Technological Advances in Tumor-On-Chip Technology: From Bench to Bedside
Abstract
:Simple Summary
Abstract
1. Introduction
2. Current Microfluidic Advances for Tumor-On-Chip Technology
2.1. Progress in Tumor-On-Chip Technology
2.2. Technological Approaches for Tumor-On-Chip Technology
2.2.1. Advances in Microfluidic Technologies
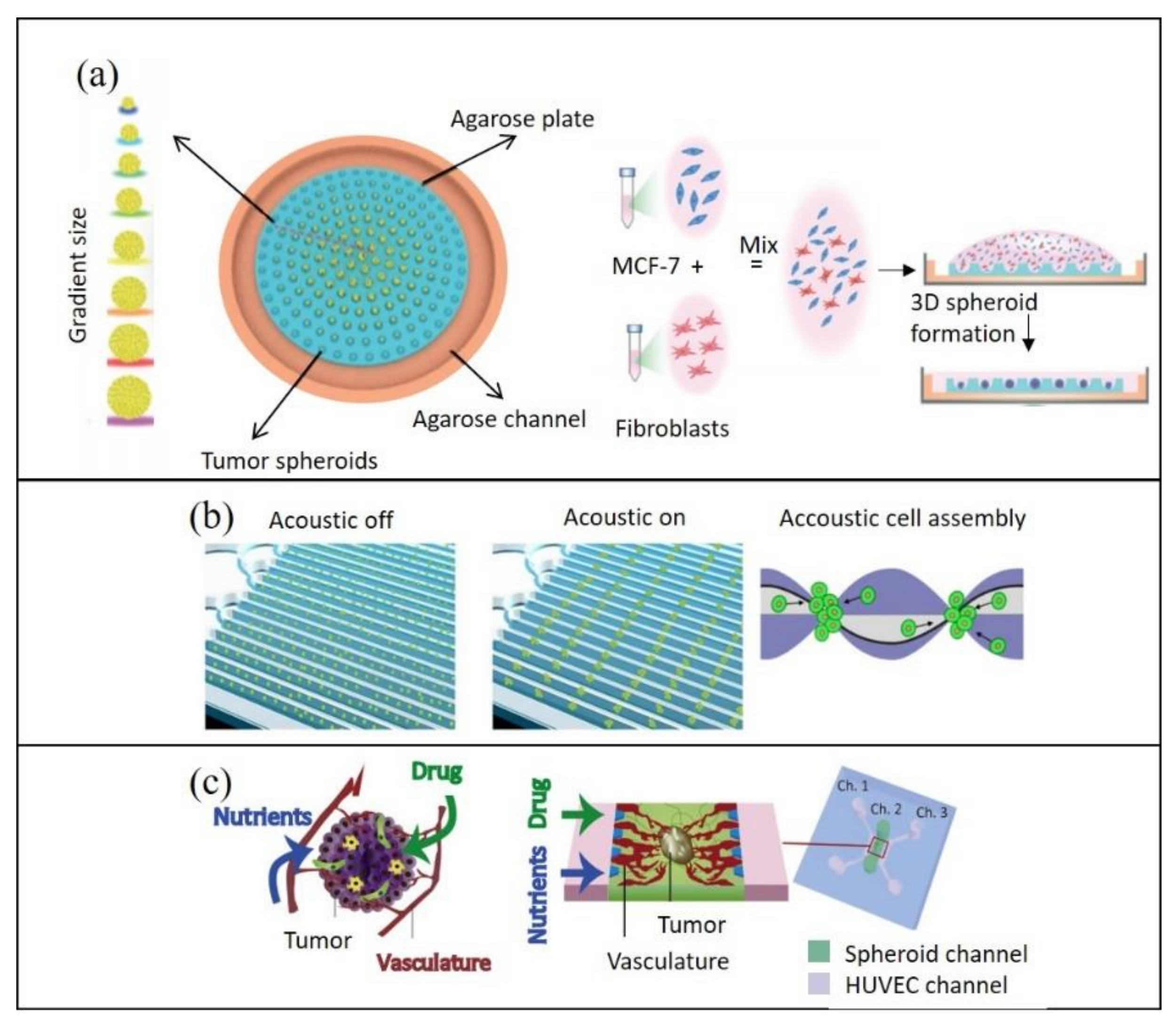
2.2.2. Reconstituting Cell–Cell Interactions On-Chip
2.2.3. Reconstituting the Biochemical Microenvironment On-Chip
3. Current Advances in Tumor-On-Chip Analyses
3.1. Physical Sensors On-Chip
3.2. On-Chip Imaging
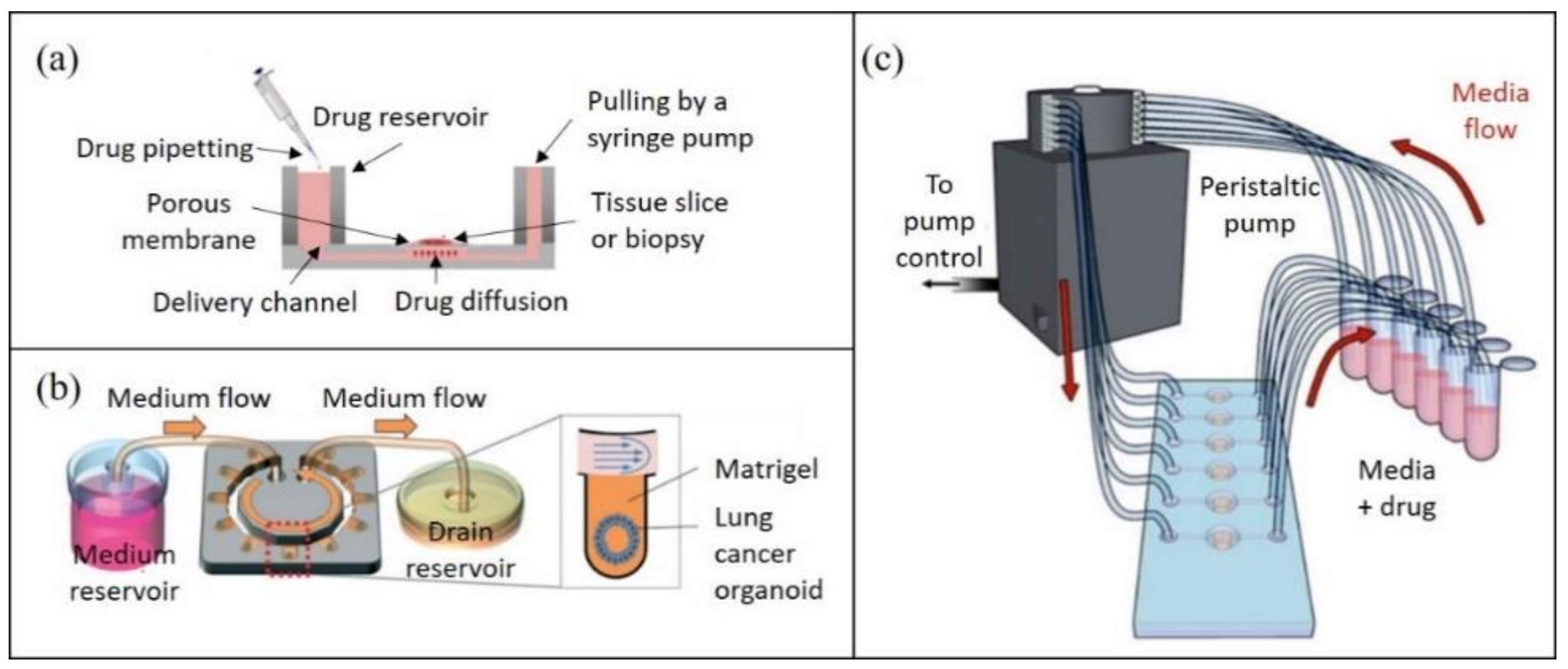
3.3. Tumor Recovery for Off-Chip Analysis
4. Applications and Clinical Aspects of Tumor-On-Chip Technology
4.1. Potential Applications of Tumor-On-Chip Technology in Personalized Medicine
4.2. Clinical Perspective of Tumor-On-Chip Technology
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wan, L.; Neumann, C.A.; LeDuc, P.R. Tumor-on-a-chip for integrating a 3D tumor microenvironment: Chemical and mechanical factors. Lab Chip 2020, 20, 873–888. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.; De Ninno, A.; Mencattini, A.; Mermet-Meillon, F.; Fornabaio, G.; Evans, S.S.; Cossutta, M.; Khira, Y.; Han, W.; Sirven, P.; et al. Dissecting Effects of Anti-cancer Drugs and Cancer-Associated Fibroblasts by On-Chip Reconstitution of Immunocompetent Tumor Microenvironments. Cell Rep. 2018, 25, 3884–3893.e3. [Google Scholar] [CrossRef] [Green Version]
- Guerin, M.V.; Finisguerra, V.; Eynde, B.J.V.D.; Bercovici, N.; Trautmann, A. Preclinical murine tumor models: A structural and functional perspective. eLife 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chanana, P.; Davila, J.I.; Hou, X.; Zanfagnin, V.; McGehee, C.D.; Goode, E.L.; Polley, E.C.; Haluska, P.; Weroha, S.J.; et al. Gene expression differences between matched pairs of ovarian cancer patient tumors and patient-derived xenografts. Sci. Rep. 2019, 9, 6314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, Y.; Ai, X.; Zhao, L.; Gao, Z.; Wang, Y.; Lu, Y.; Tu, P.; Jiang, Y. An integrated biomimetic array chip for high-throughput co-culture of liver and tumor microtissues for advanced anticancer bioactivity screening. Lab Chip 2020, 20, 2482–2494. [Google Scholar] [CrossRef] [PubMed]
- Santiago, G.T.-D.; Flores-Garza, B.G.; Negrete, J.T.; Lara-Mayorga, I.M.; González-Gamboa, I.; Zhang, Y.S.; Rojas-Martínez, A.; Ortiz-López, R.; Álvarez, M.M. The Tumor-on-Chip: Recent Advances in the Development of Microfluidic Systems to Recapitulate the Physiology of Solid Tumors. Materials 2019, 12, 2945. [Google Scholar] [CrossRef] [Green Version]
- Moshksayan, K.; Kashaninejad, N.; Warkiani, M.E.; Lock, J.; Moghadas, H.; Firoozabadi, B.; Saidi, M.S.; Nguyen, N.-T. Spheroids-on-a-chip: Recent advances and design considerations in microfluidic platforms for spheroid formation and culture. Sensors Actuators B Chem. 2018, 263, 151–176. [Google Scholar] [CrossRef] [Green Version]
- Bray, L.J.; Hutmacher, D.W.; Bock, N. Addressing Patient Specificity in the Engineering of Tumor Models. Front. Bioeng. Biotechnol. 2019, 7, 217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sontheimer-Phelps, A.; Hassell, B.A.; Ingber, D.E. Modelling cancer in microfluidic human organs-on-chips. Nat. Rev. Cancer 2019, 19, 65–81. [Google Scholar] [CrossRef]
- Patra, B.; Peng, C.-C.; Liao, W.-H.; Lee, C.-H.; Tung, Y.-C. Drug testing and flow cytometry analysis on a large number of uniform sized tumor spheroids using a microfluidic device. Sci. Rep. 2016, 6, 21061. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Wu, Y.; Ao, Z.; Cai, H.; Nunez, A.; Liu, Y.; Foley, J.; Nephew, K.; Lu, X.; Guo, F. High-throughput acoustofluidic fabrication of tumor spheroids. Lab Chip 2019, 19, 1755–1763. [Google Scholar] [CrossRef] [PubMed]
- Bradney, M.J.; Venis, S.M.; Yang, Y.; Konieczny, S.F.; Han, B.; Bradney, M.J.; Venis, S.M.; Yang, Y.; Konieczny, S.F.; Han, B. A Biomimetic Tumor Model of Heterogeneous Invasion in Pancreatic Ductal Adenocarcinoma. Small 2020, 16, 1905500. [Google Scholar] [CrossRef] [PubMed]
- Kurzrock, R.; Giles, F.J. Precision oncology for patients with advanced cancer: The challenges of malignant snowflakes. Cell Cycle 2015, 14, 2219–2221. [Google Scholar] [CrossRef]
- Eduati, F.; Utharala, R.; Madhavan, D.; Neumann, U.P.; Longerich, T.; Cramer, T.; Saez-Rodriguez, J.; Merten, C.A. A microfluidics platform for combinatorial drug screening on cancer biopsies. Nat. Commun. 2018, 9, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, R.; Kuvshinov, D.; Sdrolia, A.; Kuvshinova, E.; Hilton, K.; Crank, S.; Beavis, A.W.; Green, V.; Greenman, J. A patient tumour-on-a-chip system for personalised investigation of radiotherapy based treatment regimens. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, M.R.; Barata, D.; Teixeira, L.M.; Giselbrecht, S.; Reis, R.L.; Oliveira, J.M.; Truckenmüller, R.; Habibovic, P. Colorectal tumor-on-a-chip system: A 3D tool for precision onco-nanomedicine. Sci. Adv. 2019, 5, eaaw1317. [Google Scholar] [CrossRef] [Green Version]
- Beckwith, A.L.; Velásquez-García, L.F.; Borenstein, J.T. Microfluidic Model for Evaluation of Immune Checkpoint Inhibitors in Human Tumors. Adv. Healthc. Mater. 2019, 8, e1900289. [Google Scholar] [CrossRef]
- Misun, P.M.; Rothe, J.; Schmid, Y.R.; Hierlemann, A.; Frey, O. Multi-analyte biosensor interface for real-time monitoring of 3D microtissue spheroids in hanging-drop networks. Microsyst. Nanoeng. 2016, 2, 16022. [Google Scholar] [CrossRef] [Green Version]
- Van Meer, B.; de Vries, H.; Firth, K.; van Weerd, J.; Tertoolen, L.; Karperien, H.; Jonkheijm, P.; Denning, C.; Ijzerman, A.; Mummery, C. Small molecule absorption by PDMS in the context of drug response bioassays. Biochem. Biophys. Res. Commun. 2016, 482, 323–328. [Google Scholar] [CrossRef] [Green Version]
- Palacio-Castañeda, V.; Kooijman, L.; Venzac, B.; Verdurmen, W.; Le Gac, S. Metabolic Switching of Tumor Cells under Hypoxic Conditions in a Tumor-on-a-chip Model. Micromachines 2020, 11, 382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, A.D.; Horowitz, L.F.; Castro, K.; Kenerson, H.; Bhattacharjee, N.; Gandhe, G.; Raman, A.; Monnat, R.J.; Yeung, R.; Rostomily, R.C.; et al. A microfluidic platform for functional testing of cancer drugs on intact tumor slices. Lab Chip 2020, 20, 1658–1675. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Aleman, J.; Shin, S.R.; Kilic, T.; Kim, D.; Shaegh, S.A.M.; Massa, S.; Riahi, R.; Chae, S.; Hu, N.; et al. Multisensor-integrated organs-on-chips platform for automated and continual in situ monitoring of organoid behaviors. Proc. Natl. Acad. Sci. USA 2017, 114, E2293–E2302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, F.; Goh, Y.T.; Li, H.; Chakrapani, N.B.; Ni, M.; Xu, G.L.; Hsieh, T.-M.; Toh, Y.-C.; Cheung, C.; Iliescu, C.; et al. A vascular-liver chip for sensitive detection of nutraceutical metabolites from human pluripotent stem cell derivatives. Biomicrofluidics 2020, 14, 034108. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Deng, R.; Tong, W.H.; Huan, L.; Way, N.C.; IslamBadhan, A.; Iliescu, C.; Yu, H. A perfusion incubator liver chip for 3D cell culture with application on chronic hepatotoxicity testing. Sci. Rep. 2017, 7, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Fang, G.; Lu, H.; Law, A.; Gallego-Ortega, D.; Jin, D.; Lin, G. Gradient-sized control of tumor spheroids on a single chip. Lab Chip 2019, 19, 4093–4103. [Google Scholar] [CrossRef]
- Michael, I.J.; Kumar, S.; Oh, J.M.; Kim, D.; Kim, J.; Cho, Y.-K. Surface-Engineered Paper Hanging Drop Chip for 3D Spheroid Culture and Analysis. ACS Appl. Mater. Interfaces 2018, 10, 33839–33846. [Google Scholar] [CrossRef] [PubMed]
- Seyfoori, A.; Samiei, E.; Jalili, N.; Godau, B.; Rahmanian, M.; Farahmand, L.; Majidzadeh-A, K.; Akbari, M. Self-filling microwell arrays (SFMAs) for tumor spheroid formation. Lab Chip 2018, 18, 3516–3528. [Google Scholar] [CrossRef]
- Dong, J.; Liu, J.; Kang, G.; Xie, J.; Wang, Y. Pushing the resolution of photolithography down to 15nm by surface plasmon interference. Sci. Rep. 2014, 4, srep05618. [Google Scholar] [CrossRef] [Green Version]
- Kajtez, J.; Buchmann, S.; Vasudevan, S.; Birtele, M.; Rocchetti, S.; Pless, C.J.; Heiskanen, A.; Barker, R.A.; Martínez-Serrano, A.; Parmar, M.; et al. 3D-Printed Soft Lithography for Complex Compartmentalized Microfluidic Neural Devices. Adv. Sci. 2020, 7, 2001150. [Google Scholar] [CrossRef]
- Nashimoto, Y.; Okada, R.; Hanada, S.; Arima, Y.; Nishiyama, K.; Miura, T.; Yokokawa, R. Vascularized cancer on a chip: The effect of perfusion on growth and drug delivery of tumor spheroid. Biomaterials 2019, 229, 119547. [Google Scholar] [CrossRef]
- Ong, L.J.Y.; Islam, A.; Dasgupta, R.; Iyer, N.G.; Leo, H.L.; Toh, Y.-C. A 3D-printed microfluidic perfusion device for multicellular spheroid cultures. Biofabrication 2017, 9, 045005. [Google Scholar] [CrossRef] [PubMed]
- Beckwith, A.L.; Borenstein, J.T.; Velasquez-Garcia, L.F. Monolithic, 3D-Printed Microfluidic Platform for Recapitulation of Dynamic Tumor Microenvironments. J. Microelectromechanical Syst. 2018, 27, 1009–1022. [Google Scholar] [CrossRef]
- Van der Linden, P.J.E.M.; Popov, A.M.; Pontoni, D. Accurate and rapid 3D printing of microfluidic devices using wavelength selection on a DLP printer. Lab Chip 2020, 20, 4128–4140. [Google Scholar] [CrossRef]
- Bazaz, S.R.; Rouhi, O.; Raoufi, M.A.; Ejeian, F.; Asadnia, M.; Jin, D.; Warkiani, M.E. 3D Printing of Inertial Microfluidic Devices. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Urrios, A.; Parra-Cabrera, C.; Bhattacharjee, N.; Gonzalez-Suarez, A.; Rigat-Brugarolas, L.G.; Nallapatti, U.; Samitier, J.; DeForest, C.; Posas, F.; Garcia-Cordero, J.L.; et al. 3D-printing of transparent bio-microfluidic devices in PEG-DA. Lab Chip 2016, 16, 2287–2294. [Google Scholar] [CrossRef] [PubMed]
- Kotz, F.; Risch, P.; Helmer, D.; Rapp, B.E. Highly Fluorinated Methacrylates for Optical 3D Printing of Microfluidic Devices. Micromachines 2018, 9, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Zhang, J.M.; Liu, Y. A Modular Microfluidic Device via Multimaterial 3D Printing for Emulsion Generation. Sci. Rep. 2018, 8, 4791. [Google Scholar] [CrossRef]
- Chung, M.; Ahn, J.; Son, K.; Kim, S.; Jeon, N.L. Biomimetic Model of Tumor Microenvironment on Microfluidic Platform. Adv. Healthc. Mater. 2017, 6. [Google Scholar] [CrossRef] [Green Version]
- Jeong, S.-Y.; Lee, J.-H.; Shin, Y.; Chung, S.; Kuh, H.-J. Co-Culture of Tumor Spheroids and Fibroblasts in a Collagen Matrix-Incorporated Microfluidic Chip Mimics Reciprocal Activation in Solid Tumor Microenvironment. PLoS ONE 2016, 11, e0159013. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Hu, W.; Matulay, J.; Silva, M.V.; Owczarek, T.B.; Kim, K.; Chua, C.W.; Barlow, L.J.; Kandoth, C.; Williams, A.B.; et al. Tumor Evolution and Drug Response in Patient-Derived Organoid Models of Bladder Cancer. Cell 2018, 173, 515–528.e17. [Google Scholar] [CrossRef] [Green Version]
- Schmid, Y.R.F.; Bürgel, S.C.; Misun, P.M.; Hierlemann, A.; Frey, O. Electrical Impedance Spectroscopy for Microtissue Spheroid Analysis in Hanging-Drop Networks. ACS Sens. 2016, 1, 1028–1035. [Google Scholar] [CrossRef]
- Sart, S.; Tomasi, R.F.-X.; Amselem, G.; Baroud, C.N. Multiscale cytometry and regulation of 3D cell cultures on a chip. Nat. Commun. 2017, 8, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Sabhachandani, P.; Motwani, V.; Cohen, N.; Sarkar, S.; Torchilin, V.P.; Konry, T. Generation and functional assessment of 3D multicellular spheroids in droplet based microfluidics platform. Lab Chip 2015, 16, 497–505. [Google Scholar] [CrossRef] [Green Version]
- Sabhachandani, P.; Sarkar, S.; Mckenney, S.; Ravi, D.; Evens, A.; Konry, T. Microfluidic assembly of hydrogel-based immunogenic tumor spheroids for evaluation of anticancer therapies and biomarker release. J. Control. Release 2018, 295, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Saint-Sardos, A.; Sart, S.; Lippera, K.; Brient-Litzler, E.; Michelin, S.; Amselem, G.; Baroud, C.N. High-Throughput Measurements of Intra-Cellular and Secreted Cytokine from Single Spheroids Using Anchored Microfluidic Droplets. Small 2020, 16, e2002303. [Google Scholar] [CrossRef]
- Alhasan, L.; Qi, A.; Al-Abboodi, A.; Rezk, A.R.; Chan, P.P.Y.; Iliescu, C.; Yeo, L.Y. Rapid Enhancement of Cellular Spheroid Assembly by Acoustically Driven Microcentrifugation. ACS Biomater. Sci. Eng. 2016, 2, 1013–1022. [Google Scholar] [CrossRef]
- Bhise, N.S.; Manoharan, V.; Massa, S.; Tamayol, A.; Ghaderi, M.; Miscuglio, M.; Lang, Q.; Zhang, Y.S.; Shin, S.R.; Calzone, G.; et al. A liver-on-a-chip platform with bioprinted hepatic spheroids. Biofabrication 2016, 8, 014101. [Google Scholar] [CrossRef] [PubMed]
- Ayuso, J.M.; Virumbrales-Muñoz, M.; Lacueva, A.; Lanuza, P.M.; Checa-Chavarria, E.; Botella, P.; Fernandez, E.P.-A.; Doblare, M.; Allison, S.; Phillips, R.M.; et al. Development and characterization of a microfluidic model of the tumour microenvironment. Sci. Rep. 2016, 6, 36086. [Google Scholar] [CrossRef]
- Ko, J.; Ahn, J.; Kim, S.; Lee, Y.; Lee, J.; Park, D.; Jeon, N.L. Tumor spheroid-on-a-chip: A standardized microfluidic culture platform for investigating tumor angiogenesis. Lab Chip 2019, 19, 2822–2833. [Google Scholar] [CrossRef] [PubMed]
- Paek, J.; Park, S.E.; Lu, Q.; Park, K.-T.; Cho, M.; Oh, J.M.; Kwon, K.W.; Yi, Y.-S.; Song, J.W.; Edelstein, H.; et al. Microphysiological Engineering of Self-Assembled and Perfusable Microvascular Beds for the Production of Vascularized Three-Dimensional Human Microtissues. ACS Nano 2019, 13, 7627–7643. [Google Scholar] [CrossRef]
- Wang, H.-F.; Ran, R.; Liu, Y.; Hui, Y.; Zeng, B.; Chen, D.; Weitz, D.A.; Zhao, C.-X. Tumor-Vasculature-on-a-Chip for Investigating Nanoparticle Extravasation and Tumor Accumulation. ACS Nano 2018, 12, 11600–11609. [Google Scholar] [CrossRef]
- Cao, X.; Ashfaq, R.; Cheng, F.; Maharjan, S.; Li, J.; Ying, G.; Hassan, S.; Xiao, H.; Yue, K.; Zhang, Y.S. A Tumor-on-a-Chip System with Bioprinted Blood and Lymphatic Vessel Pair. Adv. Funct. Mater. 2019, 29. [Google Scholar] [CrossRef] [PubMed]
- Romero-López, M.; Trinh, A.; Sobrino, A.; Hatch, M.M.; Keating, M.T.; Fimbres, C.; Lewis, D.E.; Gershon, P.D.; Botvinick, E.L.; Digman, M.; et al. Recapitulating the human tumor microenvironment: Colon tumor-derived extracellular matrix promotes angiogenesis and tumor cell growth. Biomaterials 2016, 116, 118–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grist, S.M.; Nasseri, S.S.; Laplatine, L.; Schmok, J.C.; Yao, D.; Hua, J.; Chrostowski, L.; Cheung, K.C. Long-term monitoring in a microfluidic system to study tumour spheroid response to chronic and cycling hypoxia. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Rijal, G.; Li, W. A versatile 3D tissue matrix scaffold system for tumor modeling and drug screening. Sci. Adv. 2017, 3, e1700764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chhetri, A.; Chittiboyina, S.; Atrian, F.; Bai, Y.; Delisi, D.A.; Rahimi, R.; Garner, J.; Efremov, Y.; Park, K.; Talhouk, R.; et al. Cell Culture and Coculture for Oncological Research in Appropriate Microenvironments. Curr. Protoc. Chem. Biol. 2019, 11, e65. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.; Klosterhoff, B.; Han, B. Characterization of Cell-Type-Specific Drug Transport and Resistance of Breast Cancers Using Tumor-Microenvironment-on-Chip. Mol. Pharm. 2016, 13, 2214–2223. [Google Scholar] [CrossRef] [Green Version]
- Stowers, R.S.; Shcherbina, A.; Israeli, J.; Gruber, J.J.; Chang, J.; Nam, S.; Rabiee, A.; Teruel, M.N.; Snyder, M.P.; Kundaje, A.; et al. Matrix stiffness induces a tumorigenic phenotype in mammary epithelium through changes in chromatin accessibility. Nat. Biomed. Eng. 2019, 3, 1009–1019. [Google Scholar] [CrossRef]
- Costa, E.C.; Moreira, A.F.; Diogo, D.M.D.M.; Gaspar, V.; Carvalho, M.P.; Correia, I.J. 3D tumor spheroids: An overview on the tools and techniques used for their analysis. Biotechnol. Adv. 2016, 34, 1427–1441. [Google Scholar] [CrossRef] [PubMed]
- Alexander, F.; Eggert, S.; Wiest, J. A novel lab-on-a-chip platform for spheroid metabolism monitoring. Cytotechnology 2017, 70, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wei, X.; Pan, Y.; Zou, Y.; Hu, N.; Wang, P. Bionic 3D spheroids biosensor chips for high-throughput and dynamic drug screening. Biomed. Microdevices 2018, 20, 82. [Google Scholar] [CrossRef]
- Lazzari, G.; Vinciguerra, D.; Balasso, A.; Nicolas, V.; Goudin, N.; Garfa-Traore, M.; Fehér, A.; Dinnyes, A.; Nicolas, J.; Couvreur, P.; et al. Light sheet fluorescence microscopy versus confocal microscopy: In quest of a suitable tool to assess drug and nanomedicine penetration into multicellular tumor spheroids. Eur. J. Pharm. Biopharm. 2019, 142, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Lazzari, G.; Couvreur, P.; Mura, S. Multicellular tumor spheroids: A relevant 3D model for the in vitro preclinical investigation of polymer nanomedicines. Polym. Chem. 2017, 8, 4947–4969. [Google Scholar] [CrossRef] [Green Version]
- St-Georges-Robillard, A.; Masse, M.; Cahuzac, M.; Strupler, M.; Patra, B.; Orimoto, A.M.; Kendall-Dupont, J.; Peant, B.; Mes-Masson, A.-M.; Leblond, F.; et al. Fluorescence hyperspectral imaging for live monitoring of multiple spheroids in microfluidic chips. Analyst 2018, 143, 3829–3840. [Google Scholar] [CrossRef]
- St-Georges-Robillard, A.; Cahuzac, M.; Péant, B.; Fleury, H.; Lateef, M.A.; Ricard, A.; Sauriol, A.; Leblond, F.; Mes-Masson, A.-M.; Gervais, T. Long-term fluorescence hyperspectral imaging of on-chip treated co-culture tumour spheroids to follow clonal evolution. Integr. Biol. 2019, 11, 130–141. [Google Scholar] [CrossRef]
- Rodríguez-Pena, A.; Uranga-Solchaga, J.; Ortiz-De-Solórzano, C.; Cortés-Domínguez, I. Spheroscope: A custom-made miniaturized microscope for tracking tumour spheroids in microfluidic devices. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Paie, P.; Vázquez, R.M.; Osellame, R.; Bragheri, F.; Bassi, A. Microfluidic Based Optical Microscopes on Chip. Cytom. Part A 2018, 93, 987–996. [Google Scholar] [CrossRef]
- Paie, P.; Bragheri, F.; Bassi, A.; Osellame, R. Selective plane illumination microscopy on a chip. Lab Chip 2016, 16, 1556–1560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grist, S.; Nasseri, S.S.; Poon, T.; Roskelley, C.; Cheung, K.C. On-chip clearing of arrays of 3-D cell cultures and micro-tissues. Biomicrofluidics 2016, 10, 044107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santisteban, T.S.; Rabajania, O.; Kalinina, I.; Robinson, S.; Meier, M. Rapid spheroid clearing on a microfluidic chip. Lab Chip 2017, 18, 153–161. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Silva, P.N.; Syed, A.; Sindhwani, S.; Rocheleau, J.; Chan, W.C.W. Clarifying intact 3D tissues on a microfluidic chip for high-throughput structural analysis. Proc. Natl. Acad. Sci. USA 2016, 113, 14915–14920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, N.; Doty, D.; Zielstorff, M.; Kariv, I.; Moy, L.Y.; A Gimbel, A.; Chevillet, J.R.; Lowry, N.; Santos, J.; Mott, V.; et al. A multiplexed microfluidic system for evaluation of dynamics of immune-tumor interactions. Lab Chip 2018, 18, 1844–1858. [Google Scholar] [CrossRef] [PubMed]
- Fetah, K.L.; DiPardo, B.J.; Kongadzem, E.; Tomlinson, J.S.; Elzagheid, A.; Elmusrati, M.; Khademhosseini, A.; Ashammakhi, N. Cancer Modeling-on-a-Chip with Future Artificial Intelligence Integration. Small 2019, 15, e1901985. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, L.; Wang, Y.; Zhang, T.; Chen, Y.-C.; Yoon, E. Label-Free Estimation of Therapeutic Efficacy on 3D Cancer Spheres Using Convolutional Neural Network Image Analysis. Anal. Chem. 2019, 91, 14093–14100. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Xiu, J.; Liu, Y.; Zhang, T.; Pan, W.; Zheng, X.; Zhang, X. A 3D-printed Hanging Drop Dripper for Tumor Spheroids Analysis Without Recovery. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khot, M.; Levenstein, M.; Kapur, N.; Jayne, D. A Review on the Recent Advancement in “Tumour Spheroids-on-a-Chip”. J. Cancer Res. Pract. 2019, 6, 55–63. [Google Scholar] [CrossRef]
- Frey, O.; Misun, P.; Fluri, D.A.; Hengstler, J.; Hierlemann, A. Reconfigurable microfluidic hanging drop network for multi-tissue interaction and analysis. Nat. Commun. 2014, 5, 4250. [Google Scholar] [CrossRef] [Green Version]
- Lim, W.; Hoang, H.-H.; You, D.; Han, J.; Lee, J.E.; Kim, S.; Park, S. Formation of size-controllable tumour spheroids using a microfluidic pillar array (μFPA) device. Analyst 2018, 143, 5841–5848. [Google Scholar] [CrossRef]
- Zhao, L.; Shi, M.; Liu, Y.; Zheng, X.; Xiu, J.; Liu, Y.; Tian, L.; Wang, H.; Zhang, M.; Zhang, X. Systematic Analysis of Different Cell Spheroids with a Microfluidic Device Using Scanning Electrochemical Microscopy and Gene Expression Profiling. Anal. Chem. 2019, 91, 4307–4311. [Google Scholar] [CrossRef] [Green Version]
- Ayuso, J.M.; Virumbrales-Munoz, M.; McMinn, P.H.; Rehman, S.; Gomez, I.; Karim, M.R.; Trusttchel, R.; Wisinski, K.B.; Beebe, D.J.; Skala, M.C. Tumor-on-a-chip: A microfluidic model to study cell response to environmental gradients. Lab Chip 2019, 19, 3461–3471. [Google Scholar] [CrossRef] [PubMed]
- Pitingolo, G.; Nizard, P.; Riaud, A.; Taly, V.; Pitingolo, G. Beyond the on/off chip trade-off: A reversibly sealed microfluidic platform for 3D tumor microtissue analysis. Sens. Actuators B Chem. 2018, 274, 393–401. [Google Scholar] [CrossRef]
- Yuan, T.; Gao, D.; Li, S.; Jiang, Y. Co-culture of tumor spheroids and monocytes in a collagen matrix-embedded microfluidic device to study the migration of breast cancer cells. Chin. Chem. Lett. 2018, 30, 331–336. [Google Scholar] [CrossRef]
- Ma, L.-D.; Wang, Y.-T.; Wang, J.-R.; Wu, J.-L.; Meng, X.-S.; Hu, P.; Mu, X.; Liang, Q.-L.; Luo, G.-A. Design and fabrication of a liver-on-a-chip platform for convenient, highly efficient, and safe in situ perfusion culture of 3D hepatic spheroids. Lab Chip 2018, 18, 2547–2562. [Google Scholar] [CrossRef]
- Patra, B.; Lafontaine, J.; Bavoux, M.; Zerouali, K.; Glory, A.; Ahanj, M.; Carrier, J.-F.; Gervais, T.; Wong, P. On-chip combined radiotherapy and chemotherapy testing on soft-tissue sarcoma spheroids to study cell death using flow cytometry and clonogenic assay. Sci. Rep. 2019, 9, 2214. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Su, J.; Fu, X.; Zheng, L.; Yin, Z. Microfluidic device for primary tumor spheroid isolation. Exp. Hematol. Oncol. 2017, 6, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morash, M.; Mitchell, H.; Beltran, H.; Elemento, O.; Pathak, J. The Role of Next-Generation Sequencing in Precision Medicine: A Review of Outcomes in Oncology. J. Pers. Med. 2018, 8, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bower, R.; Green, V.L.; Kuvshinova, E.; Kuvshinov, D.; Karsai, L.; Crank, S.T.; Stafford, N.D.; Greenman, J. Maintenance of head and neck tumor on-chip: Gateway to personalized treatment? Futur. Sci. OA 2017, 3, FSO174. [Google Scholar] [CrossRef] [Green Version]
- Akay, M.; Hite, J.; Avci, N.G.; Fan, Y.; Akay, Y.; Lu, G.; Zhu, J.-J. Drug Screening of Human GBM Spheroids in Brain Cancer Chip. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, D.J.; Shin, T.H.; Kim, M.; Sung, C.O.; Jang, S.J.; Jeong, G.S. A one-stop microfluidic-based lung cancer organoid culture platform for testing drug sensitivity. Lab Chip 2019, 19, 2854–2865. [Google Scholar] [CrossRef] [PubMed]
- Mazzocchi, A.; Rajan, S.A.P.; Votanopoulos, K.I.; Hall, A.R.; Skardal, A. in vitro patient-derived 3D mesothelioma tumor organoids facilitate patient-centric therapeutic screening. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Eduati, F.; Jaaks, P.; Wappler, J.; Cramer, T.; A Merten, C.; Garnett, M.J.; Saez-Rodriguez, J. Patient-specific logic models of signaling pathways from screenings on cancer biopsies to prioritize personalized combination therapies. Mol. Syst. Biol. 2020, 16, e8664. [Google Scholar] [CrossRef] [PubMed]
- Aref, A.R.; Campisi, M.; Ivanova, E.; Portell, A.; Larios, D.; Piel, B.P.; Mathur, N.; Zhou, C.; Coakley, R.V.; Bartels, A.; et al. 3D microfluidic ex vivo culture of organotypic tumor spheroids to model immune checkpoint blockade. Lab Chip 2018, 18, 3129–3143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruppen, J.; Wildhaber, F.D.; Strub, C.; Hall, S.R.R.; Schmid, R.A.; Geiser, T.; Guenat, O.T. Towards personalized medicine: Chemosensitivity assays of patient lung cancer cell spheroids in a perfused microfluidic platform. Lab Chip 2015, 15, 3076–3085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Astolfi, M.; Peant, B.; Lateef, M.A.; Rousset, N.; Kendall-Dupont, J.; Carmona, E.; Monet, F.; Saad, F.; Provencher, D.; Mes-Masson, A.-M.; et al. Micro-dissected tumor tissues on chip: An ex vivo method for drug testing and personalized therapy. Lab Chip 2016, 16, 312–325. [Google Scholar] [CrossRef]
- Wong, A.H.-H.; Li, H.; Jia, Y.; Mak, P.-I.; Martins, R.; Liu, Y.; Vong, C.M.; Wong, H.C.; Wong, P.K.; Wang, H.; et al. Drug screening of cancer cell lines and human primary tumors using droplet microfluidics. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef]
- Mulholland, T.E.; McAllister, M.; Patek, S.; Flint, D.; Underwood, M.; Sim, A.; Edwards, J.; Zagnoni, M. Drug screening of biopsy-derived spheroids using a self-generated microfluidic concentration gradient. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Shirure, V.S.; Bi, Y.; Curtis, M.B.; Lezia, A.; Goedegebuure, M.M.; Goedegebuure, S.P.; Aft, R.; Fields, R.C.; George, S.C. Tumor-on-a-chip platform to investigate progression and drug sensitivity in cell lines and patient-derived organoids. Lab Chip 2018, 18, 3687–3702. [Google Scholar] [CrossRef]
- Lai, B.F.L.; Lu, R.X.Z.; Hu, Y.; Huyer, L.D.; Dou, W.; Wang, E.Y.; Radulovich, N.; Tsao, M.S.; Sun, Y.; Radisic, M. Recapitulating Pancreatic Tumor Microenvironment through Synergistic Use of Patient Organoids and Organ-on-a-Chip Vasculature. Adv. Funct. Mater. 2020, 30, 2000545. [Google Scholar] [CrossRef]
- Olubajo, F.; Achawal, S.; Greenman, J. Development of a Microfluidic Culture Paradigm for Ex Vivo Maintenance of Human Glioblastoma Tissue: A New Glioblastoma Model? Transl. Oncol. 2019, 13, 1–10. [Google Scholar] [CrossRef]
- Berg, A.V.D.; Mummery, C.L.; Passier, R.; Van Der Meer, A.D. Personalised organs-on-chips: Functional testing for precision medicine. Lab Chip 2018, 19, 198–205. [Google Scholar] [CrossRef] [Green Version]
- Rodon, J.; Soria, J.-C.; Berger, R.; Miller, W.H.; Rubin, E.; Kugel, A.; Tsimberidou, A.; Saintigny, P.; Ackerstein, A.; Brana, I.; et al. Genomic and transcriptomic profiling expands precision cancer medicine: The WINTHER trial. Nat. Med. 2019, 25, 751–758. [Google Scholar] [CrossRef] [PubMed]


| Type of Tumor Model | In Vivo/In Vitro/Ex Vivo | Dimension | Recapitulate ECM and Cell–Cell Interactions | Recapitulate TME Dynamics | Translational Value | Advantages | Disadvantages |
|---|---|---|---|---|---|---|---|
| Cancer cell line monolayers | in vitro | 2D | No | No | Low | Inexpensive Not labor-intensive and requires limited expertise High-throughput possible | Limited recapitulation of in vivo environment and subsequent translational value |
| Primary cancer cell monolayers | in vitro | 2D | No | No | Low | Inexpensive Not labor-intensive and requires limited expertise High-throughput possible Less artificial than cancer cell line monolayers | Limited recapitulation of in vivo environment and subsequent translational value |
| Cell line monocellular tumor spheroids | in vitro | 3D | No | No | Low | Inexpensive High-throughput possible | Difficult to maintain uniformity of spheroid size |
| Cell line multicellular tumor spheroids | in vitro | 3D | Yes | No | Low | Inexpensive High-throughput possible | Difficult to maintain uniformity of spheroid size |
| Microfluidic cell line multicellular tumor spheroids | in vitro | 3D | Yes | Yes | Intermediate | Can model chemical gradients High-throughput possible | More technically challenging than 2D models and cell line tumor spheroids |
| Microfluidic patient-derived tumor spheroids | ex vivo | 3D | Yes | Yes | High | May contain natural ECM Cheaper than animal models High-throughput possible | More technically challenging than cell line models May genetically vary from parent tumors Difficult to access patient tumor tissue Difficult to maintain uniformity of spheroid size |
| Microfluidic patient-derived tumor tissue | ex vivo | 3D | Yes | Yes | High | May contain natural ECM Cheaper than animal models High-throughput possible | More technically challenging than cell line models May genetically vary from parent tumors Difficult to access patient tumor tissue Difficult to maintain uniformity of spheroid size |
| Patient-derived tumor spheroids | ex vivo | 3D | Yes | No | Intermediate | Cheaper than animal models May contain natural ECM High-throughput possible | Difficult to maintain uniformity of spheroid size May genetically vary from parent tumors |
| Tumor tissue slice/explant culture | ex vivo | 3D | Yes | Yes | High | Cheaper than animal models Contain natural ECM High-throughput possible | Not easily amenable for high-throughput studies Limited amount of sample Difficult to access patient tumor tissue |
| Animal tumors | in vivo | 3D | Yes | Yes | Intermediate | Accepted by agencies as a pre-clinical platform Testing within a living organism accounts for interactions between organs | Species specific differences to treatments [1] Specific facilities and highly trained staff required Ethical approval needed Low throughput Expensive and time-consuming |
| PDX Tumors | in vivo | 3D | Yes | Yes | High | Accepted by agencies as a pre-clinical platform Testing within a living organism accounts for interactions between organs | Limited amount of samples Animal-specific immune responses limit fidelity to patient responses [3] Transcriptome may vary from that of the original tumor [4] Lack of standardization Requires specific facilities and highly trained staff Ethical approval needed Not easily amenable for high-throughput studies Expensive and time-consuming |
| Year | Sample Type | Cell Culture | Microfluidic Device | Application | Analysis | Main Outcomes | Ref. |
|---|---|---|---|---|---|---|---|
| Chemotherapy | |||||||
| 2015 | Primary human lung tumors and squamous carcinoma tissues | Dissociated cells from tissues; monocultured or co-cultured | Two PDMS parts consisting of microwells, fabricated by stereolithography epoxy molds, plasma-bonded | Drug treatment with cisplatin for 48 h | On-chip: Live–dead staining and fluorescence imaging to assess cell viability Off-chip: Flow cytometry for cell sorting, followed by caspase-3/7 activity of the supernatant to assess apoptosis | System efficiency was high with little cell loss in the microfluidic network. Primary pericytes (PCs) had a protective effect on the primary epithelial lung tumor cells (PLETCs) from the damaging effects of the chemotherapeutical drug. | [93] |
| 2016 | Primary human ovarian cancer tissues and prostate cancer tissues | Microdissected cylindrical tissues | Two PDMS replicas with 5 open channels containing 5 microwells, fabricated by micromachined PMMA master molds, plasma-bonded | Drug treatment with carboplatin for 48 h | On-chip: Fluorescence staining and imaging of live tissues to assess cell viability Off-chip: Endpoint flow cytometry analysis to assess the survival of individual cells within the microtissues | The microfluidic platform was operated using simple instruments typically found in cell biology laboratories. Drug treatment response measured in the microfluidic chip was concordant with the clinical response of the patient. | [94] |
| 2017 | Primary human nasopharyngeal tumor tissues | Dissociated cells from tissues; 100–200 cells per droplet | PDMS microchannels containing 48 droplet formation wells, fabricated by a SU-8-patterned silicon wafer molds, plasma-bonded to glass coverslips | Drug treatment with bortezomib and cisplatin for 16–24 h | On-chip: Ethidium homodimer 1 labeling during cell seeding, brightfield and red fluorescence imaging to assess cell number and viability | The microfluidic system was capable of drug-screening as few as 16,000 cells obtained from primary cancer within 24 h after tumor resection from patients. | [95] |
| 2018 | Primary human mesothelioma tumor tissues | Dissociated cells from tissues mixed with hyaluronic acid and hydrogel precursor; 20 million cells/mL were seeded | Six chambers produced in an aluminum foil–adhesive film using a cutting plotter, attached to a glass slide (bottom) and polystyrene slide (top) | Drug treatment with carboplatin–pemetrexed or cisplatin–pemetrexed for 7 d | On-chip: Live–dead staining and fluorescence imaging to assess cell viability, proliferation assays, visualization of biomarkers using IHC * | The microfluidic platform was capable of maintaining the cell viability over 14 d and key mesothelioma biomarkers in patient-derived organoids (accurate tumor phenotype). Drug response of organoids was concordant with clinical outcomes. Patient-to-patient tumor heterogeneity was demonstrated. | [90] |
| 2018 | Primary human prostate cancer biopsies | Biopsies were minced, passaged and injected into the device; 24,000 cells were seeded | Two PDMS parts containing 240 square microwells fabricated by SU-8-patterned silicon wafer molds, plasma-bonded | Drug treatment with cisplatin, docetaxel and enzalutamide for 12 h | On-chip: Live–dead staining and fluorescence imaging to assess cell viability, calcein assay to assess concentration gradient formation Off-chip: RT-qPCR to assess prostate cancer cell gene expression | Proof of concept study. The microfluidic platform was capable of forming concentration gradient and maintain its stability for 12–16 h. | [96] |
| 2018 | Primary human triple-negative breast cancer tumor biopsy | 1×105 cells/mL were seeded; ~300 spheroids on the device | Two PDMS plasma-bonded layers with pillar array fabricated by two printed transparent film masks | Drug treatment with doxorubicin or docetaxel for 72 h | On chip: Live–dead staining assay, fluorescence imaging to assess spheroid number and size Off-chip: qRT-PCR to assess cancer stem cell marker expression | Proof of concept study. The microfluidic platform was capable of controlling the spheroid size. Spheroids showed a similar differential drug response observed in the patient. | [78] |
| 2018 | Primary human triple-negative breast cancer tumor tissue biopsies | Patient-derived tumor organoids (PDTO) from sectioned tissues; 1×107 cells/mL cell suspension with Matrigel | PDMS device with 8 tumor tissue chambers fabricated by SU-8-patterned silicon wafer molds, plasma-bonded to a flat PDMS sheet | Drug treatment with paclitaxel for 48 h | On-chip: Immunostaining and fluorescence imaging to assess tumor growth, fluorescently tagged dextran perfusion via device to assess vessel permeability assessment | Proof of concept study. The microfluidic platform was capable of maintaining the viability of the primary tissue for up to 21 d. A tumor-on-a-chip device that mimics biological mass transport was designed, where 3D microvascular network was created prior to loading PDTO. | [97] |
| 2018 | Primary human glioblastoma tumor tissues | Dissociated cells from tissues; 0.5×106 cells/mL were seeded | Poly-(ethylene glycol) diacrylate (PEGDA) hydrogel layer consisting of 7 channels with 9–11 microwells per channel, fabricated by printed plastic photomasks, crosslinked between two cover glass slides | Drug treatment with combination of bevacizumab and temozolomidefor 7 d | On-chip: Immunostaining and fluorescence imaging to assess spheroid formation Off-chip: Trypan blue staining to assess cell viability | Proof of concept study. Patient-to-patient tumor heterogeneity was demonstrated. | [88] |
| 2019 | Primary human small-cell lung cancer (SCLC) biopsies | Mechanically dissociated lung cancer organoids (LCOs) mixed with Matrigel | PDMS device consisting of 29 microwells fabricated by SU-8-patterned silicon wafer molds, plasma-bonded to a cover glass | Drug treatment with cisplatin and etoposide for 72 h | On-chip: Fluorescence imaging to assess organoid size, end point live–dead staining and fluorescence imaging to assess cell viability, apoptosis analysis Off-chip: Genomic analysis to evaluate the somatic mutations, qRT-PCR to characterize the specific marker expressions for cancer stem cells | First demonstration of 3D lung cancer organoid production from small-cell lung cancer tumors. The microfluidic device was capable of culturing these organoids, as well as performing drug sensitivity tests. The centers of the organoids could survive chemotherapy-induced cell death, which may help to elucidate chemotherapy resistance mechanisms. | [89] |
| 2020 | Primary metastatic human rectal tumor tissues | Tissue slices | PMMA plate consisting of 40 wells with an integrated channel network layer, fabricated by CO2 laser micromachining, sealed with chloroform vapor | Drug treatment with combinations of FOLFOX, FOLFIRI ** and staurosporine for 48 h | Off-chip: Proliferation assay and live–dead staining to assess cell viability, fluorescence imaging to assess cell death | The microfluidic device was capable of delivering multiplexed anti-cancer drugs on tumor slices and was compatible with on-chip live–dead staining. | [21] |
| 2020 | Primary human pancreatic ductal adenocarcinoma tumor organoid | Organoids were suspended in single cells and cultured in Matrigel with human dermal fibroblasts, before introducing this suspension (6800–13,600 cells) in HUVEC (75,000–12,5000 cells) scaffold | Two PDMS molds fabricated by SU-8-patterned silicon wafer molds, between PDMS molds and a PDMS sheet, pressed and perfused with a highly elastic polyester material, plasma-bonded to a silicon wafer | Drug treatment with gemcitabine for 96 h | On-chip: Luminescence assay to assess cell viability, fluorescence imaging to assess organoid size and morphology | Tumor-derived cells cultured in the microfluidic system only underwent ECM remodeling when co-cultured with fibroblasts. These changes, as well as vascularization, decreased the efficacy of gemcitabine treatment. | [98] |
| Radiotherapy | |||||||
| 2019 | Primary human head and neck squamous cell carcinoma tumor tissue | Tumor slices | Two PEEK plate parts consisting of 1 well for 1 sample, reversibly sealed by screws | Irradiation with a photon beam; 10 Gy in 5 × 2 Gy fractions in a 72 h schema; drug treatment with cisplatin for final 48 h alongside the 5 × 2 Gy irradiation fractions | Off-chip: Trypan blue/PI staining to assess tissue viability, flow cytometry to assess cell death, IHC * staining to assess radiation response markers | The microfluidic system was capable of maintaining the viability of precision-cut tumor slices for 68 h. This system enabled monitoring of the effects of irradiation and chemoradiation on tumor slices. | [15] |
| Immunotherapy | |||||||
| 2018 | Primary human non-small-cell lung cancer biopsies | Tumor fragments | Cyclic olefin copolymer (COC) device consisting of chevron-like 12-lane channel pattern, fabricated by micromachined aluminum master mold, bonded to a COC film using a heated lamination process | Treatment with an anti-PD-1 antibody pretreated tumor-infiltrating lymphocytes (TILs), monitored daily for 7–10 d | On-chip: Fluorescence staining and imaging to assess cell growth and viability Off-chip: Magnetic cell sorting using flow cytometry to separate tumor cells | The microfluidic system was capable of studying interactions between autologous lymphocytes and biopsy sample in response to an anti-PD-1 antibody. The sample showed responder behavior, mimicking the in vivo tumor response. | [72] |
| 2018 | Primary human metastatic melanoma tumor tissues | Minced tumor tissues mixed with collagen | Cyclic olefin polymer device by AIM BIOTECH consisting of 3 chambers | Treatment with anti-PD-1 (pembrolizumab, 250 μg/mL), anti-CTLA-4 (ipilimumab, 50 μg/mL) or combination for 5–9 d | Oh-chip: Live–dead staining and fluorescence imaging to assess cell viability Off-chip: Flow cytometry for immune profiling, RNA-seq, cytokine profiling in media | The microfluidic system was capable of performing a range of on-/off-chip analyses. It demonstrated the immune checkpoint sensitivity of patient-derived tumor spheroids, which is not seen in 2D cultures. | [92] |
| 2019 | Primary human non-small-cell lung carcinoma tumor biopsies | Tumor fragments | Pro3dure GR-10 resin device fabricated by digital light projection stereolithography (DLP-SLA) 3D printing | Treatment with anti-PD-1 antibodies, monitored after 24, 48 and 72 h | On-chip: Live–dead staining and fluorescence imaging to assess cell viability, resident lymphocyte response to selected antibodies Off-chip: Fluorescence imaging to determine healthy tumor fragments | The microfluidic system was capable of culturing biopsied tumor tissue and resident lymphocytes in a dynamic perfusion system. It enabled the monitoring of tumor response to immunotherapeutic agents. Clinical correlation of the laboratory results is needed to determine its utility in guiding personalized medicine approaches. | [17] |
| Combined therapy | |||||||
| 2018 | Primary human pancreatic tumor biopsies | Dissociated cells from tumors; ~100 cells encapsulated in each plug | Droplet-based PDMS device consisting of 1140 plugs, fabricated by AZ-40XT patterned silicon wafer molds, plasma-bonded to a thin elastic PDMS membrane with integrated Braille valves | Drug treatment with chemotherapeutic drugs (oxaliplatin and gemcitabine), specific kinase targets (Cyt387, PHT-427, MK-2206, GDC0941, gefitinib, ACHP, AZD6244) and one cytokine (tumor necrosis factor-α) for up to 14 d | On-chip: Fluorescence staining and imaging to assess cell viability, caspase-3 activity to access cell apoptosis | The microfluidic system was capable of screening 62 different drug conditions on biopsy-derived cells. No drug combination had strong efficacy across all patient samples, encouraging the consideration of personalized medicine approaches to pancreatic cancer | [14] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bērziņa, S.; Harrison, A.; Taly, V.; Xiao, W. Technological Advances in Tumor-On-Chip Technology: From Bench to Bedside. Cancers 2021, 13, 4192. https://doi.org/10.3390/cancers13164192
Bērziņa S, Harrison A, Taly V, Xiao W. Technological Advances in Tumor-On-Chip Technology: From Bench to Bedside. Cancers. 2021; 13(16):4192. https://doi.org/10.3390/cancers13164192
Chicago/Turabian StyleBērziņa, Santa, Alexandra Harrison, Valérie Taly, and Wenjin Xiao. 2021. "Technological Advances in Tumor-On-Chip Technology: From Bench to Bedside" Cancers 13, no. 16: 4192. https://doi.org/10.3390/cancers13164192
APA StyleBērziņa, S., Harrison, A., Taly, V., & Xiao, W. (2021). Technological Advances in Tumor-On-Chip Technology: From Bench to Bedside. Cancers, 13(16), 4192. https://doi.org/10.3390/cancers13164192





