Low-Field NMR Relaxometry for Intraoperative Tumour Margin Assessment in Breast-Conserving Surgery
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Human Breast Cancer Samples
2.2. Relaxometric Characterisation
2.3. Reproducibility of T1 Measurements
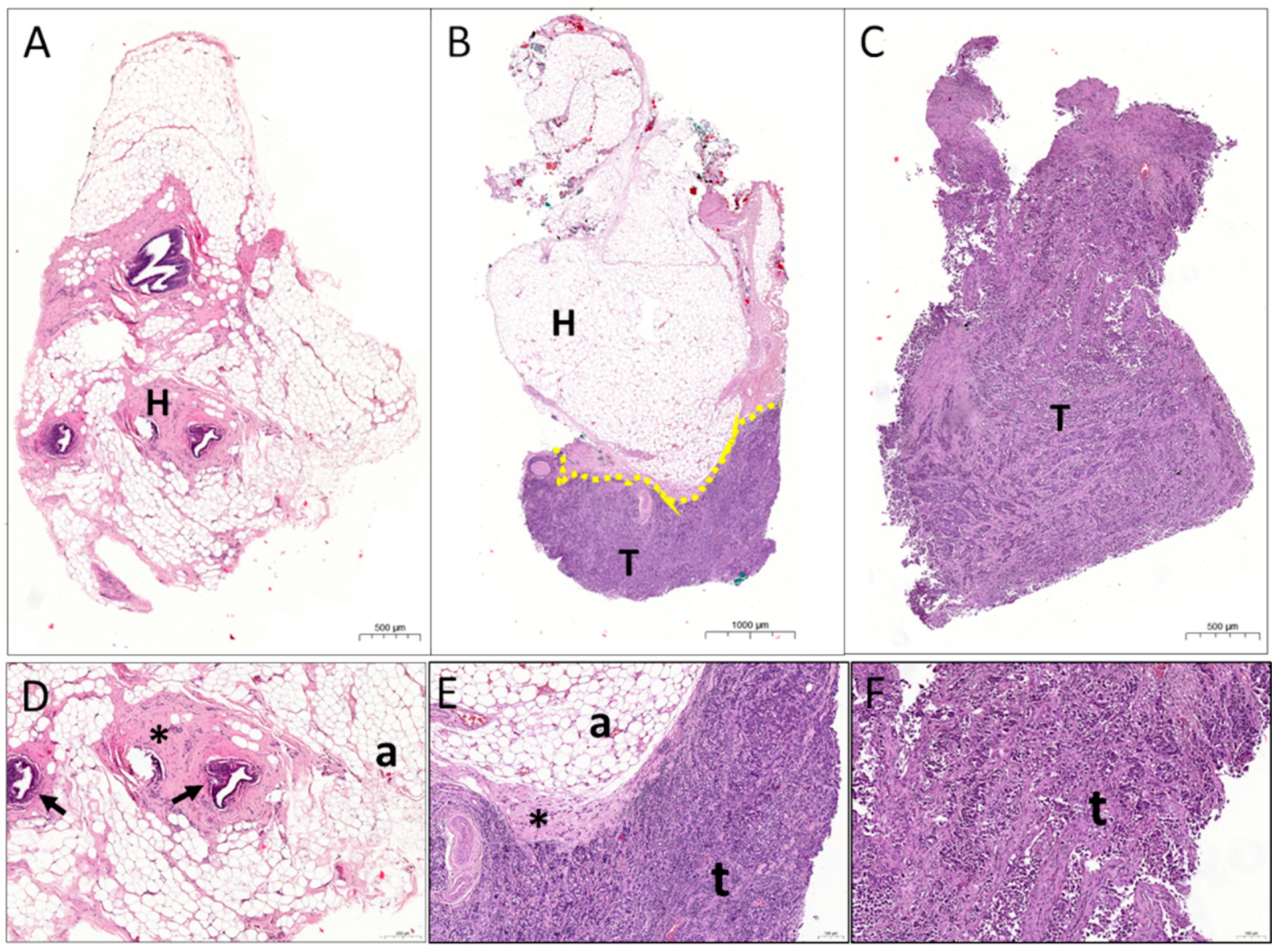
2.4. Histology Characterisation
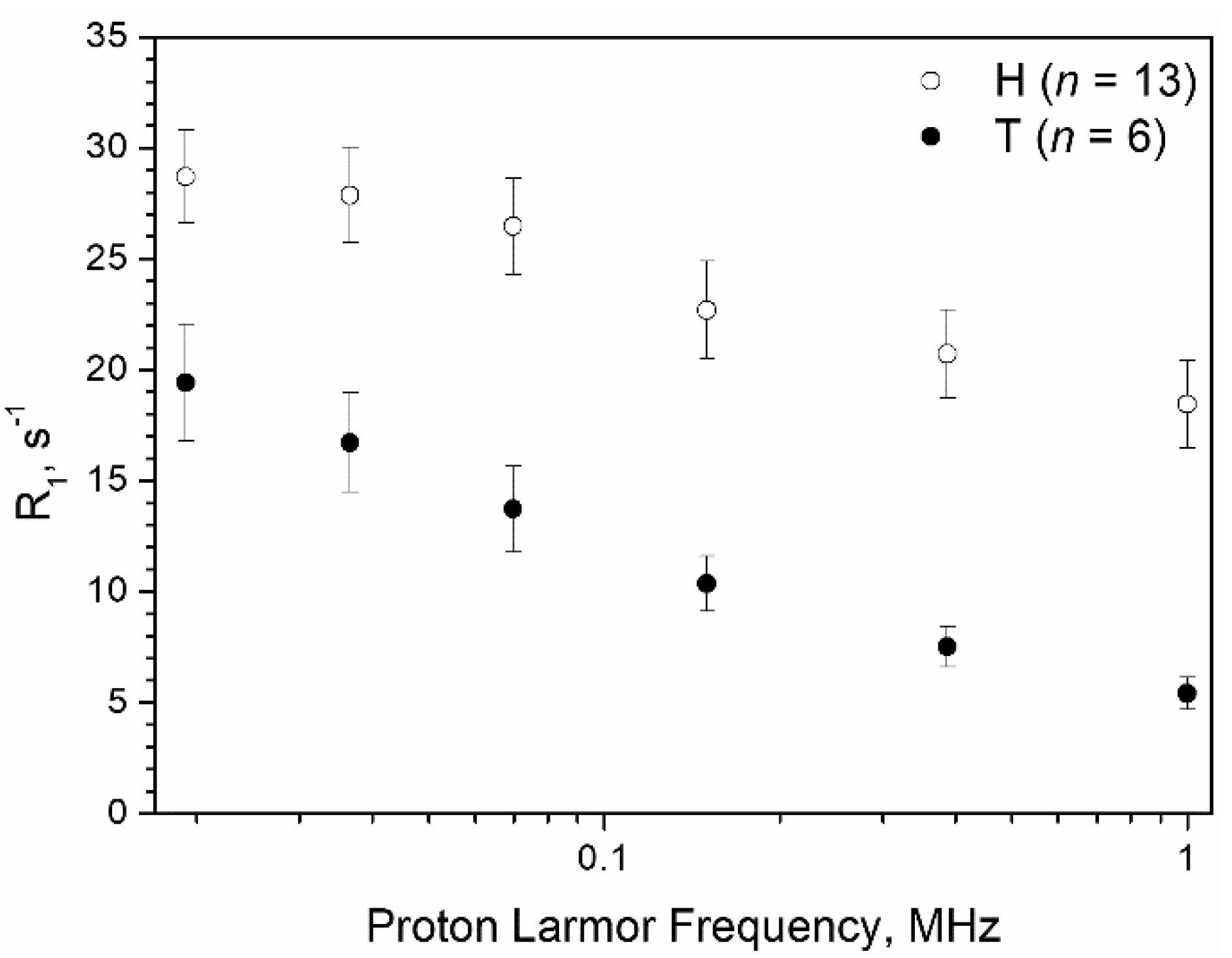
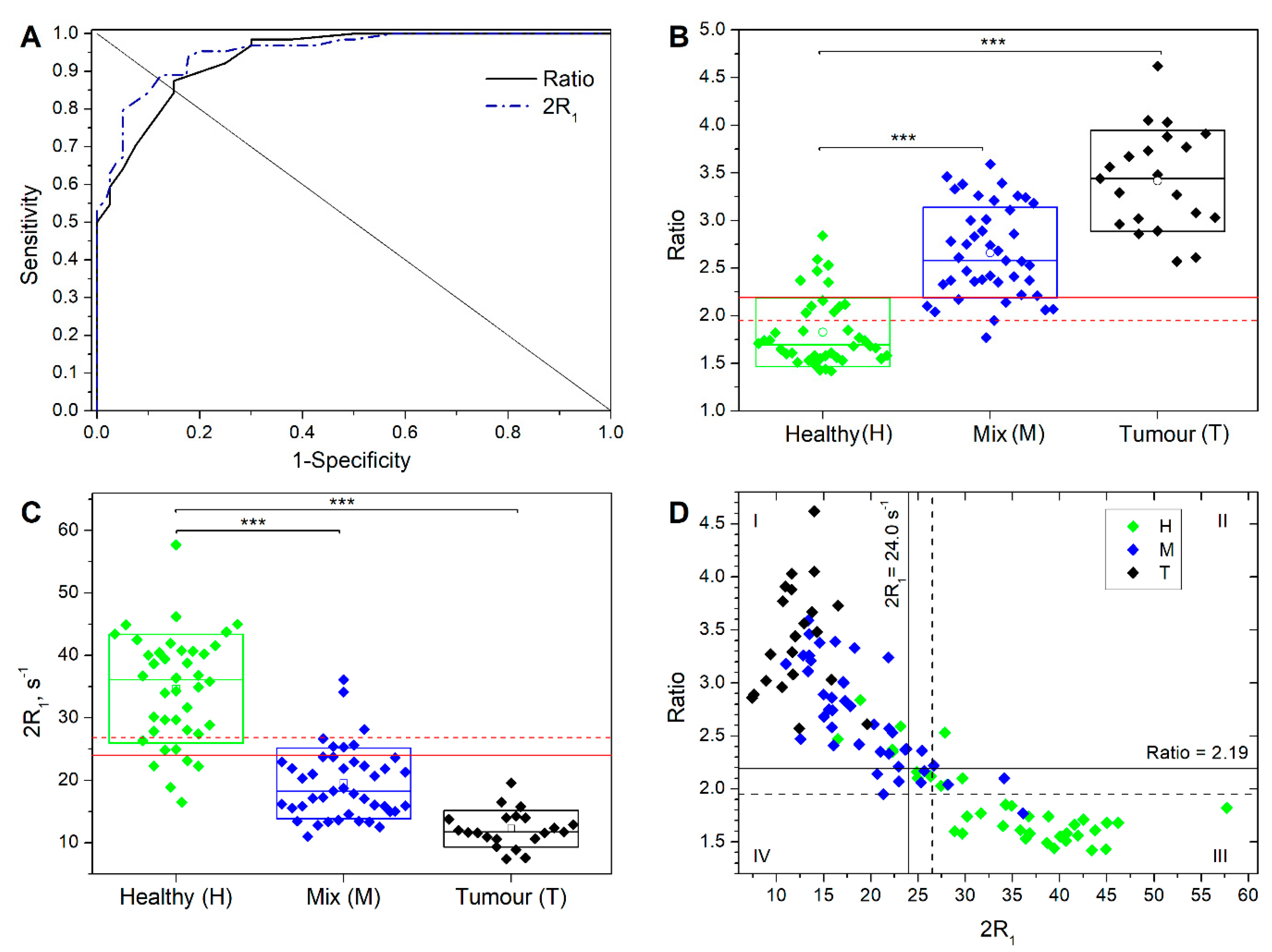
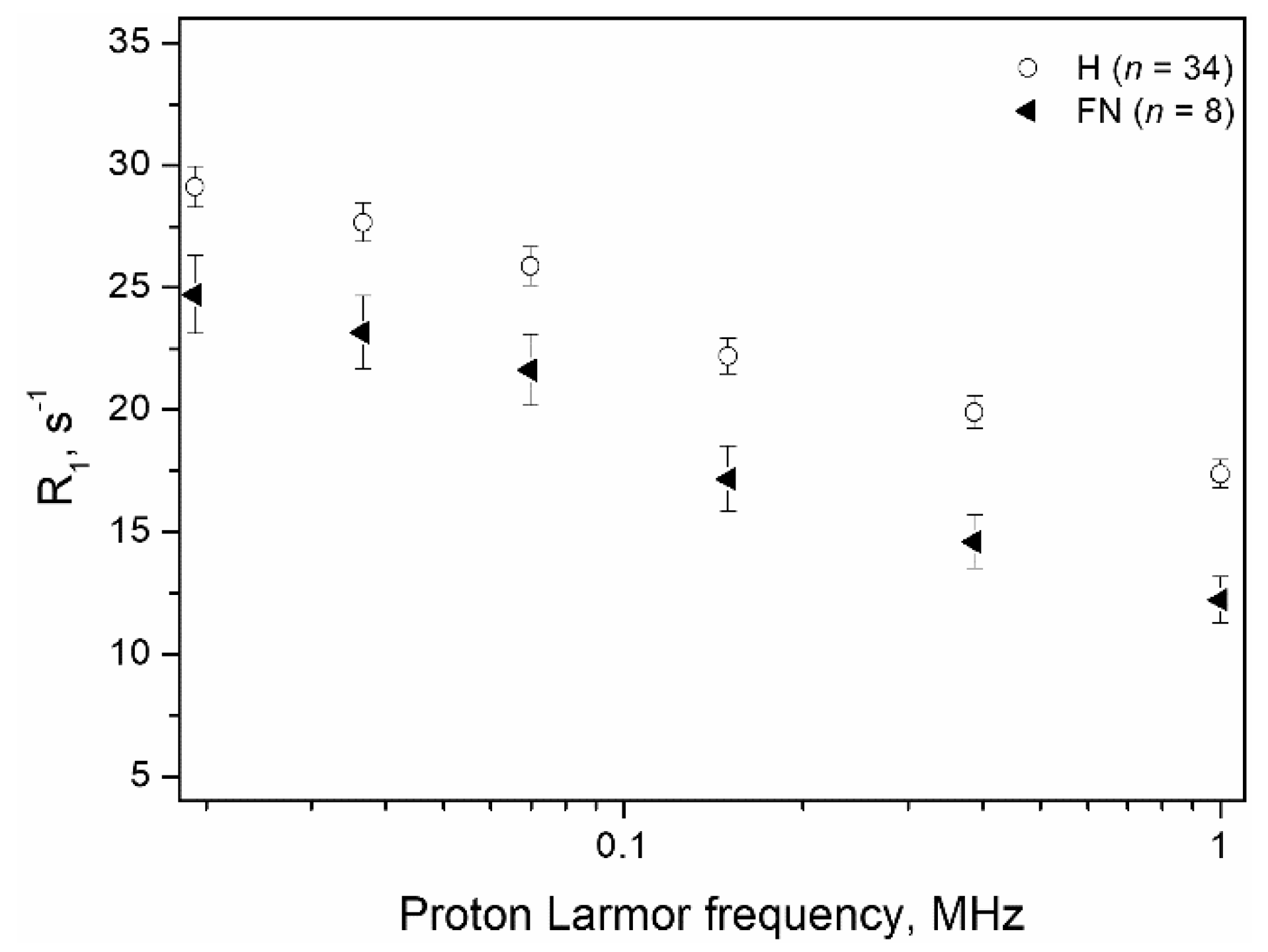
3. Results
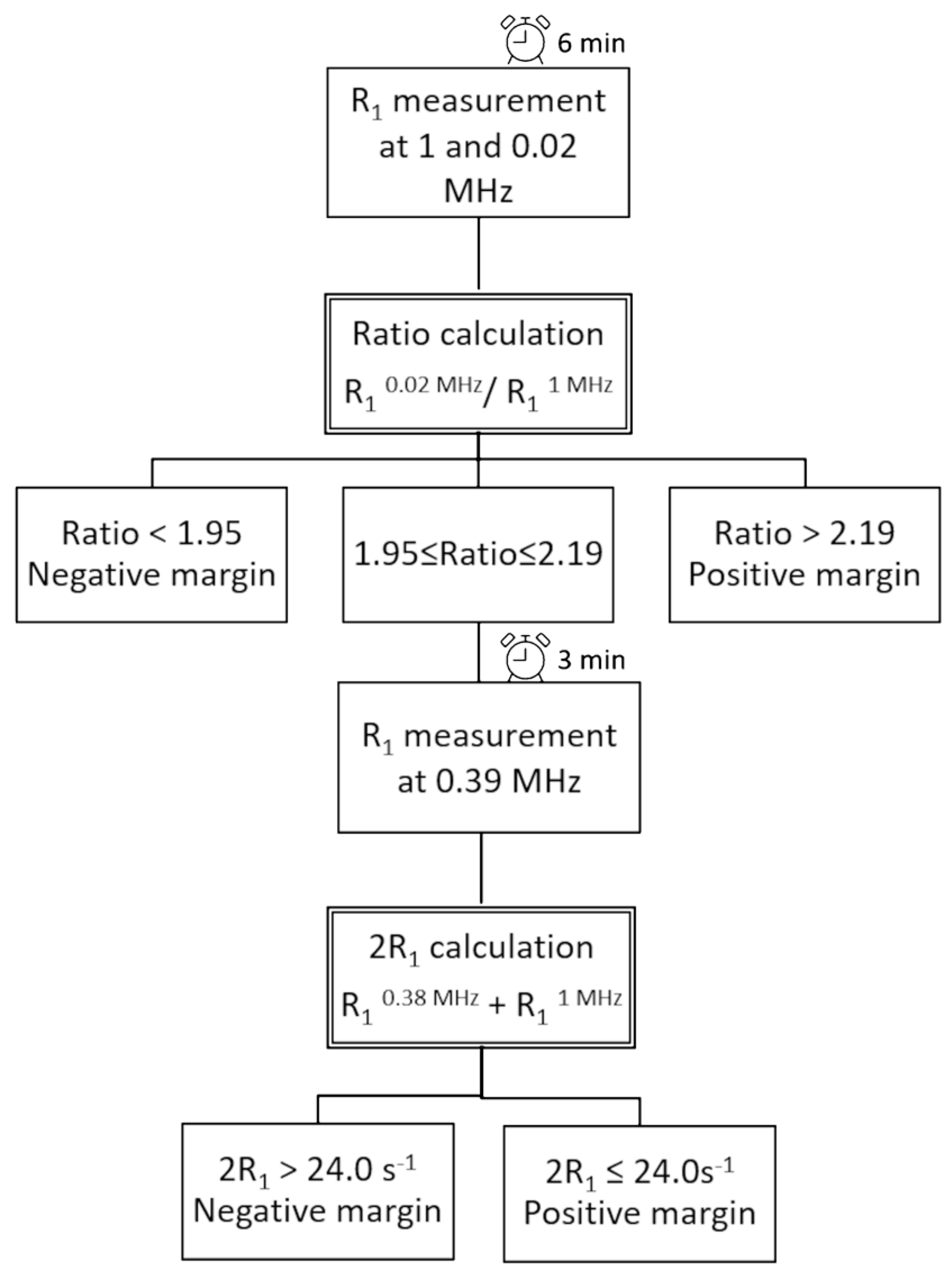
- Measurement of R1 at 0.02 MHz and 1 MHz (total time needed: 6 min);
- Calculation of the Ratio;
- Assigning samples with Ratio > 2.19 as positive and Ratio < 1.95 as negative;
- For samples showing a Ratio between 1.95 and 2.19, a further R1 acquisition at 0.39 MHz is performed (total time needed: 3 min);
- Calculation of 2R1;
- Assigning samples showing a 2R1 > 24.0 s−1 as negative and 2R1 ≤ 24.0 s−1 as positive.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Fajdic, J.; Djurovic, D.; Gotovac, N.; Hrgovic, Z. Criteria and procedures for breast conserving surgery. Acta Inform. Med. 2013, 21, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, U.; Cascinelli, N.; Mariani, L.; Greco, M.; Saccozzi, R.; Luini, A.; Aguilar, M.; Marubini, E. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N. Engl. J. Med. 2002, 347, 1227–1232. [Google Scholar] [CrossRef] [PubMed]
- Moran, M.S.; Schnitt, S.J.; Giuliano, A.E.; Harris, J.R.; Khan, S.A.; Horton, J.; Klimberg, S.; Chavez-Macgregor, M.; Freedman, G.; Houssami, N.; et al. Society of surgical oncology-American society for radiation oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Elmore, L.C.; Margenthaler, J.A. A tale of two operations: Re-excision as a quality measure. Gland Surg. 2019, 8, 593–595. [Google Scholar] [CrossRef] [PubMed]
- Olsen, M.A.; Nickel, K.B.; Margenthaler, J.A.; Wallace, A.E.; Mines, D.; Miller, J.P.; Fraser, V.J.; Warren, D.K. Increased Risk of Surgical Site Infection Among Breast-Conserving Surgery Re-excisions. Ann. Surg. Oncol. 2015, 22, 2003–2009. [Google Scholar] [CrossRef] [PubMed]
- Greenup, R.A.; Peppercorn, J.; Worni, M.; Hwang, E.S. Cost implications of the SSO-ASTRO consensus guideline on margins for breast-conserving surgery with whole breast irradiation in stage I and II invasive breast cancer. Ann. Surg. Oncol. 2014, 21, 1512–1514. [Google Scholar] [CrossRef]
- King, T.A.; Sakr, R.; Patil, S.; Gurevich, I.; Stempel, M.; Sampson, M.; Morrow, M. Clinical management factors contribute to the decision for contralateral prophylactic mastectomy. J. Clin. Oncol. 2011, 29, 2158–2164. [Google Scholar] [CrossRef]
- Maloney, B.W.; McClatchy, D.M.; Pogue, B.W.; Paulsen, K.D.; Wells, W.A.; Barth, R.J. Review of methods for intraoperative margin detection for breast conserving surgery. J. Biomed. Opt. 2018, 23, 100901. [Google Scholar] [CrossRef]
- Pradipta, A.R.; Tanei, T.; Morimoto, K.; Shimazu, K.; Noguchi, S.; Tanaka, K. Emerging Technologies for Real-Time Intraoperative Margin Assessment in Future Breast-Conserving Surgery. Adv. Sci. 2020, 7, 1901519. [Google Scholar] [CrossRef]
- Alexiou, G.A.; Vartholomatos, G.; Kobayashi, T.; Voulgaris, S.; Kyritsis, A.P. The emerging role of intraoperative flow cytometry in intracranial tumor surgery. Clin. Neurol. Neurosurg. 2020, 192, 105742. [Google Scholar] [CrossRef] [PubMed]
- Vartholomatos, G.; Harissis, H.; Andreou, M.; Tatsi, V.; Pappa, L.; Kamina, S.; Βatistatou, A.; Markopoulos, G.S.; Alexiou, G.A. Rapid Assessment of Resection Margins During Breast Conserving Surgery Using Intraoperative Flow Cytometry. Clin. Breast Cancer 2021. [Google Scholar] [CrossRef] [PubMed]
- Lamberts, L.E.; Koch, M.; de Jong, J.S.; Adams, A.L.; Glatz, U.; Kranendonk, M.E.; Terwisscha van Scheltinga, A.G.; Jansen, L.; de Vries, J.; Lub-de Hooge, M.N.; et al. Tumor-Specific Uptake of Fluorescent Bevacizumab-IRDye800CW Microdosing in Patients with Primary Breast Cancer: A Phase I Feasibility Study. Clin Cancer Res. 2017, 23, 2730–2741. [Google Scholar] [CrossRef]
- Tummers, Q.R.J.G.; Verbeek, F.P.R.; Schaafsma, B.E.; Boonstra, M.C.; Van Der Vorst, J.R.; Liefers, G.J.; Van De Velde, C.J.H.; Frangioni, J.V.; Vahrmeijer, A.L. Real-time intraoperative detection of breast cancer using near-infrared fluorescence imaging and Methylene Blue. Eur. J. Surg. Oncol. 2014, 40, 850–858. [Google Scholar] [CrossRef]
- Dintzis, S.M.; Hansen, S.; Harrington, K.M.; Tan, L.C.; Miller, D.M.; Ishak, L.; Parrish-Novak, J.; Kittle, D.; Perry, J.; Gombotz, C.; et al. Real-time visualization of breast carcinoma in pathology specimens from patients receiving fluorescent tumor-marking agent tozuleristide. Arch. Pathol. Lab. Med. 2019, 143, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- Unkart, J.T.; Chen, S.L.; Wapnir, I.L.; González, J.E.; Harootunian, A.; Wallace, A.M. Erratum to: Intraoperative Tumor Detection Using a Ratiometric Activatable Fluorescent Peptide: A First-in-Human Phase 1 Study (ANN SURG ONCOL, 10.1245/S10434-017-5991-3). Ann. Surg. Oncol. 2017, 24, 693. [Google Scholar] [CrossRef] [PubMed]
- Whitley, M.J.; Cardona, D.M.; Lazarides, A.L.; Spasojevic, I.; Ferrer, J.M.; Cahill, J.; Lee, C.L.; Snuderl, M.; Blazer, D.G.; Hwang, E.S.; et al. A mouse-human phase 1 co-clinical trial of a protease-activated fluorescent probe for imaging cancer. Sci. Transl. Med. 2016, 8, 320ra4. [Google Scholar] [CrossRef] [PubMed]
- Walker, E.; Liu, Y.; Kim, I.Y.; Biro, M.; Iyer, S.R.; Ezaldein, H.; Scott, J.; Merati, M.; Mistur, R.; Zhou, B.; et al. A protease-activated fluorescent probe allows rapid visualization of keratinocyte carcinoma during excision. Cancer Res. 2020, 80, 2045–2055. [Google Scholar] [CrossRef]
- Kennedy, K.M.; Zilkens, R.; Allen, W.M.; Foo, K.Y.; Fang, Q.; Chin, L.; Sanderson, R.W.; Anstie, J.; Wijesinghe, P.; Curatolo, A.; et al. Diagnostic accuracy of quantitative micro-elastography for margin assessment in breast-conserving surgery. Cancer Res. 2020, 80, 1773–1783. [Google Scholar] [CrossRef] [PubMed]
- Volders, J.H.; Haloua, M.H.; Krekel, N.M.; Meijer, S.; van den Tol, P.M. Current status of ultrasound-guided surgery in the treatment of breast cancer. World J. Clin. Oncol. 2016, 7, 44–53. [Google Scholar] [CrossRef]
- Kaufman, Z.; Paran, H.; Haas, I.; Malinger, P.; Zehavi, T.; Karni, T.; Pappo, I.; Sandbank, J.; Diment, J.; Allweis, T. Mapping breast tissue types by miniature radio-frequency near-field spectroscopy sensor in ex-vivo freshly excised specimens. BMC Med. Imaging 2016, 16, 1–10. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tan, A.W.; Weljie, A.M. Metabolite imaging at the margin: Visualizing metabolic tumor gradients using mass spectrometry. Cancer Res. 2020, 80, 1231–1233. [Google Scholar] [CrossRef] [PubMed]
- Orel, S.G.; Schnall, M.D. MR imaging of the breast for the detection, diagnosis, and staging of breast cancer. Radiology 2001, 220, 13–30. [Google Scholar] [CrossRef]
- Hill, M.V.; Beeman, J.L.; Jhala, K.; Holubar, S.D.; Rosenkranz, K.M.; Barth, R.J. Relationship of breast MRI to recurrence rates in patients undergoing breast-conservation treatment. Breast Cancer Res. Treat. 2017, 163, 615–622. [Google Scholar] [CrossRef]
- Gommers, J.J.J.; Duijm, L.E.M.; Bult, P.; Strobbe, L.J.A.; Kuipers, T.P.; Hooijen, M.J.H.; Mann, R.M.; Voogd, A.C. The Impact of Preoperative Breast MRI on Surgical Margin Status in Breast Cancer Patients Recalled at Biennial Screening Mammography: An Observational Cohort Study. Ann. Surg. Oncol. 2021, 1–10. [Google Scholar] [CrossRef]
- Papa, M.; Allweis, T.; Karni, T.; Sandbank, J.; Konichezky, M.; Diment, J.; Guterman, A.; Shapiro, M.; Peles, Z.; Maishar, R.; et al. An intraoperative MRI system for margin assessment in breast conserving surgery: Initial results from a novel technique. J. Surg. Oncol. 2016, 114, 22–26. [Google Scholar] [CrossRef]
- Tozaki, M.; Fukuma, E. 1 H MR Spectroscopy and Diffusion-Weighted Imaging of the Breast: Are They Useful Tools for Characterizing Breast Lesions Before Biopsy? Am. J. Roentgenol. 2009, 193, 840–849. [Google Scholar] [CrossRef] [PubMed]
- Kul, S.; Cansu, A.; Alhan, E.; Dinc, H.; Gunes, G.; Reis, A. Contribution of diffusion-weighted imaging to dynamic contrast-enhanced MRI in the characterization of breast tumors. Am. J. Roentgenol. 2011, 196, 210–217. [Google Scholar] [CrossRef]
- Koenig, S.H. Molecular basis of magnetic relaxation of water protons of tissue. Acad. Radiol. 1996, 3, 597–606. [Google Scholar] [CrossRef]
- Masiewicz, E.; Ashcroft, G.P.; Boddie, D.; Dundas, S.R.; Kruk, D.; Broche, L.M. Towards applying NMR relaxometry as a diagnostic tool for bone and soft tissue sarcomas: A pilot study. Sci. Rep. 2020, 10, 14207. [Google Scholar] [CrossRef] [PubMed]
- Korb, J.P.; Bryant, R.G. Magnetic field dependence of proton spin-lattice relaxation times. Magn. Reson. Med. 2002, 48, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Steele, R.M.; Korb, J.P.; Ferrante, G.; Bubici, S. New applications and perspectives of fast field cycling NMR relaxometry. Magn. Reson. Chem. 2016, 54, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Di Gregorio, E.; Ferrauto, G.; Lanzardo, S.; Gianolio, E.; Aime, S. Use of FCC-NMRD relaxometry for early detection and characterization of ex-vivo murine breast cancer. Sci. Rep. 2019, 9, 4624. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, M.R.; Baroni, S.; Aime, S.; Geninatti Crich, S.; Geninatti, S. Relaxometric investigations addressing the determination of intracellular water lifetime: A novel tumour biomarker of general applicability. Mol. Phys. 2018, 117, 968–974. [Google Scholar] [CrossRef]
- Ruggiero, M.R.; Baroni, S.; Pezzana, S.; Ferrante, G.; Geninatti Crich, S.; Aime, S. Evidence for the Role of Intracellular Water Lifetime as a Tumour Biomarker Obtained by In Vivo Field-Cycling Relaxometry. Angew. Chemie Int. Ed. 2018, 57, 7468–7472. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, G.; Sykora, S. Technical aspects of Fast Field Cycling. Adv. Inorg. Chem. 2005, 57, 405–470. [Google Scholar]
- Maxwell, S.E.; Delaney, H.D.; Kelley, K. Designing Experiments and Analyzing Data A Model. Comparison Perspective, 3rd ed.; Routledge, Taylor & Francis Ltd.: London, UK, 2017; ISBN 9781138892286. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Broche, L.M.; Ross, P.J.; Davies, G.R.; MacLeod, M.J.; Lurie, D.J. A whole-body Fast Field-Cycling scanner for clinical molecular imaging studies. Sci. Rep. 2019, 9, 10402. [Google Scholar] [CrossRef]
- Busch, S.; Hatridge, M.; Möãle, M.; Myers, W.; Wong, T.; Mück, M.; Chew, K.; Kuchinsky, K.; Simko, J.; Clarke, J. Measurements of T1-relaxation in ex vivo prostate tissue at 132 μt. Magn. Reson. Med. 2012, 67, 1138–1145. [Google Scholar] [CrossRef]
- Gao, R.W.; Teraphongphom, N.T.; van den Berg, N.S.; Martin, B.A.; Oberhelman, N.J.; Divi, V.; Kaplan, M.J.; Hong, S.S.; Lu, G.; Ertsey, R.; et al. Determination of tumor margins with surgical specimen mapping using near-infrared fluorescence. Cancer Res. 2018, 78, 5144–5154. [Google Scholar] [CrossRef] [PubMed]
| Evolution Field (MHz PLF) | Range of Evolution Time (s) | Time per Field | Distribution |
|---|---|---|---|
| 0.02, 0.037, 0.07 | 0.01 to 2.8 | 2′46″ | Log |
| 0.15, 0.39, 1 | 0.01 to 4 | 3′10″ | Log |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bitonto, V.; Ruggiero, M.R.; Pittaro, A.; Castellano, I.; Bussone, R.; Broche, L.M.; Lurie, D.J.; Aime, S.; Baroni, S.; Geninatti Crich, S. Low-Field NMR Relaxometry for Intraoperative Tumour Margin Assessment in Breast-Conserving Surgery. Cancers 2021, 13, 4141. https://doi.org/10.3390/cancers13164141
Bitonto V, Ruggiero MR, Pittaro A, Castellano I, Bussone R, Broche LM, Lurie DJ, Aime S, Baroni S, Geninatti Crich S. Low-Field NMR Relaxometry for Intraoperative Tumour Margin Assessment in Breast-Conserving Surgery. Cancers. 2021; 13(16):4141. https://doi.org/10.3390/cancers13164141
Chicago/Turabian StyleBitonto, Valeria, Maria Rosaria Ruggiero, Alessandra Pittaro, Isabella Castellano, Riccardo Bussone, Lionel M. Broche, David J. Lurie, Silvio Aime, Simona Baroni, and Simonetta Geninatti Crich. 2021. "Low-Field NMR Relaxometry for Intraoperative Tumour Margin Assessment in Breast-Conserving Surgery" Cancers 13, no. 16: 4141. https://doi.org/10.3390/cancers13164141
APA StyleBitonto, V., Ruggiero, M. R., Pittaro, A., Castellano, I., Bussone, R., Broche, L. M., Lurie, D. J., Aime, S., Baroni, S., & Geninatti Crich, S. (2021). Low-Field NMR Relaxometry for Intraoperative Tumour Margin Assessment in Breast-Conserving Surgery. Cancers, 13(16), 4141. https://doi.org/10.3390/cancers13164141








