Epidemiology and Clinical Aspects of Malignant Pleural Mesothelioma
Abstract
Simple Summary
Abstract
1. Introduction
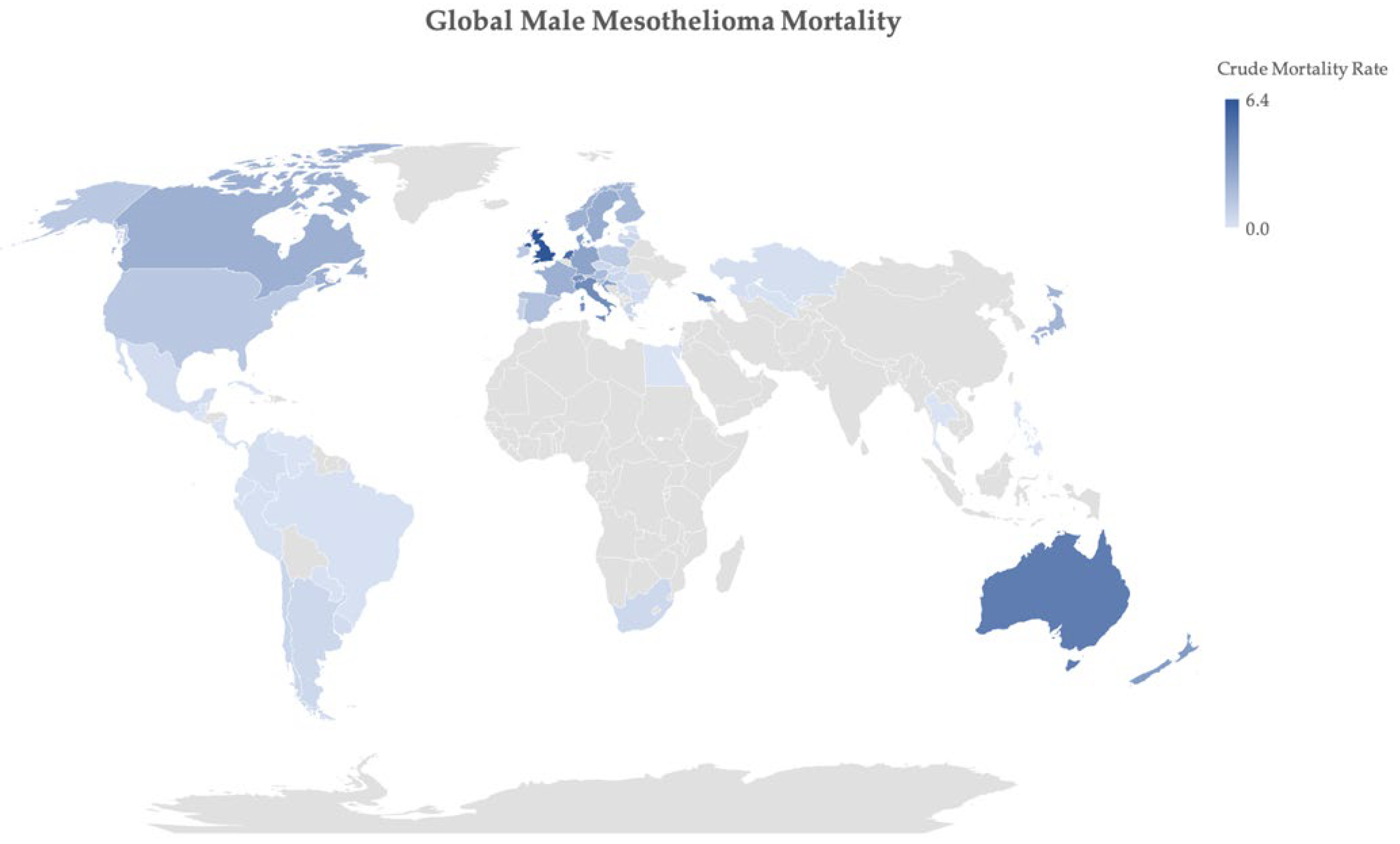
2. Epidemiology of Mesothelioma
3. Histopathology
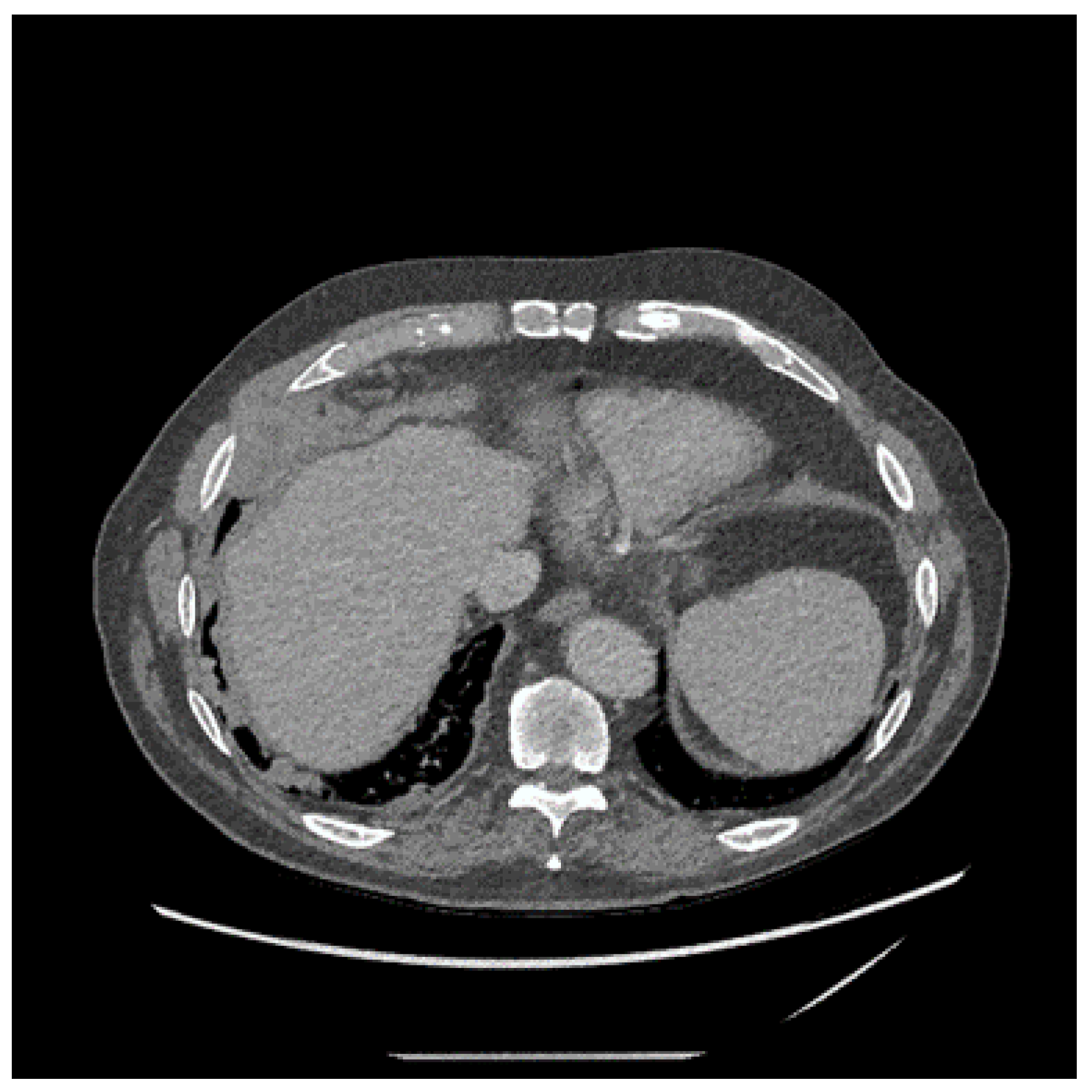
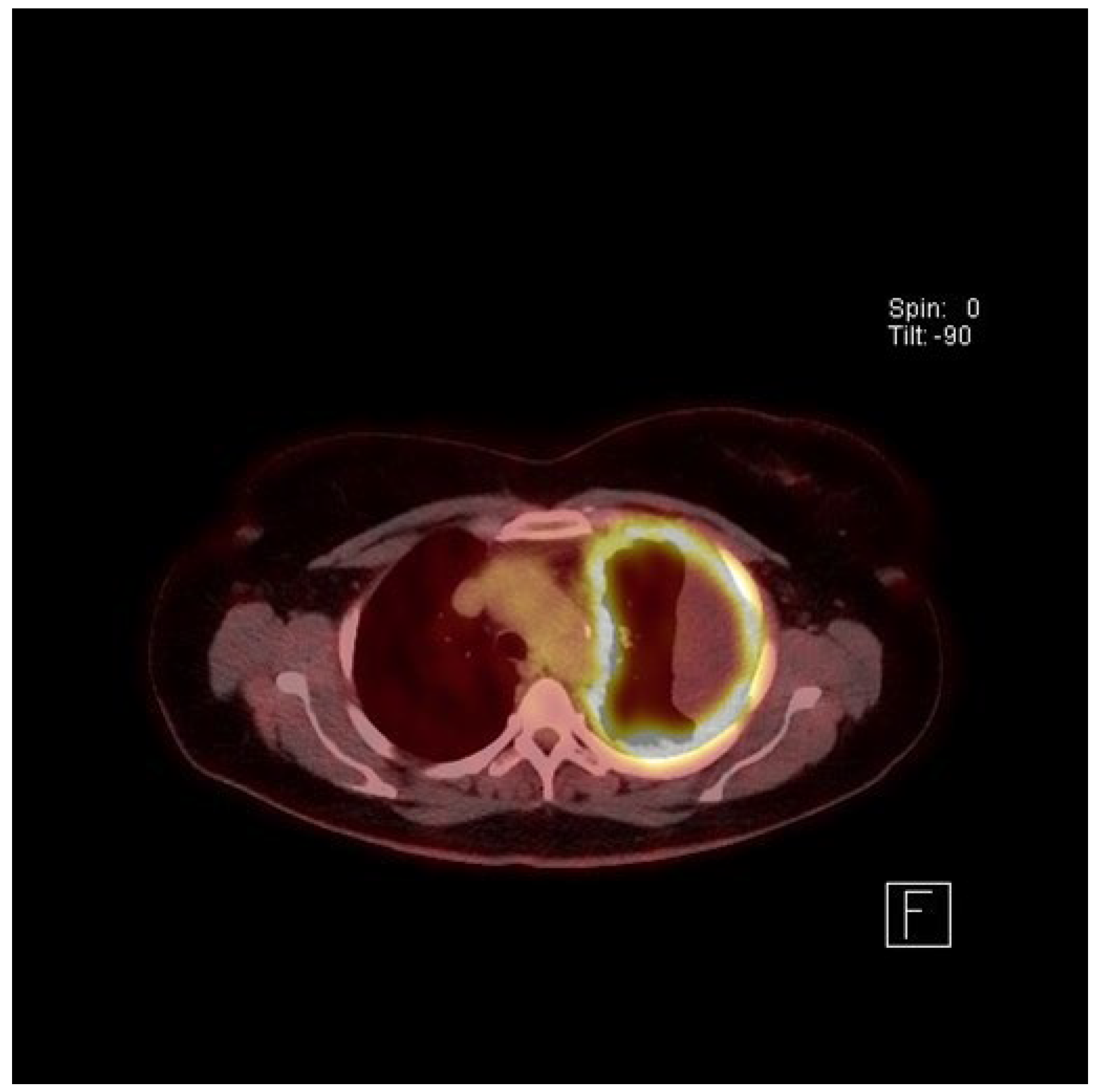
4. Clinical Presentation and Investigations
5. Treatment of Mesothelioma
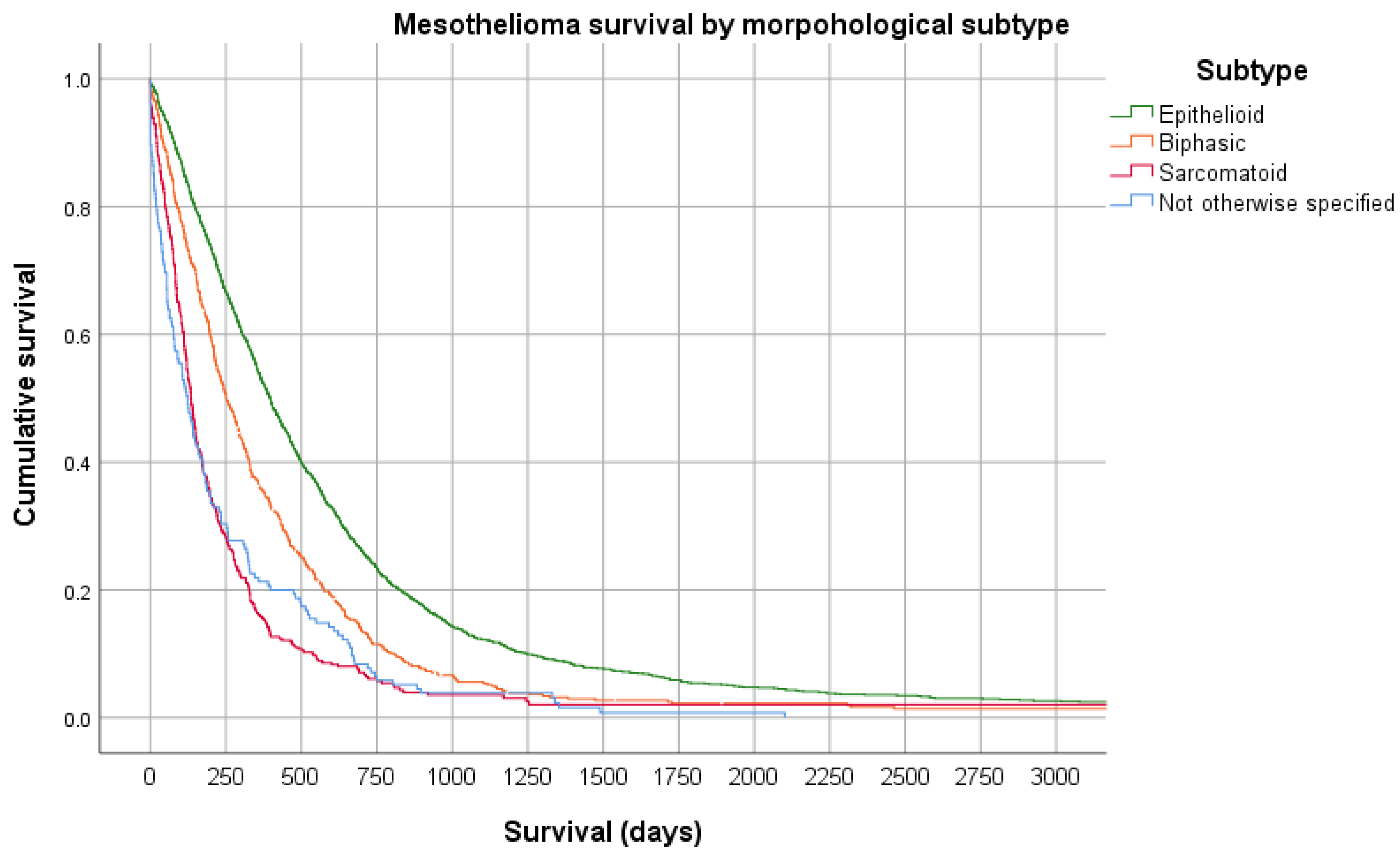
6. Prognosis
7. Conclusions
Funding
Conflicts of Interest
References
- Perry, K.M. Diseases of the lung resulting from occupational dusts other than silica. Thorax 1947, 2, 75–120. [Google Scholar] [CrossRef]
- Doll, R. Mortality from lung cancer in asbestos workers. Br. J. Ind. Med. 1955, 12, 81–86. [Google Scholar] [CrossRef]
- Wagner, J.C.; Sleggs, C.A.; Marchand, P. Diffuse pleural mesothelioma and asbestos exposure in the North Western Cape Province. Br. J. Ind. Med. 1960, 17, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Ross, M.; Nolan, R. History of asbestos discovery and use and asbestos-related disease in context with the occurrence of asbestos within ophiolite complexes. Geol. Soc. Am. Spec. Pap. 2003, 373, 447–470. [Google Scholar]
- Baris, Y.I.; Sahin, A.A.; Ozesmi, M.; Kerse, I.; Ozen, E.; Kolacan, B.; Altinors, M.; Goktepeli, A. An outbreak of pleural mesothelioma and chronic fibrosing pleurisy in the village of Karain/Urgup in Anatolia. Thorax 1978, 33, 181–192. [Google Scholar] [CrossRef]
- Delgermaa, V.; Takahashi, K.; Park, E.K.; Le, G.V.; Hara, T.; Sorahan, T. Global mesothelioma deaths reported to the World Health Organization between 1994 and 2008. Bull. World Health Organ. 2011, 89, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, O. Global trends in mortality from malignant mesothelioma: Analysis of WHO mortality database (1994–2013). Clin. Respir. J. 2018, 12, 2090–2100. [Google Scholar] [CrossRef]
- Olsen, N.J.; Franklin, P.J.; Reid, A.; de Klerk, N.H.; Threlfall, T.J.; Shilkin, K.; Musk, B. Increasing incidence of malignant mesothelioma after exposure to asbestos during home maintenance and renovation. Med. J. Aust. 2011, 195, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Odgerel, C.O.; Takahashi, K.; Sorahan, T.; Driscoll, T.; Fitzmaurice, C.; Yoko, O.M.; Sawanyawisuth, K.; Furuya, S.; Tanaka, F.; Horie, S.; et al. Estimation of the global burden of mesothelioma deaths from incomplete national mortality data. Occup. Environ. Med. 2017, 74, 851–858. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Health Organisation Cancer Mortality Database. Available online: https://www-dep.iarc.fr/WHOdb/WHOdb.htm (accessed on 24 May 2021).
- U.S. Geological Survey. Asbestos Data Sheet—Mineral Commodity Summaries 2020. Available online: https://pubs.usgs.gov/periodicals/mcs2020/mcs2020-asbestos.pdf (accessed on 11 August 2021).
- Frank, A.L. Global use of asbestos—legitimate and illegitimate issues. J. Occup. Med. Toxicol. 2020, 15. [Google Scholar] [CrossRef]
- Frank, L.A.; Joshi, T.K. The Global Spread of Asbestos. Ann. Glob. Health 2014, 80, 257. [Google Scholar] [CrossRef] [PubMed]
- Brims, F. Asbestos-a legacy and a persistent problem. J. R. Nav. Med. Serv. 2009, 95, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Landrigan, P.J. A population of children at risk of exposure to asbestos in place. Ann. N. Y. Acad. Sci. 1991, 643, 283–286. [Google Scholar] [CrossRef]
- Walters, G.I.; Robertson, A.S.; Bhomra, P.S.; Burge, P.S. Asbestosis is prevalent in a variety of construction industry trades. NPJ Prim. Care Respir. Med. 2018, 28, 11. [Google Scholar] [CrossRef]
- Peto, J.; Hodgson, J.T.; Matthews, F.E.; Jones, J.R. Continuing increase in mesothelioma mortality in Britain. Lancet 1995, 345, 535–539. [Google Scholar] [CrossRef]
- Peto, J.; Decarli, A.; La Vecchia, C.; Levi, F.; Negri, E. The European mesothelioma epidemic. Br. J. Cancer 1999, 79, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Murayama, T.; Takahashi, K.; Natori, Y.; Kurumatani, N. Estimation of future mortality from pleural malignant mesothelioma in Japan based on an age-cohort model. Am. J. Ind. Med. 2006, 49, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Montanaro, F.; Bray, F.; Gennaro, V.; Merler, E.; Tyczynski, J.E.; Parkin, D.M.; Strnad, M.; Jechov’a, M.; Storm, H.H.; Aareleid, T.; et al. Pleural mesothelioma incidence in Europe: Evidence of some deceleration in the increasing trends. Cancer Causes Control 2003, 14, 791–803. [Google Scholar] [CrossRef] [PubMed]
- Tse, L.A.; Yu, I.T.; Goggins, W.; Clements, M.; Wang, X.R.; Au, J.S.; Yu, K.S. Are current or future mesothelioma epidemics in Hong Kong the tragic legacy of uncontrolled use of asbestos in the past? Environ. Health Perspect 2010, 118, 382–386. [Google Scholar] [CrossRef]
- Reynolds, C.J.; Minelli, C.; Darnton, A.; Cullinan, P. Mesothelioma mortality in Great Britain: How much longer will dockyards dominate? Occup. Environ. Med. 2019, 76, 908–912. [Google Scholar] [CrossRef]
- Marinaccio, A.; Corfiati, M.; Binazzi, A.; Di Marzio, D.; Scarselli, A.; Ferrante, P.; Bonafede, M.; Verardo, M.; Mirabelli, D.; Gennaro, V.; et al. The epidemiology of malignant mesothelioma in women: Gender differences and modalities of asbestos exposure. Occup. Environ. Med. 2018, 75, 254–262. [Google Scholar] [CrossRef]
- Hodgson, J.T.; Darnton, A. The quantitative risks of mesothelioma and lung cancer in relation to asbestos exposure. Ann. Occup. Hyg. 2000, 44, 565–601. [Google Scholar] [CrossRef]
- Musk, A.W.; De Klerk, N.H.; Brims, F.J.H. Asbestos-Related Non-Malignant Pleural Disease and Mesothelioma. In Parkes’ Occupational Lung Disorders, 4th ed.; Newman-Taylor, A., Cullinan, P., Blanc, P., Pickering, A., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 171–187. [Google Scholar]
- Thomsen, R.W.; Riis, A.H.; Flachs, E.M.; Garabrant, D.H.; Bonde, J.P.E.; Toft Sorensen, H. Risk of asbestosis, mesothelioma, other lung disease or death among motor vehicle mechanics: A 45-year Danish cohort study. Thorax 2021. [Google Scholar] [CrossRef] [PubMed]
- Churg, A. Chrysotile, Tremolite, and Malignant Mesothelioma in Man. Chest 1988, 93, 621–628. [Google Scholar] [CrossRef]
- Mcdonald, J.C.; Armstrong, B.; Case, B.; Doell, D.; Mccaughey, W.T.E.; Mcdonald, A.D.; Sebastien, P. Mesothelioma and Asbestos Fiber Type—Evidence from Lung-Tissue Analyses. Cancer 1989, 63, 1544–1547. [Google Scholar] [CrossRef]
- Berry, G.; de Klerk, N.H.; Reid, A.; Ambrosini, G.L.; Fritschi, L.; Olsen, N.J.; Merler, E.; Musk, A.W. Malignant pleural and peritoneal mesotheliomas in former miners and millers of crocidolite at Wittenoom, Western Australia. Occup. Environ. Med. 2004, 61, e14. [Google Scholar] [CrossRef] [PubMed]
- Menon, L.; Franklin, P.; Olsen, N.; Segal, A.; De Klerk, N.H.; Musk, A.W.; Brims, F.J.H. 60 years of the Western Australian Mesothelioma Registry. In Proceedings of the Thoracic Society of Australia & New Zealand, Melbourne, Australia, April 2021; p. 93. [Google Scholar]
- Yang, H.N.; Testa, J.R.; Carbone, M. Mesothelioma Epidemiology, Carcinogenesis, and Pathogenesis. Curr. Treat. Options Oncol. 2008, 9, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Kroczynska, B.; Cutrone, R.; Bocchetta, M.; Yang, H.; Elmishad, A.G.; Vacek, P.; Ramos-Nino, M.; Mossman, B.T.; Pass, H.I.; Carbone, M. Crocidolite asbestos and SV40 are cocarcinogens in human mesothelial cells and in causing mesothelioma in hamsters. Proc. Natl. Acad. Sci. USA 2006, 103, 14128–14133. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.M.H.; Mason, C.; Rowe, P. Diffuse malignant mesothelioma of the peritoneum following abdominal radiotherapy. Eur. J. Surg. Oncol. 2001, 27, 214–215. [Google Scholar] [CrossRef]
- Travis, L.B.; Fossa, S.D.; Schonfeld, S.J.; McMaster, M.L.; Lynch, C.F.; Storm, H.; Hall, P.; Holowaty, E.; Andersen, A.; Pukkala, E.; et al. Second cancers among 40576 testicular cancer patients: Focus on long-term survivors. J. Natl. Cancer Inst. 2005, 97, 1354–1365. [Google Scholar] [CrossRef]
- Brown, L.M.; Howard, R.A.; Travis, L.B. The risk of secondary malignancies over 30 years after the treatment of non-hodgkin lymphoma. Cancer 2006, 107, 2741–2742. [Google Scholar] [CrossRef] [PubMed]
- Teta, M.J.; Lau, E.; Sceurman, B.K.; Wagner, M.E. Therapeutic radiation for lymphoma - Risk of malignant mesothelioma. Cancer 2007, 109, 1432–1438. [Google Scholar] [CrossRef] [PubMed]
- Cavazza, A.; Travis, L.B.; Travis, W.D.; Wolfe, J.T.; Foo, M.L.; Gillespie, D.J.; Weidner, N.; Colby, T.V. Post-irradiation malignant mesothelioma. Cancer 1996, 77, 1379–1385. [Google Scholar] [CrossRef]
- Andersson, M.; Wallin, H.; Jonsson, M.; Nielsen, L.L.; Visfeldt, J.; Vyberg, M.; Bennett, W.P.; Debenedetti, V.M.G.; Travis, L.B.; Storm, H.H. Lung-Carcinoma and Malignant Mesothelioma in Patients Exposed to Thorotrast—Incidence, Histology and P53 Status. Int. J. Cancer 1995, 63, 330–336. [Google Scholar] [CrossRef]
- de Klerk, N.; Alfonso, H.; Olsen, N.; Reid, A.; Sleith, J.; Palmer, L.; Berry, G.; Musk, A.B. Familial aggregation of malignant mesothelioma in former workers and residents of Wittenoom, Western Australia. Int. J. Cancer 2013, 132, 1423–1428. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.; Ferris, L.K.; Baumann, F.; Napolitano, A.; Lum, C.A.; Flores, E.G.; Gaudino, G.; Powers, A.; Bryant-Greenwood, P.; Krausz, T.; et al. BAP1 cancer syndrome: Malignant mesothelioma, uveal and cutaneous melanoma, and MBAITs. J. Transl. Med. 2012, 10, 179. [Google Scholar] [CrossRef]
- Sneddon, S.; Leon, J.S.; Dick, I.M.; Cadby, G.; Olsen, N.; Brims, F.; Allcock, R.J.; Moses, E.K.; Melton, P.E.; de Klerk, N.; et al. Absence of germline mutations in BAP1 in sporadic cases of malignant mesothelioma. Gene 2015, 563, 103–105. [Google Scholar] [CrossRef]
- Cadby, G.; Mukherjee, S.; Musk, A.W.; Reid, A.; Garlepp, M.; Dick, I.; Robinson, C.; Hui, J.; Fiorito, G.; Guarrera, S.; et al. A genome-wide association study for malignant mesothelioma risk. Lung Cancer 2013, 82, 1–8. [Google Scholar] [CrossRef]
- Finn, R.S.; Brims, F.J.; Gandhi, A.; Olsen, N.; Musk, A.W.; Maskell, N.A.; Lee, Y.C. Postmortem findings of malignant pleural mesothelioma: A two-center study of 318 patients. Chest 2012, 142, 1267–1273. [Google Scholar] [CrossRef]
- Franklin, P.; Alfonso, H.; Reid, A.; Olsen, N.; Shilkin, K.B.; Brims, F.; de Klerk, N.; Musk, A.W. Asbestos exposure and histological subtype of malignant mesothelioma. Occup. Environ. Med. 2016, 73, 749–752. [Google Scholar] [CrossRef]
- Creaney, J.; van Bruggen, I.; Hof, M.; Segal, A.; Musk, A.W.; de Klerk, N.; Horick, N.; Skates, S.J.; Robinson, B.W. Combined CA125 and mesothelin levels for the diagnosis of malignant mesothelioma. Chest 2007, 132, 1239–1246. [Google Scholar] [CrossRef]
- Creaney, J.; Olsen, N.J.; Brims, F.; Dick, I.M.; Musk, A.W.; de Klerk, N.H.; Skates, S.J.; Robinson, B.W. Serum Mesothelin for Early Detection of the Asbestos-Induced Cancer Maligant Mesothelioma. Cancer Epidemiol. Biomark. Prev. 2010. [Google Scholar] [CrossRef]
- Davies, H.E.; Sadler, R.S.; Bielsa, S.; Maskell, N.A.; Rahman, N.M.; Davies, R.J.; Ferry, B.L.; Lee, Y.C. Clinical impact and reliability of pleural fluid mesothelin in undiagnosed pleural effusions. Am. J. Respir. Crit. Care Med. 2009, 180, 437–444. [Google Scholar] [CrossRef]
- Pass, H.I.; Wali, A.; Tang, N.; Ivanova, A.; Ivanov, S.; Harbut, M.; Carbone, M.; Allard, J. Soluble mesothelin-related peptide level elevation in mesothelioma serum and pleural effusions. Ann. Thorac. Surg. 2008, 85, 265–272, discussion 272. [Google Scholar] [CrossRef] [PubMed]
- Pass, H.I.; Levin, S.M.; Harbut, M.R.; Melamed, J.; Chiriboga, L.; Donington, J.; Huflejt, M.; Carbone, M.; Chia, D.; Goodglick, L.; et al. Fibulin-3 as a blood and effusion biomarker for pleural mesothelioma. N. Engl. J. Med. 2012, 367, 1417–1427. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, G.K.; Akin, M.R.; Villanueva, R.R.; Slatnik, J. Cytopathology of malignant mesothelioma of the pleura in fine-needle aspiration biopsy. Diagn. Cytopathol. 1999, 21, 253–259. [Google Scholar] [CrossRef]
- Wolanski, K.D.; Whitaker, D.; Shilkin, K.B.; Henderson, D.W. The use of epithelial membrane antigen and silver-stained nucleolar organizer regions testing in the differential diagnosis of mesothelioma from benign reactive mesothelioses. Cancer 1998, 82, 583–590. [Google Scholar] [CrossRef]
- Segal, A.; Whitaker, D.; Henderson, D.; Shilkin, K. Pathology and mesothelioma. In Mesothelioma; Robinson, B.W.S., Chinanian, A.P., Eds.; Martin Dunitz: London, UK, 2002; pp. 143–184. [Google Scholar]
- Lechner, J.F.; Tesfaigzi, J.; Gerwin, B.I. Oncogenes and tumor-suppressor genes in mesothelioma--a synopsis. Environ. Health Perspect. 1997, 105 (Suppl. 5), 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Lindholm, P.M.; Salmenkivi, K.; Vauhkonen, H.; Nicholson, A.G.; Anttila, S.; Kinnula, V.L.; Knuutila, S. Gene copy number analysis in malignant pleural mesothelioma using oligonucleotide array CGH. Cytogenet. Genome Res. 2007, 119, 46–52. [Google Scholar] [CrossRef]
- Musti, M.; Kettunen, E.; Dragonieri, S.; Lindholm, P.; Cavone, D.; Serio, G.; Knuutila, S. Cytogenetic and molecular genetic changes in malignant mesothelioma. Cancer Genet. Cytogenet. 2006, 170, 9–15. [Google Scholar] [CrossRef]
- Chapel, D.B.; Schulte, J.J.; Berg, K.; Churg, A.; Dacic, S.; Fitzpatrick, C.; Galateau-Salle, F.; Hiroshima, K.; Krausz, T.; Le Stang, N.; et al. MTAP immunohistochemistry is an accurate and reproducible surrogate for CDKN2A fluorescence in situ hybridization in diagnosis of malignant pleural mesothelioma. Mod. Pathol 2020, 33, 245–254. [Google Scholar] [CrossRef]
- Churg, A.; Hwang, H.; Tan, L.; Qing, G.; Taher, A.; Tong, A.; Bilawich, A.M.; Dacic, S. Malignant mesothelioma in situ. Histopathology 2018, 72, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- Churg, A.; Galateau-Salle, F.; Roden, A.C.; Attanoos, R.; von der Thusen, J.H.; Tsao, M.S.; Chang, N.; De Perrot, M.; Dacic, S. Malignant mesothelioma in situ: Morphologic features and clinical outcome. Mod. Pathol 2020, 33, 297–302. [Google Scholar] [CrossRef]
- Matsumoto, S.; Hamasaki, M.; Kinoshita, Y.; Kamei, T.; Kawahara, K.; Nabeshima, K. Morphological difference between pleural mesothelioma cells in effusion smears with either BAP1 loss or 9p21 homozygous deletion and reactive mesothelial cells without the gene alterations. Pathol. Int. 2019, 69, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Hjerpe, A.; Ascoli, V.; Bedrossian, C.W.; Boon, M.E.; Creaney, J.; Davidson, B.; Dejmek, A.; Dobra, K.; Fassina, A.; Field, A.; et al. Guidelines for the Cytopathologic Diagnosis of Epithelioid and Mixed-Type Malignant Mesothelioma: A secondary publication. Cytopathology 2015, 26, 142–156. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.C.; Sheffield, B.S.; Rodriguez, S.; Thompson, K.; Tse, C.H.; Gown, A.M.; Churg, A. Utility of BAP1 Immunohistochemistry and p16 (CDKN2A) FISH in the Diagnosis of Malignant Mesothelioma in Effusion Cytology Specimens. Am. J. Surg Pathol. 2016, 40, 120–126. [Google Scholar] [CrossRef]
- Muruganandan, S.; Alfonso, H.; Franklin, P.; Shilkin, K.; Segal, A.; Olsen, N.; Reid, A.; de Klerk, N.; Musk, A.B.; Brims, F. Comparison of outcomes following a cytological or histological diagnosis of malignant mesothelioma. Br. J. Cancer 2017, 116, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Segal, A.; Sterrett, G.F.; Frost, F.A.; Shilkin, K.B.; Olsen, N.J.; Musk, A.W.; Nowak, A.K.; Robinson, B.W.; Creaney, J. A diagnosis of malignant pleural mesothelioma can be made by effusion cytology: Results of a 20 year audit. Pathology 2013, 45, 44–48. [Google Scholar] [CrossRef]
- Hoon, S.N.; Lawrie, I.; Qi, C.; Rahman, N.; Maskell, N.; Forbes, K.; Gerry, S.; Monterosso, L.; Chauhan, A.; Brims, F.J.H. Symptom Burden and Unmet Needs in Malignant Pleural Mesothelioma: Exploratory Analyses From the RESPECT-Meso Study. J. Palliat. Care 2021, 36, 113–120. [Google Scholar] [CrossRef]
- Yildirim, H.; Metintas, M.; Entok, E.; Ak, G.; Ak, I.; Dundar, E.; Erginel, S. Clinical value of fluorodeoxyglucose-positron emission tomography/computed tomography in differentiation of malignant mesothelioma from asbestos-related benign pleural disease: An observational pilot study. J. Thorac. Oncol. 2009, 4, 1480–1484. [Google Scholar] [CrossRef]
- Kruse, M.; Sherry, S.J.; Paidpally, V.; Mercier, G.; Subramaniam, R.M. FDG PET/CT in the management of primary pleural tumors and pleural metastases. AJR Am. J. Roentgenol. 2013, 201, W215–W226. [Google Scholar] [CrossRef]
- Rusch, V.W. A proposed new international TNM staging system for malignant pleural mesothelioma. From the International Mesothelioma Interest Group. Chest 1995, 108, 1122–1128. [Google Scholar] [CrossRef]
- Musk, A.W.; Dewar, J.; Shilkin, K.B.; Whitaker, D. Miliary spread of malignant pleural mesothelioma without a clinically identifiable pleural tumour. Aust. N. Z. J. Med. 1991, 21, 460–462. [Google Scholar] [CrossRef] [PubMed]
- de Gooijer, C.J.; Borm, F.J.; Scherpereel, A.; Baas, P. Immunotherapy in Malignant Pleural Mesothelioma. Front. Oncol. 2020, 10, 187. [Google Scholar] [CrossRef] [PubMed]
- Ceresoli, G.L.; Pasello, G. Immune checkpoint inhibitors in mesothelioma: A turning point. Lancet 2021, 397, 348–349. [Google Scholar] [CrossRef]
- Vogelzang, N.J.; Rusthoven, J.J.; Symanowski, J.; Denham, C.; Kaukel, E.; Ruffie, P.; Gatzemeier, U.; Boyer, M.; Emri, S.; Manegold, C.; et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J. Clin. Oncol. 2003, 21, 2636–2644. [Google Scholar] [CrossRef] [PubMed]
- Zalcman, G.; Mazieres, J.; Margery, J.; Greillier, L.; Audigier-Valette, C.; Moro-Sibilot, D.; Molinier, O.; Corre, R.; Monnet, I.; Gounant, V.; et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): A randomised, controlled, open-label, phase 3 trial. Lancet 2016, 387, 1405–1414. [Google Scholar] [CrossRef]
- Popat, S.; Curioni-Fontecedro, A.; Dafni, U.; Shah, R.; O’Brien, M.; Pope, A.; Fisher, P.; Spicer, J.; Roy, A.; Gilligan, D.; et al. A multicentre randomised phase III trial comparing pembrolizumab versus single-agent chemotherapy for advanced pre-treated malignant pleural mesothelioma: The European Thoracic Oncology Platform (ETOP 9-15) PROMISE-meso trial. Ann. Oncol. 2020, 31, 1734–1745. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.K.; Lesterhuis, W.J.; Kok, P.S.; Brown, C.; Hughes, B.G.; Karikios, D.J.; John, T.; Kao, S.C.; Leslie, C.; Cook, A.M.; et al. Durvalumab with first-line chemotherapy in previously untreated malignant pleural mesothelioma (DREAM): A multicentre, single-arm, phase 2 trial with a safety run-in. Lancet Oncol. 2020, 21, 1213–1223. [Google Scholar] [CrossRef]
- Baas, P.; Scherpereel, A.; Nowak, A.K.; Fujimoto, N.; Peters, S.; Tsao, A.S.; Mansfield, A.S.; Popat, S.; Jahan, T.; Antonia, S.; et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): A multicentre, randomised, open-label, phase 3 trial. Lancet 2021, 397, 375–386. [Google Scholar] [CrossRef]
- Brims, F.J.; Meniawy, T.M.; Duffus, I.; de Fonseka, D.; Segal, A.; Creaney, J.; Maskell, N.; Lake, R.A.; de Klerk, N.; Nowak, A.K. A Novel Clinical Prediction Model for Prognosis in Malignant Pleural Mesothelioma Using Decision Tree Analysis. J. Thorac. Oncol. 2016, 11, 573–582. [Google Scholar] [CrossRef]
- Nowak, A.K.; Francis, R.J.; Phillips, M.J.; Millward, M.J.; van der Schaaf, A.A.; Boucek, J.; Musk, A.W.; McCoy, M.J.; Segal, A.; Robins, P.; et al. A novel prognostic model for malignant mesothelioma incorporating quantitative FDG-PET imaging with clinical parameters. Clin. Cancer Res. 2010, 16, 2409–2417. [Google Scholar] [CrossRef]
- Brosseau, S.; Danel, C.; Scherpereel, A.; Mazieres, J.; Lantuejoul, S.; Margery, J.; Greillier, L.; Audigier-Valette, C.; Gounant, V.; Antoine, M.; et al. Shorter Survival in Malignant Pleural Mesothelioma Patients With High PD-L1 Expression Associated With Sarcomatoid or Biphasic Histology Subtype: A Series of 214 Cases From the Bio-MAPS Cohort. Clin. Lung Cancer 2019, 20, e564–e575. [Google Scholar] [CrossRef]
- Davies, H.E.; Mishra, E.K.; Kahan, B.C.; Wrightson, J.M.; Stanton, A.E.; Guhan, A.; Davies, C.W.; Grayez, J.; Harrison, R.; Prasad, A.; et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: The TIME2 randomized controlled trial. JAMA 2012, 307, 2383–2389. [Google Scholar] [CrossRef]
- Bhatnagar, R.; Keenan, E.K.; Morley, A.J.; Kahan, B.C.; Stanton, A.E.; Haris, M.; Harrison, R.N.; Mustafa, R.A.; Bishop, L.J.; Ahmed, L.; et al. Outpatient Talc Administration by Indwelling Pleural Catheter for Malignant Effusion. N. Engl. J. Med. 2018, 378, 1313–1322. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Fysh, E.T.H.; Smith, N.A.; Lee, P.; Kwan, B.C.H.; Yap, E.; Horwood, F.C.; Piccolo, F.; Lam, D.C.L.; Garske, L.A.; et al. Effect of an Indwelling Pleural Catheter vs Talc Pleurodesis on Hospitalization Days in Patients with Malignant Pleural Effusion. JAMA 2017, 318, 1903. [Google Scholar] [CrossRef] [PubMed]
- Muruganandan, S.; Azzopardi, M.; Fitzgerald, D.B.; Shrestha, R.; Kwan, B.C.H.; Lam, D.C.L.; De Chaneet, C.C.; Rashid Ali, M.R.S.; Yap, E.; Tobin, C.L.; et al. Aggressive versus symptom-guided drainage of malignant pleural effusion via indwelling pleural catheters (AMPLE-2): An open-label randomised trial. Lancet Respir. Med. 2018, 6, 671–680. [Google Scholar] [CrossRef]
- Agarwal, P.P.; Seely, J.M.; Matzinger, F.R.; MacRae, R.M.; Peterson, R.A.; Maziak, D.E.; Dennie, C.J. Pleural mesothelioma: Sensitivity and incidence of needle track seeding after image-guided biopsy versus surgical biopsy. Radiology 2006, 241, 589–594. [Google Scholar] [CrossRef]
- Bayman, N.; Appel, W.; Ashcroft, L.; Baldwin, D.R.; Bates, A.; Darlison, L.; Edwards, J.G.; Ezhil, V.; Gilligan, D.; Hatton, M.; et al. Prophylactic Irradiation of Tracts in Patients with Malignant Pleural Mesothelioma: An Open-Label, Multicenter, Phase III Randomized Trial. J. Clin. Oncol. 2019, 37, 1200–1208. [Google Scholar] [CrossRef]
- Clive, A.O.; Taylor, H.; Dobson, L.; Wilson, P.; de Winton, E.; Panakis, N.; Pepperell, J.; Howell, T.; Stewart, S.A.; Penz, E.; et al. Prophylactic radiotherapy for the prevention of procedure-tract metastases after surgical and large-bore pleural procedures in malignant pleural mesothelioma (SMART): A multicentre, open-label, phase 3, randomised controlled trial. Lancet Oncol. 2016, 17, 1094–1104. [Google Scholar] [CrossRef]
- Bertoglio, P.; Waller, D.A. The role of thoracic surgery in the management of mesothelioma: An expert opinion on the limited evidence. Expert Rev. Respir. Med. 2016, 10, 663–672. [Google Scholar] [CrossRef]
- Treasure, T.; Lang-Lazdunski, L.; Waller, D.; Bliss, J.M.; Tan, C.; Entwisle, J.; Snee, M.; O’Brien, M.; Thomas, G.; Senan, S.; et al. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: Clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. Lancet Oncol. 2011, 12, 763–772. [Google Scholar] [CrossRef]
- McRonald, F.; Baldwin, D.R.; Devaraj, A.; Brain, K.; Eisen, T.; Holeman, J.; Ledson, M.; Screaton, N.; Rintoul, R.C.; Yadegarfar, G.; et al. The uniqueness of the United Kingdom Lung Cancer Screening trial (UKLS)—A population screening study. Lung Cancer 2013, 79, S28–S29. [Google Scholar] [CrossRef]
- Hasani, A.; Alvarez, J.M.; Wyatt, J.M.; Bydder, S.; Millward, M.; Byrne, M.; Musk, A.W.; Nowak, A.K. Outcome for patients with malignant pleural mesothelioma referred for Trimodality therapy in Western Australia. J. Thorac. Oncol. 2009, 4, 1010–1016. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C. Surgical resection of mesothelioma: An evidence-free practice. Lancet 2014, 384, 1080–1081. [Google Scholar] [CrossRef]
- Ricciardi, S.; Cardillo, G.; Zirafa, C.C.; Carleo, F.; Facciolo, F.; Fontanini, G.; Mutti, L.; Melfi, F. Surgery for malignant pleural mesothelioma: An international guidelines review. J. Thorac. Dis. 2018, 10, S285–S292. [Google Scholar] [CrossRef]
- Rintoul, R.C.; Ritchie, A.J.; Edwards, J.G.; Waller, D.A.; Coonar, A.S.; Bennett, M.; Lovato, E.; Hughes, V.; Fox-Rushby, J.A.; Sharples, L.D.; et al. Efficacy and cost of video-assisted thoracoscopic partial pleurectomy versus talc pleurodesis in patients with malignant pleural mesothelioma (MesoVATS): An open-label, randomised, controlled trial. Lancet 2014, 384, 1118–1127. [Google Scholar] [CrossRef]
- Brims, F.; Gunatilake, S.; Lawrie, I.; Marshall, L.; Fogg, C.; Qi, C.; Creech, L.; Holtom, N.; Killick, S.; Yung, B.; et al. Early specialist palliative care on quality of life for malignant pleural mesothelioma: A randomised controlled trial. Thorax 2019, 74, 354–361. [Google Scholar] [CrossRef]
- Sinclair, C.; Auret, K.A.; Evans, S.F.; Williamson, F.; Dormer, S.; Wilkinson, A.; Greeve, K.; Koay, A.; Price, D.; Brims, F. Advance care planning uptake among patients with severe lung disease: A randomised patient preference trial of a nurse-led, facilitated advance care planning intervention. BMJ Open 2017, 7, e013415. [Google Scholar] [CrossRef] [PubMed]
- Neumann, V.; Rutten, A.; Scharmach, M.; Muller, K.M.; Fischer, M. Factors influencing long-term survival in mesothelioma patients--results of the German mesothelioma register. Int. Arch. Occup. Environ. Health 2004, 77, 191–199. [Google Scholar] [CrossRef]
- Musk, A.W.; Olsen, N.; Alfonso, H.; Reid, A.; Mina, R.; Franklin, P.; Sleith, J.; Hammond, N.; Threlfall, T.; Shilkin, K.B.; et al. Predicting survival in malignant mesothelioma. Eur. Respir. J. 2011, 38, 1420–1424. [Google Scholar] [CrossRef] [PubMed]
- Brims, F.J.; Maskell, N.A. Prognostic factors for malignant pleural mesothelioma. Curr. Respir. Care Rep. 2013, 2, 100–108. [Google Scholar] [CrossRef][Green Version]
- Mirarabshahii, P.; Pillai, K.; Chua, T.C.; Pourgholami, M.H.; Morris, D.L. Diffuse malignant peritoneal mesothelioma—An update on treatment. Cancer Treat. Rev. 2012, 38, 605–612. [Google Scholar] [CrossRef]
- Creaney, J.; Francis, R.J.; Dick, I.M.; Musk, A.W.; Robinson, B.W.; Byrne, M.J.; Nowak, A.K. Serum soluble mesothelin concentrations in malignant pleural mesothelioma: Relationship to tumor volume, clinical stage and changes in tumor burden. Clin. Cancer Res. 2011, 17, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Grigoriu, B.D.; Scherpereel, A.; Devos, P.; Chahine, B.; Letourneux, M.; Lebailly, P.; Gregoire, M.; Porte, H.; Copin, M.C.; Lassalle, P. Utility of osteopontin and serum mesothelin in malignant pleural mesothelioma diagnosis and prognosis assessment. Clin. Cancer Res. 2007, 13, 2928–2935. [Google Scholar] [CrossRef]
- Hollevoet, K.; Nackaerts, K.; Gosselin, R.; De Wever, W.; Bosquee, L.; De Vuyst, P.; Germonpre, P.; Kellen, E.; Legrand, C.; Kishi, Y.; et al. Soluble mesothelin, megakaryocyte potentiating factor, and osteopontin as markers of patient response and outcome in mesothelioma. J. Thorac. Oncol. 2011, 6, 1930–1937. [Google Scholar] [CrossRef]
- Creaney, J.; Dick, I.M.; Meniawy, T.M.; Leong, S.L.; Leon, J.S.; Demelker, Y.; Segal, A.; Bill Musk, A.W.; Lee, Y.C.; Skates, S.J.; et al. Comparison of fibulin-3 and mesothelin as markers in malignant mesothelioma. Thorax 2014, 69, 895–902. [Google Scholar] [CrossRef]
- Kadota, K.; Suzuki, K.; Colovos, C.; Sima, C.S.; Rusch, V.W.; Travis, W.D.; Adusumilli, P.S. A nuclear grading system is a strong predictor of survival in epitheloid diffuse malignant pleural mesothelioma. Mod. Pathol. 2012, 25, 260–271. [Google Scholar] [CrossRef]
- Kao, S.C.; Pavlakis, N.; Harvie, R.; Vardy, J.L.; Boyer, M.J.; van Zandwijk, N.; Clarke, S.J. High blood neutrophil-to-lymphocyte ratio is an indicator of poor prognosis in malignant mesothelioma patients undergoing systemic therapy. Clin. Cancer Res. 2010, 16, 5805–5813. [Google Scholar] [CrossRef]
- Kao, S.C.; Klebe, S.; Henderson, D.W.; Reid, G.; Chatfield, M.; Armstrong, N.J.; Yan, T.D.; Vardy, J.; Clarke, S.; van Zandwijk, N.; et al. Low calretinin expression and high neutrophil-to-lymphocyte ratio are poor prognostic factors in patients with malignant mesothelioma undergoing extrapleural pneumonectomy. J. Thorac. Oncol. 2011, 6, 1923–1929. [Google Scholar] [CrossRef]
- Yeap, B.Y.; De Rienzo, A.; Gill, R.R.; Oster, M.E.; Dao, M.N.; Dao, N.T.; Levy, R.D.; Vermilya, K.; Gustafson, C.E.; Ovsak, G.; et al. Mesothelioma Risk Score: A New Prognostic Pretreatment, Clinical-Molecular Algorithm for Malignant Pleural Mesothelioma. J. Thorac. Oncol. 2021. [Google Scholar] [CrossRef]
- Curran, D.; Sahmoud, T.; Therasse, P.; van Meerbeeck, J.; Postmus, P.E.; Giaccone, G. Prognostic factors in patients with pleural mesothelioma: The European Organization for Research and Treatment of Cancer experience. J. Clin. Oncol. 1998, 16, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Herndon, J.E.; Green, M.R.; Chahinian, A.P.; Corson, J.M.; Suzuki, Y.; Vogelzang, N.J. Factors predictive of survival among 337 patients with mesothelioma treated between 1984 and 1994 by the Cancer and Leukemia Group B. Chest 1998, 113, 723–731. [Google Scholar] [CrossRef] [PubMed]
- van der Bij, S.; Koffijberg, H.; Burgers, J.A.; Baas, P.; van de Vijver, M.J.; de Mol, B.A.; Moons, K.G. Prognosis and prognostic factors of patients with mesothelioma: A population-based study. Br. J. Cancer 2012, 107, 161–164. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Milano, M.T.; Zhang, H. Malignant pleural mesothelioma: A population-based study of survival. J. Thorac. Oncol. 2010, 5, 1841–1848. [Google Scholar] [CrossRef] [PubMed]
- Nojiri, S.; Gemba, K.; Aoe, K.; Kato, K.; Yamaguchi, T.; Sato, T.; Kubota, K.; Kishimoto, T. Survival and prognostic factors in malignant pleural mesothelioma: A retrospective study of 314 patients in the west part of Japan. Jpn. J. Clin. Oncol. 2011, 41, 32–39. [Google Scholar] [CrossRef]
- Montanaro, F.; Rosato, R.; Gangemi, M.; Roberti, S.; Ricceri, F.; Merler, E.; Gennaro, V.; Romanelli, A.; Chellini, E.; Pascucci, C.; et al. Survival of pleural malignant mesothelioma in Italy: A population-based study. Int. J. Cancer 2009, 124, 201–207. [Google Scholar] [CrossRef]
- Marinaccio, A.; Nesti, M.; Regional Operational, C. Analysis of survival of mesothelioma cases in the Italian register (ReNaM). Eur. J. Cancer 2003, 39, 1290–1295. [Google Scholar] [CrossRef]
- Woolhouse, I.; Bishop, L.; Darlison, L.; De Fonseka, D.; Edey, A.; Edwards, J.; Faivre-Finn, C.; Fennell, D.A.; Holmes, S.; Kerr, K.M.; et al. British Thoracic Society Guideline for the investigation and management of malignant pleural mesothelioma. Thorax 2018, 73, i1–i30. [Google Scholar] [CrossRef] [PubMed]
- Harris, E.J.A.; Kao, S.; McCaughan, B.; Nakano, T.; Kondo, N.; Hyland, R.; Nowak, A.K.; de Klerk, N.H.; Brims, F.J.H. Prediction modelling using routine clinical parameters to stratify survival in Malignant Pleural Mesothelioma patients undergoing cytoreductive surgery. J. Thorac. Oncol. 2019, 14, 288–293. [Google Scholar] [CrossRef]
- Bibby, A.C.; Dorn, P.; Psallidas, I.; Porcel, J.M.; Janssen, J.; Froudarakis, M.; Subotic, D.; Astoul, P.; Licht, P.; Schmid, R.; et al. ERS/EACTS statement on the management of malignant pleural effusions. Eur. Respir. J. 2018, 52. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brims, F. Epidemiology and Clinical Aspects of Malignant Pleural Mesothelioma. Cancers 2021, 13, 4194. https://doi.org/10.3390/cancers13164194
Brims F. Epidemiology and Clinical Aspects of Malignant Pleural Mesothelioma. Cancers. 2021; 13(16):4194. https://doi.org/10.3390/cancers13164194
Chicago/Turabian StyleBrims, Fraser. 2021. "Epidemiology and Clinical Aspects of Malignant Pleural Mesothelioma" Cancers 13, no. 16: 4194. https://doi.org/10.3390/cancers13164194
APA StyleBrims, F. (2021). Epidemiology and Clinical Aspects of Malignant Pleural Mesothelioma. Cancers, 13(16), 4194. https://doi.org/10.3390/cancers13164194





