The Role of Positron Emission Tomography/Computed Tomography (PET/CT) for Staging and Disease Response Assessment in Localized and Locally Advanced Pancreatic Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. 18F-Fluorodeoxiglucose (18F-FDG) PET/CT for PC Diagnosis
3. The Role of 18F-FDG PET/CT in Surgical Management of PC
3.1. The Role of 18F-FDG PET/CT in PC Differential Diagnosis
3.2. Pre-Surgical Tumors Staging and Grading
3.3. Assessing Clincal Management
3.4. Assessing Resectability after Neoadjuvant Treatment
4. 18F-FDG PET Radiomics Analysis in PC
5. Future Directions in Preclinical PET Imaging for PC
6. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Israel, O.; Kuten, A. Early detection of cancer recurrence: 18F-FDG PET/CT can make a difference in diagnosis and patient care. J. Nucl. Med. 2007, 48 (Suppl. 1), 28S–35S. [Google Scholar] [PubMed]
- Kaur, S.; Baine, M.J.; Jain, M.; Sasson, A.R.; Batra, S.K. Early diagnosis of pancreatic cancer: Challenges and new developments. Biomark. Med. 2012, 6, 597–612. [Google Scholar] [CrossRef]
- Jemal, A.; Siegel, R.; Ward, E.; Hao, Y.; Xu, J.; Thun, M.J. Cancer statistics. CA Cancer J. Clin. 2009, 59, 225–249. [Google Scholar] [CrossRef]
- Duan, H.; Baratto, L.; Iagaru, A. The Role of PET/CT in the Imaging of Pancreatic Neoplasms. Semin. Ultrasound CT MRI 2019, 40, 500–508. [Google Scholar] [CrossRef]
- Zakharova, O.P.; Karmazanovsky, G.G.; Egorov, V.I. Pancreatic adenocarcinoma: Outstanding problems. World J. Gastrointest. Surg. 2012, 4, 104–113. [Google Scholar] [CrossRef]
- Bang, S.; Chung, H.W.; Park, S.W.; Chung, J.B.; Yun, M.; Lee, J.D.; Song, S.Y. The clinical usefulness of 18-fluorodeoxyglucose positron emission tomography in the differential diagnosis, staging, and response evaluation after concurrent chemoradiotherapy for pancreatic cancer. J. Clin. Gastroenterol. 2006, 40, 923–929. [Google Scholar] [CrossRef]
- Buchs, N.C.; Buhler, L.; Bucher, P.; Willi, J.P.; Frossard, J.L.; Roth, A.D.; Addeo, P.; Rosset, A.; Terraz, S.; Becker, C.D.; et al. Value of contrast-enhanced 18F-fluorodeoxyglucose positron emission tomography/computed tomography in detection and presurgical assessment of pancreatic cancer: A prospective study. J. Gastroenterol. Hepatol. 2011, 26, 657–662. [Google Scholar] [CrossRef]
- Gambhir, S.S.; Czernin, J.; Schwimmer, J.; Silverman, D.H.; Coleman, R.E.; Phelps, M.E. A tabulated summary of the FDG PET literature. J. Nucl. Med. 2001, 42, 1S–93S. [Google Scholar]
- Zhang, J.; Zuo, C.J.; Jia, N.Y.; Wang, J.H.; Hu, S.P.; Yu, Z.F.; Zheng, Y.; Zhang, A.Y.; Feng, X.Y. Cross-modality PET/CT and contrast-enhanced CT imaging for pancreatic cancer. World J. Gastroenterol. 2015, 21, 2988–2996. [Google Scholar] [CrossRef] [PubMed]
- Bipat, S.; Phoa, S.S.; van Delden, O.M.; Bossuyt, P.M.; Gouma, D.J.; Lameris, J.S.; Stoker, J. Ultrasonography, computed tomography and magnetic resonance imaging for diagnosis and determining resectability of pancreatic adenocarcinoma: A meta-analysis. J. Comput. Assist. Tomogr. 2005, 29, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Kauhanen, S.P.; Komar, G.; Seppanen, M.P.; Dean, K.I.; Minn, H.R.; Kajander, S.A.; Rinta-Kiikka, I.; Alanen, K.; Borra, R.J.; Puolakkainen, P.A.; et al. A prospective diagnostic accuracy study of 18F-fluorodeoxyglucose positron emission tomography/computed tomography, multidetector row computed tomography, and magnetic resonance imaging in primary diagnosis and staging of pancreatic cancer. Ann. Surg. 2009, 250, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Zimny, M.; Bares, R.; Fass, J.; Adam, G.; Cremerius, U.; Dohmen, B.; Klever, P.; Sabri, O.; Schumpelick, V.; Buell, U. Fluorine-18 fluorodeoxyglucose positron emission tomography in the differential diagnosis of pancreatic carcinoma: A report of 106 cases. Eur. J. Nucl. Med. 1997, 24, 678–682. [Google Scholar] [CrossRef] [PubMed]
- Taveira, M. Comparison of (68)Ga-FAPI versus (18)F-FDG PET/CT for Initial Cancer Staging. Radiol. Imaging Cancer 2021, 3, e219007. [Google Scholar] [CrossRef] [PubMed]
- Rohrich, M.; Naumann, P.; Giesel, F.L.; Choyke, P.L.; Staudinger, F.; Wefers, A.; Liew, D.P.; Kratochwil, C.; Rathke, H.; Liermann, J.; et al. Impact of (68)Ga-FAPI PET/CT Imaging on the Therapeutic Management of Primary and Recurrent Pancreatic Ductal Adenocarcinomas. J. Nucl. Med. 2021, 62, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Asagi, A.; Ohta, K.; Nasu, J.; Tanada, M.; Nadano, S.; Nishimura, R.; Teramoto, N.; Yamamoto, K.; Inoue, T.; Iguchi, H. Utility of contrast-enhanced FDG-PET/CT in the clinical management of pancreatic cancer: Impact on diagnosis, staging, evaluation of treatment response, and detection of recurrence. Pancreas 2013, 42, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.S.; Choi, S.H.; Lee, Y.; Kim, K.H.; Park, J.Y.; Song, S.Y.; Cho, A.; Yun, M.; Lee, J.D.; Seong, J. Clinical usefulness of (1)(8)F-fluorodeoxyglucose-positron emission tomography in patients with locally advanced pancreatic cancer planned to undergo concurrent chemoradiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Strobel, K.; Heinrich, S.; Bhure, U.; Soyka, J.; Veit-Haibach, P.; Pestalozzi, B.C.; Clavien, P.A.; Hany, T.F. Contrast-enhanced 18F-FDG PET/CT: 1-stop-shop imaging for assessing the resectability of pancreatic cancer. J. Nucl. Med. 2008, 49, 1408–1413. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, S.; Goerres, G.W.; Schafer, M.; Sagmeister, M.; Bauerfeind, P.; Pestalozzi, B.C.; Hany, T.F.; von Schulthess, G.K.; Clavien, P.A. Positron emission tomography/computed tomography influences on the management of resectable pancreatic cancer and its cost-effectiveness. Ann. Surg. 2005, 242, 235–243. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Pancreatic Adenocarcinoma Guidelines Version 2.2021, 2.2021 ed.; National Comprehensive Cancer Network: Plymouth, PA, USA, 2021. [Google Scholar]
- Ducreux, M.; Cuhna, A.S.; Caramella, C.; Hollebecque, A.; Burtin, P.; Goere, D.; Seufferlein, T.; Haustermans, K.; Van Laethem, J.L.; Conroy, T.; et al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26 (Suppl. 5), v56–v68. [Google Scholar] [CrossRef]
- Associazione Italiana di Oncologia Medica. Linee Guida Carcinoma del Pancreas Esocrino; Associazione Italiana di Oncologia Medica: Milan, Italy, 2020. [Google Scholar]
- Katz, M.H.; Fleming, J.B.; Bhosale, P.; Varadhachary, G.; Lee, J.E.; Wolff, R.; Wang, H.; Abbruzzese, J.; Pisters, P.W.; Vauthey, J.N.; et al. Response of borderline resectable pancreatic cancer to neoadjuvant therapy is not reflected by radiographic indicators. Cancer 2012, 118, 5749–5756. [Google Scholar] [CrossRef]
- Orlando, G.; Pilone, V.; Vitiello, A.; Gervasi, R.; Lerose, M.A.; Silecchia, G.; Puzziello, A. Gastric cancer following bariatric surgery: A review. Surg. Laparosc. Endosc. Percutaneous Tech. 2014, 24, 400–405. [Google Scholar] [CrossRef]
- Panda, A.; Garg, I.; Truty, M.J.; Kline, T.L.; Johnson, M.P.; Ehman, E.C.; Suman, G.; Anaam, D.A.; Kemp, B.J.; Johnson, G.B.; et al. Borderline Resectable and Locally Advanced Pancreas Cancer: FDG PET/MRI and CT Tumor Metrics for Assessment of Neoadjuvant Therapy Pathologic Response and Prediction of Survival. AJR Am. J. Roentgenol. 2020, 1–11. [Google Scholar] [CrossRef]
- Best, L.M.; Rawji, V.; Pereira, S.P.; Davidson, B.R.; Gurusamy, K.S. Imaging modalities for characterising focal pancreatic lesions. Cochrane Database Syst. Rev. 2017, 4, CD010213. [Google Scholar] [CrossRef] [PubMed]
- Ergul, N.; Gundogan, C.; Tozlu, M.; Toprak, H.; Kadioglu, H.; Aydin, M.; Cermik, T.F. Role of (18)F-fluorodeoxyglucose positron emission tomography/computed tomography in diagnosis and management of pancreatic cancer; comparison with multidetector row computed tomography, magnetic resonance imaging and endoscopic ultrasonography. Rev. Esp. Med. Nucl. Imagen Mol. 2014, 33, 159–164. [Google Scholar] [CrossRef]
- Krishnaraju, V.S.; Kumar, R.; Mittal, B.R.; Sharma, V.; Singh, H.; Nada, R.; Bal, A.; Rohilla, M.; Singh, H.; Rana, S.S. Differentiating benign and malignant pancreatic masses: Ga-68 PSMA PET/CT as a new diagnostic avenue. Eur. Radiol. 2020, 31, 2199–2208. [Google Scholar] [CrossRef]
- Akcam, A.T.; Teke, Z.; Saritas, A.G.; Ulku, A.; Guney, I.B.; Rencuzogullari, A. The efficacy of (18)F-FDG PET/CT in the preoperative evaluation of pancreatic lesions. Ann. Surg. Treat. Res. 2020, 98, 184–189. [Google Scholar] [CrossRef]
- Santhosh, S.; Mittal, B.R.; Bhasin, D.; Srinivasan, R.; Rana, S.; Das, A.; Nada, R.; Bhattacharya, A.; Gupta, R.; Kapoor, R. Role of (18)F-fluorodeoxyglucose positron emission tomography/computed tomography in the characterization of pancreatic masses: Experience from tropics. J. Gastroenterol. Hepatol. 2013, 28, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, M.J.; McPhail, M.J.; Possamai, L.; Dhar, A.; Vlavianos, P.; Monahan, K.J. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: A meta-analysis. Gastrointest. Endosc. 2012, 75, 319–331. [Google Scholar] [CrossRef]
- Lai, J.P.; Yue, Y.; Zhang, W.; Zhou, Y.; Frishberg, D.; Jamil, L.H.; Mirocha, J.M.; Guindi, M.; Balzer, B.; Bose, S.; et al. Comparison of endoscopic ultrasound guided fine needle aspiration and PET/CT in preoperative diagnosis of pancreatic adenocarcinoma. Pancreatology 2017, 17, 617–622. [Google Scholar] [CrossRef]
- Yeh, R.; Dercle, L.; Garg, I.; Wang, Z.J.; Hough, D.M.; Goenka, A.H. The Role of 18F-FDG PET/CT and PET/MRI in Pancreatic Ductal Adenocarcinoma. Abdom. Radiol. 2018, 43, 415–434. [Google Scholar] [CrossRef] [PubMed]
- Ghaneh, P.; Hanson, R.; Titman, A.; Lancaster, G.; Plumpton, C.; Lloyd-Williams, H.; Yeo, S.T.; Edwards, R.T.; Johnson, C.; Abu Hilal, M.; et al. PET-PANC: Multicentre prospective diagnostic accuracy and health economic analysis study of the impact of combined modality 18fluorine-2-fluoro-2-deoxy-d-glucose positron emission tomography with computed tomography scanning in the diagnosis and management of pancreatic cancer. Health Technol. Assess. 2018, 22, 1–114. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.H.; Melstrom, L.G. Use of imaging as staging and surgical planning for pancreatic surgery. Hepatobiliary Surg. Nutr. 2020, 9, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Lemke, A.J.; Niehues, S.M.; Hosten, N.; Amthauer, H.; Boehmig, M.; Stroszczynski, C.; Rohlfing, T.; Rosewicz, S.; Felix, R. Retrospective digital image fusion of multidetector CT and 18F-FDG PET: Clinical value in pancreatic lesions-a prospective study with 104 patients. J. Nucl. Med. 2004, 45, 1279–1286. [Google Scholar] [PubMed]
- Lee, J.W.; Choi, M.; Choi, J.Y. Impact of F-18 Fluorodeoxyglucose PET/CT and PET/MRI on Initial Staging and Changes in Management of Pancreatic Ductal Adenocarcinoma: A Systemic Review and Meta-Analysis. Diagnostics 2020, 10, 952. [Google Scholar] [CrossRef]
- Barina, A.R.; Bashir, M.R.; Howard, B.A.; Hanks, B.A.; Salama, A.K.; Jaffe, T.A. Isolated recto-sigmoid colitis: A new imaging pattern of ipilimumab-associated colitis. Abdom. Radiol. 2016, 41, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Shi, H.; Yang, F.; Teng, X.; Jiang, B. The value of (18)F-FDG PET/CT and carbohydrate antigen 19–9 in predicting lymph node micrometastases of pancreatic cancer. Abdom. Radiol. 2019, 44, 4057–4062. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, T.; Tateishi, U.; Endo, I.; Inoue, T. Staging accuracy of pancreatic cancer: Comparison between non-contrast-enhanced and contrast-enhanced PET/CT. Eur. J. Radiol. 2014, 83, 1734–1739. [Google Scholar] [CrossRef]
- Santhosh, S.; Mittal, B.R.; Bhasin, D.K.; Rana, S.S.; Gupta, R.; Das, A.; Nada, R. Fluorodeoxyglucose-positron emission tomography/computed tomography performs better than contrast-enhanced computed tomography for metastasis evaluation in the initial staging of pancreatic adenocarcinoma. Ann. Nucl. Med. 2017, 31, 575–581. [Google Scholar] [CrossRef]
- Pergolini, I.; Crippa, S.; Salgarello, M.; Belfiori, G.; Partelli, S.; Ruffo, G.; Pucci, A.; Zamboni, G.; Falconi, M. SUVmax after (18)fluoro-deoxyglucose positron emission tomography/computed tomography: A tool to define treatment strategies in pancreatic cancer. Dig. Liver Dis. 2018, 50, 84–90. [Google Scholar] [CrossRef]
- Moon, D.; Kim, H.; Han, Y.; Byun, Y.; Choi, Y.; Kang, J.; Kwon, W.; Jang, J.Y. Preoperative carbohydrate antigen 19–9 and standard uptake value of positron emission tomography-computed tomography as prognostic markers in patients with pancreatic ductal adenocarcinoma. J. Hepatobiliary Pancreat. Sci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.; Prithviraj, G.; Kothari, N.; Springett, G.; Malafa, M.; Hodul, P.; Kim, J.; Yue, B.; Morse, B.; Mahipal, A. PET/CT Fusion Scan Prevents Futile Laparotomy in Early Stage Pancreatic Cancer. Clin. Nucl. Med. 2015, 40, e501–e505. [Google Scholar] [CrossRef] [PubMed]
- Joo, I.; Lee, J.M.; Lee, E.S.; Ahn, S.J.; Lee, D.H.; Kim, S.W.; Ryu, J.K.; Oh, D.Y.; Kim, K.; Lee, K.B.; et al. Preoperative MDCT Assessment of Resectability in Borderline Resectable Pancreatic Cancer: Effect of Neoadjuvant Chemoradiation Therapy. AJR Am. J. Roentgenol. 2018, 210, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Morgan, D.E.; Waggoner, C.N.; Canon, C.L.; Lockhart, M.E.; Fineberg, N.S.; Posey, J.A., 3rd; Vickers, S.M. Resectability of pancreatic adenocarcinoma in patients with locally advanced disease downstaged by preoperative therapy: A challenge for MDCT. AJR Am. J. Roentgenol. 2010, 194, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Heilbrun, L.K.; Venkatramanamoorthy, R.; Lawhorn-Crews, J.M.; Zalupski, M.M.; Shields, A.F. Using 18F-fluorodeoxyglucose positron emission tomography to monitor clinical outcomes in patients treated with neoadjuvant chemo-radiotherapy for locally advanced pancreatic cancer. Am. J. Clin. Oncol. 2010, 33, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Shaban, E. Added value of 18-F-FDG-PET/CT in patients with pancreatic cancer: Initial observation. Egypt. J. Radiol. Nucl. Med. 2016, 47, 1275–1282. [Google Scholar] [CrossRef][Green Version]
- Yokose, T.; Kitago, M.; Matsusaka, Y.; Masugi, Y.; Shinoda, M.; Yagi, H.; Abe, Y.; Oshima, G.; Hori, S.; Endo, Y.; et al. Usefulness of (18) F-fluorodeoxyglucose positron emission tomography/computed tomography for predicting the prognosis and treatment response of neoadjuvant therapy for pancreatic ductal adenocarcinoma. Cancer Med. 2020, 9, 4059–4068. [Google Scholar] [CrossRef]
- Zimmermann, C.; Distler, M.; Jentsch, C.; Blum, S.; Folprecht, G.; Zophel, K.; Polster, H.; Troost, E.G.C.; Abolmaali, N.; Weitz, J.; et al. Evaluation of response using FDG-PET/CT and diffusion weighted MRI after radiochemotherapy of pancreatic cancer: A non-randomized, monocentric phase II clinical trial-PaCa-DD-041 (Eudra-CT 2009–011968–11). Strahlenther. Onkol. 2021, 197, 19–26. [Google Scholar] [CrossRef]
- Itchins, M.; Chua, T.C.; Arena, J.; Jamieson, N.B.; Nahm, C.B.; O’Connell, R.L.; Bailey, E.A.; Schembri, G.P.; Gill, A.J.; Kneebone, A.; et al. Evaluation of Fluorodeoxyglucose Positron Emission Tomography Scanning in the Neoadjuvant Therapy Paradigm in Pancreatic Ductal Adenocarcinoma. Pancreas 2020, 49, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef]
- Van Timmeren, J.E.; Cester, D.; Tanadini-Lang, S.; Alkadhi, H.; Baessler, B. Radiomics in medical imaging “how-to” guide and critical reflection. Insights Imaging 2020, 11, 91. [Google Scholar] [CrossRef]
- Alongi, P.; Laudicella, R.; Stefano, A.; Caobelli, F.; Comelli, A.; Vento, A.; Sardina, D.; Ganduscio, G.; Toia, P.; Ceci, F.; et al. Choline PET/CT features to predict survival outcome in high risk prostate cancer restaging: A preliminary machine-learning radiomics study. Q. J. Nucl. Med. Mol. Imaging 2020. [Google Scholar] [CrossRef]
- Fan, Y.; Feng, M.; Wang, R. Application of Radiomics in Central Nervous System Diseases: A Systematic literature review. Clin. Neurol. Neurosurg. 2019, 187, 105565. [Google Scholar] [CrossRef] [PubMed]
- Mapelli, P.; Partelli, S.; Salgarello, M.; Doraku, J.; Pasetto, S.; Rancoita, P.M.V.; Muffatti, F.; Bettinardi, V.; Presotto, L.; Andreasi, V.; et al. Dual tracer 68Ga-DOTATOC and 18F-FDG PET/computed tomography radiomics in pancreatic neuroendocrine neoplasms: An endearing tool for preoperative risk assessment. Nucl. Med. Commun. 2020, 41, 896–905. [Google Scholar] [CrossRef]
- Thawani, R.; McLane, M.; Beig, N.; Ghose, S.; Prasanna, P.; Velcheti, V.; Madabhushi, A. Radiomics and radiogenomics in lung cancer: A review for the clinician. Lung Cancer 2018, 115, 34–41. [Google Scholar] [CrossRef]
- Yue, Y.; Osipov, A.; Fraass, B.; Sandler, H.; Zhang, X.; Nissen, N.; Hendifar, A.; Tuli, R. Identifying prognostic intratumor heterogeneity using pre- and post-radiotherapy 18F-FDG PET images for pancreatic cancer patients. J. Gastrointest. Oncol. 2017, 8, 127–138. [Google Scholar] [CrossRef]
- Toyama, Y.; Hotta, M.; Motoi, F.; Takanami, K.; Minamimoto, R.; Takase, K. Prognostic value of FDG-PET radiomics with machine learning in pancreatic cancer. Sci. Rep. 2020, 10, 17024. [Google Scholar] [CrossRef]
- Mori, M.; Passoni, P.; Incerti, E.; Bettinardi, V.; Broggi, S.; Reni, M.; Whybra, P.; Spezi, E.; Vanoli, E.G.; Gianolli, L.; et al. Training and validation of a robust PET radiomic-based index to predict distant-relapse-free-survival after radio-chemotherapy for locally advanced pancreatic cancer. Radiother. Oncol. 2020, 153, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.H.; Cho, Y.S.; Choi, J.Y.; Lee, K.H.; Lee, J.K.; Min, J.H.; Hyun, S.H. Imaging phenotype using (18)F-fluorodeoxyglucose positron emission tomography-based radiomics and genetic alterations of pancreatic ductal adenocarcinoma. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2113–2122. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, C.; Liu, Z.; Pan, G.; Sun, G.; Yang, X.; Zuo, C. [Differentiation of autoimmune pancreatitis and pancreatic ductal adenocarcinoma based on multi-modality texture features in (18)F-FDG PET/CT]. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi 2019, 36, 755–762. [Google Scholar] [CrossRef]
- Gao, J.; Huang, X.; Meng, H.; Zhang, M.; Zhang, X.; Lin, X.; Li, B. Performance of Multiparametric Functional Imaging and Texture Analysis in Predicting Synchronous Metastatic Disease in Pancreatic Ductal Adenocarcinoma Patients by Hybrid PET/MR: Initial Experience. Front. Oncol. 2020, 10, 198. [Google Scholar] [CrossRef]
- Abunahel, B.M.; Pontre, B.; Kumar, H.; Petrov, M.S. Pancreas image mining: A systematic review of radiomics. Eur. Radiol. 2020, 31, 3447–3467. [Google Scholar] [CrossRef]
- Cornelissen, B.; Knight, J.C.; Mukherjee, S.; Evangelista, L.; Xavier, C.; Caobelli, F.; Del Vecchio, S.; Rbah-Vidal, L.; Barbet, J.; de Jong, M.; et al. Translational molecular imaging in exocrine pancreatic cancer. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2442–2455. [Google Scholar] [CrossRef]
- Yonezawa, S.; Higashi, M.; Yamada, N.; Goto, M. Precursor lesions of pancreatic cancer. Gut Liver 2008, 2, 137–154. [Google Scholar] [CrossRef]
- Tummers, W.S.; Farina-Sarasqueta, A.; Boonstra, M.C.; Prevoo, H.A.; Sier, C.F.; Mieog, J.S.; Morreau, J.; van Eijck, C.H.; Kuppen, P.J.; van de Velde, C.J.; et al. Selection of optimal molecular targets for tumor-specific imaging in pancreatic ductal adenocarcinoma. Oncotarget 2017, 8, 56816–56828. [Google Scholar] [CrossRef]
- Hezel, A.F.; Deshpande, V.; Zimmerman, S.M.; Contino, G.; Alagesan, B.; O’Dell, M.R.; Rivera, L.B.; Harper, J.; Lonning, S.; Brekken, R.A.; et al. TGF-β and αvβ6 integrin act in a common pathway to suppress pancreatic cancer progression. Cancer Res. 2012, 72, 4840–4845. [Google Scholar] [CrossRef] [PubMed]
- Ui, T.; Ueda, M.; Higaki, Y.; Kamino, S.; Sano, K.; Kimura, H.; Saji, H.; Enomoto, S. Development and characterization of a (68)Ga-labeled A20FMDV2 peptide probe for the PET imaging of αvβ6 integrin-positive pancreatic ductal adenocarcinoma. Bioorg. Med. Chem. 2020, 28, 115189. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yuan, L.; Long, Y.; Fang, H.; Li, M.; Liu, Q.; Xia, X.; Qin, C.; Zhang, Y.; Lan, X.; et al. Synthesis and Preclinical Evaluation of a (68)Ga-Radiolabeled Peptide Targeting Very Late Antigen-3 for PET Imaging of Pancreatic Cancer. Mol. Pharm. 2020, 17, 3000–3008. [Google Scholar] [CrossRef] [PubMed]
- Rosty, C.; Christa, L.; Kuzdzal, S.; Baldwin, W.M.; Zahurak, M.L.; Carnot, F.; Chan, D.W.; Canto, M.; Lillemoe, K.D.; Cameron, J.L.; et al. Identification of hepatocarcinoma-intestine-pancreas/pancreatitis-associated protein I as a biomarker for pancreatic ductal adenocarcinoma by protein biochip technology. Cancer Res. 2002, 62, 1868–1875. [Google Scholar] [PubMed]
- Van den Berg, Y.W.; Osanto, S.; Reitsma, P.H.; Versteeg, H.H. The relationship between tissue factor and cancer progression: Insights from bench and bedside. Blood 2012, 119, 924–932. [Google Scholar] [CrossRef]
- Muller, M.; Altmann, A.; Sauter, M.; Lindner, T.; Jager, D.; Rathke, H.; Herold-Mende, C.; Marme, F.; Babich, J.; Mier, W.; et al. Preclinical evaluation of peptide-based radiotracers for integrin αvβ6-positive pancreatic carcinoma. Nuklearmedizin 2019, 58, 309–318. [Google Scholar] [CrossRef] [PubMed]
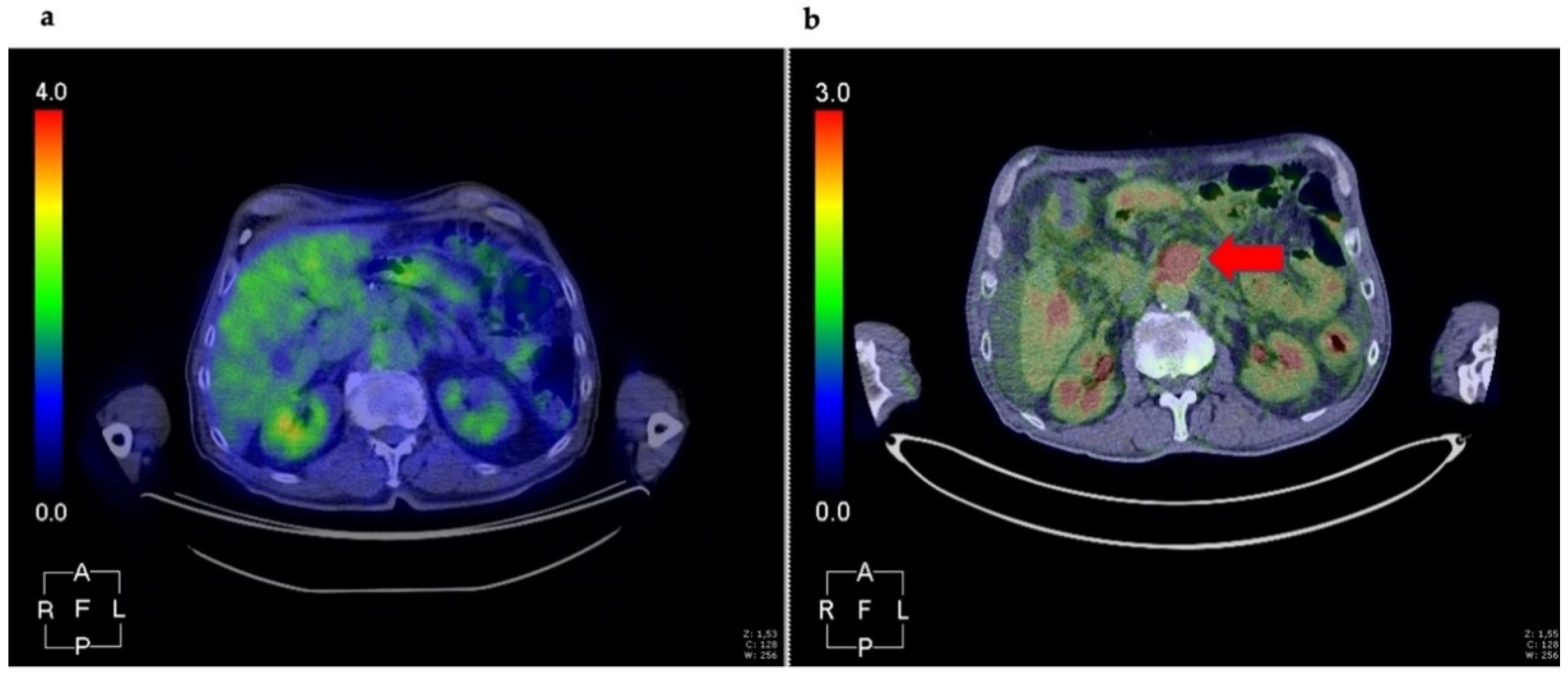
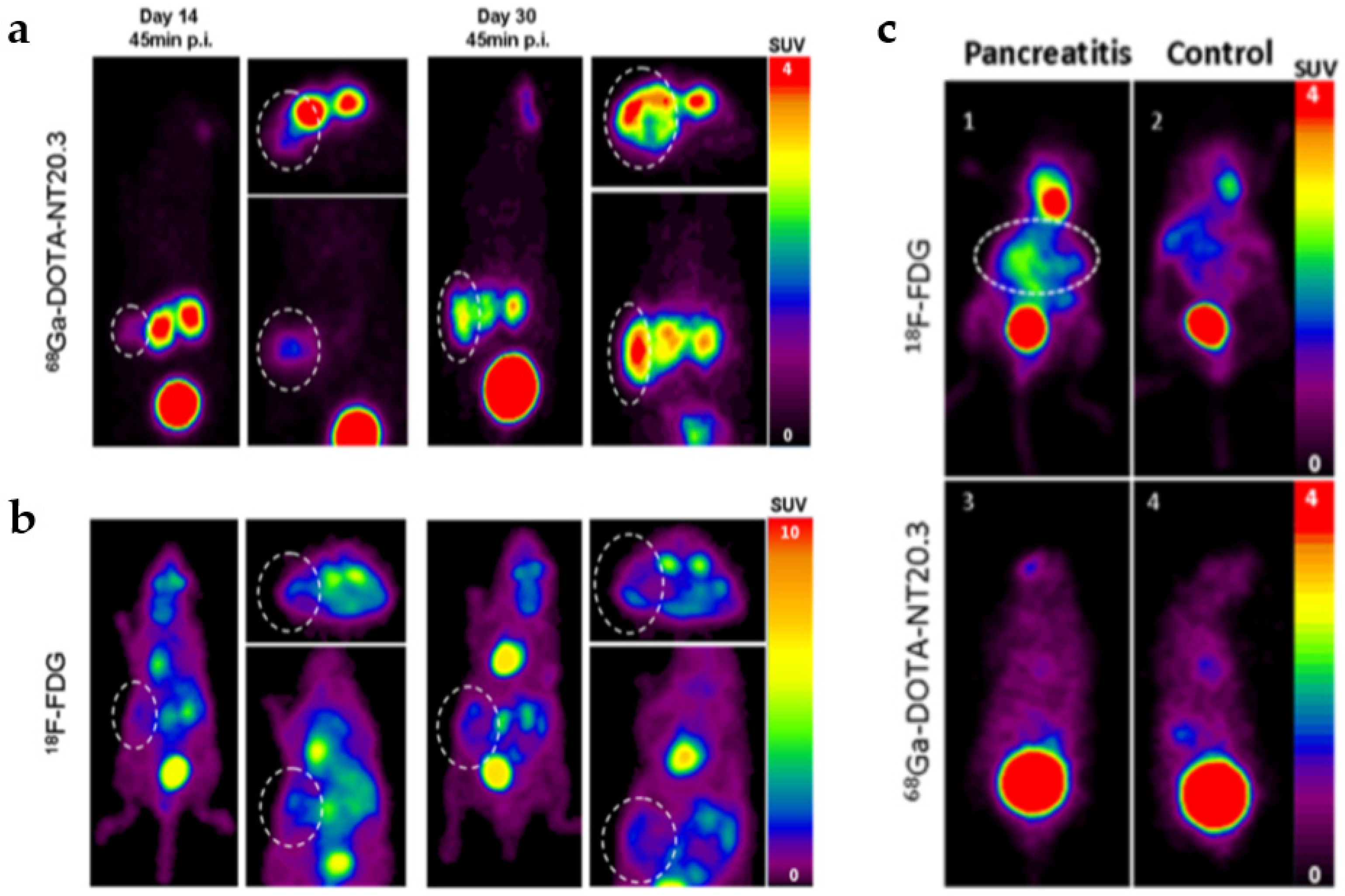
- Korner, M.; Waser, B.; Strobel, O.; Buchler, M.; Reubi, J.C. Neurotensin receptors in pancreatic ductal carcinomas. EJNMMI Res. 2015, 5, 17. [Google Scholar] [CrossRef]
- Prignon, A.; Provost, C.; Alshoukr, F.; Wendum, D.; Couvelard, A.; Barbet, J.; Forgez, P.; Talbot, J.N.; Gruaz-Guyon, A. Preclinical Evaluation of (68)Ga-DOTA-NT-20.3: A Promising PET Imaging Probe To Discriminate Human Pancreatic Ductal Adenocarcinoma from Pancreatitis. Mol. Pharm. 2019, 16, 2776–2784. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, H.; Wang, H.; Feng, H.; Deng, H.; Wu, Z.; Lu, H.; Li, Z. Development of [(18)F]AlF-NOTA-NT as PET Agents of Neurotensin Receptor-1 Positive Pancreatic Cancer. Mol. Pharm. 2018, 15, 3093–3100. [Google Scholar] [CrossRef] [PubMed]
- Iovanna, J.L.; Dagorn, J.C. The multifunctional family of secreted proteins containing a C-type lectin-like domain linked to a short N-terminal peptide. Biochim. Biophys. Acta 2005, 1723, 8–18. [Google Scholar] [CrossRef]
- Xie, M.J.; Motoo, Y.; Iovanna, J.L.; Su, S.B.; Ohtsubo, K.; Matsubara, F.; Sawabu, N. Overexpression of pancreatitis-associated protein (PAP) in human pancreatic ductal adenocarcinoma. Dig. Dis. Sci. 2003, 48, 459–464. [Google Scholar] [CrossRef] [PubMed]
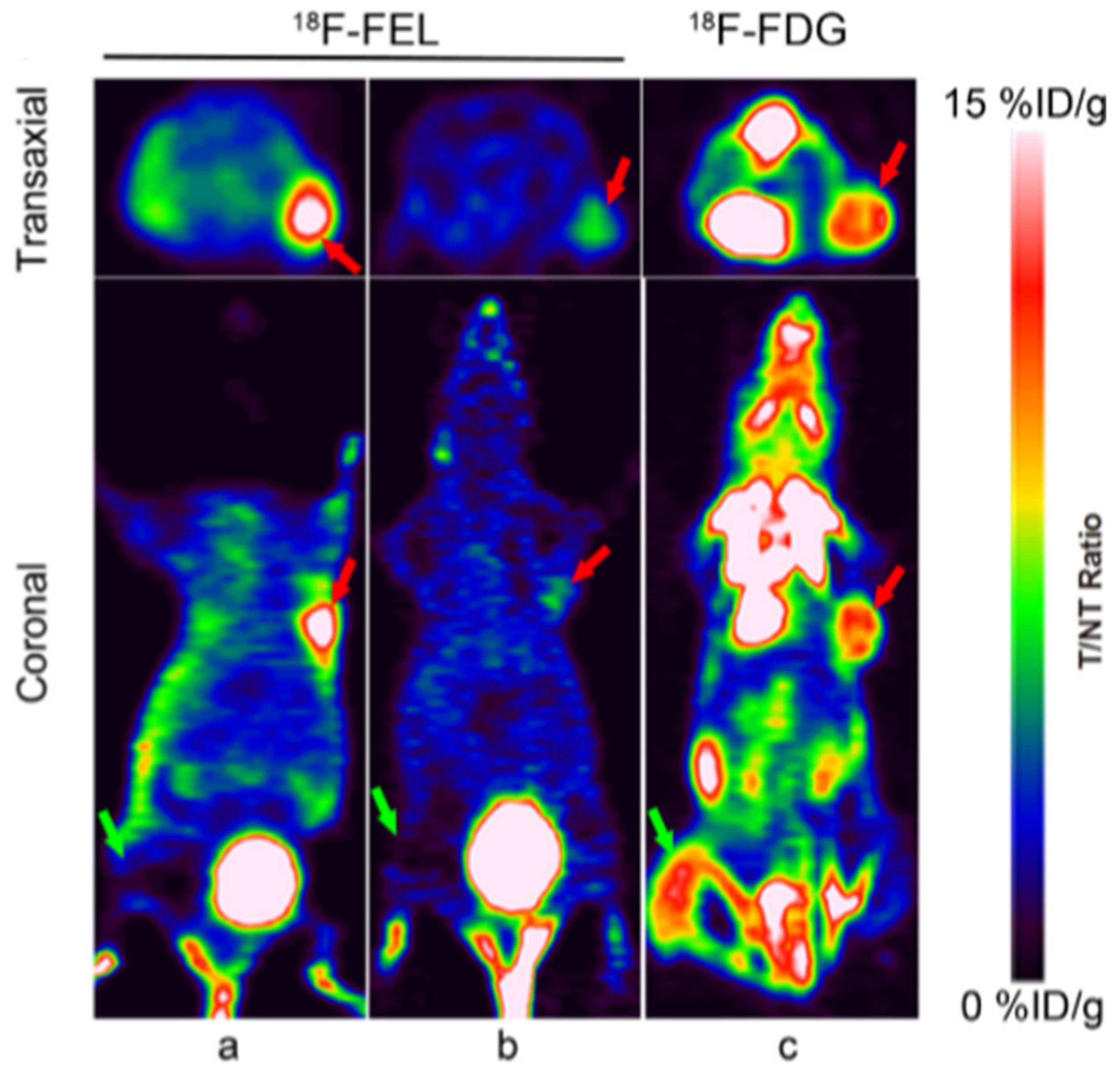
- Yao, S.; Luo, Y.; Zhang, Z.; Hu, G.; Zhu, Z.; Li, F. Preclinical PET imaging of HIP/PAP using 1′-(18)F-fluoroethyl-beta-D-lactose. Oncotarget 2017, 8, 75162–75173. [Google Scholar] [CrossRef]
- Demaugre, F.; Philippe, Y.; Sar, S.; Pileire, B.; Christa, L.; Lasserre, C.; Brechot, C. HIP/PAP, a C-type lectin overexpressed in hepatocellular carcinoma, binds the RII α regulatory subunit of cAMP-dependent protein kinase and alters the cAMP-dependent protein kinase signalling. Eur. J. Biochem. 2004, 271, 3812–3820. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, N.; Koopmann, J.; Sato, N.; Prasad, N.; Carvalho, R.; Leach, S.D.; Hruban, R.H.; Goggins, M. Gene expression alterations in the non-neoplastic parenchyma adjacent to infiltrating pancreatic ductal adenocarcinoma. Mod. Pathol. 2005, 18, 779–787. [Google Scholar] [CrossRef]
- Nitori, N.; Ino, Y.; Nakanishi, Y.; Yamada, T.; Honda, K.; Yanagihara, K.; Kosuge, T.; Kanai, Y.; Kitajima, M.; Hirohashi, S. Prognostic significance of tissue factor in pancreatic ductal adenocarcinoma. Clin. Cancer Res. 2005, 11, 2531–2539. [Google Scholar] [CrossRef]
- Zhou, L.; Yu, L.; Ding, G.; Chen, W.; Zheng, S.; Cao, L. Overexpressions of DLL4 and CD105 are Associated with Poor Prognosis of Patients with Pancreatic Ductal Adenocarcinoma. Pathol. Oncol. Res. 2015, 21, 1141–1147. [Google Scholar] [CrossRef]
- Luo, H.; England, C.G.; Shi, S.; Graves, S.A.; Hernandez, R.; Liu, B.; Theuer, C.P.; Wong, H.C.; Nickles, R.J.; Cai, W. Dual Targeting of Tissue Factor and CD105 for Preclinical PET Imaging of Pancreatic Cancer. Clin. Cancer Res. 2016, 22, 3821–3830. [Google Scholar] [CrossRef]
| Guidelines | Version | CT | MRI | 18F-FDG PET/TC |
|---|---|---|---|---|
| NCCN [17] | 1.2021 | Recommended for Initial Diagnosis | Recommended for Problem Solving | Not Univocally Defined |
| CT of abdomen preferred. MDCT angiography with sub-millimeter, axial sections (pancreatic and portal venous phases) is the preferred imaging tool | RMN preferred as a problem-solving tool, particularly for characterization of CT-indeterminate liver lesion and when suspected PC is not visible on CT. | Role of PET/CT remains unclear. PET scan may be considered after formal pancreatic CT protocol in high-risk patients (borderline resectable disease, markedly elevated CA 19.9, large primary tumor, large regional nodes) to detect extra-pancreatic metastases. Not substitute for high-quality CT. | ||
| ESMO [18] | 2015 | Recommended for Initial Diagnosis | Recommended for Problem Solving | Not Univocally Defined |
| Radiological studies should include CT angiography at the pancreatic arterial (40–50 s) and portal venous (65–70 s) phases | When assessing vessel involvement, use of MRI left to expert discretion. It shows equal benefit to CT with no superiority. MRI is useful for detection of hepatic lesions that cannot be characterized by CT | PET/CT does not add staging information in resectable disease and cannot be recommended. | ||
| AIOM [19] | 2020 | Recommended for Initial Diagnosis | Recommended for Problem Solving (Liver) | Not Reported |
| In patients with suspected PC, multislice CT scan of the chest and abdomen is the first-choice | MRI improves liver staging in patients with potentially resectable PC |
| Molecular Target | Tracer | Animal Model | Clinical Relevance | Reference |
|---|---|---|---|---|
| Integrin αvβ6 | [68Ga]Ac-CG6 | AsPC-1 MIA PaCa-2 | Specific affinity to αvβ6 | Ui T, Bioorganic and Medicinal Chemistry, 2020 [66] |
| Integrin αvβ6 | [68Ga]DOTA-SFLAP3 | Capan-2 | Specific affinity to αvβ6 | Muller M, Nuklearmedizin 2019 [67] |
| Integrin α3β1 | [68Ga]NOTA-CK11 | SW1990 | Specific affinity to α3β1 | Li H, Mol Pharm 2020 [68] |
| Neurotensin receptor 1 (NTS1) | [68Ga]DOTA-NT20.3 | AsPC-1 | Differential diagnosis for tumour and not for inflammatory lesion | Prignon A, Mol Pharm 2019 [69] |
| Hepatocarcinomaintestine-pancreas and pancreatitis-associated protein (HIP/PAP) | [18F]FEL | T3M4 | Differentiating tumors from aseptic inflammation | Yao S, Oncotarget 2017 [70] |
| Tissue factor (TF) CD105 | [64Cu]-NOTA-heterodimer | BxPC-3 (TF/CD105+/+) | Specific affinity to TF and CD105 | Luo H, Clin Cancer Res. 2016 [71] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghidini, M.; Vuozzo, M.; Galassi, B.; Mapelli, P.; Ceccarossi, V.; Caccamo, L.; Picchio, M.; Dondossola, D. The Role of Positron Emission Tomography/Computed Tomography (PET/CT) for Staging and Disease Response Assessment in Localized and Locally Advanced Pancreatic Cancer. Cancers 2021, 13, 4155. https://doi.org/10.3390/cancers13164155
Ghidini M, Vuozzo M, Galassi B, Mapelli P, Ceccarossi V, Caccamo L, Picchio M, Dondossola D. The Role of Positron Emission Tomography/Computed Tomography (PET/CT) for Staging and Disease Response Assessment in Localized and Locally Advanced Pancreatic Cancer. Cancers. 2021; 13(16):4155. https://doi.org/10.3390/cancers13164155
Chicago/Turabian StyleGhidini, Michele, Marta Vuozzo, Barbara Galassi, Paola Mapelli, Virginia Ceccarossi, Lucio Caccamo, Maria Picchio, and Daniele Dondossola. 2021. "The Role of Positron Emission Tomography/Computed Tomography (PET/CT) for Staging and Disease Response Assessment in Localized and Locally Advanced Pancreatic Cancer" Cancers 13, no. 16: 4155. https://doi.org/10.3390/cancers13164155
APA StyleGhidini, M., Vuozzo, M., Galassi, B., Mapelli, P., Ceccarossi, V., Caccamo, L., Picchio, M., & Dondossola, D. (2021). The Role of Positron Emission Tomography/Computed Tomography (PET/CT) for Staging and Disease Response Assessment in Localized and Locally Advanced Pancreatic Cancer. Cancers, 13(16), 4155. https://doi.org/10.3390/cancers13164155








