Molecularly Targeted Therapies for Gastric Cancer. State of the Art
Abstract
Simple Summary
Abstract
1. Introduction
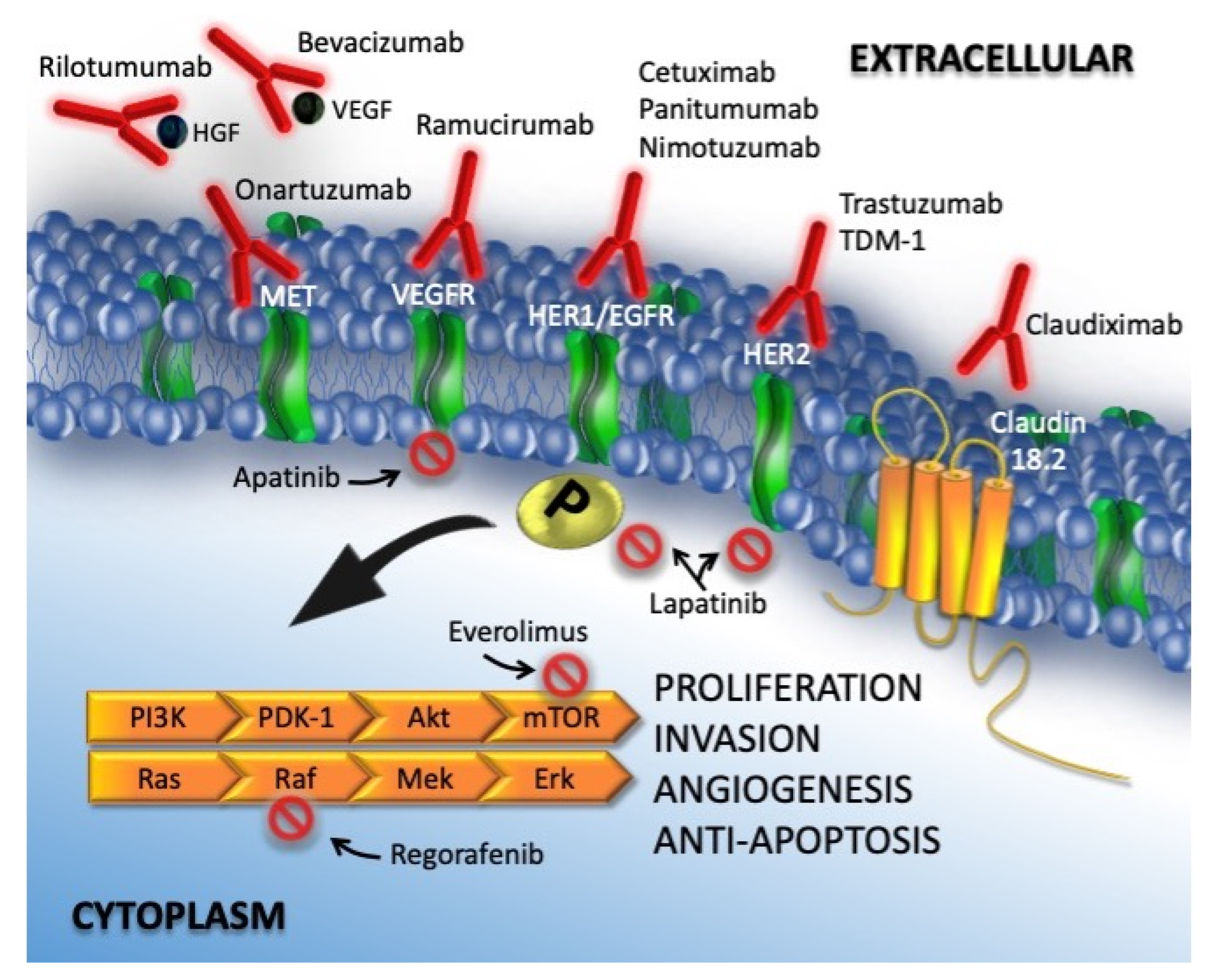
2. Molecular Targets and Target Agents
2.1. Epidermal Growth Factor Receptor
2.1.1. Anti-HER1
2.1.2. Anti HER2
2.2. Vascular Endothelial Growth Factor
2.3. Mammalian Target of Rapamycin
2.4. Hepatocyte Growth Factor Receptor
2.5. Preclinical Trials
3. Materials and Methods
3.1. Inclusion and Exclusion Criteria
3.2. Outcomes
3.3. Search Strategy
3.4. Data Selection
3.5. Quality Assessment
3.6. Statistical Analysis
4. Results
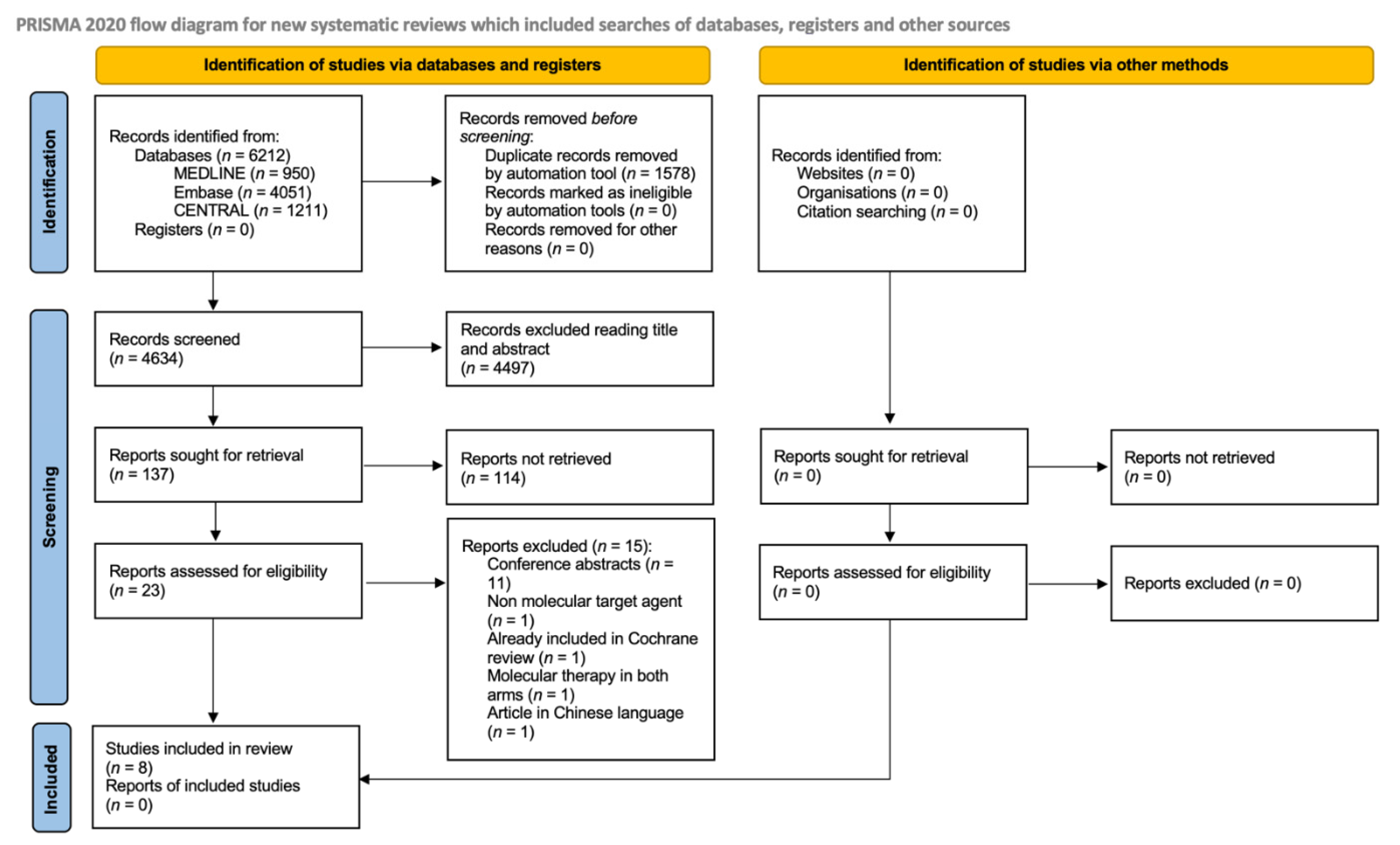
4.1. Literature Searches
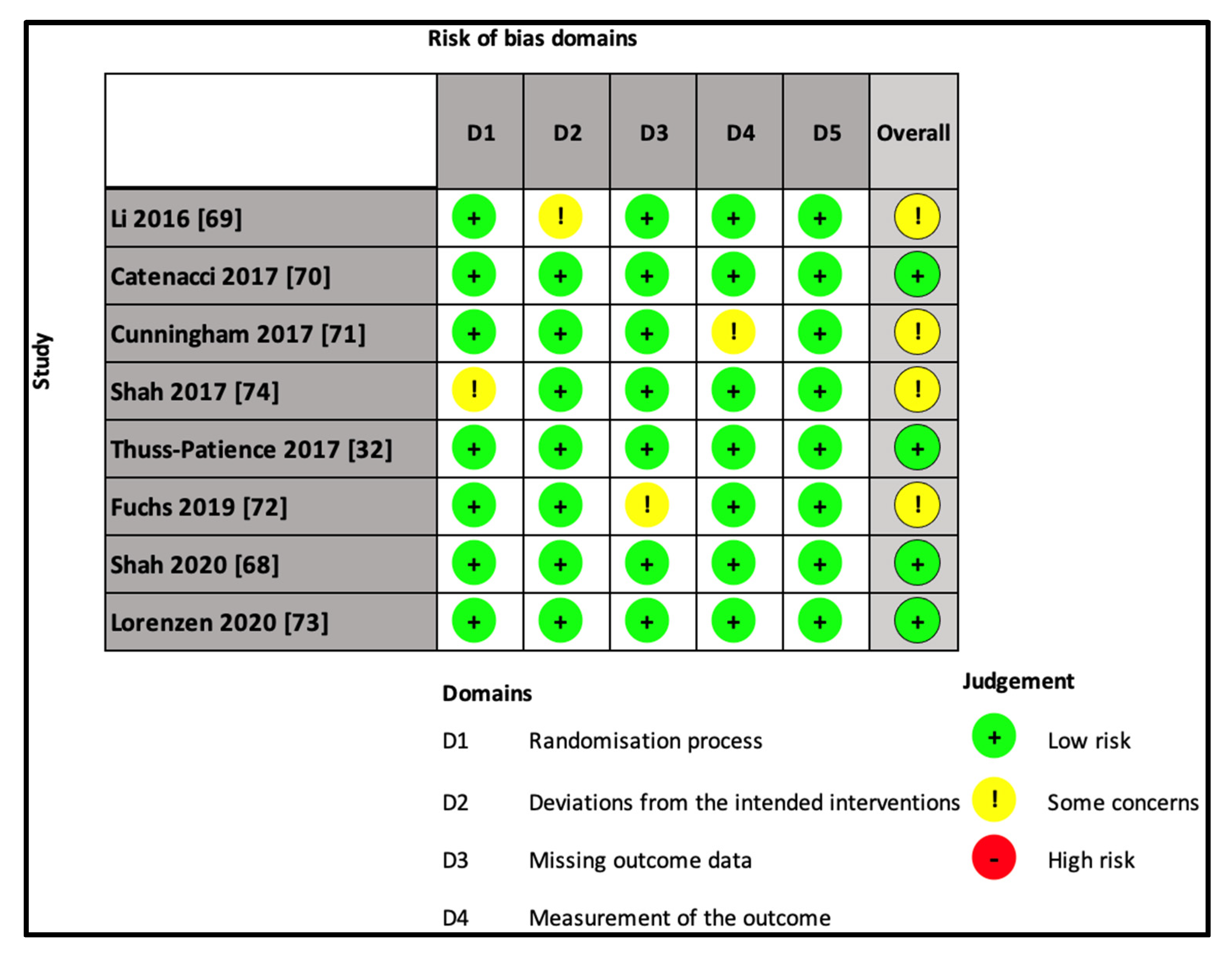
4.2. Risk of Bias in the Included Studies
4.2.1. Study Characteristics
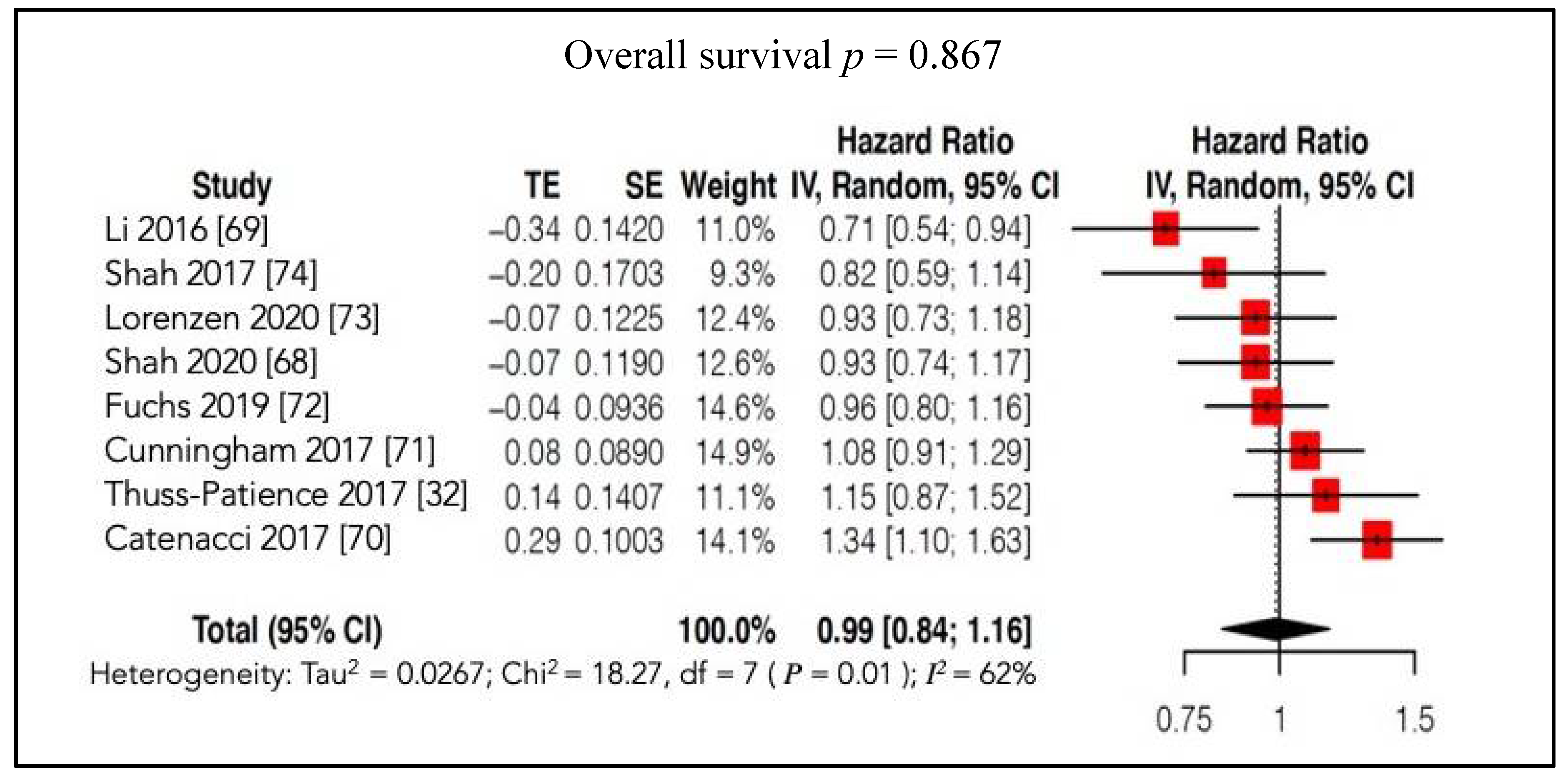
4.2.2. Survival Outcomes
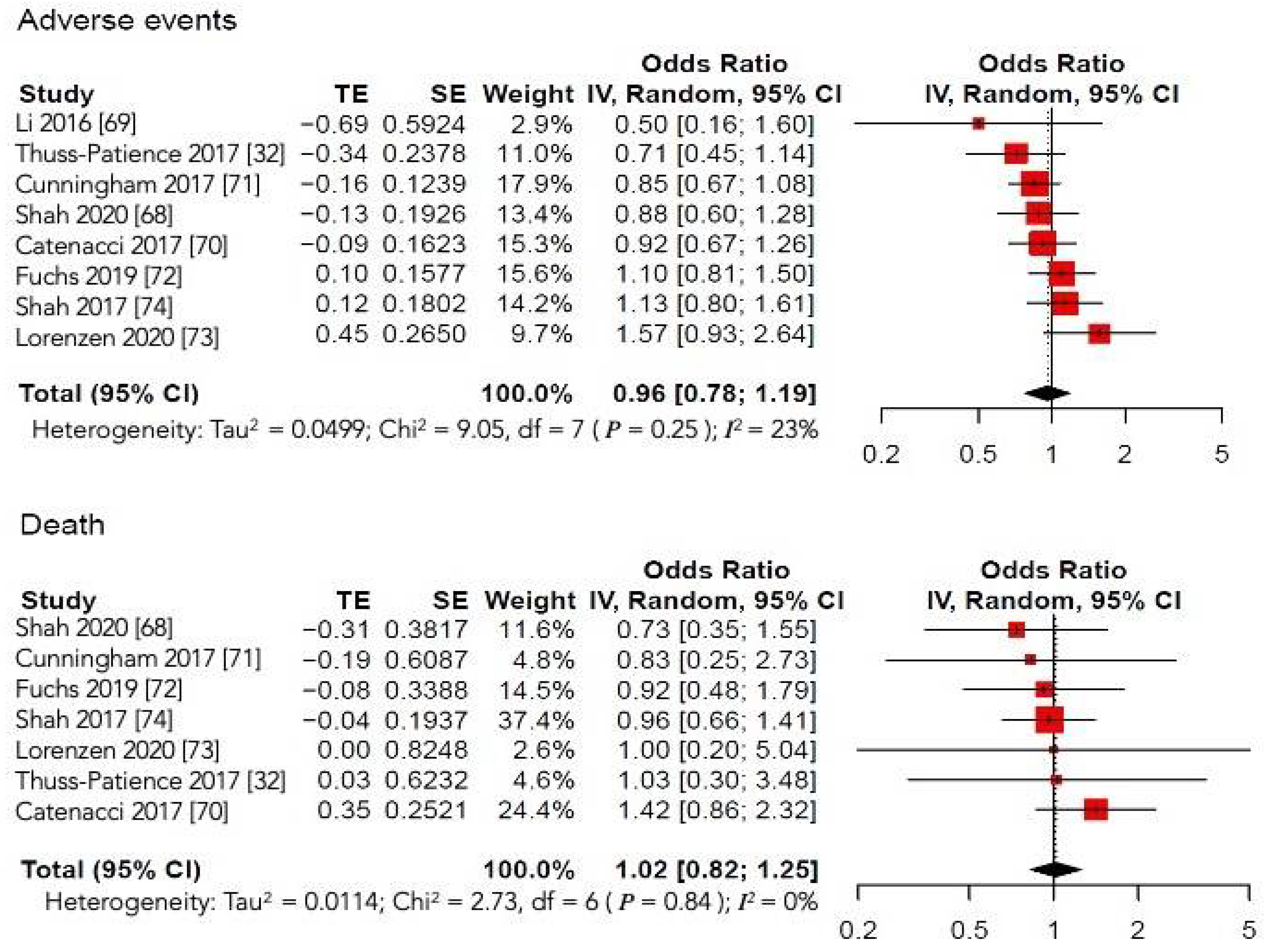
4.2.3. Secondary Outcomes
Overall Response Rate
Quality of Life
Serious Adverse Effects
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- The Italian Gastric Cancer Study Group. Randomized clinical trial comparing survival after D1 or D2 gastrectomy for gastric cancer. Br. J. Surg. 2014, 101, 23–31. [Google Scholar] [CrossRef]
- Songun, I.; Putter, H.; Kranenbarg, E.M.-K.; Sasako, M.; van de Velde, C.J. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010, 11, 439–449. [Google Scholar] [CrossRef]
- Degiuli, M.; Sasako, M.; Ponti, A.; Calvo, F. Survival results of a multicentre phase II study to evaluate D2 gastrectomy for gastric cancer. Br. J. Cancer 2004, 90, 1727–1732. [Google Scholar] [CrossRef]
- Hartgrink, H.; Van De Velde, C.; Putter, H.; Bonenkamp, J.; Kranenbarg, E.M.-K.; Songun, V.; Welvaart, K.; Van Krieken, J.; Meijer, S.; Plukker, J.; et al. Extended Lymph Node Dissection for Gastric Cancer: Who May Benefit? Final Results of the Randomized Dutch Gastric Cancer Group Trial. J. Clin. Oncol. 2004, 22, 2069–2077. [Google Scholar] [CrossRef]
- Degiuli, M.; Reddavid, R.; Tomatis, M.; Ponti, A.; Morino, M.; Sasako, M.; Rebecchi, F.; Garino, M.; Vigano, L.; Scaglione, D.; et al. D2 dissection improves disease-specific survival in advanced gastric cancer patients: 15-year follow-up results of the Italian Gastric Cancer Study Group D1 versus D2 randomised controlled trial. Eur. J. Cancer 2021, 150, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Reddavid, R.; Sofia, S.; Chiaro, P.; Colli, F.; Trapani, R.; Esposito, L.; Solej, M.; Degiuli, M. Neoadjuvant chemotherapy for gastric cancer. Is it a must or a fake? World J. Gastroenterol. 2018, 24, 274–289. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network Gastric Cancer (Version 3.2020). Available online: https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf (accessed on 5 December 2020).
- Smyth, E.C.; Verheij, M.; Allum, W.; Cunningham, D.; Cervantes, A.; Arnold, D. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, v38–v49. [Google Scholar] [CrossRef] [PubMed]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer 2020, 24, 1–21. [Google Scholar] [CrossRef]
- Atlass, A.J.; Thorsson, V.; Shmulevich, I.; Reynolds, S.M.; Miller, M.; Bernard, B.; HiNoue, T.; Laird, P.W.; Curtis, C.; Shen, H.; et al. The Cancer Genome Atlas Research Network Comprehensive molecular characterization of gastric adenocarcinoma. Nat. Cell Biol. 2014, 513, 202–209. [Google Scholar] [CrossRef]
- Bang, Y.-J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef]
- Jüttner, S.; Wiβmann, C.; Jöns, T.; Vieth, M.; Hertel, J.; Gretschel, S.; Schlag, P.M.; Kemmner, W.; Höcker, M. Vascular Endothelial Growth Factor-D and Its Receptor VEGFR-3: Two Novel Independent Prognostic Markers in Gastric Adenocarcinoma. J. Clin. Oncol. 2006, 24, 228–240. [Google Scholar] [CrossRef]
- Corso, S.; Isella, C.; Bellomo, S.E.; Apicella, M.; Durando, S.; Migliore, C.; Ughetto, S.; D’Errico, L.; Menegon, S.; Rull, D.M.; et al. A Comprehensive PDX Gastric Cancer Collection Captures Cancer Cell–Intrinsic Transcriptional MSI Traits. Cancer Res. 2019, 79, 5884–5896. [Google Scholar] [CrossRef]
- Reddavid, R.; Corso, S.; Moya-Rull, D.; Giordano, S.; Degiuli, M. Patient-Derived Orthotopic Xenograft models in gastric cancer: A systematic review. Updates Surg. 2020, 72, 951–966. [Google Scholar] [CrossRef]
- Song, H.; Zhu, J.; Lu, D. Molecular-targeted first-line therapy for advanced gastric cancer. Cochrane Database Syst. Rev. 2016, 7, CD011461. [Google Scholar] [CrossRef]
- Zhang, Z.; Tang, H.; Lin, J.; Hu, Y.; Luo, G.; Luo, Z.; Cheng, C.; Wang, P. Clinicopathologic and prognostic significance of human epidermal growth factor receptor in patients with gastric cancer: An updated meta-analysis. Oncotarget 2017, 8, 17202–17215. [Google Scholar] [CrossRef]
- Navarini, D.; Gurski, R.R.; Madalosso, C.; Aita, L.; Meurer, L.; Fornari, F. Epidermal Growth Factor Receptor Expression in Esophageal Adenocarcinoma: Relationship with Tumor Stage and Survival after Esophagectomy. Gastroenterol. Res. Pract. 2012, 2012, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Hara, M.; Nakanishi, H.; Tsujimura, K.; Matsui, M.; Yatabe, Y.; Manabe, T.; Tatematsu, M. Interleukin-2 potentiation of cetuximab antitumor activity for epidermal growth factor receptor-overexpressing gastric cancer xenografts through antibody-dependent cellular cytotoxicity. Cancer Sci. 2008, 99, 1471–1478. [Google Scholar] [CrossRef]
- Lordick, F.; Allum, W.; Carneiro, F.; Mitry, E.; Tabernero, J.; Tan, P.; Van Cutsem, E.; van de Velde, C.; Cervantes, A. Unmet needs and challenges in gastric cancer: The way forward. Cancer Treat. Rev. 2014, 40, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Rojo, F.; Tabernero, J.; Albanell, J.; Van Cutsem, E.; Ohtsu, A.; Doi, T.; Koizumi, W.; Shirao, K.; Takiuchi, H.; Cajal, S.R.; et al. Pharmacodynamic Studies of Gefitinib in Tumor Biopsy Specimens From Patients With Advanced Gastric Carcinoma. J. Clin. Oncol. 2006, 24, 4309–4316. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.P.; Adelstein, D.J.; Rice, T.W.; Rybicki, L.A.; Videtic, G.M.M.; Saxton, J.P.; Murthy, S.C.; Mason, D.P.; Ives, D.I. A Phase II Study of Perioperative Concurrent Chemotherapy, Gefitinib, and Hyperfractionated Radiation Followed by Maintenance Gefitinib in Locoregionally Advanced Esophagus and Gastroesophageal Junction Cancer. J. Thorac. Oncol. 2010, 5, 229–235. [Google Scholar] [CrossRef]
- Dragovich, T.; McCoy, S.; Fenoglio-Preiser, C.M.; Wang, J.; Benedetti, J.K.; Baker, A.F.; Hackett, C.B.; Urba, S.G.; Zaner, K.S.; Blanke, C.D.; et al. Phase II Trial of Erlotinib in Gastroesophageal Junction and Gastric Adenocarcinomas: SWOG 0127. J. Clin. Oncol. 2006, 24, 4922–4927. [Google Scholar] [CrossRef]
- Maron, S.B.; Alpert, L.; Kwak, H.A.; Lomnicki, S.; Chase, L.; Xu, D.; O’Day, E.; Nagy, R.J.; Lanman, R.B.; Cecchi, F.; et al. Targeted Therapies for Targeted Populations: Anti-EGFR Treatment for EGFR-Amplified Gastroesophageal Adenocarcinoma. Cancer Discov. 2018, 8, 696–713. [Google Scholar] [CrossRef]
- Corso, S.; Pietrantonio, F.; Apicella, M.; Migliore, C.; Conticelli, D.; Petrelli, A.; D’Errico, L.; Durando, S.; Moya-Rull, D.; Bellomo, S.E.; et al. Optimized EGFR Blockade Strategies in EGFR Addicted Gastroesophageal Adenocarcinomas. Clin. Cancer Res. 2021, 27, 3126–3140. [Google Scholar] [CrossRef]
- Hynes, N.E.; Stern, D.F. The biology of erbB-2/nue/HER-2 and its role in cancer. BBA Rev. Cancer 1994, 1198, 165–184. [Google Scholar] [CrossRef]
- Satoh, T.; Xu, R.-H.; Chung, H.; Sun, G.-P.; Doi, T.; Xu, J.-M.; Tsuji, A.; Omuro, Y.; Li, J.; Wang, J.-W.; et al. Lapatinib Plus Paclitaxel Versus Paclitaxel Alone in the Second-Line Treatment ofHER2-Amplified Advanced Gastric Cancer in Asian Populations: TyTAN—A Randomized, Phase III Study. J. Clin. Oncol. 2014, 32, 2039–2049. [Google Scholar] [CrossRef]
- Hughes, J.B.; Berger, C.; Rødland, M.S.; Hasmann, M.; Stang, E.; Madshus, I.H. Pertuzumab increases epidermal growth factor receptor down-regulation by counteracting epidermal growth factor receptor-ErbB2 heterodimerization. Mol. Cancer Ther. 2009, 8, 1885–1892. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, J.; Hoff, P.; Shen, L.; Ohtsu, A.; Shah, M.; Siddiqui, A.; Heeson, S.; Wu, H.; Restuccia, E.; Kang, Y.-K. 1423MO End-of-study analysis from JACOB: A phase III study of pertuzumab (P) + trastuzumab (H) and chemotherapy (CT) in HER2-positive metastatic gastric or gastro-esophageal junction cancer (mGC/GEJC). Ann. Oncol. 2020, 31, S900–S901. [Google Scholar] [CrossRef]
- Ughetto, S.; Migliore, C.; Pietrantonio, F.; Apicella, M.; Petrelli, A.; D’Errico, L.; Durando, S.; Moya-Rull, D.; Bellomo, S.E.; Rizzolio, S.; et al. Personalized therapeutic strategies in HER2-driven gastric cancer. Gastric Cancer 2021, 24, 897–912. [Google Scholar] [CrossRef]
- Krop, I.; Winer, E.P. Trastuzumab Emtansine: A Novel Antibody–Drug Conjugate for HER2-Positive Breast Cancer. Clin. Cancer Res. 2013, 20, 15–20. [Google Scholar] [CrossRef]
- Thuss-Patience, P.C.; Shah, M.A.; Ohtsu, A.; Van Cutsem, E.; Ajani, J.A.; Castro, H.; Mansoor, W.; Chung, H.C.; Bodoky, G.; Shitara, K.; et al. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): An international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol. 2017, 18, 640–653. [Google Scholar] [CrossRef]
- Shitara, K.; Bang, Y.-J.; Iwasa, S.; Sugimoto, N.; Ryu, M.-H.; Sakai, D.; Chung, H.-C.; Kawakami, H.; Yabusaki, H.; Lee, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Gastric Cancer. N. Engl. J. Med. 2020, 382, 2419–2430. [Google Scholar] [CrossRef]
- Jung, Y.; Mansfield, P.; Akagi, M.; Takeda, A.; Liu, W.; Bucana, C.; Hicklin, D.; Ellis, L. Effects of combination anti-vascular endothelial growth factor receptor and anti-epidermal growth factor receptor therapies on the growth of gastric cancer in a nude mouse model. Eur. J. Cancer 2002, 38, 1133–1140. [Google Scholar] [CrossRef]
- Maeda, K.; Chung, Y.-S.; Ogawa, Y.; Kang, S.-M.; Ogawa, M.; Sawada, T.; Sowa, M. Prognostic value of vascular endothelial growth factor expression in gastric carcinoma. Cancer 1996, 77, 858–863. [Google Scholar] [CrossRef]
- Presta, L.G.; Chen, H.; O’Connor, S.J.; Chisholm, V.; Meng, Y.G.; Krummen, L.; Winkler, M.; Ferrara, N. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997, 57, 4593–4599. [Google Scholar] [PubMed]
- Miller, K.; Wang, M.; Gralow, J.; Dickler, M.; Cobleigh, M.; Perez, E.A.; Shenkier, T.; Cella, D.; Davidson, N.E. Paclitaxel plus Bevacizumab versus Paclitaxel Alone for Metastatic Breast Cancer. N. Engl. J. Med. 2007, 357, 2666–2676. [Google Scholar] [CrossRef]
- Hurwitz, H.; Fehrenbacher, L.; Novotny, W.; Cartwright, T.; Hainsworth, J.; Heim, W.; Berlin, J.; Baron, A.; Griffing, S.; Holmgren, E.; et al. Bevacizumab plus Irinotecan, Fluorouracil, and Leucovorin for Metastatic Colorectal Cancer. N. Engl. J. Med. 2004, 350, 2335–2342. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.; Haworth, L.; Sherry, R.M.; Hwu, P.; Schwartzentruber, D.J.; Topalian, S.L.; Steinberg, S.M.; Chen, H.X.; Rosenberg, S.A. A Randomized Trial of Bevacizumab, an Anti–Vascular Endothelial Growth Factor Antibody, for Metastatic Renal Cancer. N. Engl. J. Med. 2003, 349, 427–434. [Google Scholar] [CrossRef]
- Ohtsu, A.; Shah, M.A.; Van Cutsem, E.; Rha, S.Y.; Sawaki, A.; Park, S.R.; Lim, H.Y.; Yamada, Y.; Wu, J.; Langer, B.; et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: A randomized, double-blind, placebo-controlled phase iii study. J. Clin. Oncol. 2011, 29, 3968–3976. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.A.; Jhawer, M.; Ilson, D.H.; Lefkowitz, R.A.; Robinson, E.; Capanu, M.; Kelsen, D.P. Phase II Study of modified docetaxel, cisplatin, and fluorouracil with bevacizumab in patients with metastatic gastroesophageal adenocarcinoma. J. Clin. Oncol. 2011, 29, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; De Haas, S.; Kang, Y.-K.; Ohtsu, A.; Tebbutt, N.C.; Xu, J.M.; Yong, W.P.; Langer, B.; Delmar, P.; Scherer, S.J.; et al. Bevacizumab in Combination With Chemotherapy As First-Line Therapy in Advanced Gastric Cancer: A Biomarker Evaluation From the AVAGAST Randomized Phase III Trial. J. Clin. Oncol. 2012, 30, 2119–2127. [Google Scholar] [CrossRef]
- Bang, Y.-J.; Kang, Y.-K.; Kang, W.K.; Boku, N.; Chung, H.; Chen, J.-S.; Doi, T.; Sun, Y.; Shen, L.; Qin, S.; et al. Phase II study of sunitinib as second-line treatment for advanced gastric cancer. Investig. New Drugs 2010, 29, 1449–1458. [Google Scholar] [CrossRef]
- Sun, W.; Powell, M.; O’Dwyer, P.J.; Catalano, P.; Ansari, R.H.; Benson, A.B. Phase II Study of Sorafenib in Combination With Docetaxel and Cisplatin in the Treatment of Metastatic or Advanced Gastric and Gastroesophageal Junction Adenocarcinoma: ECOG 5203. J. Clin. Oncol. 2010, 28, 2947–2951. [Google Scholar] [CrossRef] [PubMed]
- Spratlin, J.L.; Cohen, R.B.; Eadens, M.; Gore, L.; Camidge, D.R.; Diab, S.; Leong, S.; O’Bryant, C.; Chow, L.Q.; Serkova, N.J.; et al. Phase I Pharmacologic and Biologic Study of Ramucirumab (IMC-1121B), a Fully Human Immunoglobulin G1Monoclonal Antibody Targeting the Vascular Endothelial Growth Factor Receptor-2. J. Clin. Oncol. 2010, 28, 780–787. [Google Scholar] [CrossRef]
- Fuchs, C.S.; Tomasek, J.; Yong, C.J.; Dumitru, F.; Passalacqua, R.; Goswami, C.; Safran, H.; dos Santos, L.V.; Aprile, G.; Ferry, D.R.; et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): An international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2013, 383, 31–39. [Google Scholar] [CrossRef]
- Wilke, H.; Muro, K.; Van Cutsem, E.; Oh, S.-C.; Bodoky, G.; Shimada, Y.; Hironaka, S.; Sugimoto, N.; Lipatov, O.; Kim, T.-Y.; et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): A double-blind, randomised phase 3 trial. Lancet Oncol. 2014, 15, 1224–1235. [Google Scholar] [CrossRef]
- Bjornsti, M.-A.; Houghton, P.J. The tor pathway: A target for cancer therapy. Nat. Rev. Cancer 2004, 4, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Cejka, D.; Preusser, M.; Woehrer, A.; Sieghart, W.; Strommer, S.; Werzowa, J.; Fuereder, T.; Wacheck, V. Everolimus (RAD001) and anti-angiogenic cyclophosphamide show long-term control of gastric cancer growth in vivo. Cancer Biol. Ther. 2008, 7, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Scagliotti, G.V.; Novello, S.; von Pawel, J. The emerging role of MET/HGF inhibitors in oncology. Cancer Treat. Rev. 2013, 39, 793–801. [Google Scholar] [CrossRef]
- Graziano, F.; Galluccio, N.; Lorenzini, P.; Ruzzo, A.; Canestrari, E.; D’Emidio, S.; Catalano, V.; Sisti, V.; Ligorio, C.; Andreoni, F.; et al. Genetic Activation of the MET Pathway and Prognosis of Patients with High-Risk, Radically Resected Gastric Cancer. J. Clin. Oncol. 2011, 29, 4789–4795. [Google Scholar] [CrossRef]
- Okamoto, W.; Okamoto, I.; Arao, T.; Kuwata, K.; Hatashita, E.; Yamaguchi, H.; Sakai, K.; Yanagihara, K.; Nishio, K.; Nakagawa, K. Antitumor Action of the MET Tyrosine Kinase Inhibitor Crizotinib (PF-02341066) in Gastric Cancer Positive for MET Amplification. Mol. Cancer Ther. 2012, 11, 1557–1564. [Google Scholar] [CrossRef] [PubMed]
- Waddell, T.; Moorcraft, S.Y.; Cunningham, D. Potential role of rilotumumab in the treatment of gastric cancer. Immunotherapy 2014, 6, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- Merchant, M.; Ma, X.; Maun, H.R.; Zheng, Z.; Peng, J.; Romero, M.; Huang, A.; Yang, N.-Y.; Nishimura, M.; Greve, J.; et al. Monovalent antibody design and mechanism of action of onartuzumab, a MET antagonist with anti-tumor activity as a therapeutic agent. Proc. Natl. Acad. Sci. USA 2013, 110, E2987–E2996. [Google Scholar] [CrossRef]
- Waddell, T.; Chau, I.; Cunningham, D.; Gonzalez, D.; Okines, A.F.C.; Wotherspoon, A.; Saffery, C.; Middleton, G.; Wadsley, J.; Ferry, D.; et al. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): A randomised, open-label phase 3 trial. Lancet Oncol. 2013, 14, 481–489. [Google Scholar] [CrossRef]
- Sahin, U.; Türeci, Ö.; Manikhas, G.; Lordick, F.; Rusyn, A.; Vynnychenko, I.; Dudov, A.; Bazin, I.; Bondarenko, I.; Melichar, B.; et al. FAST: A randomised phase II study of zolbetuximab (IMAB362) plus EOX versus EOX alone for first-line treatment of advanced CLDN18.2-positive gastric and gastro-oesophageal adenocarcinoma. Ann. Oncol. 2021, 32, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Pavlakis, N.; Sjoquist, K.; Martin, A.J.; Tsobanis, E.; Yip, S.; Kang, Y.-K.; Bang, Y.-J.; Alcindor, T.; O’Callaghan, C.J.; Burnell, M.J.; et al. Regorafenib for the Treatment of Advanced Gastric Cancer (INTEGRATE): A Multinational Placebo-Controlled Phase II Trial. J. Clin. Oncol. 2016, 34, 2728–2735. [Google Scholar] [CrossRef] [PubMed]
- Hecht, J.R.; Bang, Y.-J.; Qin, S.K.; Chung, H.; Xu, J.M.; Park, J.O.; Jeziorski, K.; Shparyk, Y.; Hoff, P.M.; Sobrero, A.; et al. Lapatinib in Combination with Capecitabine Plus Oxaliplatin in Human Epidermal Growth Factor Receptor 2–Positive Advanced or Metastatic Gastric, Esophageal, or Gastroesophageal Adenocarcinoma: TRIO-013/LOGiC—A Randomized Phase III Trial. J. Clin. Oncol. 2016, 34, 443–451. [Google Scholar] [CrossRef]
- Satoh, T.; Lee, K.H.; Rha, S.Y.; Sasaki, Y.; Park, S.H.; Komatsu, Y.; Yasui, H.; Kim, T.-Y.; Yamaguchi, K.; Fuse, N.; et al. Randomized phase II trial of nimotuzumab plus irinotecan versus irinotecan alone as second-line therapy for patients with advanced gastric cancer. Gastric Cancer 2014, 18, 824–832. [Google Scholar] [CrossRef]
- Ohtsu, A.; Ajani, J.A.; Bai, Y.-X.; Bang, Y.-J.; Chung, H.; Pan, H.-M.; Sahmoud, T.; Shen, L.; Yeh, K.-H.; Chin, K.; et al. Everolimus for Previously Treated Advanced Gastric Cancer: Results of the Randomized, Double-Blind, Phase III GRANITE-1 Study. J. Clin. Oncol. 2013, 31, 3935–3943. [Google Scholar] [CrossRef]
- Lordick, F.; Kang, Y.-K.; Chung, H.; Salman, P.; Oh, S.C.; Bodoky, G.; Kurteva, G.; Volovat, C.; Moiseyenko, V.; Gorbunova, V.; et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): A randomised, open-label phase 3 trial. Lancet Oncol. 2013, 14, 490–499. [Google Scholar] [CrossRef]
- Gomez-Martin, C.; Plaza, J.C.; Pazo-Cid, R.A.; Salud, A.; Pons, F.; Fonseca, P.; Leon, A.; Alsina, M.; Visa, L.; Rivera, F.; et al. Level of HER2 Gene Amplification Predicts Response and Overall Survival in HER2-Positive Advanced Gastric Cancer Treated With Trastuzumab. J. Clin. Oncol. 2013, 31, 4445–4452. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.; Therasse, P.; Bogaerts, J.; Schwartz, L.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; De Haes, J.C.J.M.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A Quality-of-Life Instrument for Use in International Clinical Trials in Oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (Updated February 2021); Cochrane: London, UK, 2021. [Google Scholar]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.A.; Ruiz, E.P.Y.; Bodoky, G.; Starodub, A.; Cunningham, D.; Yip, D.; Wainberg, Z.A.; Bendell, J.C.; Thai, D.; Bhargava, P.; et al. A phase III, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of andecaliximab combined with mFOLFOX6 as first-line treatment in patients with advanced gastric or gastroesophageal junction adenocarcinoma (GAMMA-1). J. Clin. Oncol. 2019, 37, 4. [Google Scholar] [CrossRef]
- Li, J.; Qin, S.; Xu, J.; Xiong, J.; Wu, C.; Bai, Y.; Liu, W.; Tong, J.; Liu, Y.; Xu, R.; et al. Randomized, Double-Blind, Placebo-Controlled Phase III Trial of Apatinib in Patients With Chemotherapy-Refractory Advanced or Metastatic Adenocarcinoma of the Stomach or Gastroesophageal Junction. J. Clin. Oncol. 2016, 34, 1448–1454. [Google Scholar] [CrossRef]
- Catenacci, D.V.T.; Tebbutt, N.C.; Davidenko, I.; Murad, A.M.; Al-Batran, S.-E.; Ilson, D.H.; Tjulandin, S.; Gotovkin, E.; Karaszewska, B.; Bondarenko, I.; et al. Rilotumumab plus epirubicin, cisplatin, and capecitabine as first-line therapy in advanced MET-positive gastric or gastro-oesophageal junction cancer (RILOMET-1): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1467–1482. [Google Scholar] [CrossRef]
- Cunningham, D.; Stenning, S.P.; Smyth, E.C.; Okines, A.F.; Allum, W.H.; Rowley, S.; Stevenson, L.; Grabsch, H.I.; Alderson, D.; Crosby, T.; et al. Peri-operative chemotherapy with or without bevacizumab in operable oesophagogastric adenocarcinoma (UK Medical Research Council ST03): Primary analysis results of a multicentre, open-label, randomised phase 2–3 trial. Lancet Oncol. 2017, 18, 357–370. [Google Scholar] [CrossRef]
- Fuchs, C.S.; Shitara, K.; Di Bartolomeo, M.; Lonardi, S.; Al-Batran, S.-E.; Van Cutsem, E.; Ilson, D.H.; Alsina, M.; Chau, I.; Lacy, J.; et al. Ramucirumab with cisplatin and fluoropyrimidine as first-line therapy in patients with metastatic gastric or junctional adenocarcinoma (RAINFALL): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 420–435. [Google Scholar] [CrossRef]
- Lorenzen, S.; Knorrenschild, J.R.; Pauligk, C.; Hegewisch-Becker, S.; Seraphin, J.; Thuss-Patience, P.; Kopp, H.; Dechow, T.; Vogel, A.; Luley, K.B.; et al. Phase III randomized, double-blind study of paclitaxel with and without everolimus in patients with advanced gastric or esophagogastric junction carcinoma who have progressed after therapy with a fluoropyrimidine/platinum-containing regimen (RADPAC). Int. J. Cancer 2020, 147, 2493–2502. [Google Scholar] [CrossRef]
- Shah, M.A.; Bang, Y.-J.; Lordick, F.; Alsina, M.; Chen, M.; Hack, S.P.; Bruey, J.M.; Smith, D.; McCaffery, I.; Shames, D.S.; et al. Effect of Fluorouracil, Leucovorin, and Oxaliplatin with or Without Onartuzumab in HER2-Negative, MET-Positive Gastroesophageal Adenocarcinoma. JAMA Oncol. 2017, 3, 620–627. [Google Scholar] [CrossRef]
- Shah, M.A.; Cho, J.-Y.; Tan, I.B.; Tebbutt, N.C.; Yen, C.-J.; Kang, A.; Shames, D.S.; Bu, L.; Kang, Y.-K. A Randomized Phase II Study of FOLFOX with or Without the MET Inhibitor Onartuzumab in Advanced Adenocarcinoma of the Stomach and Gastroesophageal Junction. Oncologist 2016, 21, 1085–1090. [Google Scholar] [CrossRef]
- Eatock, M.M.; Tebbutt, N.C.; Bampton, C.L.; Strickland, A.H.; Valladares-Ayerbes, M.; Swieboda-Sadlej, A.; Van Cutsem, E.; Nanayakkara, N.; Sun, Y.N.; Zhong, Z.D.; et al. Phase II randomized, double-blind, placebo-controlled study of AMG 386 (trebananib) in combination with cisplatin and capecitabine in patients with metastatic gastro-oesophageal cancer. Ann. Oncol. 2013, 24, 710–718. [Google Scholar] [CrossRef]
- Iveson, T.; Donehower, R.C.; Davidenko, I.; Tjulandin, S.; Deptala, A.; Harrison, M.; Nirni, S.; Lakshmaiah, K.; Thomas, A.; Jiang, Y.; et al. Rilotumumab in combination with epirubicin, cisplatin, and capecitabine as first-line treatment for gastric or oesophagogastric junction adenocarcinoma: An open-label, dose de-escalation phase 1b study and a double-blind, randomised phase 2 study. Lancet Oncol. 2014, 15, 1007–1018. [Google Scholar] [CrossRef]
- Koizumi, W.; Yamaguchi, K.; Hosaka, H.; Takinishi, Y.; Nakayama, N.; Hara, T.; Muro, K.; Baba, H.; Sasaki, Y.; Nishina, T.; et al. Randomised phase II study of S-1/cisplatin plus TSU-68 vs S-1/cisplatin in patients with advanced gastric cancer. Br. J. Cancer 2013, 109, 2079–2086. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Starling, N.; Cunningham, D.; Sumpter, K.; Gilligan, D.; Ruhstaller, T.; Valladares-Ayerbes, M.; Wilke, H.; Archer, C.; Kurek, R.; et al. Matuzumab plus epirubicin, cisplatin and capecitabine (ECX) compared with epirubicin, cisplatin and capecitabine alone as first-line treatment in patients with advanced oesophago-gastric cancer: A randomised, multicentre open-label phase II study. Ann. Oncol. 2010, 21, 2213–2219. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Li, J.; Xu, J.; Pan, H.; Dai, G.; Qin, S.; Wang, L.; Wang, J.; Yang, Z.; Shu, Y.; et al. Bevacizumab plus capecitabine and cisplatin in Chinese patients with inoperable locally advanced or metastatic gastric or gastroesophageal junction cancer: Randomized, double-blind, phase III study (AVATAR study). Gastric Cancer 2014, 18, 168–176. [Google Scholar] [CrossRef]
- Zhang, Z.D.; Kong, Y.; Yang, W.; Zhang, B.; Zhang, Y.L.; Ma, E.M.; Liu, H.X.; Chen, X.B.; Hua, Y.W. Clinical evaluation of cetuximab combined with an S-1 and oxaliplatin regimen for Chinese patients with advanced gastric cancer. World J. Surg. Oncol. 2014, 12, 115. [Google Scholar] [CrossRef]
- Chen, H.-D.; Zhou, J.; Wen, F.; Zhang, P.-F.; Zhou, K.-X.; Zheng, H.-R.; Yang, Y.; Li, Q. Cost-effectiveness analysis of apatinib treatment for chemotherapy-refractory advanced gastric cancer. J. Cancer Res. Clin. Oncol. 2017, 143, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Xu, Y.; Wu, B. Cost-effectiveness and budget impact analysis of apatinib for advanced metastatic gastric cancer from the perspective of health insurance system. Gastroenterol. Res. Pract. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Peng, L.; Tan, C.; Zeng, X.; Wan, X.; Luo, X.; Yi, L.; Li, J. Cost-Effectiveness of ramucirumab plus paclitaxel as a second-line therapy for advanced gastric or gastro-oesophageal cancer in China. PLoS ONE 2020, 15, e0232240. [Google Scholar] [CrossRef]
- Saito, S.; Muneoka, Y.; Ishikawa, T.; Akazawa, K. Cost-effectiveness of Paclitaxel + Ramucirumab Combination Therapy for Advanced Gastric Cancer Progressing After First-line Chemotherapy in Japan. Clin. Ther. 2017, 39, 2380–2388. [Google Scholar] [CrossRef]
- Apicella, M.; Corso, S.; Giordano, S. Targeted therapies for gastric cancer: Failures and hopes from clinical trials. Oncotarget 2017, 8, 57654–57669. [Google Scholar] [CrossRef]
- Liu, X.; Meltzer, S.J. Gastric Cancer in the Era of Precision Medicine. CMGH 2017, 3, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Kwak, E.L.; LoRusso, P.; Hamid, O.; Janku, F.; Kittaneh, M.; Catenacci, D.V.T.; Chan, E.; Bekaii-Saab, T.S.; Amore, B.; Hwang, Y.C.; et al. Clinical activity of AMG 337, an oral MET kinase inhibitor, in adult patients (pts) with MET-amplified gastroesophageal junction (GEJ), gastric (G), or esophageal (E) cancer. J. Clin. Oncol. 2015, 33, 1. [Google Scholar] [CrossRef]
- Asioli, S.; Maletta, F.; Verdun Di Cantogno, L.; Satolli, M.A.; Schena, M.; Pecchioni, C.; Botta, C.; Chiusa, L.; Molinaro, L.; Conti, L.; et al. Approaching heterogeneity of human epidermal growth factor receptor 2 in surgical specimens of gastric cancer. Hum. Pathol. 2012, 43, 2070–2079. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gerlinger, M.; Rowan, A.J.; Horswell, S.; Larkin, J.; Endesfelder, D.; Gronroos, E.; Martinez, P.; Matthews, N.; Stewart, A.; Tarpey, P.; et al. Intratumor Heterogeneity and Branched Evolution Revealed by Multiregion Sequencing. N. Engl. J. Med. 2012, 366, 883–892. [Google Scholar] [CrossRef]
- Apicella, M.; Migliore, C.; Capelôa, T.; Menegon, S.; Cargnelutti, M.; Degiuli, M.; Sapino, A.; Sottile, A.; Sarotto, I.; Casorzo, L.; et al. Dual MET/EGFR therapy leads to complete response and resistance prevention in a MET-amplified gastroesophageal xenopatient cohort. Oncogene 2017, 36, 1200–1210. [Google Scholar] [CrossRef] [PubMed]
- Smolen, G.A.; Sordella, R.; Muir, B.; Mohapatra, G.; Barmettler, A.; Archibald, H.; Kim, W.J.; Okimoto, R.A.; Bell, D.W.; Sgroi, D.C.; et al. Amplification of MET may identify a subset of cancers with extreme sensitivity to the selective tyrosine kinase inhibitor PHA-665752. Proc. Natl. Acad. Sci. USA 2006, 103, 2316–2321. [Google Scholar] [CrossRef] [PubMed]
- Corso, S.; Ghiso, E.; Cepero, V.; Sierra, J.R.; Migliore, C.; Bertotti, A.; Trusolino, L.; Comoglio, P.M.; Giordano, S. Activation of HER family members in gastric carcinoma cells mediates resistance to MET inhibition. Mol. Cancer 2010, 9, 121. [Google Scholar] [CrossRef] [PubMed]
- Corso, S.; Comoglio, P.M.; Giordano, S. Cancer therapy: Can the challenge be MET? Trends Mol. Med. 2005, 11, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, M.; Amant, F.; Biankin, A.V.; Budinská, E.; Byrne, A.T.; Caldas, C.; Clarke, R.B.; de Jong, S.; Jonkers, J.; Mælandsmo, G.M.; et al. Patient-derived Xenograft models: An emerging platform for translational cancer research. Cancer Discov. 2014, 4, 998–1013. [Google Scholar] [CrossRef] [PubMed]
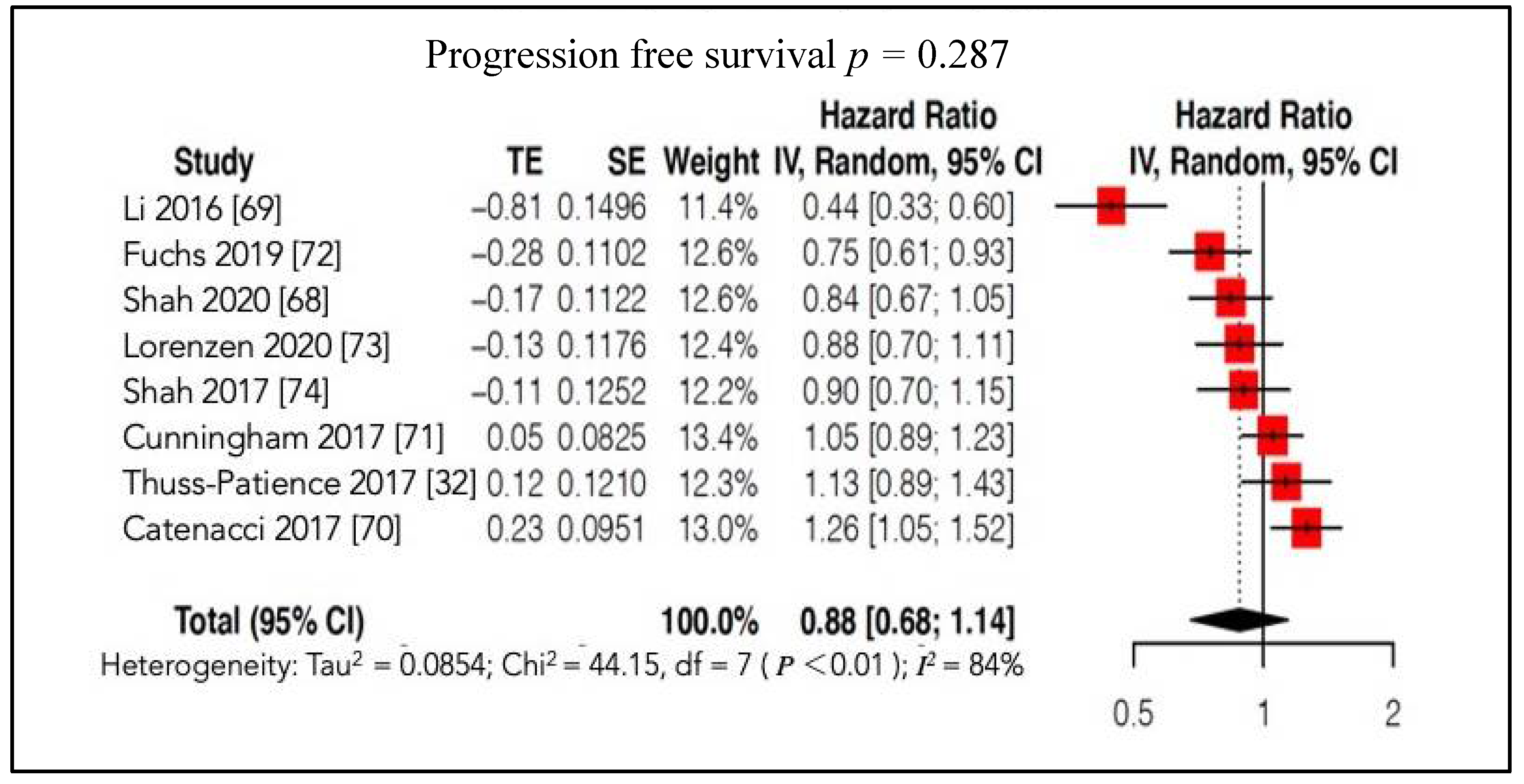
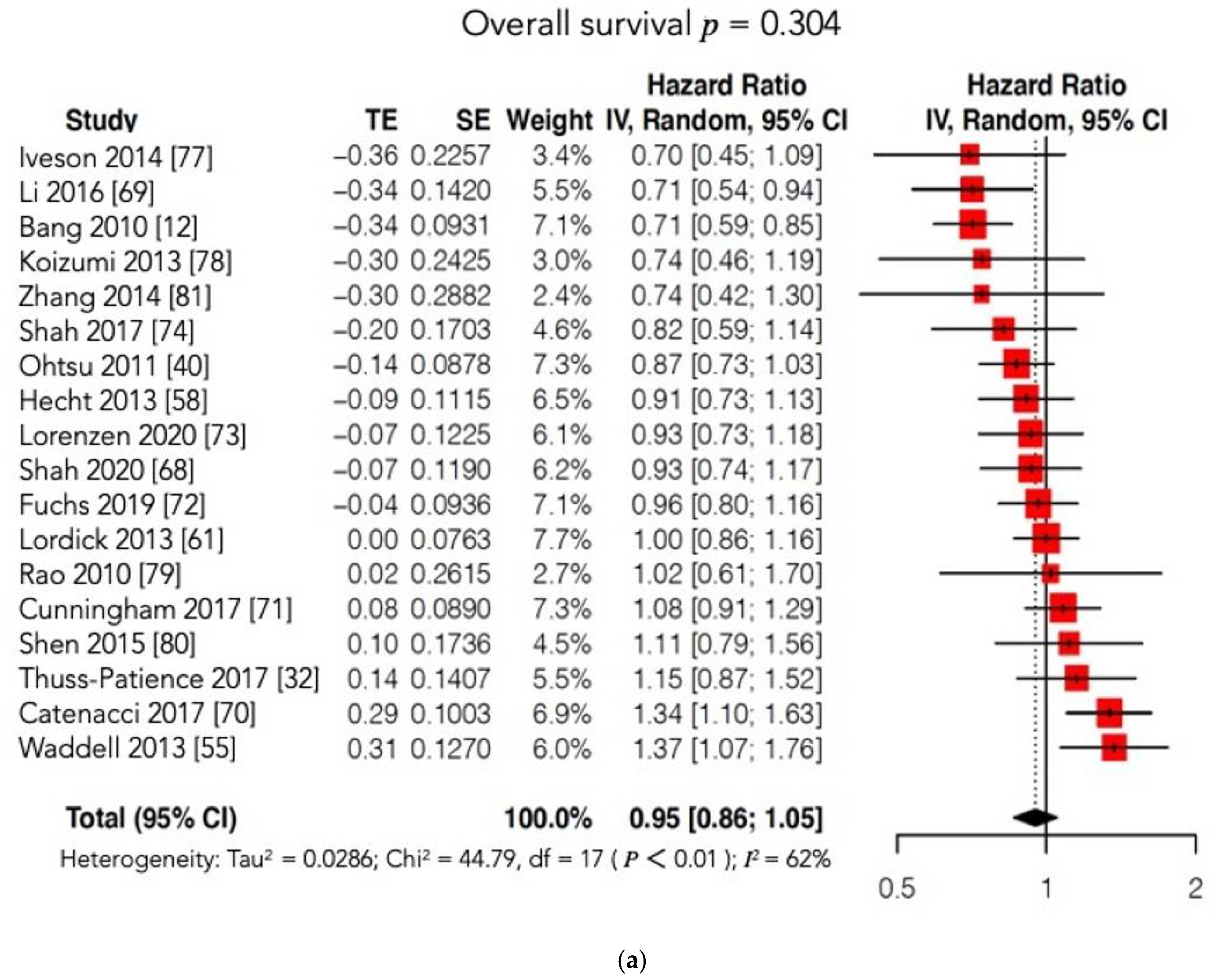
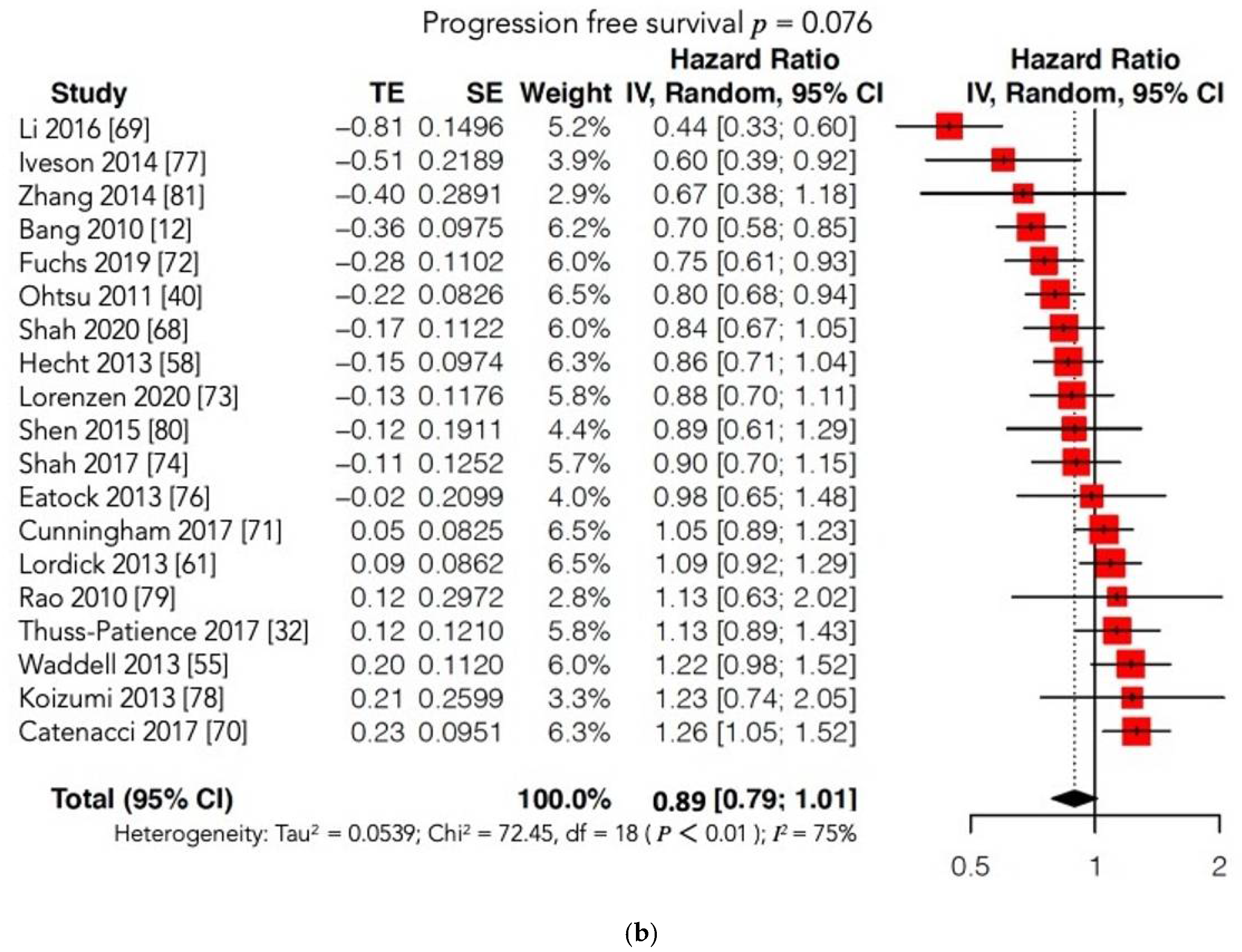
| Trial, Year | EXP Arm | CTR Arm | Molecular Target | Nr Total Pts (EXP/CTR) | Treatment Line | Phase | Median OS (Months) | Median PFS (Months) | Results |
|---|---|---|---|---|---|---|---|---|---|
| REAL-3 [55], 2009 | EOC + Panitumumab | EOC | EGFR | 553 | I | III | 11.3 CTR arm 8.8 EXP arm 95%CI: 1.07–1.76 p = 0.013 HR = 1.37 | 7.4 CTR arm 6.0 EXP arm 95%CI: 0.98–1.52 p = 0.068 HR = 1.22 |  |
| AVAGAST [40], 2012 | XP + Bevacizumab | XP + Placebo | VEGF | 774 | I | III | 10.1 CTR arm 12.1 EXP arm 95%CI: 0.73–1.03 p = 0.1002 HR = 0.87 | 5.3 CTR arm 6.7 EXP arm 95%CI: 0.68–0.93 p = 0.0037 HR = 0.80 |  |
| FAST [56], 2012 | EOX + Claudiximab | EOX | Claudin 18.2 | 161 | I | II | 8.4 CTR arm 13.4 EXP arm 95%CI: 0.36–0.73 p < 0.001 HR = 0.51 | 4.8 CTR arm 7.9 EXP arm 95%CI: 0.31–0.70 p = 0.0001 HR = 0.47 |  |
| INTEGRATE [57], 2012 | Regorafenib | Placebo | VEGF, RET, RAF | 147 | II III | II | 4.5 CTR arm 5.3 EXP arm 95%CI: 0.51–1.08 p = 0.147 HR = 0.74 | 0.9 CTR arm 2.6 EXP arm 95%CI: 0.28–0.59 p < 0.001 HR = 0.4 |  |
| ENRICH (NCT01813253), 2013 | Irinotecan + Nimotuzumab | Irinotecan | EGFR | 400 | II | III | NO RESULT POSTED | NO RESULT POSTED |  |
| LOGiC [58], 2013 | XELOX + Lapatinib | XELOX + Placebo | HER2 | 545 | I | III | 10.5 CTR arm 12.2 EXP arm 95%CI: 0.73–1.12 p = 0.3492 HR = 0.91 | 5.4 CTR arm 6.0 EXP arm 95%CI: 0.68–1 p = 0.0381 HR = 0.82 |  |
| JapicCTI-090849 [59], 2014 | Irinotecan + Nimotuzumab | Irinotecan | EGFR | 83 | II | II | 7.7 CTR arm 8.4 EXP arm 95%CI: 0.618–1.599 p = 0.9778 HR = 0.994 | 2.9 CTR arm 2.4 EXP arm 95%CI: 0.516–1.435 p = 0.5668 HR = 0.860 |  |
| RAINBOW [47], 2014 | Paclitaxel + Ramucirumab | Paclitaxel + Placebo | VEGFR2 | 665 | II | III | 7.36 CTR arm 9.63 EXP arm 95%CI: 0.678–0.962 p = 0.0169 HR = 0.807 | 2.86 CTR arm 4.4 EXP arm 95%CI: 0.536–0.752 p < 0.0001 HR = 0.635 |  |
| REGARD [46], 2014 | Ramucirumab | Placebo | VEGFR2 | 355 | II | III | 3.8 CTR arm 5.2 EXP arm 95%CI: 0.603–0.998 p = 0.047 HR = 0.776 | 1.3 CTR arm 2.1 EXP arm 95%CI: 0.376–0.620 p < 0.0001 HR = 0.483 |  |
| ToGA [12], 2014 | FP/XP + Trastuzumab | FP/XP | HER2 | 594 | I | III | 11.1 CTR arm 13.8 EXP arm 95%CI: 0.60–0.91 p = 0.0046 HR = 0.74 | 5.5 CTR arm 6.7 EXP arm 95%CI: 0.59–0.85 p = 0.0002 HR = 0.71 |  |
| TyTAN [27], 2014 | PTX + Lapatinib | PTX | HER2 | 261 | II | III | 8.9 CTR arm 11 EXP arm 95%CI: 0.64–1.11 p = 0.1044 HR = 0.84 | 4.4 CTR arm 5.4 EXP arm 95%CI: 0.63–1.13 p = 0.2241 HR = 0.85 |  |
| GRANITE-1 [60], 2015 | Everolimus | Placebo | mTOR | 656 | II III | III | 4.34 CTR arm 5.39 EXP arm 95%CI: 0.75–1.08 p = 0.1244 HR = 0.90 | 1.41 CTR arm 1.68 EXP arm 95%CI: 0.56–0.78 p < 0.0001 HR = 0.66 |  |
| EXPAND [61], 2016 | XP + Cetuximab | XP | EGFR | 904 | I | III | 10.7 CTR arm 9.4 EXP arm 95%CI: 0.87–1.17 p = 0.95 HR = 1 | 5.6 CTR arm 4.4 EXP arm 95%CI: 0.92–1.29 p = 0.32 HR = 1.09 |  |
 positive study.
positive study.  partially positive study.
partially positive study.  negative study.
negative study.| Author, Year, Acronym | Nr Total Pts (EXP/CTR) | Treatment Line (%) | Primary Endpoint | Setting | Molecular Target | EXP Arm | CTR Arm | Tumor Stage (%) | Tumor Site (%) | Notes |
|---|---|---|---|---|---|---|---|---|---|---|
| Li [69], 2016 | 267 (176/91) | III (65.1) IV(34.8) | OS, PFS | Adj | VEGFR-2 | Apatinib | Placebo | III IV | Stomach (41.9) GEJ (13.5) | |
| Shah [74], 2017, METGastric | 562 (279/283) | I (81.7) II (18.3) | OS | Adj | MET | Onartuzumab + FOLFOX6 | Placebo + FOLFOX6 | IV | Stomach (76.9) GEJ (23.1) | Enrollment was stopped early due to sponsor decision, which was agreed with the IDMC, due to a lack of efficacy in a phase II trial also assessing mFOLFOX6 plus onartuzumab |
| Thuss-Patience [32], 2017, GATSBY | 345 (228/117) | II | OS | Adj | HER-2 | Trastuzumab + Emtasine | Taxane | III (4) IV (95.9) | Stomach (68.1) GEJ (31.9) | |
| Catenacci [70], 2017RILOMET-1 | 609 (304/305) | I | OS | Adj | HGF | Rilotumumab + ECX | Placebo + ECX | IV (93.1) III (6.9) | Stomach (69.3) GEJ (20.4) Distal esophagus (10.3) | Study treatment was stopped early after a higher number of deaths in the rilotumab group. |
| Cunningham [71], 2017, UK Medical Research Council ST03 | 1063 (533/530) | I | OS | Periop | VEGF | Bevacizumab + ECX | ECX | Early (0.6) Advanced (91.7) Metastatic (0.19) | Stomach (55.7) GEJ (30.8) Distal esophagus (13.5) | EGJ type III was classified as gastric cancer |
| Fuchs [72], 2019, RAINFALL | 645 (326/319) | I | PFS | Adj | VEGFR-2 | Ramucirumab + Fluoropyrimidine + Cisplatin | Placebo + Fluoropyrimidine + cisplatin | IV (100) | Stomach (74.6) EGJ (25.3) | |
| Lorenzen [73], 2020, RADPAC | 300(150/150) | II (57.7) III (31.7) IV (10.7) | OS | Adj | mTOR | Paclitaxel + Everolimus | Placebo + Paclitaxel | III IV | Stomach (41) GEJ (58.7) | |
| Shah [68], 2020 GAMMA-1 | 432 (218/214) | I | OS | Adj | MMP9 | Andecaliximab + mFOLFOX6 | Placebo + mFOLFOX6 | IIIIV | Stomach (66) GEJ (34) | |
| Summary of Findings | 4223 (2214/2009) | I (76) II (14.7) III (6.4) IV (3) | OS (87.5%) PFS (25%) | Adj 9 (87.5%) Periop 1 (12.5) | Stomach (63.5) EGJ (28.7) Esophagus (4.9) | 2 trials were stopped early |
| OS | PFS | Results | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author, Year, Acronym | EXP | CTR | Nr | HR | Low | High | p Value | HR | Low | High | p Value | |
| Li [69], 2016 | Apatinib | Placebo | 267 (176/91) | 0.709 | 0.537 | 0.937 | 0.015 | 0.444 | 0.331 | 0.595 | <0.001 |  |
| Shah [74], 2017, METGastric | Onartuzumab + FOLFOX6 | Placebo + FOLFOX6 | 562 (279/283) | 0.82 | 0.59 | 1.15 | 0.24 | 0.90 | 0.71 | 1.16 | 0.43 |  |
| Thuss-Patience [32], 2017, GATSBY | Trastuzumab + Emtasine | Taxane | 345 (228/117) | 1.15 | 0.87 | 1.51 | 0.86 | 1.13 | 0.89 | 1.43 | 0.31 |  |
| Catenacci [70], 2017, RILOMET-1 | Rilotumab + ECX | Placebo + ECX | 609 (304/305) | 1.34 | 1.10 | 1.63 | 0.003 | 1.26 | 1.04 | 1.51 | 0.016 |  |
| Cunningham [71], 2017, UK Medical Research Council ST03 | Bevacizumab + ECX | ECX | 1063 (533/530) | 1.08 | 0.91 | 1.29 | 0.36 | 1.05 | 0.89 | 1.23 | 0.56 |  |
| Fuchs [72], 2019, RAINFALL | Ramucirumab + Fluoropyrimidine + Cisplatin | Placebo + Fluoropyrimidine + Cisplatin | 645 (326/319) | 0.962 | 0.801 | 1.156 | 0.68 | 0.753 | 0.607 | 0.935 | 0.011 |  |
| Lorenzen [73], 2020, RADPAC | Paclitaxel + Everolimus | Placebo + Paclitaxel | 300 (150/150) | 0.93 | 0.73 | 1.18 | 0.544 | 0.88 | 0.70 | 1.11 | 0.273 |  |
| Shah [68], 2020 GAMMA-1 | Andecaliximab + mFOLFOX6 | Placebo + mFOLFOX6 | 432 (218/214) | 0.93 | 0.74 | 1.18 | 0.56 | 0.84 | 0.67 | 1.04 | 0.10 |  |
 positive study.
positive study.  negative study.
negative study.| Author, Year, Acronym | Overall Response Rate | Quality of Life EORTC QLQ-C30 | ||
|---|---|---|---|---|
| EXP arm (%) | CTR arm (%) | p value | ||
| Li [69], 2016 | 2.84 | 0.0 | 0.1695 | No differences (p > 0.05) |
| Shah [74], 2017, METGastric | 40.6 | 46.1 | 0.25 | nd |
| Thuss-Patience [32], 2017, GATSBY | 20.6 | 19.6 | 0.8406 | nd |
| Catenacci [70], 2017, RILOMET-1 | 29.8 | 44.6 | 0.0005 | nd |
| Cunningham [71], 2017, UK Medical Research Council ST03 | 41 | 42 | 0.70 | nd |
| Fuchs [72], 2019, RAINFALL | 41.1 | 36.4 | 0.17 | HR 1.029 (0.786, 1.347) |
| Lorenzen [73], 2020, RADPAC | 8 | 7.3 | nd | nd |
| Shah [68], 2020, GAMMA-1 | 50.5 | 41.1 | 0.049 | nd |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reddavid, R.; Dagatti, S.; Franco, C.; Puca, L.; Tomatis, M.; Corso, S.; Giordano, S.; Degiuli, M. Molecularly Targeted Therapies for Gastric Cancer. State of the Art. Cancers 2021, 13, 4094. https://doi.org/10.3390/cancers13164094
Reddavid R, Dagatti S, Franco C, Puca L, Tomatis M, Corso S, Giordano S, Degiuli M. Molecularly Targeted Therapies for Gastric Cancer. State of the Art. Cancers. 2021; 13(16):4094. https://doi.org/10.3390/cancers13164094
Chicago/Turabian StyleReddavid, Rossella, Simona Dagatti, Caterina Franco, Lucia Puca, Mariano Tomatis, Simona Corso, Silvia Giordano, and Maurizio Degiuli. 2021. "Molecularly Targeted Therapies for Gastric Cancer. State of the Art" Cancers 13, no. 16: 4094. https://doi.org/10.3390/cancers13164094
APA StyleReddavid, R., Dagatti, S., Franco, C., Puca, L., Tomatis, M., Corso, S., Giordano, S., & Degiuli, M. (2021). Molecularly Targeted Therapies for Gastric Cancer. State of the Art. Cancers, 13(16), 4094. https://doi.org/10.3390/cancers13164094







