Discontinuation of Imatinib in Children with Chronic Myeloid Leukemia: A Study from the International Registry of Childhood CML
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rowley, J.D. A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature 1973, 243, 290–293. [Google Scholar] [CrossRef]
- Heisterkamp, N.; Stephenson, J.R.; Groffen, J.; Hansen, P.F.; De Klein, A.; Bartram, C.R.; Grosveld, G. Localization of the c-abl oncogene adjacent to a translocation break point in chronic myelocytic leukaemia. Nature 1983, 306, 239–242. [Google Scholar] [CrossRef]
- Druker, B.J.; Guilhot, F.; O’Brien, S.G.; Gathmann, I.; Kantarjian, H.M.; Gattermann, N.; Deininger, M.W.; Silver, R.T.; Goldman, J.M.; Stone, R.M.; et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N. Engl. J. Med. 2006, 355, 2408–2417. [Google Scholar] [CrossRef] [PubMed]
- Millot, F.; Baruchel, A.; Guilhot, J.; Petit, A.; Leblanc, T.; Bertrand, Y.; Mazingue, F.; Lutz, P.; Vérité, C.; Berthou, C.; et al. Imatinib is effective in children with previously untreated chronic myelogenous leukemia in early chronic phase: Results of the French national phase IV trial. J. Clin. Oncol. 2011, 29, 2827–2832. [Google Scholar] [CrossRef] [Green Version]
- Suttorp, M.; Schulze, P.; Glauche, I.; Göhring, G.; Von Neuhoff, N.; Metzler, M.; Sedlacek, P.; De Bont, E.S.J.M.; Balduzzi, A.C.; Lausen, B.; et al. Front-line imatinib treatment in children and adolescents with chronic myeloid leukemia: Results from a phase III trial. Leukemia 2018, 32, 1657–1669. [Google Scholar] [CrossRef]
- Millot, F.; Guilhot, J.; Baruchel, A.; Petit, A.; Leblanc, T.; Bertrand, Y.; Mazingue, F.; Lutz, P.; Vérité, C.; Berthou, C.; et al. Growth deceleration in children treated with imatinib for chronic myeloid leukaemia. Eur. J. Cancer 2014, 50, 3206–3211. [Google Scholar] [CrossRef]
- Mahon, F.X.; Rea, D.; Guilhot, J.; Guilhot, F.; Huguet, F.; Nicolini, F.; Legros, L.; Charbonnier, L.; Guerci, A.; Varet, B.; et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: The prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010, 11, 1029–1035. [Google Scholar] [CrossRef]
- Rousselot, P.; Charbonnier, A.; Cony-Makhoul, P.; Agape, P.; Nicolini, F.E.; Varet, B.; Gardembas, M.; Etienne, G.; Réa, D.; Roy, L.; et al. Loss of major molecular response as a trigger for restarting tyrosine kinase inhibitor therapy in patients with chronic-phase chronic myelogenous leukemia who have stopped imatinib after durable undetectable disease. J. Clin. Oncol. 2014, 32, 424–430. [Google Scholar] [CrossRef]
- Rea, D.; Nicolini, F.; Tulliez, M.; Guilhot, F.; Guilhot, J.; Guerci-Bresler, A.; Gardembas, M.; Coiteux, V.; Guillerm, G.; Legros, L.; et al. Discontinuation of dasatinib or nilotinib in chronic myeloid leukemia: Interim analysis of the STOP 2G-TKI study. Blood 2017, 129, 846–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saussele, S.; Richter, J.; Guilhot, J.; Gruber, F.X.; Hjorth-Hansen, H.; Almeida, A.; Janssen, J.J.W.M.; Mayer, J.; Koskenvesa, P.; Panayiotidis, P.; et al. Discontinuation of tyrosine kinase inhibitor therapy in chronic myeloid leukaemia (EURO-SKI): A prespecified interim analysis of a prospective, multicentre, non-randomised, trial. Lancet Oncol. 2018, 19, 747–757. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.K.; Du, T.F.; Xiong, P.S.; Fan, G.H.; Yang, W. Discontinuation of tyrosine kinase inhibitors in chronic myeloid leukemia with losing major molecular response as a definition for molecular relapse: A systematic review and meta-Analysis. Front. Oncol. 2019, 9, 372. [Google Scholar] [CrossRef]
- Hochhaus, A.; Baccarani, M.; Silver, R.T.; Schiffer, C.; Apperley, J.F.; Cervantes, F.; Clark, R.E.; Cortes, J.E.; Deininger, M.W.; Guilhot, F. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia 2020, 34, 966–984. [Google Scholar] [CrossRef] [Green Version]
- Deininger, M.W.; Shah, N.P.; Altman, J.K.; Berman, E.; Bhatia, R.; Bhatnagar, B.; DeAngelo, D.J.; Gotlib, J.; Hobbs, G.; Maness, L. Chronic myeloid leukemia, version 2.2021, NCCN clinical practice guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2020, 18, 1385–1415. [Google Scholar] [CrossRef]
- Millot, F.; Traore, P.; Guilhot, J.; Nelken, B.; Leblanc, T.; Leverger, G.; Plantaz, D.; Bertrand, Y.; Bordigoni, P.; Guilhot, F. Clinical and biological features at diagnosis in 40 children with chronic myeloid leukemia. Pediatrics 2005, 116, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Suttorp, M.; Millot, F.; Sembill, S.; Deutsch, H.; Metzler, M. Definition, epidemiology, pathophysiology, and essential criteria for diagnosis of pediatric chronic myeloid leukemia. Cancers 2021, 13, 798. [Google Scholar] [CrossRef] [PubMed]
- Millot, F.; Claviez, A.; Leverger, G.; Corbaciglu, S.; Groll, A.H.; Suttorp, M. Imatinib cessation in children and adolescents with chronic myeloid leukemia in chronic phase. Pediatr. Blood Cancer 2014, 61, 355–357. [Google Scholar] [CrossRef] [PubMed]
- De Bruijn, C.M.A.; Millot, F.; Suttorp, M.; Borisevich, M.; Brons, P.; Lausen, B.; De Bont, E.S.J.M. Discontinuation of imatinib in children with chronic myeloid leukaemia in sustained deep molecular remission: Results of the STOP IMAPED study. Br. J. Haematol. 2019, 185, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Shima, H.; Kada, A.; Tanizawa, A.; Yuza, Y.; Watanabe, A.; Ito, M.; Uryu, H.; Koh, K.; Imai, C.; Yoshida, N.; et al. Discontinuation of tyrosine kinase inhibitor in children with chronic myeloid leukemia (JPLSG STKI-14 study) (abstract). Blood 2019, 134 (Suppl. 1), 25. [Google Scholar] [CrossRef]
- Suttorp, M.; Metzler, M.; Millot, F. Horn of plenty: Value of the international registry for pediatric chronic myeloid leukemia. World J. Clin. Oncol. 2020, 11, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Baccarani, M.; Deininger, M.W.; Rosti, G.; Hochhaus, A.; Soverini, S.; Apperley, J.F.; Cervantes, F.; Clark, R.E.; Cortes, J.E.; Guilhot, F.; et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia. Blood 2013, 122, 872–884. [Google Scholar] [CrossRef]
- Sokal, J.E.; Baccarani, M.; Tura, S.; Fiacchini, M.; Cervantes, F.; Rozman, C.; Gomez, G.A.; Galton, D.A.G.; Canellos, G.P.; Braun, T.J. Prognostic discrimination among younger patients with chronic granulocytic leukemia: Relevance to bone marrow transplantation. Blood 1985, 66, 1352–1357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfirrmann, M.; Baccarani, M.; Saussele, S.; Guilhot, J.; Cervantes, F.; Ossenkoppele, G.; Hoffmann, V.S.; Castagnetti, F.; Hasford, J.; Hehlmann, R.; et al. Prognosis of long-term survival considering disease-specific death in patients with chronic myeloid leukemia. Leukemia 2016, 30, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.; Deininger, M.; Hochhaus, A.; Branford, S.; Radich, J.; Kaeda, J.; Baccarani, M.; Cortes, J.; Cross, N.C.P.; Druker, B.J.; et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: Review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood 2006, 108, 28–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Etienne, G.; Guilhot, J.; Rea, D.; Rigal-Huguet, F.; Nicolini, F.; Charbonnier, A.; Guerci-Bresler, A.; Legros, L.; Varet, B.; Gardembas, M.; et al. Long-term follow-up of the French Stop Imatinib (STIM1) study in patients with chronic myeloid leukemia. J. Clin. Oncol. 2017, 35, 298–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hochhaus, A.; Masszi, T.; Giles, F.T.; Radich, J.P.; Ross, D.M.; Gómez Casares, M.T.; Hellmann, A.; Stentoft, J.; Conneally, E.; García-Gutiérrez, V.; et al. Treatment-free remission following frontline nilotinib in patients with chronic myeloid leukemia in chronic phase: Results from the ENESTfreedom study. Leukemia 2017, 31, 1525–1531. [Google Scholar] [CrossRef]
- Campiotti, M.; Suter, M.B.; Guasti, L.; Piazza, R.; Gambacorti-Passerini, C.; Grandi, A.M.; Squizzato, A. Imatinib discontinuation in chronic myeloid leukaemia patients with undetectable BCR-ABL transcript level: A systematic review and a meta-analysis. Eur. J. Cancer 2017, 77, 48–56. [Google Scholar] [CrossRef]
- Richter, J.; Söderlund, S.; Lübking, A.; Dreimane, A.; Lotfi, K.; Markevärn, B.; Själander, A.; Saussele, S.; Olsson-Strömberg, U.; Stenke, L. Musculoskeletal pain in patients with chronic myeloid leukemia after discontinuation of imatinib: A tyrosine kinase inhibitor withdrawal syndrome? J. Clin. Oncol. 2014, 32, 2821–2823. [Google Scholar] [CrossRef]
- Berger, M.G.; Pereira, B.; Rousselot, P.; Cony-Makhoul, P.; Gardembas, M.; Legros, L.; Escoffre-Barbe, M.; Nicolini, F.; Saugues, S.; Lambert, C.; et al. Longer treatment duration and history of osteoarticular symptoms predispose to tyrosine kinase inhibitor withdrawal syndrome. Br. J. Haematol. 2019, 187, 337–346. [Google Scholar] [CrossRef]
- Lee, S.E.; Choi, S.Y.; Song, H.Y.; Kim, S.Y.; Choi, M.Y.; Park, J.S.; Kim, H.J.; Kim, S.H.; Zang, D.Y.; Oh, S.; et al. Imatinib withdrawal syndrome and longer duration of imatinib have a close association with a lower molecular relapse after treatment discontinuation: The KID study. Haematologica 2016, 101, 717–723. [Google Scholar] [CrossRef] [Green Version]
- Clark, R.E.; Polydoros, F.; Apperley, J.F.; Milojkovic, D.; Pocock, C.; Smith, G.; Byrne, J.L.; De Lavallade, H.; O’Brien, S.G.; Coffey, T.; et al. De-escalation of tyrosine kinase inhibitor dose in patients with chronic myeloid leukaemia with stable major molecular response (DESTINY): An interim analysis of a non-randomised, phase 2 trial. Lancet Haematol. 2017, 4, e310–e316. [Google Scholar] [CrossRef] [Green Version]
- Legros, L.; Nicolini, F.E.; Etienne, G.; Rousselot, P.; Rea, D.; Giraudier, S.; Guerci-Bresle, A.; Huguet, F.; Gardembas, M.; Escoffre, M.; et al. Second tyrosine kinase inhibitor discontinuation attempt in patients with chronic myeloid leukemia. Cancer 2017, 123, 4403–4410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legros, L.; Nicolini, F.; Etienne, G.; Rousselot, P.; Delphine, R.; Giraudier, S.; Guerci, A.; Huguet, F.; Gardembas, M.; Ianotto, J.C.; et al. The TKI-free duration after a first discontinuation attempt that failed in CP CML patients is a predictive factor of TKI-free remission after a second attempt (abstract). Blood 2019, 134 (Suppl. 1), 28. [Google Scholar] [CrossRef]
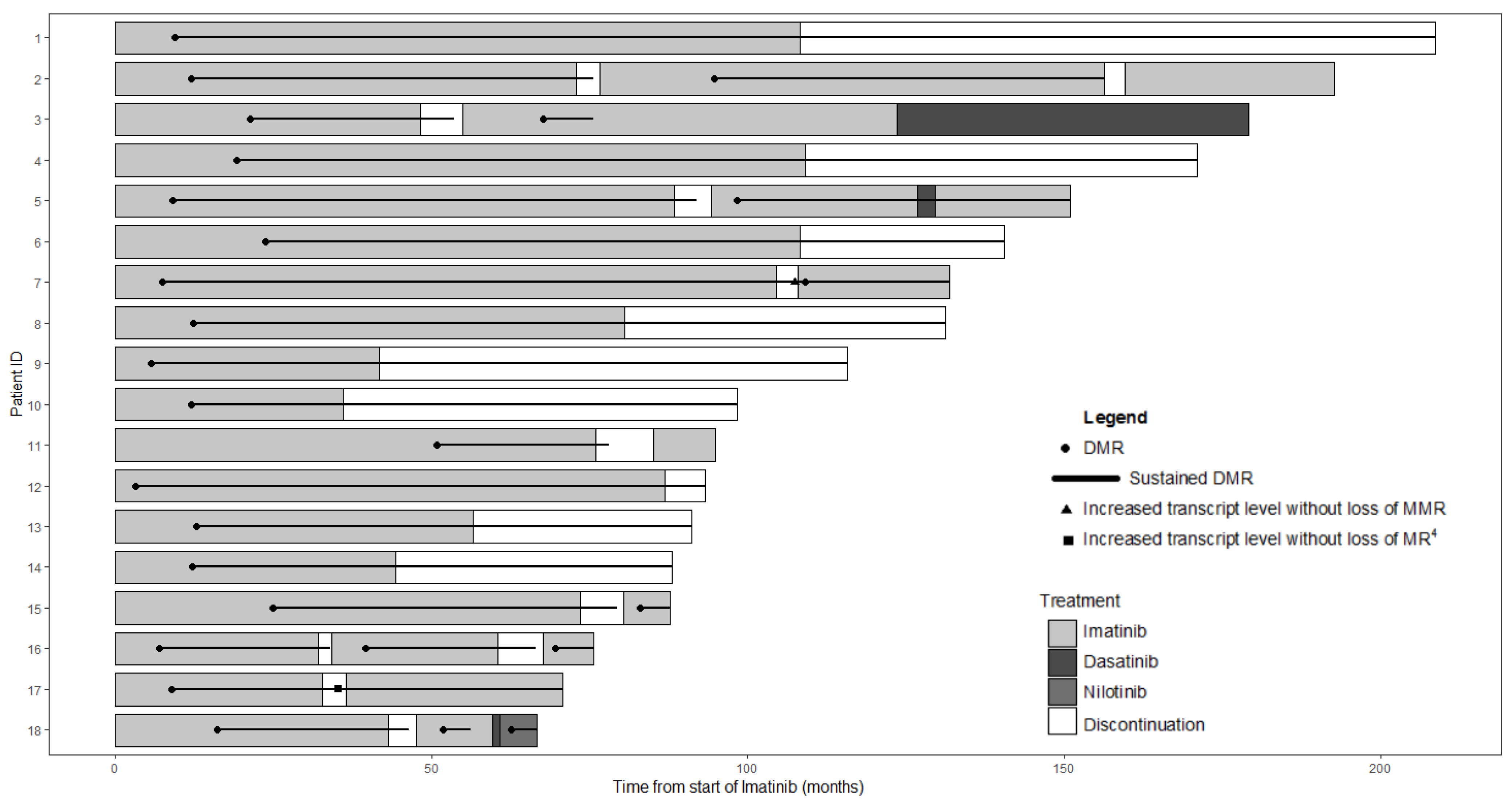
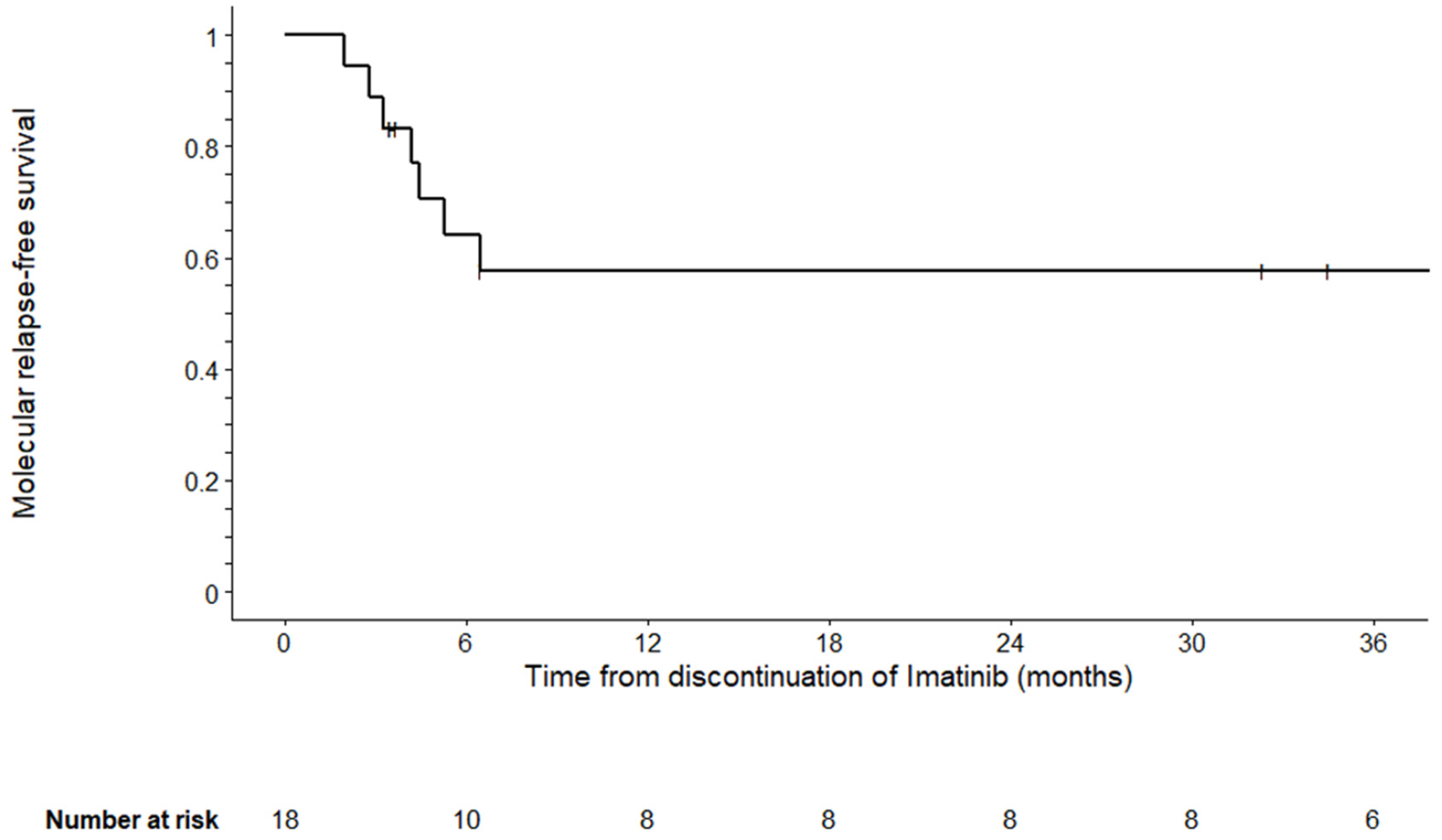
| Characteristics | N = 18 |
| Sex, N | |
| Boys | 11 |
| Girls | 7 |
| Median age at diagnosis of CML, years | 11.9 (range, 2.3–15.8) |
| Median white blood cell count at diagnosis, 10−9/L | 77.8 (19.2–352.7) |
| Transcript type | |
| b3a2 | 10 |
| b2a2 | 1 |
| b3a2 and b2a2 | 3 |
| Unknown | 4 |
| Sokal risk score (<45 years), N | |
| Low | 6 |
| Intermediate | 4 |
| High | 5 |
| Missing | 3 |
| ELTS score, N | |
| Low | 12 |
| Intermediate | 2 |
| High | 2 |
| Missing | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Millot, F.; Suttorp, M.; Ragot, S.; Leverger, G.; Dalle, J.-H.; Thomas, C.; Cheikh, N.; Nelken, B.; Poirée, M.; Plat, G.; et al. Discontinuation of Imatinib in Children with Chronic Myeloid Leukemia: A Study from the International Registry of Childhood CML. Cancers 2021, 13, 4102. https://doi.org/10.3390/cancers13164102
Millot F, Suttorp M, Ragot S, Leverger G, Dalle J-H, Thomas C, Cheikh N, Nelken B, Poirée M, Plat G, et al. Discontinuation of Imatinib in Children with Chronic Myeloid Leukemia: A Study from the International Registry of Childhood CML. Cancers. 2021; 13(16):4102. https://doi.org/10.3390/cancers13164102
Chicago/Turabian StyleMillot, Frédéric, Meinolf Suttorp, Stéphanie Ragot, Guy Leverger, Jean-Hugues Dalle, Caroline Thomas, Nathalie Cheikh, Brigitte Nelken, Marilyne Poirée, Geneviève Plat, and et al. 2021. "Discontinuation of Imatinib in Children with Chronic Myeloid Leukemia: A Study from the International Registry of Childhood CML" Cancers 13, no. 16: 4102. https://doi.org/10.3390/cancers13164102
APA StyleMillot, F., Suttorp, M., Ragot, S., Leverger, G., Dalle, J.-H., Thomas, C., Cheikh, N., Nelken, B., Poirée, M., Plat, G., Versluys, B., Lausen, B., & Borisevich, M. (2021). Discontinuation of Imatinib in Children with Chronic Myeloid Leukemia: A Study from the International Registry of Childhood CML. Cancers, 13(16), 4102. https://doi.org/10.3390/cancers13164102






