Fatty Acid Receptor CD36 Functions as a Surrogate Parameter for Lymph Node Metastasis in Oral Squamous Cell Carcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Cohort
2.2. Study Variables
2.3. Immunohistochemistry
2.4. Statistical Analysis
3. Results
3.1. Description of Study Population
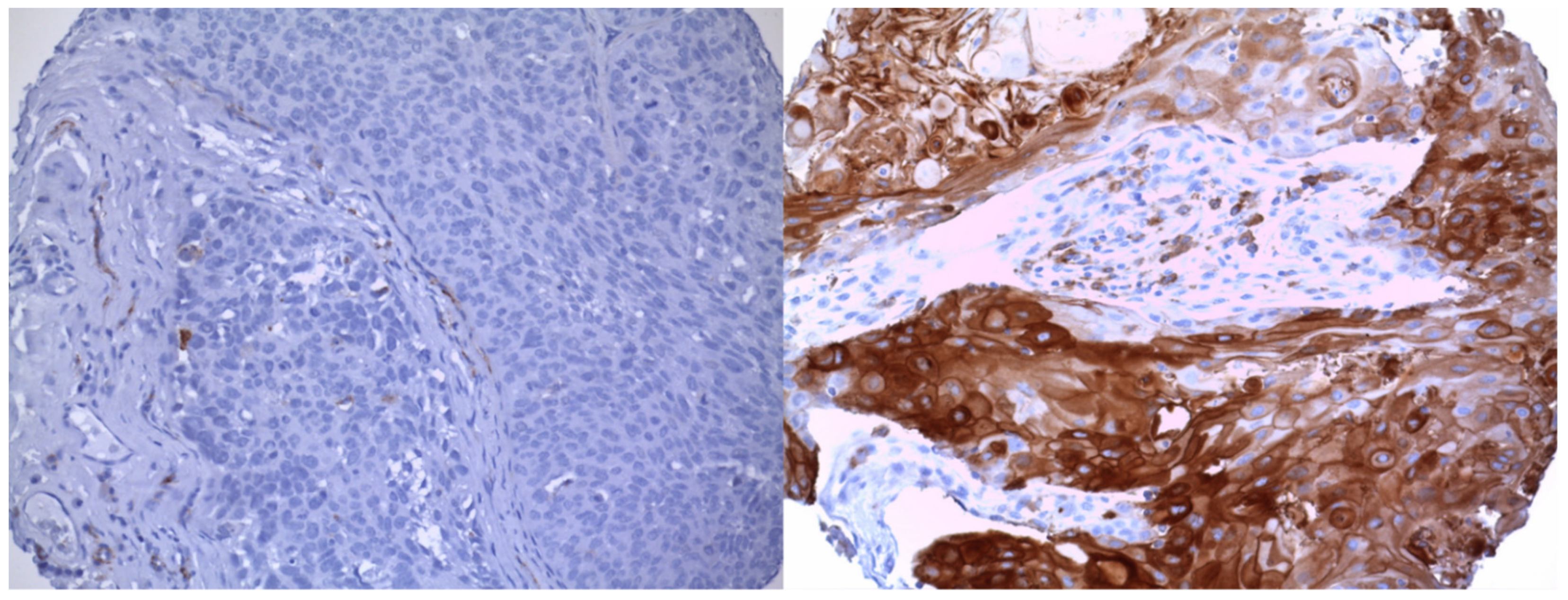
3.2. Association between CD36 Expression and Lymph Node Metastasis
3.3. Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Bavle, R.M.; Venugopal, R.; Konda, P.; Muniswamappa, S.; Makarla, S. Molecular Classification of Oral Squamous Cell Carcinoma. J. Clin. Diagn. Res. JCDR 2016, 10, ZE18. [Google Scholar] [CrossRef]
- Scully, C.; Bagan, J. Oral squamous cell carcinoma overview. Oral Oncol. 2009, 45, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Troeltzsch, M.; Haidari, S.; Boser, S.; Troeltzsch, M.; Probst, F.A.; Ehrenfeld, M.; Otto, S. What Factors Are Associated With Regional Recurrence After Operative Treatment of Oral Squamous Cell Carcinoma? J. Oral Maxillofac. Surg. 2018, 76, 2650–2659. [Google Scholar] [CrossRef] [PubMed]
- Boxberg, M.; Gotz, C.; Haidari, S.; Dorfner, C.; Jesinghaus, M.; Drecoll, E.; Boskov, M.; Wolff, K.D.; Weichert, W.; Haller, B.; et al. Immunohistochemical expression of CD44 in oral squamous cell carcinoma in relation to histomorphological parameters and clinicopathological factors. Histopathology 2018, 73, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Sumino, J.; Uzawa, N.; Ohyama, Y.; Michi, Y.; Kawamata, A.; Mizutani, M.; Yamashiro, M. First signs of late-presenting cervical lymph node metastasis in oral cancers during follow-up. Int. J. Oral Maxillofac. Surg. 2017, 46, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Mermod, M.; Jourdan, E.F.; Gupta, R.; Bongiovanni, M.; Tolstonog, G.; Simon, C.; Clark, J.; Monnier, Y. Development and validation of a multivariable prediction model for the identification of occult lymph node metastasis in oral squamous cell carcinoma. Head Neck 2020, 42, 1811–1820. [Google Scholar] [CrossRef] [PubMed]
- Nobis, C.P.; Otto, S.; Grigorieva, T.; Alnaqbi, M.; Troeltzsch, M.; Schope, J.; Wagenpfeil, S.; Ehrenfeld, M.; Wolff, K.D.; Kesting, M.R. Elective neck dissection in unilateral carcinomas of the tongue: Unilateral versus bilateral approach. J. Craniomaxillofac. Surg. 2017, 45, 579–584. [Google Scholar] [CrossRef]
- Troeltzsch, M.; Probst, F.A.; Rominger, A.; Muller-Lisse, U.; Probst, M.; Obermeier, K.; Ehrenfeld, M.; Otto, S. Comorbidity Assessment in Patients With Oral Squamous Cell Carcinoma: Can Imaging Techniques (Fludeoxyglucose Positron-Emission Tomographic Computed Tomography and Contrast-Enhanced Computed tomography) Provide Additional Information? J. Oral Maxillofac. Surg. 2018, 76, 190–198. [Google Scholar] [CrossRef]
- Cariati, P.; Cabello-Serrano, A.; Monsalve-Iglesias, F.; Fernadez-Solis, J.; Martinez-Lara, I. Is a “watch and wait strategy” safe to manage clinically N0 squamous cell carcinoma of the upper jaw? Curr. Probl. Cancer 2019, 43, 336–343. [Google Scholar] [CrossRef]
- Moratin, J.; Fuchs, A.; Zeidler, C.; Muller-Richter, U.D.A.; Brands, R.C.; Hartmann, S.; Kubler, A.C.; Linz, C. Squamous cell carcinoma of the maxilla: Analysis of clinicopathological predictors for disease recurrence and metastatic behavior. J. Craniomaxillofac. Surg. 2018, 46, 611–616. [Google Scholar] [CrossRef]
- Capote-Moreno, A.; Brabyn, P.; Munoz-Guerra, M.F.; Sastre-Perez, J.; Escorial-Hernandez, V.; Rodriguez-Campo, F.J.; Garcia, T.; Naval-Gias, L. Oral squamous cell carcinoma: Epidemiological study and risk factor assessment based on a 39-year series. Int. J. Oral Maxillofac. Surg. 2020, 49, 1525–1534. [Google Scholar] [CrossRef]
- Massague, J.; Obenauf, A.C. Metastatic colonization by circulating tumour cells. Nature 2016, 529, 298–306. [Google Scholar] [CrossRef]
- Mani, S.A.; Guo, W.; Liao, M.J.; Eaton, E.N.; Ayyanan, A.; Zhou, A.Y.; Brooks, M.; Reinhard, F.; Zhang, C.C.; Shipitsin, M.; et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008, 133, 704–715. [Google Scholar] [CrossRef] [Green Version]
- Grigore, A.D.; Jolly, M.K.; Jia, D.; Farach-Carson, M.C.; Levine, H. Tumor Budding: The Name is EMT. Partial EMT. J. Clin. Med. 2016, 5, 51. [Google Scholar] [CrossRef]
- Fabian, A.; Barok, M.; Vereb, G.; Szollosi, J. Die hard: Are cancer stem cells the Bruce Willises of tumor biology? Cytom. A 2009, 75, 67–74. [Google Scholar] [CrossRef]
- Brown, M.; Assen, F.P.; Leithner, A.; Abe, J.; Schachner, H.; Asfour, G.; Bago-Horvath, Z.; Stein, J.V.; Uhrin, P.; Sixt, M.; et al. Lymph node blood vessels provide exit routes for metastatic tumor cell dissemination in mice. Science 2018, 359, 1408–1411. [Google Scholar] [CrossRef] [Green Version]
- Pascual, G.; Avgustinova, A.; Mejetta, S.; Martin, M.; Castellanos, A.; Attolini, C.S.; Berenguer, A.; Prats, N.; Toll, A.; Hueto, J.A.; et al. Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature 2017, 541, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Y. CD36 tango in cancer: Signaling pathways and functions. Theranostics 2019, 9, 4893–4908. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, G.B.; Ali, A.; Luengo, A.; Kodack, D.P.; Deik, A.; Abbott, K.L.; Bezwada, D.; Blanc, L.; Prideaux, B.; Jin, X.; et al. Fatty Acid Synthesis Is Required for Breast Cancer Brain Metastasis. Nat. Cancer 2021, 2, 414–428. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Lei, P.J.; Padera, T.P. Progression of Metastasis through Lymphatic System. Cells 2021, 10, 627. [Google Scholar] [CrossRef]
- Ladanyi, A.; Mukherjee, A.; Kenny, H.A.; Johnson, A.; Mitra, A.K.; Sundaresan, S.; Nieman, K.M.; Pascual, G.; Benitah, S.A.; Montag, A.; et al. Adipocyte-induced CD36 expression drives ovarian cancer progression and metastasis. Oncogene 2018, 37, 2285–2301. [Google Scholar] [CrossRef] [PubMed]
- Kubo, M.; Gotoh, K.; Eguchi, H.; Kobayashi, S.; Iwagami, Y.; Tomimaru, Y.; Akita, H.; Asaoka, T.; Noda, T.; Takeda, Y.; et al. Impact of CD36 on Chemoresistance in Pancreatic Ductal Adenocarcinoma. Ann. Surg. Oncol. 2020, 27, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.W.; Wilkins, O.; Bang, S.; Ung, M.; Li, J.; An, J.; Del Genio, C.; Canfield, K.; DiRenzo, J.; Wells, W.; et al. CD36-Mediated Metabolic Rewiring of Breast Cancer Cells Promotes Resistance to HER2-Targeted Therapies. Cell Rep. 2019, 29, 3405–3420.e5. [Google Scholar] [CrossRef] [Green Version]
- Jiang, M.; Wu, N.; Xu, B.; Chu, Y.; Li, X.; Su, S.; Chen, D.; Li, W.; Shi, Y.; Gao, X.; et al. Fatty acid-induced CD36 expression via O-GlcNAcylation drives gastric cancer metastasis. Theranostics 2019, 9, 5359–5373. [Google Scholar] [CrossRef] [PubMed]
- Nath, A.; Li, I.; Roberts, L.R.; Chan, C. Elevated free fatty acid uptake via CD36 promotes epithelial-mesenchymal transition in hepatocellular carcinoma. Sci. Rep. 2015, 5, 14752. [Google Scholar] [CrossRef] [Green Version]
- Deng, M.; Cai, X.; Long, L.; Xie, L.; Ma, H.; Zhou, Y.; Liu, S.; Zeng, C. CD36 promotes the epithelial-mesenchymal transition and metastasis in cervical cancer by interacting with TGF-beta. J. Transl. Med. 2019, 17, 352. [Google Scholar] [CrossRef]
- Sakurai, K.; Tomihara, K.; Yamazaki, M.; Heshiki, W.; Moniruzzaman, R.; Sekido, K.; Tachinami, H.; Ikeda, A.; Imaue, S.; Fujiwara, K.; et al. CD36 expression on oral squamous cell carcinoma cells correlates with enhanced proliferation and migratory activity. Oral Dis. 2020, 26, 745–755. [Google Scholar] [CrossRef]
- El-Naggar, A.; Chan, J.; Grandis, J.; Takata, T.; Slootweg, P. WHO Classification of Head and Neck Tumours; IARC: Lyon, France, 2017. [Google Scholar]
- Venables, W.N.; Ripley, B.D.; Venables, W.N. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002; 495p. [Google Scholar]
- Yoshida, T.; Yokobori, T.; Saito, H.; Kuriyama, K.; Kumakura, Y.; Honjo, H.; Hara, K.; Sakai, M.; Miyazaki, T.; Obinata, H.; et al. CD36 Expression Is Associated with Cancer Aggressiveness and Energy Source in Esophageal Squamous Cell Carcinoma. Ann. Surg. Oncol. 2021, 28, 1217–1227. [Google Scholar] [CrossRef]
- Lee, J.; Park, M.; Ko, Y.; Kim, B.; Kim, O.; Hyun, H.; Kim, D.; Sohn, H.; Moon, Y.L.; Lim, W. Ectopic overexpression of CD133 in HNSCC makes it resistant to commonly used chemotherapeutics. Tumour Biol. 2017, 39, 1010428317695534. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Han, H.; Liu, L.; Duan, Y.; Yang, X.; Ma, C.; Zhu, Y.; Han, J.; Li, X.; Chen, Y. CD36 plays a critical role in proliferation, migration and tamoxifen-inhibited growth of ER-positive breast cancer cells. Oncogenesis 2018, 7, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hale, J.S.; Otvos, B.; Sinyuk, M.; Alvarado, A.G.; Hitomi, M.; Stoltz, K.; Wu, Q.; Flavahan, W.; Levison, B.; Johansen, M.L.; et al. Cancer stem cell-specific scavenger receptor CD36 drives glioblastoma progression. Stem Cells 2014, 32, 1746–1758. [Google Scholar] [CrossRef] [Green Version]
- Watt, M.J.; Clark, A.K.; Selth, L.A.; Haynes, V.R.; Lister, N.; Rebello, R.; Porter, L.H.; Niranjan, B.; Whitby, S.T.; Lo, J.; et al. Suppressing fatty acid uptake has therapeutic effects in preclinical models of prostate cancer. Sci. Transl. Med. 2019, 11, eaau5758. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.K.; Jeong, S.H.; Jang, C.; Bae, H.; Kim, Y.H.; Park, I.; Kim, S.K.; Koh, G.Y. Tumor metastasis to lymph nodes requires YAP-dependent metabolic adaptation. Science 2019, 363, 644–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Pretreatment | Heat Treatment with p ProTaqs EDTA Buffer 1 mM pH 8.0 (Fa.Quartett, 400500192) |
| Incubation | Incubation with primary antibody (CD36, (mouse monoclonal, clone OTI3F4)), 60 min RT, dilution 1:50 |
| Detection system | ImmPRESS Anti-Mouse IgG Polymer Kit (Fa.Vector, MP-7402) |
| Chromogen | DAB + (Fa.Agilent Technologies, K3468) |
| Counterstaining | Hematoxylin Gill’s Formula (Fa.Vector, H-3401) |
| Characteristic | N | Overall, N = 83 | Low, N = 45 | High, N = 38 | p-Value |
|---|---|---|---|---|---|
| Age of patient (at time of surgery) [years] | 83 | 64 (53, 78) | 64 (52, 76) | 66 (53, 81) | 0.6 |
| Sex | 83 | 0.7 | |||
| Female | 33 (40%) | 17 (38%) | 16 (42%) | ||
| Male | 50 (60%) | 28 (62%) | 22 (58%) | ||
| T classification | 83 | 0.046 | |||
| T1 | 28 (34%) | 21 (47%) | 7 (18%) | ||
| T2 | 19 (23%) | 9 (20%) | 10 (26%) | ||
| T3 | 12 (14%) | 6 (13%) | 6 (16%) | ||
| T4 | 24 (29%) | 9 (20%) | 15 (39%) | ||
| N classification | 83 | <0.001 | |||
| N0 | 43 (52%) | 37 (82%) | 6 (16%) | ||
| N1 | 19 (23%) | 6 (13%) | 13 (34%) | ||
| N2 | 15 (18%) | 2 (4.4%) | 13 (34%) | ||
| N3 | 6 (7.2%) | 0 (0%) | 6 (16%) | ||
| M classification | 83 | 0.017 | |||
| M0 | 78 (94%) | 45 (100%) | 33 (87%) | ||
| M1 | 5 (6.0%) | 0 (0%) | 5 (13%) | ||
| Grading | 83 | 0.005 | |||
| G1 | 16 (19%) | 14 (31%) | 2 (5.3%) | ||
| G2 | 52 (63%) | 26 (58%) | 26 (68%) | ||
| G3 | 15 (18%) | 5 (11%) | 10 (26%) |
| N Stage | Total | |||
|---|---|---|---|---|
| N0 | N+ | |||
| CD36 | Low | 37 | 8 | 45 |
| High | 6 | 32 | 38 | |
| Total | 43 | 40 | 83 | |
| Characteristic | OR | 95% CI | p-Value |
|---|---|---|---|
| CD36 expression | |||
| Low | |||
| High | 44.7 | 10.0, 316 | <0.001 |
| Sex | |||
| Female | |||
| Male | 2.61 | 0.66, 12.6 | 0.2 |
| Age of patient (at time of surgery) [years] | 0.99 | 0.94, 1.03 | 0.5 |
| T classification | |||
| T1 | |||
| T2 | 0.92 | 0.11, 6.51 | >0.9 |
| T3 | 0.57 | 0.06, 4.66 | 0.6 |
| T4 | 0.36 | 0.05, 2.17 | 0.3 |
| Grading | |||
| G1 | |||
| G2 | 3.17 | 0.51, 28.6 | 0.2 |
| G3 | 47.3 | 4.01, 1015 | 0.005 |
| Characteristic | HR | 95% CI | p-Value |
|---|---|---|---|
| CD36 expression | |||
| Low | |||
| High | 1.35 | 0.55, 3.35 | 0.5 |
| Sex | |||
| Female | |||
| Male | 2.59 | 1.15, 5.86 | 0.022 |
| Age of patient (at time of surgery) [years] | 1.05 | 1.02, 1.08 | <0.001 |
| T classification | |||
| T1 | |||
| T2 | 1.20 | 0.41, 3.50 | 0.7 |
| T3 | 2.36 | 0.82, 6.76 | 0.11 |
| T4 | 1.92 | 0.72, 5.13 | 0.2 |
| N classification | |||
| N0 | |||
| N1 | 2.42 | 0.91, 6.42 | 0.076 |
| N2 | 4.42 | 1.55, 12.6 | 0.006 |
| N3 | 8.12 | 1.67, 39.4 | 0.009 |
| Grading | |||
| G1 | |||
| G2 | 1.54 | 0.52, 4.58 | 0.4 |
| G3 | 0.99 | 0.26, 3.75 | >0.9 |
| N Stage for T1 and T2 | Total | |||
|---|---|---|---|---|
| N0 | N+ | |||
| CD36 | Low | 25 | 5 | 30 |
| High | 2 | 15 | 17 | |
| Total | 27 | 20 | 47 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haidari, S.; Tröltzsch, M.; Knösel, T.; Liokatis, P.; Kasintsova, A.; Eberl, M.; Ortner, F.; Otto, S.; Fegg, F.; Boskov, M.; et al. Fatty Acid Receptor CD36 Functions as a Surrogate Parameter for Lymph Node Metastasis in Oral Squamous Cell Carcinoma. Cancers 2021, 13, 4125. https://doi.org/10.3390/cancers13164125
Haidari S, Tröltzsch M, Knösel T, Liokatis P, Kasintsova A, Eberl M, Ortner F, Otto S, Fegg F, Boskov M, et al. Fatty Acid Receptor CD36 Functions as a Surrogate Parameter for Lymph Node Metastasis in Oral Squamous Cell Carcinoma. Cancers. 2021; 13(16):4125. https://doi.org/10.3390/cancers13164125
Chicago/Turabian StyleHaidari, Selgai, Matthias Tröltzsch, Thomas Knösel, Paris Liokatis, Anastasia Kasintsova, Marian Eberl, Florian Ortner, Sven Otto, Florian Fegg, Marko Boskov, and et al. 2021. "Fatty Acid Receptor CD36 Functions as a Surrogate Parameter for Lymph Node Metastasis in Oral Squamous Cell Carcinoma" Cancers 13, no. 16: 4125. https://doi.org/10.3390/cancers13164125
APA StyleHaidari, S., Tröltzsch, M., Knösel, T., Liokatis, P., Kasintsova, A., Eberl, M., Ortner, F., Otto, S., Fegg, F., Boskov, M., & Probst, F. A. (2021). Fatty Acid Receptor CD36 Functions as a Surrogate Parameter for Lymph Node Metastasis in Oral Squamous Cell Carcinoma. Cancers, 13(16), 4125. https://doi.org/10.3390/cancers13164125






