The Role of Total Parenteral Nutrition in Patients with Peritoneal Carcinomatosis: A Systematic Review and Meta-Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
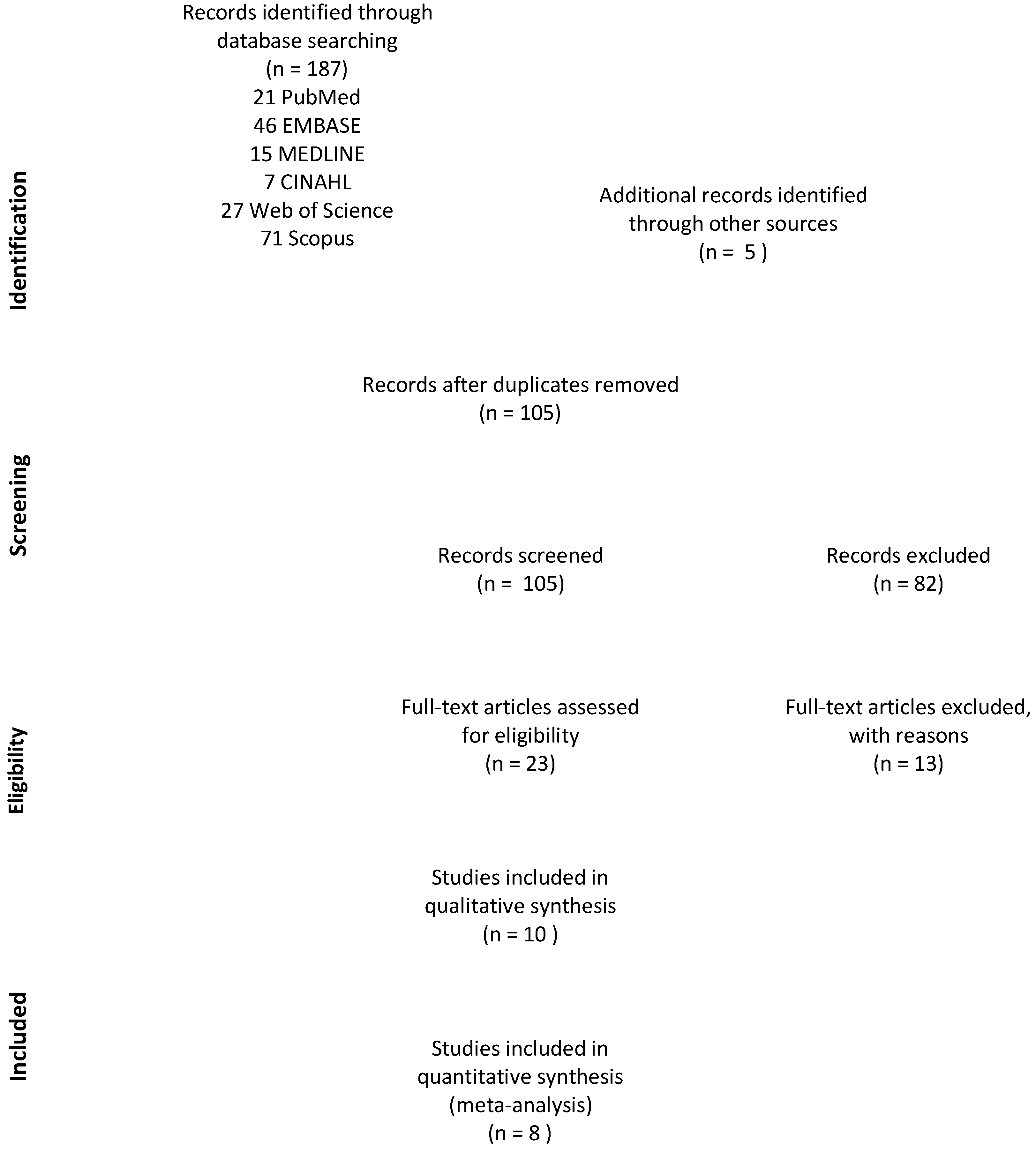
2. Materials and Methods
3. Results
3.1. Study Selection and Participant Characteristics
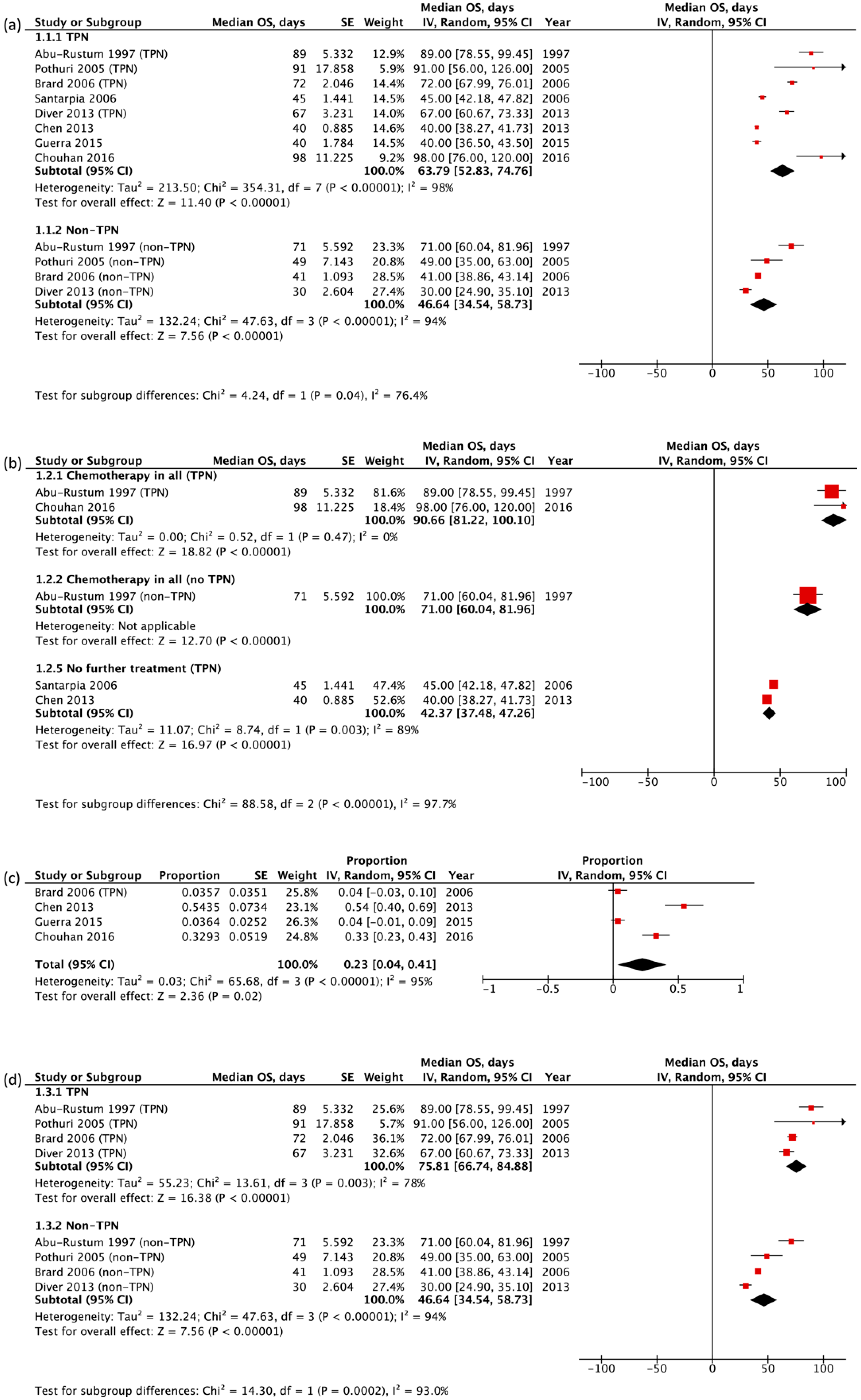
3.2. Primary Outcome Measures: Overall Survival
3.3. Additional Outcome Measures: Complications, Quality of Life (QOL)
3.4. Sensitivity Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Glockzin, G.; Schlitt, H.J.; Piso, P. Peritoneal Carcinomatosis: Patients Selection, Perioperative Complications and Quality of Life Related to Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. World J. Surg. Oncol. 2009, 7, 5. [Google Scholar] [CrossRef] [Green Version]
- McQuellon, R.; Gavazzi, C.; Piso, P.; Swain, D.; Levine, E. Quality of Life and Nutritional Assessment in Peritoneal Surface Malignancy (PSM): Recommendations for Care. J. Surg. Oncol. 2008, 98, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Correia, M.I.T.D.; Waitzberg, D.L. The Impact of Malnutrition on Morbidity, Mortality, Length of Hospital Stay and Costs Evaluated through a Multivariate Model Analysis. Clin. Nutr. 2003, 22, 235–239. [Google Scholar] [CrossRef]
- Bozzetti, F.; Yu, W.; Baratti, D.; Kusamura, S.; Deraco, M. Locoregional Treatment of Peritoneal Carcinomatosis from Gastric Cancer. J. Surg. Oncol. 2008, 98, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Coccolini, F.; Gheza, F.; Lotti, M.; Virzì, S.; Iusco, D.; Ghermandi, C.; Melotti, R.; Baiocchi, G.; Giulini, S.M.; Ansaloni, L.; et al. Peritoneal Carcinomatosis. World J. Gastroenterol. 2013, 19, 6979–6994. [Google Scholar] [CrossRef] [PubMed]
- Klaver, Y.L.B.; Lemmens, V.E.P.P.; Creemers, G.J.; Rutten, H.J.T.; Nienhuijs, S.W.; Hingh, I.H.J.T. de Population-Based Survival of Patients with Peritoneal Carcinomatosis from Colorectal Origin in the Era of Increasing Use of Palliative Chemotherapy. Ann. Oncol. 2011, 22, 2250–2256. [Google Scholar] [CrossRef]
- de Boer, N.L.; Hagemans, J.A.W.; Schultze, B.T.A.; Brandt-Kerkhof, A.R.M.; Madsen, E.V.E.; Verhoef, C.; Burger, J.W.A. Acute Malignant Obstruction in Patients with Peritoneal Carcinomatosis: The Role of Palliative Surgery. Eur. J. Surg. Oncol. 2019, 45, 389–393. [Google Scholar] [CrossRef]
- Du Bois, A.; Sehouli, J.; Vergote, I.; Ferron, G.; Reuss, A.; Meier, W.; Greggi, S.; Jensen, P.T.; Selle, F.; Guyon, F.; et al. Randomized Phase III Study to Evaluate the Impact of Secondary Cytoreductive Surgery in Recurrent Ovarian Cancer: Final Analysis of AGO DESKTOP III/ENGOT-Ov20. JCO 2020, 38, 6000. [Google Scholar] [CrossRef]
- Coleman, R.L.; Spirtos, N.M.; Enserro, D.; Herzog, T.J.; Sabbatini, P.; Armstrong, D.K.; Kim, J.-W.; Park, S.-Y.; Kim, B.-G.; Nam, J.-H.; et al. Secondary Surgical Cytoreduction for Recurrent Ovarian Cancer. N. Engl. J. Med. 2019, 381, 1929–1939. [Google Scholar] [CrossRef]
- Alyami, M.; Mercier, F.; Siebert, M.; Bonnot, P.-E.; Laplace, N.; Villeneuve, L.; Passot, G.; Glehen, O.; Bakrin, N.; Kepenekian, V. Unresectable Peritoneal Metastasis Treated by Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) Leading to Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. Eur. J. Surg. Oncol. 2019, 47, 128–133. [Google Scholar] [CrossRef]
- Auer, R.C.; Sivajohanathan, D.; Biagi, J.; Conner, J.; Kennedy, E.; May, T. Indications for Hyperthermic Intraperitoneal Chemotherapy with Cytoreductive Surgery: A Systematic Review. Eur. J. Cancer 2020, 127, 76–95. [Google Scholar] [CrossRef]
- Tempfer, C.; Giger-Pabst, U.; Hilal, Z.; Dogan, A.; Rezniczek, G.A. Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) for Peritoneal Carcinomatosis: Systematic Review of Clinical and Experimental Evidence with Special Emphasis on Ovarian Cancer. Arch. Gynecol. Obs. 2018, 298, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Laval, G.; Marcelin-Benazech, B.; Guirimand, F.; Chauvenet, L.; Copel, L.; Durand, A.; Francois, E.; Gabolde, M.; Mariani, P.; Rebischung, C.; et al. Recommendations for Bowel Obstruction With Peritoneal Carcinomatosis. J. Pain Symptom Manag. 2014, 48, 75–91. [Google Scholar] [CrossRef]
- Raspé, C.; Flöther, L.; Schneider, R.; Bucher, M.; Piso, P. Best Practice for Perioperative Management of Patients with Cytoreductive Surgery and HIPEC. Eur. J. Surg. Oncol. 2017, 43, 1013–1027. [Google Scholar] [CrossRef]
- Diver, E.; O’Connor, O.; Garrett, L.; Boruta, D.; Goodman, A.; Del Carmen, M.; Schorge, J.; Mueller, P.; Growdon, W. Modest Benefit of Total Parenteral Nutrition and Chemotherapy after Venting Gastrostomy Tube Placement. Gynecol. Oncol. 2013, 129, 332–335. [Google Scholar] [CrossRef]
- Mercadante, S. Nutrition in Cancer Patients. Support. Care Cancer 1996, 4, 10–20. [Google Scholar] [CrossRef]
- Ripamonti, C.; Twycross, R.; Baines, M.; Bozzetti, F.; Capri, S.; De Conno, F.; Gemlo, B.; Hunt, T.M.; Krebs, H.B.; Mercadante, S.; et al. Clinical-Practice Recommendations for the Management of Bowel Obstruction in Patients with End-Stage Cancer. Support. Care Cancer 2001, 9, 223–233. [Google Scholar] [CrossRef]
- Bozzetti, F.; Arends, J.; Lundholm, K.; Micklewright, A.; Zurcher, G.; Muscaritoli, M. ESPEN Guidelines on Parenteral Nutrition: Non-Surgical Oncology. Clin. Nutr. 2009, 28, 445–454. [Google Scholar] [CrossRef]
- Jeejeebhoy, K.N. Enteral Nutrition versus Parenteral Nutrition—the Risks and Benefits. Nat. Clin. Pract. Gastroenterol. Hepatol. 2007, 4, 260–265. [Google Scholar] [CrossRef]
- Weimann, A.; Braga, M.; Carli, F.; Higashiguchi, T.; Hübner, M.; Klek, S.; Laviano, A.; Ljungqvist, O.; Lobo, D.N.; Martindale, R.; et al. ESPEN Guideline: Clinical Nutrition in Surgery. Clin. Nutr. 2017, 36, 623–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Argilés, J.M.; Busquets, S.; Stemmler, B.; López-Soriano, F.J. Cancer Cachexia: Understanding the Molecular Basis. Nat. Rev. Cancer 2014, 14, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Bozzetti, F. Is There a Place for Nutrition in Palliative Care? Support. Care Cancer 2020, 28, 4069–4075. [Google Scholar] [CrossRef]
- Sugarbaker, P.H. Peritoneal Metastases from Gastrointestinal Cancer. Curr. Oncol. Rep. 2018, 20, 62. [Google Scholar] [CrossRef] [PubMed]
- Elekonawo, F.M.K.; van Der Meeren, M.M.D.; Simkens, G.A.; de Wil, J.H.W.; de Hingh, I.H.; Bremers, N.J.A. Comparison of 2 Perioperative Management Protocols and Their Influence on Postoperative Recovery after Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy: Standard Parenteral Nutrition, Selective Bowel Decontamination and Suprapubic Catheters? Dig. Surg. 2019, 36, 394–401. [Google Scholar] [CrossRef]
- Sowerbutts, A.M.; Lal, S.; Sremanakova, J.; Clamp, A.; Todd, C.; Jayson, G.C.; Teubner, A.; Raftery, A.-M.; Sutton, E.J.; Hardy, L.; et al. Home Parenteral Nutrition for People with Inoperable Malignant Bowel Obstruction. Cochrane Database Syst. Rev. 2018. [Google Scholar] [CrossRef] [Green Version]
- Naghibi, M.; Smith, T.R.; Elia, M. A Systematic Review with Meta-Analysis of Survival, Quality of Life and Cost-Effectiveness of Home Parenteral Nutrition in Patients with Inoperable Malignant Bowel Obstruction. Clin. Nutr. 2015, 34, 825–837. [Google Scholar] [CrossRef] [PubMed]
- O’Hanlon, F.J.; Fragkos, K.C.; Fini, L.; Patel, P.S.; Mehta, S.J.; Rahman, F.; Caro, S.D. Home Parenteral Nutrition in Patients with Advanced Cancer: A Systematic Review and Meta-Analysis. Nutr. Cancer 2020, 3, 943–955. [Google Scholar] [CrossRef]
- Tobberup, R.; Thoresen, L.; Falkmer, U.G.; Yilmaz, M.K.; Solheim, T.S.; Balstad, T.R. Effects of Current Parenteral Nutrition Treatment on Health-Related Quality of Life, Physical Function, Nutritional Status, Survival and Adverse Events Exclusively in Patients with Advanced Cancer: A Systematic Literature Review. Crit. Rev. Oncol. Hematol. 2019, 139, 96–107. [Google Scholar] [CrossRef]
- Gramlich, L.; Kichian, K.; Pinilla, J.; Rodych, N.J.; Dhaliwal, R.; Heyland, D.K. Does Enteral Nutrition Compared to Parenteral Nutrition Result in Better Outcomes in Critically Ill Adult Patients? A Systematic Review of the Literature. Nutrition 2004, 20, 843–848. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, T.P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Patterson, P.D.; Weaver, M.; Clark, S.; Yealy, D.M. Case Reports and Case Series in Prehospital Emergency Care Research. Emerg. Med. J. 2010, 27, 807–809. [Google Scholar] [CrossRef]
- Abu-Zidan, F.; Abbas, A.; Hefny, A. Clinical “Case Series”: A Concept Analysis. Afr. Health Sci. 2012, 12, 557–562. [Google Scholar] [CrossRef] [Green Version]
- Fayers, P.; Bottomley, A. Quality of Life Research within the EORTC—the EORTC QLQ-C30. Eur. J. Cancer 2002, 38, 125–133. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O’Connell, D.; Robertson, J.; Peterson, J.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analysis; Ottawa Hospital Research Institute: Ottawa, ON, Canada, 2014. [Google Scholar]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [Green Version]
- Lueza, B.; Mauguen, A.; Pignon, J.-P.; Rivero-Arias, O.; Bonastre, J.; Group, M.-L.C. Difference in Restricted Mean Survival Time for Cost-Effectiveness Analysis Using Individual Patient Data Meta-Analysis: Evidence from a Case Study. PLoS ONE 2016, 11, e0150032. [Google Scholar] [CrossRef]
- Wei, Y.; Royston, P.; Tierney, J.F.; Parmar, M.K.B. Meta-Analysis of Time-to-Event Outcomes from Randomized Trials Using Restricted Mean Survival Time: Application to Individual Participant Data. Stat. Med. 2015, 34, 2881–2898. [Google Scholar] [CrossRef]
- Review Manager (RevMan) [Computer Program], Version 5.4; The Nordic Cochrane Centre, The Cochrane Collaboration: Copenhagen, Denmark, 2020.
- Abu-Rustum, N.R.; Barakat, R.R.; Venkatraman, E.; Spriggs, D. Chemotherapy and Total Parenteral Nutrition for Advanced Ovarian Cancer with Bowel Obstruction. Gynecol. Oncol. 1997, 64, 493–495. [Google Scholar] [CrossRef]
- Pothuri, B.; Montemarano, M.; Gerardi, M.; Shike, M.; Ben-Porat, L.; Sabbatini, P.; Barakat, R.R. Percutaneous Endoscopic Gastrostomy Tube Placement in Patients with Malignant Bowel Obstruction Due to Ovarian Carcinoma. Gynecol. Oncol. 2005, 96, 330–334. [Google Scholar] [CrossRef]
- Brard, L.; Weitzen, S.; Strubel-Lagan, S.L.; Swamy, N.; Gordinier, M.E.; Moore, R.G.; Granai, C.O. The Effect of Total Parenteral Nutrition on the Survival of Terminally Ill Ovarian Cancer Patients. Gynecol. Oncol. 2006, 103, 176–180. [Google Scholar] [CrossRef]
- Chen, C.-J.; Shih, S.-C.; Wang, H.-Y.; Sun, F.-J.; Lu, S.-C.; Chu, C.-H.; Wang, T.-E.; Chen, M.-J. Clinical Application of Total Parenteral Nutrition in Patients with Peritoneal Carcinomatosis. Eur. J. Cancer Care (Engl.) 2013, 22, 468–473. [Google Scholar] [CrossRef]
- Chouhan, J.; Gupta, R.; Ensor, J.; Raghav, K.; Fogelman, D.; Wolff, R.A.; Fisch, M.; Overman, M.J. Retrospective Analysis of Systemic Chemotherapy and Total Parenteral Nutrition for the Treatment of Malignant Small Bowel Obstruction. Cancer Med. 2016, 5, 239–247. [Google Scholar] [CrossRef] [Green Version]
- Santarpia, L.; Alfonsi, L.; Pasanisi, F.; De Caprio, C.; Scalfi, L.; Contaldo, F. Predictive Factors of Survival in Patients with Peritoneal Carcinomatosis on Home Parenteral Nutrition. Nutrition 2006, 22, 355–360. [Google Scholar] [CrossRef]
- Solassol, C.; Joyeux, H.; Dubois, J.-B. Total Parenteral Nutrition (TPN) with Complete Nutritive Mixtures: An Artificial Gut in Cancer Patients. Nutr. Cancer 1979, 1, 13–18. [Google Scholar] [CrossRef]
- Ansari, N.; Chandrakumaran, K.; Dayal, S.; Mohamed, F.; Cecil, T.D.; Moran, B.J. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy in 1000 Patients with Perforated Appendiceal Epithelial Tumours. Eur. J. Surg. Oncol. 2016, 42, 1035–1041. [Google Scholar] [CrossRef]
- Guerra, E.M.; Cortés-Salgado, A.; Mateo-Lobo, R.; Nattero, L.; Riveiro, J.; Vega-Piñero, B.; Valbuena, B.; Carabaña, F.; Carrero, C.; Grande, E.; et al. Role of Parenteral Nutrition in Oncologic Patients with Intestinal Occlusion and Peritoneal Carcinomatosis. Nutr. Hosp. 2015, 32, 1222–1227. [Google Scholar] [CrossRef]
- Tsai, M.-S.; Liang, J.-T. Surgery Is Justified in Patients with Bowel Obstruction Due to Radiation Therapy. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 2006, 10, 575–582. [Google Scholar] [CrossRef]
- Fajardo, A.D.; Tan, B.; Reddy, R.; Fleshman, J. Delayed Repeated Intraperitoneal Chemotherapy after Cytoreductive Surgery for Colorectal and Appendiceal Carcinomatosis. Dis. Colon Rectum 2012, 55, 1044–1052. [Google Scholar] [CrossRef]
- Halkia, E.; Papantziala, A.; Vassiliadou, D.; Tsochrinis, A.; Efstathiou, E.; Giassas, S.; Spiliotis, J. Short Bowel Syndrome after Cytoreductive Surgery and HIPEC: Nutritional Considerations. J BUON 2014, 19, 549–553. [Google Scholar]
- Dineen, S.P.; Robinson, K.A.; Roland, C.L.; Beaty, K.A.; Rafeeq, S.; Mansfield, P.F.; Royal, R.E.; Fournier, K.F. Feeding Tube Placement during Cytoreductive Surgery and Heated Intraperitoneal Chemotherapy Does Not Improve Postoperative Nutrition and Is Associated with Longer Length of Stay and Higher Readmission Rates. J. Surg. Res. 2016, 200, 158–163. [Google Scholar] [CrossRef]
- Shannon, N.B.; Tan, G.H.C.; Chia, C.S.; Soo, K.C.; Teo, M.C. Does Having a Gastrectomy Delay Time to Feeding and Prolong Hospital Stay in Patients Undergoing Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy? Int. J. Hyperth. 2018, 34, 518–523. [Google Scholar] [CrossRef] [Green Version]
- Bekhor, E.; Carr, J.; Hofstedt, M.; Sullivan, B.; Solomon, D.; Leigh, N.; Bolton, N.; Golas, B.; Sarpel, U.; Labow, D.; et al. The Safety of Iterative Cytoreductive Surgery and HIPEC for Peritoneal Carcinomatosis: A High Volume Center Prospectively Maintained Database Analysis. Ann. Surg. Oncol. 2020, 27, 1448–1455. [Google Scholar] [CrossRef] [PubMed]
- Vashi, P.G.; Gupta, D.; Lammersfeld, C.A.; Braun, D.P.; Popiel, B.; Misra, S.; Brown, K.C. The Relationship between Baseline Nutritional Status with Subsequent Parenteral Nutrition and Clinical Outcomes in Cancer Patients Undergoing Hyperthermic Intraperitoneal Chemotherapy. Nutr. J. 2013, 12, 118. [Google Scholar] [CrossRef] [Green Version]
- Morris, R.S.; Gani, F.; Hammad, A.Y.; Peltier, W.; Gamblin, T.C.; Turaga, K.K.; Johnston, F.M. Factors Associated with Palliative Care Use in Patients Undergoing Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. J. Surg. Res. 2017, 1, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Swain, D.R.; Yates, A.L.; Mohamed, F.; Dayal, S.P.; Tzivanakis, A.; Cecil, T.D.; Moran, B.J. Do Patients Undergoing Cytoreductive Surgery and HIPEC for Peritoneal Malignancy Need Parenteral Nutrition? Pleura Peritoneum 2018, 3. [Google Scholar] [CrossRef]
- Kubi, B.; Gunn, J.; Fackche, N.; Cloyd, J.M.; Abdel-Misih, S.; Grotz, T.; Leiting, J.; Fournier, K.; Lee, A.J.; Dineen, S.; et al. Predictors of Nonhome Discharge after Cytoreductive Surgery and HIPEC. J. Surg. Res. 2020, 255, 475–485. [Google Scholar] [CrossRef]
- Hara, H.; Kadowaki, S.; Asayama, M.; Ooki, A.; Yamada, T.; Yoshii, T.; Yamaguchi, K. First-Line Bolus 5-Fluorouracil plus Leucovorin for Peritoneally Disseminated Gastric Cancer with Massive Ascites or Inadequate Oral Intake. Int. J. Clin. Oncol. 2018, 23, 275–280. [Google Scholar] [CrossRef]
- Osumi, H.; Takahari, D.; Chin, K.; Ogura, M.; Ichimura, T.; Wakatsuki, T.; Nakayama, I.; Ota, Y.; Suenaga, M.; Shinozaki, E.; et al. First-Line MFOLFOX6 for Peritoneally Disseminated Gastric Cancer with Massive Ascites or Inadequate Oral Intake. Ann. Oncol. 2018, 29, v23. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Bozzetti, F. The Role of Parenteral Nutrition in Patients with Malignant Bowel Obstruction. Support. Care Cancer 2019, 27, 4393–4399. [Google Scholar] [CrossRef] [PubMed]
- Ozols, R.F. Optimum Chemotherapy for Ovarian Cancer. Int. J. Gynecol. Cancer 2000, 10, 33–37. [Google Scholar] [CrossRef]
- Sugarbaker, P.H. Preface. In Peritoneal Carcinomatosis: Principles of Management; Cancer Treatment and Research; Sugarbaker, P.H., Ed.; Springer US: Boston, MA, USA, 1996; p. xvii. ISBN 978-1-4613-1247-5. [Google Scholar]
- Feuer, D.D.; Broadley, K.E. Surgery for the Resolution of Symptoms in Malignant Bowel Obstruction in Advanced Gynaecological and Gastrointestinal Cancer. Cochrane Database Syst. Rev. 2000. [Google Scholar] [CrossRef]
- Matulonis, U.A.; Sood, A.K.; Fallowfield, L.; Howitt, B.E.; Sehouli, J.; Karlan, B.Y. Ovarian Cancer. Nat. Rev. Dis. Primers 2016, 2, 1–22. [Google Scholar] [CrossRef]
- Chu, D.Z.; Lang, N.P.; Thompson, C.; Osteen, P.K.; Westbrook, K.C. Peritoneal Carcinomatosis in Nongynecologic Malignancy. A Prospective Study of Prognostic Factors. Cancer 1989, 63, 364–367. [Google Scholar] [CrossRef]
- Davies, J.M.; O’Neil, B. Peritoneal Carcinomatosis of Gastrointestinal Origin: Natural History and Treatment Options. Expert Opin. Investig. Drugs 2009, 18, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, B.; Arvieux, C.; Glehen, O.; Beaujard, A.C.; Rivoire, M.; Baulieux, J.; Fontaumard, E.; Brachet, A.; Caillot, J.L.; Faure, J.L.; et al. Peritoneal Carcinomatosis from Non-Gynecologic Malignancies: Results of the EVOCAPE 1 Multicentric Prospective Study. Cancer 2000, 88, 358–363. [Google Scholar] [CrossRef]
- Chua, T.C.; Moran, B.J.; Sugarbaker, P.H.; Levine, E.A.; Glehen, O.; Gilly, F.N.; Baratti, D.; Deraco, M.; Elias, D.; Sardi, A.; et al. Early- and Long-Term Outcome Data of Patients With Pseudomyxoma Peritonei From Appendiceal Origin Treated by a Strategy of Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. J. Clin. Oncol. 2012. [Google Scholar] [CrossRef]
- Sibeoni, J.; Picard, C.; Orri, M.; Labey, M.; Bousquet, G.; Verneuil, L.; Revah-Levy, A. Patients’ Quality of Life during Active Cancer Treatment: A Qualitative Study. BMC Cancer 2018, 18, 951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Leeuwen, M.; Husson, O.; Alberti, P.; Arraras, J.I.; Chinot, O.L.; Costantini, A.; Darlington, A.-S.; Dirven, L.; Eichler, M.; Hammerlid, E.B.; et al. Understanding the Quality of Life (QOL) Issues in Survivors of Cancer: Towards the Development of an EORTC QOL Cancer Survivorship Questionnaire. Health Qual. Life Outcomes 2018, 16, 114. [Google Scholar] [CrossRef] [Green Version]
- Buchman, A.L. Must Every Cancer Patient Die with a Central Venous Catheter? Clin. Nutr. 2002, 21, 269–271. [Google Scholar] [CrossRef]
- Mullady, D.K.; O’Keefe, S.J.D. Treatment of Intestinal Failure: Home Parenteral Nutrition. Nat. Clin. Pract. Gastroenterol. Hepatol. 2006, 3, 492–504. [Google Scholar] [CrossRef]
- Reece, L.; Dragicevich, H.; Lewis, C.; Rothwell, C.; Fisher, O.M.; Carey, S.; Alzahrani, N.A.; Liauw, W.; Morris, D.L. Preoperative Nutrition Status and Postoperative Outcomes in Patients Undergoing Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. Ann. Surg. Oncol. 2019, 26, 2622–2630. [Google Scholar] [CrossRef]
- Maciver, A.H.; Al-Sukhni, E.; Esquivel, J.; Skitzki, J.J.; Kane, J.M.; Francescutti, V.A. Current Delivery of Hyperthermic Intraperitoneal Chemotherapy with Cytoreductive Surgery (CS/HIPEC) and Perioperative Practices: An International Survey of High-Volume Surgeons. Ann. Surg. Oncol. 2017, 24, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Franke, A.J.; Iqbal, A.; Starr, J.S.; Nair, R.M.; GeorgeJr, T.J. Management of Malignant Bowel Obstruction Associated with GI Cancers. J. Oncol. Pract. 2017. [Google Scholar] [CrossRef] [PubMed]
- Hoda, D.; Jatoi, A.; Burnes, J.; Loprinzi, C.; Kelly, D. Should Patients with Advanced, Incurable Cancers Ever Be Sent Home with Total Parenteral Nutrition? Cancer 2005, 103, 863–868. [Google Scholar] [CrossRef]
- Philip, J.; Depczynski, B. The Role of Total Parenteral Nutrition for Patients with Irreversible Bowel Obstruction Secondary to Gynecological Malignancy. J. Pain Symptom Manag. 1997, 13, 104–111. [Google Scholar] [CrossRef]
| Author | Country | Study Design | Sample Size (n) | TPN (n) | Median OS (Days) | Cointerventions (n) | Complications (n) |
|---|---|---|---|---|---|---|---|
| Abu-Rustum et al., 1997 [39] | USA | Retrospective cohort study | 21 | Yes: 52% (11) | 89 | Drainage gastrostomy tube 100% (21) Chemotherapy 100% (21)
| Gastrostomy-related complications in 33% (7) Replacement of new drainage tube required in 24% (5) Chemotherapy-related complications, nadir fever or sepsis requiring readmission in 24% (5) |
| No: 48% (10) | 71 | ||||||
| Pothuri et al., 2005 [40] | USA | Retrospective cohort study | 94 | Yes: 15% (14) | 91 | PEG tube 100% (94) Chemotherapy 31% (29) | PEG tube placement-related complications in 18% (17) |
| No: 85% (80) | 49 | ||||||
| Brard et al., 2006 [41] | USA | Retrospective cohort study | 55 | Yes: 51% (28) | 72 | Concurrent chemotherapy in patients receiving TPN Yes: 64% (18) No: 36% (10) | Line sepsis in 4% (1) Gastrostomy tube replacement required in 12.5% (2) |
| No: 49% (27) | 42 | Concurrent chemotherapy in patients not receiving TPN Yes: 24% (7) No: 76% (20) | |||||
| Diver et al., 2013 [15] | USA | Retrospective cohort study | 115 | Yes: 36% (41) | 67 | Concurrent chemotherapy in patients receiving TPN Yes: 54% (22) No: 46% (19) | Gastrostomy-related complications in 45% (51) |
| No: 63% (74) | 30 | Concurrent chemotherapy in patients not receiving TPN Yes: 31% (23) No: 69% (51) | |||||
| Guerra et al., 2015 [47] | Spain | Prospective case series | 55 | Yes: 100% (55) | 40 | Able to further receive chemotherapy after TPN Yes: 51% (28) No: 49% (27) | Catheter-related bloodstream infections in 3.6% (2) No thrombotic episodes/severe metabolic complications |
| Chouhan et al., 2016 [43] | USA | Retrospective case series | 82 | Yes: 100% (82) | 93 | Chemotherapy 100% (82) | Line infections in 20.7% (17) Hyperbilirubinemia in 12.2% (10) Bowel perforation in 4.9% (4) |
| Solassol et al., 1979 [45] | France | Randomized controlled trial | 40 | Yes: 53% (21) | 46 (mean) | (Steroids (symptomatic) only) | - |
| No: 47% (19) | 7 (mean) | ||||||
| Santarpia et al., 2006 [44] | Italy | Retrospective case series | 152 | Yes: 100% (152) | 45 | Analgesics 44.1% (67) Antiemetics 27% (41) Nasogastric tube 8.6% (13) | - |
| Chen et al., 2013 [42] | Taiwan | Retrospective cohort study * | 46 | Yes: 100% (46) | 40 | - | Sepsis related to TPN in 54.3% (25)
|
| Ansari et al., 2016 [46] | UK | Prospective cohort study * | 980 | Yes: 100% (980) | CCRS: 3102 MTD: 1596 | CCRS + HIPEC 75.3% (738) MTD ± HIPEC 24.7% (242) | Clavien–Dindo grade 30 days post-op CCRS:
|
| Author | Period of Treatment | Median Age, Years | Gender, % Female | Disease Characteristics Site of Primary Tumor (n) Tumor Histology (n) Stage of Cancer (n) | Prior Treatment Received (n) | Performance Indicators | Nutrition Status |
|---|---|---|---|---|---|---|---|
| Abu-Rustum et al., 1997 [39] | 1990–1995 | 54.5 (mean) | 100 | Site Gynecological: epithelial ovarian (21) Histology Poorly differentiated adenocarcinoma (14) Moderately differentiated tumor (7) Stage Stage IIB (1) Stage IIIC (16) Stage IV (3) Not surgically staged (1) | Chemotherapy (18)
| - | - |
| Pothuri et al., 2005 [40] | 1995–2002 | 56 (mean) | 100 | Site Gynecological: ovarian (94) Stage Stage I (1) Stage II (2) Stage III (66) Stage IV (25) | Previous lines of chemotherapy
| - | - |
| Brard et al., 2006 [41] | 1994–2002 | 56.4 (mean) | 100 | Site Gynecological: epithelial ovarian (55) Stage Stage IIIC/ IV (55) | CRS at time of original diagnosis (55) Platinum-based chemotherapy (paclitaxel/ platinum) (55) | ECOG (n) TPN: 1 (1) 2 (23) 3 (14) No TPN: 1 (0) 2 (24) 3 (3) | Albumin (g/dL), mean (SD) All: 2.47 (0.72) TPN: 2.52 (0.74) No TPN: 2.41 (0.71) |
| Diver et al., 2013 [15] | 2000–2008 | 57 | 100 | Site Gynecological
| No. of lines of chemotherapy (115)
| - | - |
| Guerra et al., 2015 [47] | 2007–2012 | 60 (mean) | - | Site Gastrointestinal (28) Gynecological (10) Others (7) | Previous lines of chemotherapy, mean (SD)
| Baseline ECOG, mean (SD): 1.5 (0.5) | BMI (kg/m2), mean (SD): 21.6 (±4.3) Malnutrition in 85% using MUST |
| Chouhan et al., 2016 [43] | 2005–2013 | 55 | 62.2 | Site Gastrointestinal (49)
Histology Carcinoma (71) Non-carcinoma (11) | Abdominal surgery (59) Previous lines of chemotherapy
| - | BMI (kg/m2), median (range): 23.9 (14.3–38.0), n = 81 Albumin (g/dL), median (range): 2.8 (1.6–4.4), n = 79 |
| Solassol et al., 1979 [45] | 1976–1977 | 52.3 (mean) | 62.5 | Site Gastrointestinal (23) Gynecological: ovarian (17) Stage “advanced malignant disease”; no lung/liver metastases | - | - | - |
| Santarpia et al., 2006 [44] | 1996–2003 | 57.8 (mean) | 70.4 | Site Gastrointestinal (90)
Stage “advanced” | - | KPS ≤ 40 | Weight (kg), mean (SD), median: 53.4 (±10.9), 50.2 BMI (kg/m2), mean (SD), median: 20.1 (±3.6), 19.6 Albumin (g/dL), mean (SD), median: 3.1 (±0.6), 3.1 |
| Chen et al., 2013 [42] | 2013 | 56.5 (mean) | 47.8 | Site Gastrointestinal (35)
Others (4) Stage “advanced/terminal” | - | - | All malnutritioned based on weight, BMI, % of standard mid-upper arm circumference and triceps skinfold thickness BMI (kg/m2), mean (SD): TPN: 18.6 (±3.3) No TPN: 19.5 (±3.2) Albumin (g/dL): TPN: 26 (±7.0) No TPN: 26 (±6.0) |
| Ansari et al., 2016 [46] (CCRS group) | 2016 | 56 | 34 | Site Gastrointestinal: appendiceal (718) Histology Low-grade mucinous (575) High-grade (115) Adenocarcinoma (28) | - | - | - |
| Ansari et al., 2016 [46] (MTD group) | 2016 | 60 | 48.7 | Site Gastrointestinal: appendiceal (231) Histology Low-grade mucinous (163) High-grade (50) Adenocarcinoma (18) | - | - | - |
| Study | Reason(s) for Exclusion |
|---|---|
| Tsai et al., 2006 [48] | TPN dependency used as a measure of complications/outcome; intervention investigated surgery for bowel obstruction |
| Fajardo et al., 2012 [49] | TPN dependency used as a measure of complications/outcome, not intervention |
| Halkia et al., 2014 [50] | TPN dependency used as a measure of complications/outcome; intervention investigated consequences of short bowel syndrome (SBS) from CRS-HIPEC |
| Dineen et al., 2016 [51] | TPN dependency used as a measure of complications/outcome; intervention investigated feeding tube placement during CRS-HIPEC |
| Shannon et al., 2018 [52] | TPN dependency used as a measure of complications/outcome; intervention investigated gastrectomy in CRS-HIPEC |
| Bekhor et al., 2020 [53] | TPN dependency used as a measure of complications/outcome; intervention investigated safety of multiple reiterations of CRS-HIPEC |
| Vashi et al., 2013 [54] | Relationship between TPN and outcomes data could not be determined: “study not designed to investigate a causative relationship between PN and clinical outcomes” |
| Morris et al., 2017 [55] | Relationship between TPN and outcomes data could not be determined: TPN was investigated as a factor contributing to palliative care referral |
| Swain et al., 2018 [56] | No data for overall survival; complication outcomes not related to TPN |
| Elekonawo et al., 2019 [24] | Relationship between TPN and outcome data could not be determined: “setup of study did not allow for a fair comparison of TPN vs. early enteral feeding” |
| Kubi et al., 2020 [57] | Relationship between TPN and outcomes data could not be determined: TPN and surgical complications as factors of nonhome discharge |
| Hara et al., 2018 [58] | Relationship between TPN and outcomes data could not be determined |
| Osumi et al., 2018 [59] | Relationship between TPN and outcomes data could not be determined |
| Study | Selection | Comparability | Outcome | Total | Quality |
|---|---|---|---|---|---|
| Abu-Rustum et al., 1997 [39] | *** | * | *** | ******* | High |
| Pothuri et al., 2005 [40] | *** | * | *** | ******* | High |
| Brard et al., 2006 [41] | *** | ** | *** | ******** | High |
| Santarpia et al., 2006 [44] | *** | n/a | *** | ****** | Moderate |
| Chen et al., 2013 [42] | *** | n/a | *** | ****** | Moderate |
| Diver et al., 2013 [15] | *** | ** | *** | ******** | High |
| Guerra et al., 2015 [47] | *** | n/a | *** | ****** | Moderate |
| Ansari et al., 2016 [46] | ** | n/a | *** | ***** | Moderate |
| Chouhan et al., 2016 [43] | *** | n/a | *** | ****** | Moderate |
| Study | Risk of Bias Arising From | Risk-of-Bias Judgement |
|---|---|---|
| Solassol et al., 1979 [45] | The randomization process | Some concerns |
| Deviations from the intended intervention | Some concerns | |
| Missing outcome data | Low | |
| Measurement of outcome | Low | |
| Selection of the reported result | Low | |
| Overall | Some concerns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ong, X.-Y.S.; Sultana, R.; Tan, J.W.-S.; Tan, Q.X.; Wong, J.S.M.; Chia, C.S.; Ong, C.-A.J. The Role of Total Parenteral Nutrition in Patients with Peritoneal Carcinomatosis: A Systematic Review and Meta-Analysis. Cancers 2021, 13, 4156. https://doi.org/10.3390/cancers13164156
Ong X-YS, Sultana R, Tan JW-S, Tan QX, Wong JSM, Chia CS, Ong C-AJ. The Role of Total Parenteral Nutrition in Patients with Peritoneal Carcinomatosis: A Systematic Review and Meta-Analysis. Cancers. 2021; 13(16):4156. https://doi.org/10.3390/cancers13164156
Chicago/Turabian StyleOng, Xing-Yi Sarah, Rehena Sultana, Joey Wee-Shan Tan, Qiu Xuan Tan, Jolene Si Min Wong, Claramae Shulyn Chia, and Chin-Ann Johnny Ong. 2021. "The Role of Total Parenteral Nutrition in Patients with Peritoneal Carcinomatosis: A Systematic Review and Meta-Analysis" Cancers 13, no. 16: 4156. https://doi.org/10.3390/cancers13164156
APA StyleOng, X.-Y. S., Sultana, R., Tan, J. W.-S., Tan, Q. X., Wong, J. S. M., Chia, C. S., & Ong, C.-A. J. (2021). The Role of Total Parenteral Nutrition in Patients with Peritoneal Carcinomatosis: A Systematic Review and Meta-Analysis. Cancers, 13(16), 4156. https://doi.org/10.3390/cancers13164156







