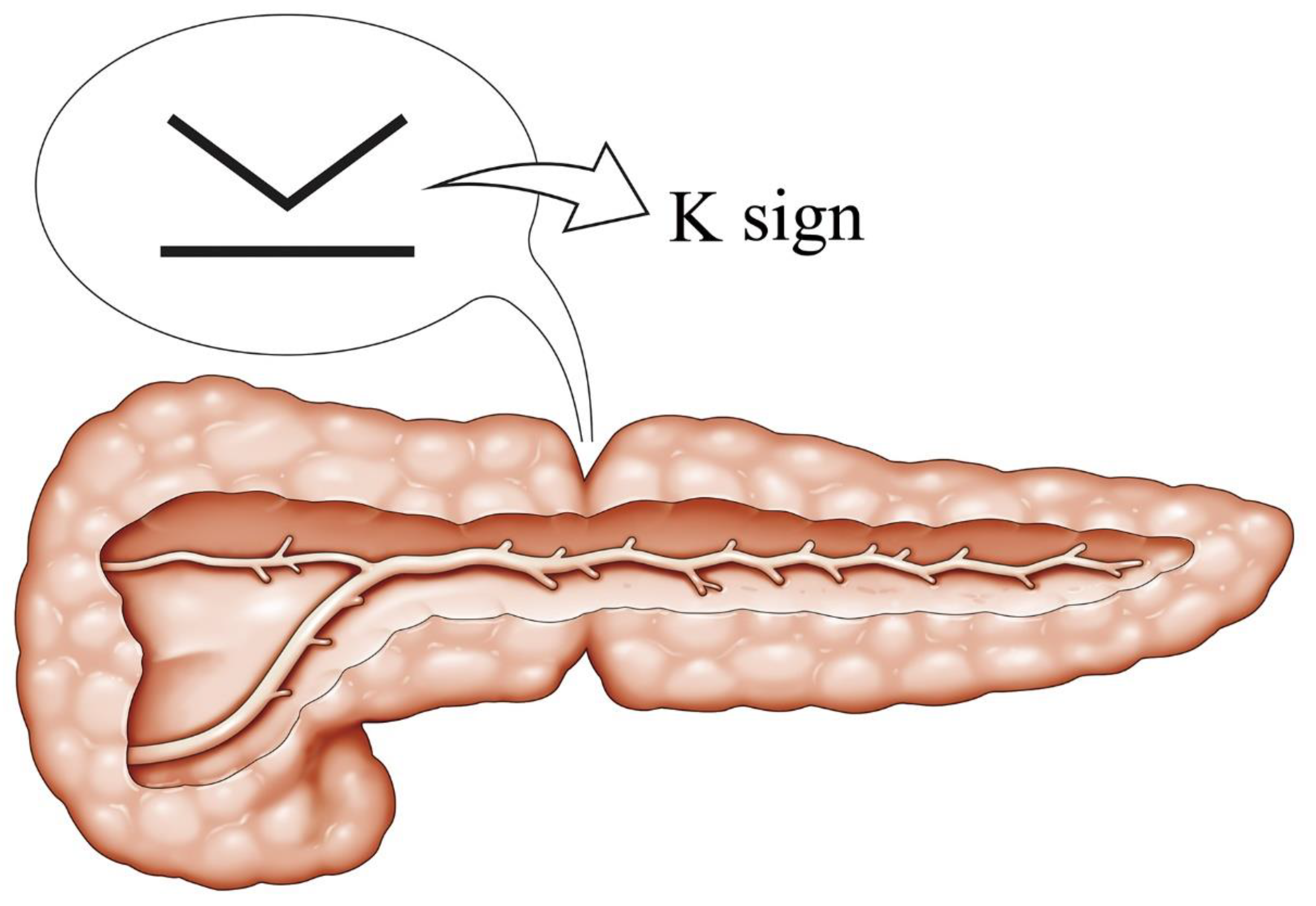
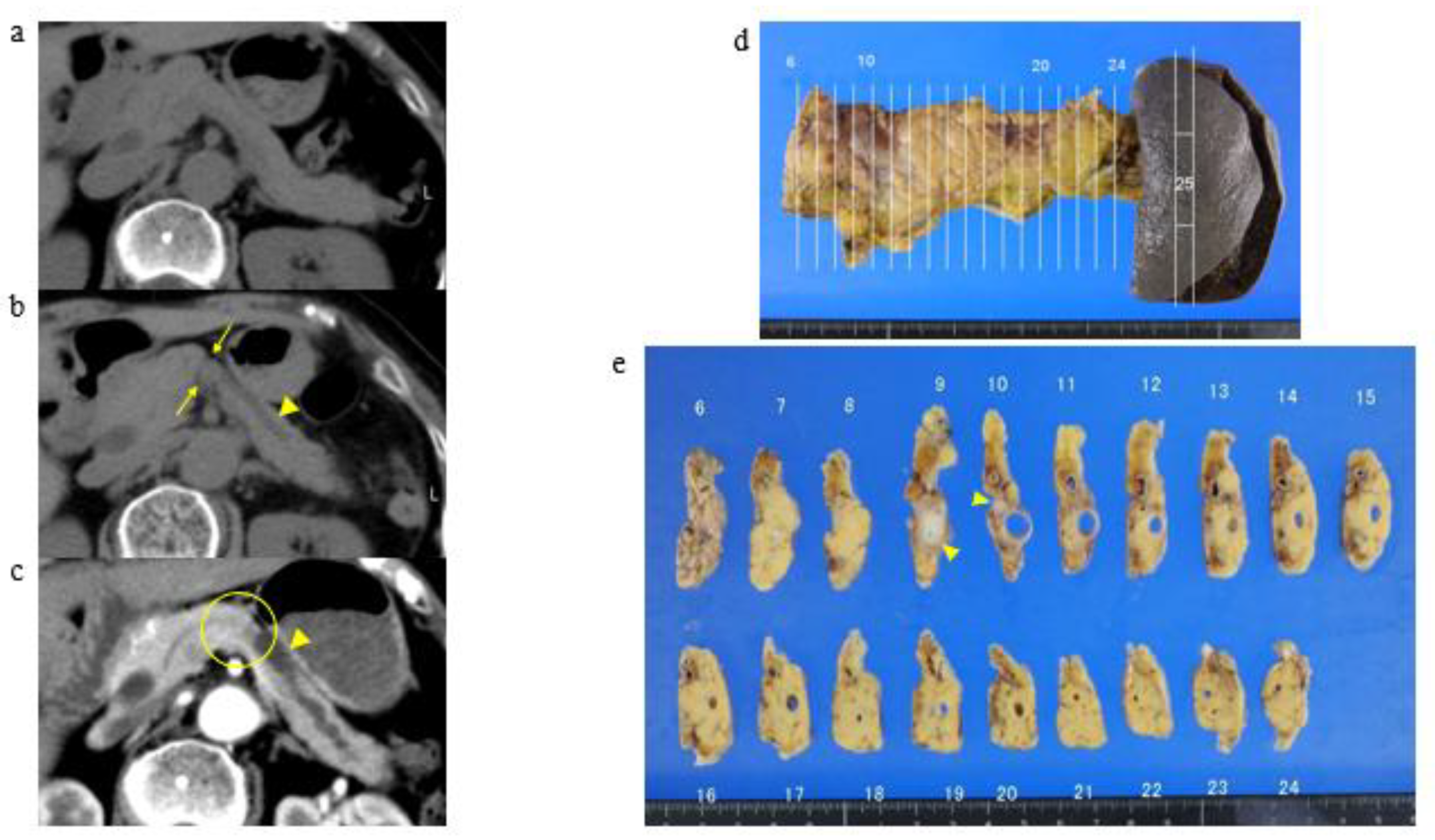
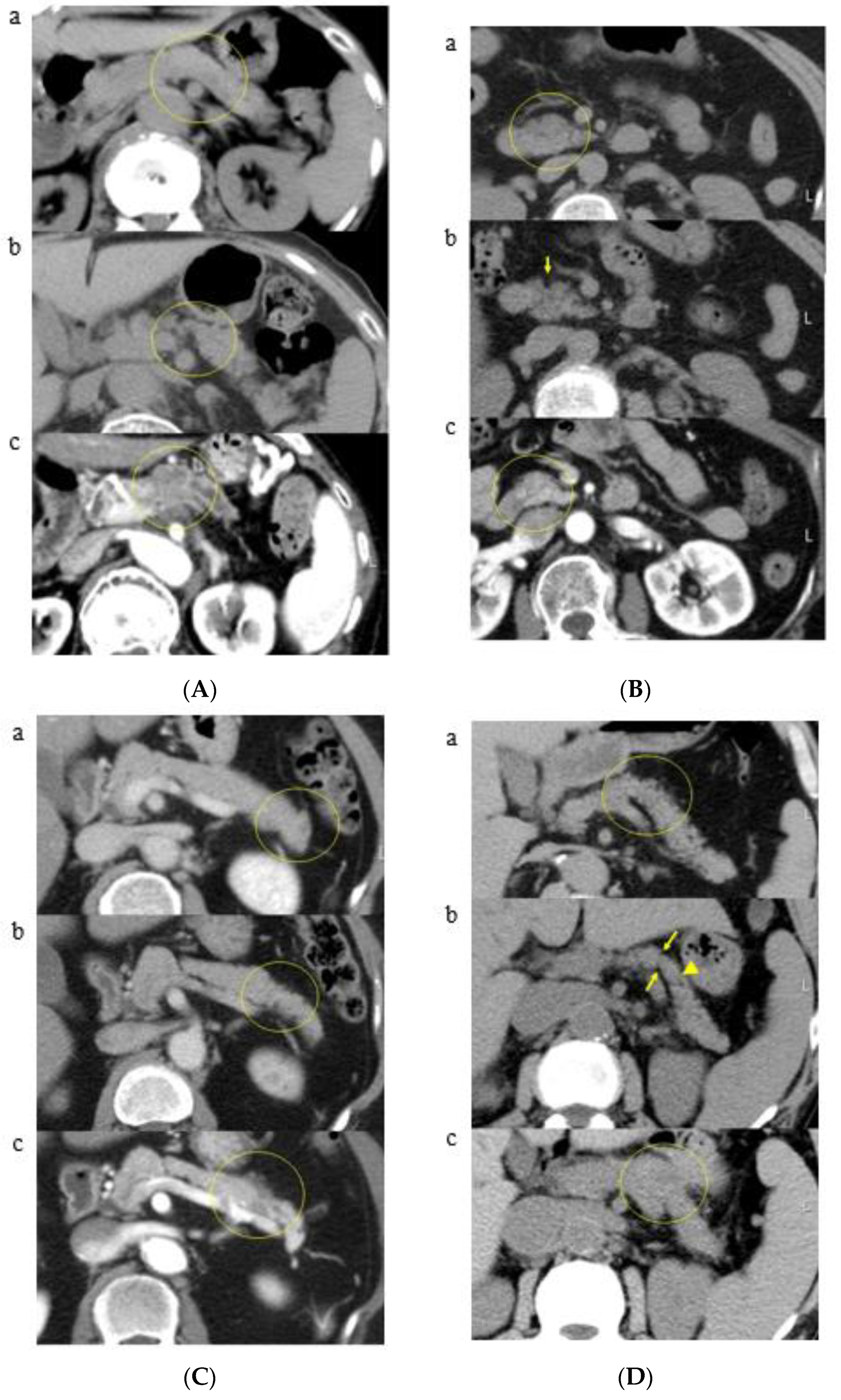
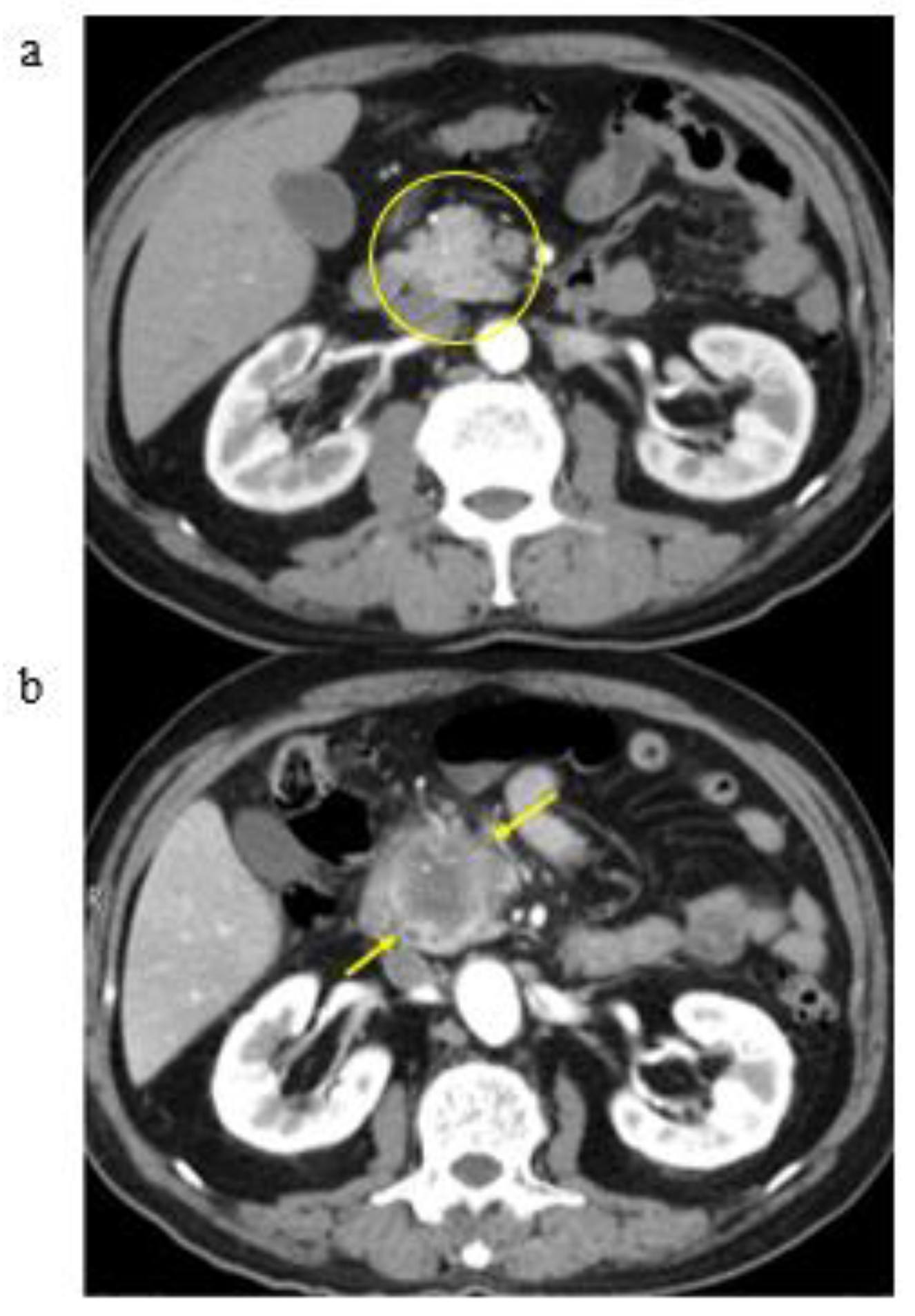
3.2. A Representative Case and Classification by Specific Abnormality of the Pancreas
3.3. Time to Development of Pancreatic Cancer
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vital Statistics of Japan—Ministry of Health, Labour and Welfare. Available online: https://www.mhlw.go.jp/english/database/db-hw/vs01.html (accessed on 2 June 2021).
- Matsuda, T.; Ajiki, W.; Marugawa, T.; Ioka, A.; Tsukuma, H.; Sobue, T. Population-based Survival of Cancer Patients Diagnosed Between 1993 and 1999 in Japan: A Chronological and International Comparative Study. Jpn. J. Clin. Oncol. 2011, 41, 40–51. [Google Scholar] [CrossRef]
- Eagawa, S.; Toma, H.; Ohigashi, H.; Okusaka, T.; Nakao, A.; Hatori, T.; Maguchi, H.; Yanagisawa, A.; Tanaka, M. A digest of the Pancreatic Cancer Registry Report 2007. Pancreas 2008, 23, 105–123. (In Japanese) [Google Scholar] [CrossRef][Green Version]
- Kikukawa, M.; Kajikawa, T.; Kuruma, S.; Chiba, K.; Kawaguchi, S.; Terada, S.; Satoh, T. Early diagnosis to improve the poor prognosis of pancreatic cancer. Cancers 2018, 10, 48. [Google Scholar]
- Scaglione, M.; Pinto, A.; Romano, S.; Scialpi, M.; Volterrani, L.; Rotondo, A.; Romano, L. Using multidetector row computed tomography to diagnose and stage pancreatic carcinoma: The problems and possibilities. JOP 2005, 6, 1–5. [Google Scholar]
- Kamisawa, T.; Wood, L.D.; Itoi, T.; Takaori, K. Pancreatic cancer. Lancet 2016, 388, 73–85. [Google Scholar] [CrossRef]
- Egawa, S.; Takeda, K.; Fukuyama, S.; Motoi, F.; Sunamura, M.; Matsuno, S. Clinicopathological aspects of small pancreatic cancer. Pancreas 2004, 28, 235–240. [Google Scholar] [CrossRef]
- Kaur, S.; Baine, M.J.; Jain, M.; Sasson, A.R.; Batra, S.K. Early diagnosis of pancreatic cancer: Challenges and new developments. Biomark Med. 2012, 6, 597–612. [Google Scholar] [CrossRef]
- Zhou, B.; Xu, J.; Cheng, Y.; Gao, J.; Hu, S.; Wang, L.; Zhan, H. Early detection of pancreatic cancer: Where are we now and where are we going? Int. J. Cancer 2017, 141, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Iiboshi, T.; Hanada, K.; Hirano, N.; Yamao, K.; Teraoka, Y.; Amano, M.; Imagawa, H.; Hashimoto, Y.; Fukumoto, A.; Onogawa, S.; et al. The clue of the imaging in the pickup of the pancreatic carcinoma in situ. Syokakinaika 2012, 55, 116–123. (In Japanese) [Google Scholar]
- Yachida, S.; Jones, S.; Bozic, I.; Antal, T.; Leary, R.; Fu, B.; Kamiyama, M.; Hruban, R.H.; Eshleman, J.R.; Nowak, M.A.; et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature 2010, 467, 1114–1117. [Google Scholar] [CrossRef] [PubMed]
- Kanno, A.; Masamune, A.; Hanada, K.; Maguchi, H.; Shimizu, Y.; Ueki, T.; Hasebe, O.; Ohtsuka, T.; Nakamura, M.; Takenaka, M.; et al. Multicenter study of early pancreatic cancer in Japan. Pancreatology 2018, 18, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Okusaka, T.; Shimizu, K.; Furuse, J.; Ito, Y.; Hanada, K.; Shimosegawa, T.; Okazaki, K. Clinical Practice Guidelines for Pancreatic Cancer 2016 From the Japan Pancreas Society: A Synopsis. Pancreas 2017, 46, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Pannala, R.; Basu, A.; Petersen, G.M.; Chari, S.T. New-onset diabetes: A potential clue to the early diagnosis of pancreatic cancer. Lancet Oncol. 2009, 10, 88–95. [Google Scholar] [CrossRef]
- Canto, M.I.; Hruban, R.H.; Fishman, E.K.; Kamel, I.R.; Schulick, R.; Zhang, Z.; Topazian, M.; Takahashi, N.; Fletcher, J.; Petersen, G.; et al. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology 2012, 142, 796–804. [Google Scholar] [CrossRef]
- Langer, P.; Kann, P.H.; Fendrich, V.; Habbe, N.; Schneider, M.; Sina, M.; Slater, E.P.; Heverhagen, J.T.; Gress, T.M.; Rothmund, M.; et al. Five years of prospective screening of high-risk individuals from families with familial pancreatic cancer. Gut 2009, 58, 1410–1418. [Google Scholar] [CrossRef]
- Nazli, O.; Bozdag, A.D.; Tansug, T.; Kir, R.; Kaymak, K. The diagnostic importance of CEA and CA 19-9 for the early diagnosis of pancreatic carcinoma. Hepatogastroenterology 2000, 47, 1750–1752. [Google Scholar]
- Kim, J.; Bamlet, W.R.; Oberg, A.L.; Chaffee, K.G.; Donahue, G.; Cao, X.J.; Chari, S.; Garcia, B.A.; Petersen, G.M.; Zaret, K.S. Detection of early pancreatic ductal adenocarcinoma with thrombospondin-2 and CA19-9 blood markers. Sci. Transl. Med. 2017, 9, 398. [Google Scholar] [CrossRef] [PubMed]
- Gangi, S.; Fletcher, J.G.; Nathan, M.A.; Christensen, J.A.; Harmsen, W.S.; Crownhart, B.S.; Chari, S.T. Time interval between abnormalities seen on CT and the clinical diagnosis of pancreatic cancer: Retrospective review of CT scans obtained before diagnosis. AJR Am. J. Roentgenol. 2004, 182, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.S.; Kim, M.; Choi, J.; Hong, H.; Chung, Y.E.; Lim, J.S. Indicative findings of pancreatic cancer in prediagnostic CT. Eur. Radiol. 2009, 19, 2448–2455. [Google Scholar] [CrossRef] [PubMed]
- Masamune, A.; Shimosegawa, T. Pancreatic stellate cells: A dynamic player of the intercellular communication in pancreatic cancer. Clin. Res. Hepatol. Gastroenterol. 2015, 39, S98–S103. [Google Scholar] [CrossRef]
- Hori, M.; Onaya, H.; Hiraoka, N.; Yamaji, T.; Kobayashi, H.; Takahashi, M.; Mutoh, M.; Shimada, K.; Nakagama, H. Evaluation of the degree of pancreatic fatty infiltration by area-based assessment of CT images: Comparison with histopathology-based and CT attenuation index-based assessments. Jpn. J. Radiol. 2016, 34, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Izumi, Y.; Hanada, K.; Okazaki, A.; Minami, T.; Hirano, N.; Ikemoto, J.; Kanemitsu, K.; Nakadoi, K.; Shishido, T.; Katamura, Y.; et al. Endoscopic ultrasound findings and pathological features of pancreatic carcinoma in situ. Endosc. Int. Open 2019, 7, E585–E593. [Google Scholar] [CrossRef] [PubMed]
- Vargas, R.; Nino-Murcia, M.; Trueblood, W.; Jeffrey, R.B., Jr. MDCT in Pancreatic adenocarcinoma: Prediction of vascular invasion and resectability using a multiphasic technique with curved planar reformations. AJR Am. J. Roentgenol. 2004, 182, 419–425. [Google Scholar] [CrossRef]
- Pietryga, J.A.; Morgan, D.E. Imaging preoperatively for pancreatic adenocarcinoma. J. Gastrointest. Oncol. 2015, 6, 343–357. [Google Scholar] [PubMed]
- Kulkarni, N.M.; Hough, D.M.; Tolat, P.P.; Soloff, E.V.; Kambadakone, A.R. Pancreatic adenocarcinoma: Cross-sectional imaging techniques. Abdom. Radiol. 2018, 43, 253–263. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | n = 41 |
|---|---|
| Age, y, mean (±SD) | 74.8 (±10.5) |
| Female gender, n (%) | 21 (51.2) |
| Location of the pancreatic tumor, n (%) | |
| Pancreatic head | 18 (43.9) |
| Pancreatic body | 15 (36.6) |
| Pancreatic uncinate | 5 (12.2) |
| Pancreatic tail | 3 (7.3) |
| Group | CT Finding | n = 41 (%) | Location of Pancreatic Cancer (%) | |||
|---|---|---|---|---|---|---|
| Head | Body | Uncinate | Tail | |||
| Group A | K-sign | 15 (36.6) | 5 (33.3) | 7 (46.7) | 1 (6.7) | 2 (13.3) |
| K-sign + pancreatic duct patency | 9 (21.9) | 4 (44.4) | 5 (55.6) | 0 (0) | 0 (0) | |
| Group B | Localized fatty change | 7 (17.0) | 4 (57.1) | 0 (0) | 3 (42.9) | 0 (0) |
| Localized fatty change + pancreatic duct patency | 1 (2.4) | 0 (0) | 0 (0) | 1 (100) | 0 (0) | |
| Group C | No abnormality | 9 (21.9) | 4 (44.4) | 3 (33.3) | 1 (11.1) | 1 (11.1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobashi, Y.; Uchiyama, M.; Matsui, J. The “K-Sign”—A Novel CT Finding Suggestive before the Appearance of Pancreatic Cancer. Cancers 2021, 13, 4222. https://doi.org/10.3390/cancers13164222
Kobashi Y, Uchiyama M, Matsui J. The “K-Sign”—A Novel CT Finding Suggestive before the Appearance of Pancreatic Cancer. Cancers. 2021; 13(16):4222. https://doi.org/10.3390/cancers13164222
Chicago/Turabian StyleKobashi, Yuko, Masateru Uchiyama, and Junichi Matsui. 2021. "The “K-Sign”—A Novel CT Finding Suggestive before the Appearance of Pancreatic Cancer" Cancers 13, no. 16: 4222. https://doi.org/10.3390/cancers13164222
APA StyleKobashi, Y., Uchiyama, M., & Matsui, J. (2021). The “K-Sign”—A Novel CT Finding Suggestive before the Appearance of Pancreatic Cancer. Cancers, 13(16), 4222. https://doi.org/10.3390/cancers13164222





