Simple Summary
Pheochromocytoma/paraganglioma (PPGL) has been recognised as one of the most frequent inherited tumours with genetic heterogeneity based on studies in Caucasian populations. Early identification of germline variants is crucial for accurate treatment and follow-up in affected patients and relatives. However, there are only a few large cohort studies in Asia and none from the Japanese population. In this first comprehensive study of Japanese patients with PPGL, we found one in four PPGLs with apparently sporadic presentation harboured germline variant in any of the seven susceptibility genes (MAX, SDHB, SDHC, SDHD, TMEM127, VHL, and RET). SDHB was the most frequently mutated gene and was strongly associated with metastatic PPGLs. Our findings emphasise the importance of genetic testing in determining appropriate treatment and follow-up strategies for patients and relatives.
Abstract
The high incidence of germline variants in pheochromocytoma and paraganglioma (PPGL) has been reported mainly in Europe, but not among Japanese populations in Asia. We aimed to study the prevalence of germline variants in Japanese PPGL patients and the genotype–phenotype correlation. We examined 370 PPGL probands, including 43 patients with family history and/or syndromic presentation and 327 patients with apparently sporadic (AS) presentation. Clinical data and blood samples were collected, and the seven major susceptibility genes (MAX, SDHB, SDHC, SDHD, TMEM127, VHL, and RET) were tested using Sanger sequencing. Overall, 120/370 (32.4%) patients had pathogenic or likely pathogenic variants, with 81/327 (24.8%) in AS presentation. SDHB was the most frequently mutated gene (57, 15.4%), followed by SDHD (27, 7.3%), and VHL (18, 4.9%). The incidence of metastatic PPGL was high in SDHB carriers (21/57, 36.8%). A few unique recurrent variants (SDHB c.137G>A and SDHB c.470delT) were detected in this Japanese cohort, highlighting ethnic differences. In summary, almost a quarter of patients with apparently sporadic PPGL in Japan harboured germline variants of the targeted genes. This study reinforces the recommendation in Western guidelines to perform genetic testing for PPGL and genotype-based clinical decision-making in the Japanese population.
1. Introduction
Pheochromocytoma (PCC) and paraganglioma (PGL) are neuroendocrine tumours derived from the chromaffin cells in the adrenal medulla and autonomic nervous system ganglia, respectively. Despite the anatomical distinction, PCC and PGL share a common pathological basis and genetic background and are collectively referred to as pheochromocytoma/paraganglioma (PPGL) [1,2]. PPGL can secrete catecholamines, the plasma or urinary metabolites which are essential for biochemical diagnosis [3], while excess catecholamines can cause cardio- or cerebrovascular complications. All PPGLs have the potential to metastasise to non-chromaffin tissues including bone, lung, liver, and lymph nodes; thus, the prefix term “benign” was abandoned for these tumours in the 2017 World Health Organization (WHO) classification of endocrine tumours [4].
PPGL is now considered to be the most frequent inherited tumour with genetic heterogeneity. Germline variants are present in around 30% of PPGLs [5,6,7,8]; more than 20 susceptibility genes have been identified over the last two decades [9,10]. Even in PPGL with apparently sporadic (AS) presentation (no familial/syndromic [FS] characteristics), pathogenic variants have been found with a frequency of 11–24% [11,12,13]. In this context, current guidelines recommend that germline genetic testing should be considered in all patients with PPGLs regardless of family history [2,14,15]. Identification of a predisposing germline variant enables risk assessment of distant metastases in probands and clinical surveillance of variant carriers in healthy relatives [1,2,9,16].
The genetic aetiology and genotype-phenotype relationship of PPGL have been studied extensively in Caucasian populations [5,6,11,17,18,19]. Recently, a large population study of 719 Chinese and 919 European patients demonstrated broad Sino-European differences in the genetic landscape and clinical presentation of PPGL [20]. This study suggested that the genetic background of PPGL and the associated genotype–phenotype relationship established in Caucasian populations may not apply to Asian populations. It is also worth noting that in the few cohort studies of PPGL in Asia [21,22,23], the profiles of the most common mutated gene and the recurring SDHB variants vary widely by ethnicity even within Asia. In several case reports and case series from Japan, characteristic variants with PPGL have been reported [24,25,26,27,28]; however, there are no comprehensive national studies. We aimed to investigate the prevalence of germline variants in the major susceptibility genes (MAX, SDHB, SDHC, SDHD, TMEM127, VHL, and RET) in the Japanese population with PPGL. Furthermore, we evaluated variant classification based on several updated databases and in silico meta-prediction tools and summarised the clinical and genetic features of patients with PPGLs in Japan.
2. Results
2.1. Clinical Characteristics of the Study Population
Overall, 370 probands were enrolled in the study (166 males and 204 females; mean age: 43.3 years; range: 6–83). Table 1 summarises the clinical characteristics for the whole study cohort and the FS and AS groups. PCC was present in 153 (41.4%) patients, and 31 of these patients had a bilateral PCC. There were 194 (53.0%) patients with extra-adrenal PGL; 79 (21.4%) had head and neck PGL (HNPGL), of which 6 were bilateral HNPGLs; and 116 (31.4%) had abdominal/thoracic PGL (ATPGL), of which 10 were multiple. Twenty-two (5.9%) patients had multifocal tumours, of which 17 had comorbid tumours of PCC and ATPGL, three had both PCC and HNPGL, and two had both HNPGL and ATPGL. Metastatic PPGL was observed in 63 (17.0%) patients. A positive FS presentation was observed in 43 (11.6%) patients in the entire study cohort. The other 327 (88.4%) patients had an AS presentation. The FS group were diagnosed at a younger age than the AS group (36.0 ± 13.9 vs. 44.3 ± 15.8 years, p = 0.001) and more often had bilateral PCC (25.6% vs. 6.1%), whereas the AS group had a greater proportion of unilateral PCC (35.2% vs. 16.3%).

Table 1.
Clinical characteristics of patients with PPGL in the study.
2.2. Classification of Profiled Variants
Of the 370 probands, 63 distinct germline variants were identified in 129 probands, excluding benign (B) or likely benign (LB) variants (Table 2). We assessed the pathogenicity of these variants according to the American College of Medical Genetics (ACMG) and the Association for Molecular Pathology (AMP) guidelines [29] (see Materials and Methods). As a result, 24 variants were classified as pathogenic (P), 29 variants as likely pathogenic (LP), and 9 variants as a variant of uncertain significance (VUS). We found nine novel variants, which were not previously reported or registered in any disease database. Among them, 6/9 variants were classified as pathogenic or likely pathogenic, three variants remained VUS.

Table 2.
List of identified germline variants.
2.3. Frequency of Germline Variants
In our cohort of 370 probands, 120 subjects were found to harbour P/LP variants, thus the prevalence rate of germline variants was 32.4% (Figure 1A). The most common variants were SDHB detected in 57 probands (accounting for 47.5% of P/LP variants detected), SDHD in 27 probands (22.5%), VHL in 18 probands (15.0%), RET in 8 probands (6.7%), MAX in 5 probands (4.2%), TMEM127 in 3 probands (2.5%), and SDHC in 2 probands (1.7%). Among the 327 AS presentations, 81 probands had P/LP variants, with a prevalence of the germline variant of 24.8% (Figure 1B). Compared to the AS presentation, the FS presentation had a higher percentage of SDHD (30.2% vs. 4.3%) and VHL (27.9% vs. 1.8%) variants (Table S1). Almost half (29/63, 46.0%) of the probands who developed metastatic PPGL carried a P/LP germline variant (Figure 1C). Metastatic PPGL were mostly by SDHB variant (21/29, 72.4%), followed by SDHD, RET, VHL, and MAX.
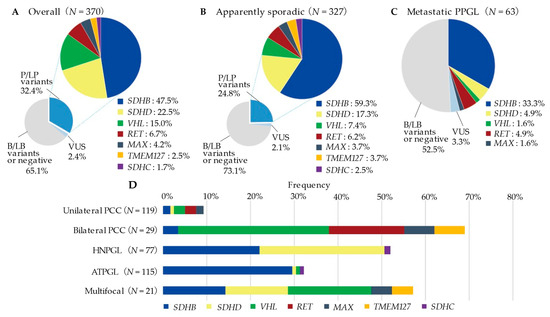
Figure 1.
Prevalence and overview of mutated genes by presentation or tumour locations. P/LP, pathogenic/likely pathogenic variants; VUS, variant of uncertain significance; B/LB, benign/likely benign variants; PCC, pheochromocytoma; HNPGL, head and neck paraganglioma; ATPGL, abdominal and thoracic paraganglioma. (A) Prevalence and overview of germline variants in the overall PPGL probands (B) Prevalence and overview of germline variants in apparently sporadic presentations (C) Prevalence and overview of germline variants in the Metastatic PPGL (D) Prevalence of P/LP variants by tumour location (VUS was excluded from analysis).
The frequency of germline variants according to tumour location is shown in Figure 1D (see Table S3 for details). Among the 119 unilateral PCC patients, 11 (9.2%) had the germline variants in either 5 genes (SDHB: n = 2, SDHD: 1, VHL: 3, RET: 3, MAX: 2). In contrast, the variant rate was remarkably high in bilateral PCC (20/29, 69.0%), with VHL (10/20, 50.0%) being the most frequently mutated gene. In 77 HNPGL patients, 51.9% (40/77) of them had germline variants. The most frequently mutated genes were SDHD (22/40, 55.0%) and SDHB (17/40, 42.5%), with one case of SDHC variants. Thirty-three per cent of the ATPGL (37/115) patients had germline variants. The majority of these variants were in the SDHB (34/37, 91.9%) gene, with one case each of SDHD, VHL, and SDHC variants also detected. In 21 patients with multifocal PPGL, 57.1% (12/21) of the patients had germline variants; mutated genes including VHL (4/12), SDHB (3/12), SDHD (3/12), MAX (1/12), and TMEM127 (1/12). On the other hand, 9/21 (42.9%) cases of multifocal PPGL, 11/28 (39.3%) cases of bilateral PCC, 1/6 (16.7%) cases of bilateral/multiple HNPGL, and 6/10 (60.0%) cases of multiple ATPGL were variant negative (VUS was excluded).
Table S2 shows the age-based frequencies of the germline variants detected at the diagnosis. The distribution of the number of patients in each age group showed a symmetrical distribution with a peak at 31–50 years. While almost all VHL variants (15/18, 83.3%) were diagnosed before the age of 40, SDHB/SDHD variant positive patients had a wider age distribution including in older patients. Of the 61 patients diagnosed with PPGL even after the age of 60, 25% (15/61) were found to harbour P/LP variants.
2.4. Clinical Characteristics of Probands with P/LP Variants
Probands with a P/LP variant were younger at the age of diagnosis than the variant negative group (38.2. ± 15.2 vs. 46.0 ± 15.6 years, p < 0.001; Table 3). The variant-negative group had a significantly greater proportion of unilateral PCC compared to the P/LP positive group (108/241 (44.8%) vs. 11/120 (9.2%), p < 0.00278). In contrast, the ratio of bilateral PCC to single HNPGL was significantly higher in the P/LP variant-positive group (20/120 (16.7%) vs. 9/241 (3.7%), p < 0.00278). The P/LP variant group were more likely to develop metastatic PPGL than the variant negative group (24.2% vs. 13.4%, p = 0.029).

Table 3.
Clinical phenotype based on the seven genotypes (excluding VUS).
2.5. Genetic and Clinical Characteristics by Specific Susceptibility Genes
2.5.1. SDHB Variants
Fifty-seven SDHB variant-positive probands had 15 distinct variants classified as P/LP (missense: 5, nonsense: 4, splice site variant: 2, small deletion: 1, deletion-insertion across the intron/exon border: 1, large deletions: 2; Table 2). A heterozygous duplication of exon 1 in single probands was classified as VUS. The missense variant c.137G>A (p.Arg46Gln) and the frameshift deletion c.470delT (p.Leu157Ter) were the two most frequent variants occurring in 14/57 (24.6%) probands and 13/57 (22.8%) probands, respectively. Most SDHB variant-positive patients belonged to the AS presentation (48/57, 84.2%; Table 3). Of the 57 patients, 54 (94.7%) had PGL (single HNPGL: 17, single ATPGL: 30, multiple ATPGL: 4, multifocal of PCC/ATPGL: 3), and only 3 (5.3%) patients had PCC. The SDHB variants group had a significantly greater proportion of metastatic PPGL compared with a variant negative group (14.3% vs. 35.8%, p < 0.00625 after Bonferroni correction).
We also investigated associations between SDHB variant type (truncating or missense) and patient phenotype (Table S3). There were no significant differences in age at diagnosis, tumour size, or frequency of metastatic PPGL between the truncating and missense variants. We only noticed that truncated variants of the SDHB gene tended to show high frequencies of HNPGL (14/33, 42.4%) as well as ATPGL (17/33, 51.5%).
2.5.2. SDHD Variants
We identified 14 distinct P/LP variants and three VUS in the SDHD gene (Table 2). Interestingly, three deletion variants of 10–15 bases were found in exon 3 of the SDHD gene, among which, c.285_296del was found in several families with distant origins. We found 13/27 (48.1%) SDHD variant carriers with a family history (Table S1). Among them, nine had a family history of PPGL on the paternal side, the remaining four had a brother/sister or son/daughter with PPGL, and none had a family history on the maternal side. The majority of probands with SDHD variants presented with HNPGL or HNPGL combined multifocal tumours (25/27 patients, 92.6%). Eleven per cent (3/27) of patients with SDHD variants were metastatic forms, comparable to those in the variants negative group (13.4%, 32/241). There were no significant differences in age at diagnosis, tumour size, or frequency of metastatic PPGL between the truncating (n = 9) and missense variants (n = 13) (Table S3).
2.5.3. VHL Variants
The P/LP variants of VHL in 18 probands, consisted of 12 missense and a deleterious synonymous variant (c.414A>G, p.Pro138Pro). The p.Pro138Pro has been reported to confer PCC susceptibility by promoting exon 2 skipping and consequently repressing the expression of the full-length VHL transcript [32]. The three probands with syndromic presentations had hemangioblastoma, pancreatic cysts, and pancreatic endocrine tumours, respectively. The mean age of VHL variant-positive probands was 27.1 ± 13.8 (range: 10–64) years. Patients with VHL variants were younger than those in the variant negative group (Table 3). The tumours were bilateral PCC in 10 patients (55.6%), and four (22.2%) had the multifocal disease (PCC with ATPGL). Only one case showed distant metastasis.
2.5.4. Minor Variants Genes
The four RET missense variants in eight probands were located at the hot-spot codons 631 and 634 in exon 11. Of the five patients with FS presentations, three had a family history of PPGL, and two had prior medullary thyroid carcinoma. The tumours with RET patients were always adrenal and often bilateral (5/8, 62.5%). Three of the eight patients had been diagnosed with metastatic PCC. Five distinct MAX variants, four TMEM127 variants, and two SDHC variants were detected (including 3 VUS; See Table 2). Bilateral PCC was often observed as a phenotype of the variants in MAX or TMEM127 genes. Of the two patients with the SDHC variant, one had multiple HNPGLs, the other had a single ATPGL.
3. Discussion
The present study revealed five major findings. First, in previously unstudied Japanese patients with PPGL, we comprehensively profiled the P/LP germline variants in 32.4% of the total and 24.8% of the AS presentations. Second, heritable PPGL could not be ruled out even at an older age of diagnosis (60–70 years), and the prevalence of germline variants was high (32.2–67.8%) in all tumour locations except unilateral PCC (9.2%). Third, as in the previous reports in European populations, the most frequently mutated gene was SDHB (47.5%). However, several SDHB variants common in the probands of distinct Japanese families differed from those in other races. Fourth, the incidence of metastatic PPGL in P/LP variant carriers was high (24.2%), especially in SDHB carriers (36.8%). Finally, we described nine novel variants, six of which were classified as P/LP.
The overall frequency of germline variants in our study cohort (32.4%) is consistent with that in the reported European cohorts (27.4–32.9%) [5,6,7] and with recent reports in Asians (32.6–34.1%) [22,23,31]. The prevalence of variants in AS presentations vary widely (11.0–36.6%) [6,11,12,18,21,22,23] depending on the definition of “sporadic” and the number of genes investigated. Applying the simple criterion of non-familial and non-syndromic presentation as AS presentation, we found a 24.8% frequency of germline variants in anyof the seven major susceptibility genes (MAX, SDHB, SDHC, SDHD, TMEM127, VHL, and RET). Our findings also established that even patients with PPGL diagnosed over the age of 60 may harbour germline variants such as SDHB and SDHD genes (Table S2). It should be noted that false-negative family history in patients with PPGL can occur due to patients’ lack of awareness of their relatives’ medical history, the presence of multiple genes with low penetrance in hereditary PPGL [33], and the possibility of transmission by maternal imprinting, which is characteristic of SDHD-related PPGL [34]. Nevertheless, the present study performed in the one of the scanty large cohorts in Asia shows that more than one in four PPGL patients had a germline variant, supporting the recommendation for genetic testing in all patients with PPGLs, regardless of age at presentation and family history [2]. Early detection of predisposing germline variants is an important step to detect potentially variant carriers in relatives, as well as the potential for improved outcomes through surveillance of SDHB and VHL variant carriers [35].
Our cohort confirms the high prevalence of heritability in all tumour locations except unilateral PCC (Figure 1). Of note, bilateral PCC (69.0%) and multifocal PPGL (57.1%) had the strongest association with the presence of a germline variant. These prevalence data are in line with almost all major studies showing that 56.3–90.0% of patients with bilateral PCC and 68.8–85.7% patients with multifocal PPGL have a germline variant [6,18,19,22,23]. In these strongly suspected hereditary bilateral PCC or multifocal PPGL, the most commonly mutated gene was VHL, consistent with the priority set by the genetic testing decision algorithm [6,23]. Conversely, unilateral PCC was characterized by a 9.2% frequency of variants, notably lower than that in other tumour locations. From a cost-effectiveness perspective, the value of genetic testing in patients with unilateral PCC lacking symptomatic or metastatic features and without positive family history has not been established [14]. However, in a seminal study, Sbardella et al. showed the usefulness of routine genetic screening with multi-gene panels in PCC patients with genetic heterogeneity [36]. Furthermore, NGS has been recently validated to reduce the processing time and cost compared to conventional Sanger sequencing for each exon.
The detailed breakdown of the genes found to be mutated in our study was unique compared to the non-Japanese cohort studies. Our study showed that SDHB was the most mutated gene (47.5% of patients with positive variant and 15.4% of all cases studied), followed by SDHD (22.5%, 7.3% of all). While a recent study has suggested a Sino-European difference in the frequency of SDHB variants with a lower frequency in East Asian than in Caucasian populations [20], there are other reports which suggest that SDHB is the most common variant in Asia [21,22]. These inconsistent results might be attributed to both the differences in criteria for patient selection and the geographic origins of the studies. The present study has the advantage of having a relatively higher number of patients with functional or non-functional HNPGL (79 patients, 21.4% of all PPGL) than other Asian cohort studies (9–39 patients, 2.9–38.6%) [21,22,23]. We found 22/77 (28.6%) of the subjects with HNPGL had SDHD variants, and 17/77 (220.1%) had SDHB variants (excluding VUS, see Figure 1, Table S3). These results confirmed previous reports of a high prevalence of SDHD variants in HNPGL [37,38] and showed that SDHB variants were present at almost comparable frequency. Notably, the truncating variants of the SDHB gene showed a high frequency of presentation of HNPGL as well as ATPGL (Table S4). Further extensive cohort studies and molecular genetics research are required to clarify the association between HNPGL and truncated variants of SDHB.
In our cohorts, SDHB variants were the most frequently associated variants with distant metastasis occurring in 21/63 (33.3%; Figure 1C). Furthermore, the SDHB variant group showed a significantly higher percentage of metastatic PPGL than the variant-negative group (Table 3). These results have been depicted across almost major studies with SDHB variants associated with an increased risk of distant metastasis [5,39,40,41]. Of note, there are several recurrent variants among the 16 distinct SDHB variants in this study. c.137G>A was the most frequent variant (14/59, 23.7%), followed by c.470delT, which was the second most frequent (13/59, 22.0%; Table 2). In a large UK cohort reported in 2018, SDHB intragenic variants were detected in 237 probands, of which c.137G>A was found in 22 cases (9.3%), and this variant was the second most frequent variant in SDHB [17]. In the most recent study in the Chinese population, c.137G>A was found in 2/46 (4.3%) of the SDHB variant-positive patients, whereas c.136C>T, the most recurrent SDHB variant in China (6/46, 13.0%), was not found in our Japanese PPGL population [22]. Inversely, c.470delT, which was reported as a novel variant associated with metastatic PGL in Japan in 2009 [28], has not been profiled in any other recent cohort studies. Although haplotype analysis is required for conclusive determination, a founder effect is highly suspected for c.470delT because this variant is found only in a limited ethnic population. The accumulation and sharing of the phenotypical knowledge (such as the frequency of metastasis and tumour localization) of recurrent variants in each population may lead to the development of variant-specific personalised disease-risk management.
The detection of vast numbers of genetic variants with the recent evolution of sequencing technology in cancer testing has highlighted the importance of standardizing the interpretation and classification of variants among laboratories. Internationally recognised guidelines have been reported by the ACMG and the AMP [29], providing a five-tier classification system based on the combination of multiple lines of evidence with variable rank. In silico prediction of pathogenicity is one of the evidence categories recommended by the ACMG/AMP guidelines. Recently, several in silico meta-prediction tools have been developed based on the analysis of multiple individual scores. Among them, Rare Exome Variant Ensemble Learner (REVEL) has been confirmed to be an excellent predictive tool for assessing the pathogenicity of missense variants [42,43,44]. We used this reliable in silico tool and the latest disease and population databases to facilitate the identification of disease-causing variants. We also profiled nine VUS, including novel variants. Although VUS is excluded from the analysis of the relationship between genotype and phenotype, it is necessary to repeatedly follow the latest database because the class may change by the re-evaluation of VUS by PPGL experts [45].
The present study is the first comprehensive study profiling the prevalence of germline variants in Japanese subjects with PPGL. However, it is essential to acknowledge the study’s limitations. First, due to financial constraints, we could analyse only seven genes using the Sanger sequencing technique. Therefore, it remains possible to carry another minor susceptibility gene, especially in variant-negative multiple PPGLs. However, these seven genes are reported to account for the majority of the germline variants in PPGL [9,45] and exhaust the list of inherited PPGL genes for which secondary findings are required to be reported in the latest ACMG statement (with the exception of the extremely rare SDHAF2 gene) [46]. Second, this study has the potential for selection bias, thereby falsely increasing the frequencies of germline variants. However, this bias is minimised by the fact that our cohort included more than 50% of subjects with only isolated unilateral benign PCC or ATPGL. Third, we were not able to mention the relationship between the catecholamine profile or SDHB-negative immunohistochemistry and the variant status. This was due to variations in the ability to investigate PPGL systematically among the facilities which provided the samples. Finally, the variant classification to interpret pathogenicity is incomplete because we were not able to perform genetic tests on both parents in all subjects, thereby preventing the determination of the de novo nature of the annotated variants. However, we were able to enhance the accuracy of pathogenicity assessment by reviewing the allele frequencies in multiple population databases, up-to-date disease databases, and utilizing in silico prediction tools.
4. Materials and Methods
4.1. Patients
The study subjects included Japanese probands clinically and pathologically diagnosed with PPGL referred to the University of Tsukuba Hospital seeking genetic testing during the February 2007–March 2020 period. Genetic testing was performed on patients older than 16 years of age for ethical reasons, although patients diagnosed before the age of 16 years were included. Blood samples and clinical information, including sex, age at diagnosis, tumour location, and extra-paraganglionic metastases, were collected. The study was conducted in accordance with the Declaration of Helsinki and Ethical Guidelines for Human Genome/Gene Analysis Research of Japan. Written informed consent was obtained from all patients. We offered genetic counselling before and after the genetic test, as appropriate.
FS (familial/syndromic) presentation was characterised by the presence of clinical features of multiple endocrine neoplasia type 2 (MEN2) or Von Hippel–Lindau (VHL) disease in the proband or their family members (syndromic) or history of PPGL in the family members (familial), or in combination. The absence of FS features was considered to be AS (apparently sporadic) presentation. Multifocal PPGL was defined as the coexistence of adrenal PCC and PGL or the presence of PGL across multiple areas of the head and neck and abdomen/thorax. Metastatic PPGL was defined by the presence of extra-paraganglionic metastases, including in the lung, liver, bone, and lymph nodes.
4.2. Genetic Analysis
Genomic DNA samples were extracted from 10 mL of ethylenediaminetetraacetic acid (EDTA)-treated peripheral blood samples using the Nucleosopin Blood Mini Kit (MACHEREY–NAGEL GmbH & Co. KG, Düren, Germany) according to the manufacturer’s instructions. The following seven genes were analysed: MAX (NM_002382.5), SDHB (NM_003000.3), SDHC (NM_003001.5), SDHD (NM_003002.4), TMEM127 (NM_017849.4), VHL (NM_000551.3), and exons 10, 11 and 13–16 of the RET (NM_020630.4) proto-oncogene. A priority order was used for genetic analysis according to previous recommendations [5,6]. For instance, VHL and RET genes were analysed with priority for bilateral PCC, SDHB gene for retroperitoneum PGL or metastatic PCC/PGL, and SDHD gene for head and neck PGL. When a pathogenic variant was found in one of these genes, no further testing was performed in the remaining genes. After performing PCR using gel electrophoresis for each primer pair, Sanger sequencing was outsourced to a commercial service provider (Eurofins Genomics, Ota-ku, Tokyo, Japan) to detect variants, and the results were analysed at our department. The primers used for the PCR amplification are listed in Table S5. When no P/LP variant was found by the Sanger sequencing, all exons of the SDHB, SDHC, and SDHD gene were reanalysed using multiplex ligation-dependent probe amplification (MLPA) to examine copy number variation. The MLPA assay was carried out by a commercial service provider (FALCO Biosystems Ltd., Kumiyama, Kyoto, Japan). The sequence results were analysed using Sequence Scanner Software v2.0 (Thermo Fisher Scientific, Waltham, MA, USA) and CLC Sequence Viewer 8.0 software (QIAGEN, Aarhus C, Denmark).
4.3. Variant Classification
ACMG/AMP guidelines were used to classify variant pathogenicity [29]. The detected variations were assessed for pathogenic potential using the following in silico tools: Rare Exome Variant Ensemble Learner (REVEL) (https://sites.google.com/site/revelgenomics/downloads, accessed on 21 May 2021), and Human Splicing Finder (https://www.genomnis.com/access-hsf, accessed on 21 May 2021). REVEL is a recently developed in silico variant meta-predictor, which scores rare missense variants on a scale ranging from 0 to 1, with higher scores indicating a greater likelihood of being a disease-causing variant [44]. While REVEL does not suggest a strict threshold for variant categorisation, a score above 0.5 was used for supporting pathogenic variants (ACMG/AMP codes; PP3) with reference to the previously reported criteria [47]. Human Splicing Finder provides information on changes in scores caused by splice site variants in donor and acceptor sites. A reduction of >10% in the predicted score was used as the pathogenic variant in accordance with the original article [48]. Reported interpretations for known variants were obtained through the disease databases such as Human Gene Mutation Database (HGMD) (accessed on 20 May 2021) (http://www.hgmd.cf.ac.uk/ac/index.php; accessed on 20 May 2021) and ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/; accessed on 20 May 2021). In addition, genome aggregation database (gnomAD v3.1.1 (non-cancer); https://gnomad.broadinstitute.org/, accessed on 10 June 2021) and Japanese Multi Omics Reference Panel (jMorp; Genome variation 8.3K JPN (v20200831); https://jmorp.megabank.tohoku.ac.jp/202102/variants, accessed on 10 June 2021) were used as the population database for the estimation of mutant allele frequency (MAF). Only annotated variants with MAF < 0.01 in the gnomAD v3.1.1 (non-cancer) database was retained. All variants absent, or extremely rare, (MAF ≤ 0.00002) in both gnomAD and jMorp were encoded as PM2 in ACMG/AMP. Frameshift, nonsense, deletion of exon(s), and canonical splicing-site change were grouped as protein-truncating variants (Table S4).
4.4. Statistical Analysis
All data were analysed using the SPSS Statistics Version 26 software (IBM Japan Ltd., Chuo-ku, Tokyo, Japan). Data are reported as mean ± S.D., actual numbers, or percentages. Categorical variables were presented as frequency counts and percentages. Comparison of categorical data was performed using the chi-squared test or Fisher’s exact test as appropriate. For data with normal distributions, between-group differences were analysed using the Student’s t-test or one-way analysis of variance (ANOVA). For statistical comparison of nonnormally distributed data, Kruskal–Wallis tests were used. In all statistical tests, two-sided testing was used, and a p value of less than 0.05 was considered statistically significant. For multiple comparisons, Bonferroni’s correction was used to adjust the critical p-value.
5. Conclusions
In this first large-scale examination of the Japanese PPGL cohort, almost a quarter of patients with apparently sporadic PPGL harboured germline variants. This study reinforces the recommendation in Western guidelines to perform genetic testing for PPGL and genotype-based clinical decision-making in the Japanese population.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cancers13164014/s1, Table S1: Frequency of germline variants according to the presentation; Table S2: Frequency of germline variant according to the age at diagnosis; Table S3: Comparison of clinical characteristics based on the presence of germline variants and tumour site in probands with PPGL; Table S4: Comparison of clinical characteristic between truncating vs. missense variants in SDHB and SDHD variant-positive probands; Table S5: Primers used for the PCR amplification of genes.
Author Contributions
Conceptualization, K.T. (Kazuhiro Takekoshi); methodology, M.Y., Y.A., T.S. and K.T. (Kazuhiro Takekoshi); formal analysis, M.Y., K.W. and Y.A.; investigation, M.Y.; resources, K.T. (Katsutoshi Takahashi), H.S., H.N., M.N., T.O., T.M., S.K., K.H., A.T., A.W., N.K., E.N., A.S., K.S. and K.T. (Kazuhiro Takekoshi); data curation, M.Y. and Y.A.; writing—original draft preparation, M.Y.; writing—review and editing, K.W., T.S., K.T. (Katsutoshi Takahashi), M.N., A.W., N.K. and K.T. (Kazuhiro Takekoshi); visualization, M.Y.; supervision, K.W. and Y.K.; project administration, T.S., Y.K. and K.T. (Kazuhiro Takekoshi); funding acquisition, K.T. (Kazuhiro Takekoshi), M.N., H.H., Y.K. and E.N. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported in part by Grants-in-Aid from Japan Society for the Promotion of Science (JSPS KAKENHI Grant Numbers 201128060B to M.N.,21591168 to K. Takekoshi, 23591889 to H.H., 25460671 to Y.K., 16K08961 to Y.K., and 17H04328 to E.N.). The funders had no role in the study design, data collection and analysis, decision to publish, or the preparation of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Medical Ethics Committee (approval #119, date of approval: 5 February 2007) and the Institutional Review Board of the University of Tsukuba Hospital (approval #H28–134, date of approval: 29 September 2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The authors confirm that the datasets analysed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
We are grateful to all the patients who participated and the collaborating research facilities for providing samples from all over Japan. We would like to thank Sumiko Niisato and Seiko Ono for their technical support. We would also like to thank Masaya Hoshino for help with variant classification.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Favier, J.; Amar, L.; Gimenez-Roqueplo, A.P. Paraganglioma and phaeochromocytoma: From genetics to personalized medicine. Nat. Rev. Endocrinol. 2015, 11, 101–111. [Google Scholar] [CrossRef]
- Muth, A.; Crona, J.; Gimm, O.; Elmgren, A.; Filipsson, K.; Stenmark Askmalm, M.; Sandstedt, J.; Tengvar, M.; Tham, E. Genetic testing and surveillance guidelines in hereditary pheochromocytoma and paraganglioma. J. Intern. Med. 2019, 285, 187–204. [Google Scholar] [CrossRef] [PubMed]
- Eisenhofer, G.; Prejbisz, A.; Peitzsch, M.; Pamporaki, C.; Masjkur, J.; Rogowski-Lehmann, N.; Langton, K.; Tsourdi, E.; Pęczkowska, M.; Fliedner, S.; et al. Biochemical Diagnosis of Chromaffin Cell Tumors in Patients at High and Low Risk of Disease: Plasma versus Urinary Free or Deconjugated O-Methylated Catecholamine Metabolites. Clin. Chem. 2018, 64, 1646–1656. [Google Scholar] [CrossRef]
- Lloyd, R.V.; Osamura, R.Y.; Kloppel, G.; Rosai, J. WHO Classification of Tumours: Pathology and Genetics of Tumours of Endocrine Organs, 4th ed.; International Agency for Research on Cancer (IARC): Lyon, France, 2017; Volume 10. [Google Scholar]
- Amar, L.; Bertherat, J.; Baudin, E.; Ajzenberg, C.; Bressac-de Paillerets, B.; Chabre, O.; Chamontin, B.; Delemer, B.; Giraud, S.; Murat, A.; et al. Genetic testing in pheochromocytoma or functional paraganglioma. J. Clin. Oncol. 2005, 23, 8812–8818. [Google Scholar] [CrossRef]
- Mannelli, M.; Castellano, M.; Schiavi, F.; Filetti, S.; Giacchè, M.; Mori, L.; Pignataro, V.; Bernini, G.; Giachè, V.; Bacca, A.; et al. Clinically guided genetic screening in a large cohort of italian patients with pheochromocytomas and/or functional or nonfunctional paragangliomas. J. Clin. Endocrinol. Metab. 2009, 94, 1541–1547. [Google Scholar] [CrossRef]
- Jafri, M.; Whitworth, J.; Rattenberry, E.; Vialard, L.; Kilby, G.; Kumar, A.V.; Izatt, L.; Lalloo, F.; Brennan, P.; Cook, J.; et al. Evaluation of SDHB, SDHD and VHL gene susceptibility testing in the assessment of individuals with non-syndromic phaeochromocytoma, paraganglioma and head and neck paraganglioma. Clin. Endocrinol. 2013, 78, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Fishbein, L.; Leshchiner, I.; Walter, V.; Danilova, L.; Robertson, A.G.; Johnson, A.R.; Lichtenberg, T.M.; Murray, B.A.; Ghayee, H.K.; Else, T.; et al. Comprehensive Molecular Characterization of Pheochromocytoma and Paraganglioma. Cancer Cell 2017, 31, 181–193. [Google Scholar] [CrossRef]
- Buffet, A.; Burnichon, N.; Favier, J.; Gimenez-Roqueplo, A.P. An overview of 20 years of genetic studies in pheochromocytoma and paraganglioma. Best Pract. Res. Clin. Endocrinol. Metab. 2020, 34, 101416. [Google Scholar] [CrossRef] [PubMed]
- Koopman, K.; Gaal, J.; de Krijger, R.R. Pheochromocytomas and Paragangliomas: New Developments with Regard to Classification, Genetics, and Cell of Origin. Cancers 2019, 11, 1070. [Google Scholar] [CrossRef]
- Neumann, H.P.; Bausch, B.; McWhinney, S.R.; Bender, B.U.; Gimm, O.; Franke, G.; Schipper, J.; Klisch, J.; Altehoefer, C.; Zerres, K.; et al. Germ-line mutations in nonsyndromic pheochromocytoma. N. Engl. J. Med. 2002, 346, 1459–1466. [Google Scholar] [CrossRef]
- Brito, J.P.; Asi, N.; Bancos, I.; Gionfriddo, M.R.; Zeballos-Palacios, C.L.; Leppin, A.L.; Undavalli, C.; Wang, Z.; Domecq, J.P.; Prustsky, G.; et al. Testing for germline mutations in sporadic pheochromocytoma/paraganglioma: A systematic review. Clin. Endocrinol. 2015, 82, 338–345. [Google Scholar] [CrossRef]
- Lee, C.H.; Cheung, C.Y.; Chow, W.S.; Woo, Y.C.; Yeung, C.Y.; Lang, B.H.; Fong, C.H.; Kwok, K.H.; Chen, S.P.; Mak, C.M.; et al. Genetics of Apparently Sporadic Pheochromocytoma and Paraganglioma in a Chinese Population. Horm. Metab. Res. 2015, 47, 833–838. [Google Scholar] [CrossRef][Green Version]
- Lenders, J.W.; Duh, Q.Y.; Eisenhofer, G.; Gimenez-Roqueplo, A.P.; Grebe, S.K.; Murad, M.H.; Naruse, M.; Pacak, K.; Young, W.F., Jr. Pheochromocytoma and paraganglioma: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2014, 99, 1915–1942. [Google Scholar] [CrossRef] [PubMed]
- Plouin, P.F.; Amar, L.; Dekkers, O.M.; Fassnacht, M.; Gimenez-Roqueplo, A.P.; Lenders, J.W.; Lussey-Lepoutre, C.; Steichen, O. European Society of Endocrinology Clinical Practice Guideline for long-term follow-up of patients operated on for a phaeochromocytoma or a paraganglioma. Eur. J. Endocrinol. 2016, 174, G1–G10. [Google Scholar] [CrossRef]
- Neumann, H.P.H.; Young, W.F., Jr.; Eng, C. Pheochromocytoma and Paraganglioma. N. Engl. J. Med. 2019, 381, 552–565. [Google Scholar] [CrossRef]
- Andrews, K.A.; Ascher, D.B.; Pires, D.E.V.; Barnes, D.R.; Vialard, L.; Casey, R.T.; Bradshaw, N.; Adlard, J.; Aylwin, S.; Brennan, P.; et al. Tumour risks and genotype-phenotype correlations associated with germline variants in succinate dehydrogenase subunit genes SDHB, SDHC and SDHD. J. Med. Genet. 2018, 55, 384–394. [Google Scholar] [CrossRef]
- Buffet, A.; Venisse, A.; Nau, V.; Roncellin, I.; Boccio, V.; Le Pottier, N.; Boussion, M.; Travers, C.; Simian, C.; Burnichon, N.; et al. A decade (2001–2010) of genetic testing for pheochromocytoma and paraganglioma. Horm. Metab. Res. 2012, 44, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Cascón, A.; Pita, G.; Burnichon, N.; Landa, I.; López-Jiménez, E.; Montero-Conde, C.; Leskelä, S.; Leandro-García, L.J.; Letón, R.; Rodríguez-Antona, C.; et al. Genetics of pheochromocytoma and paraganglioma in Spanish patients. J. Clin. Endocrinol. Metab. 2009, 94, 1701–1705. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, J.; Pang, Y.; Bechmann, N.; Li, M.; Monteagudo, M.; Calsina, B.; Gimenez-Roqueplo, A.P.; Nölting, S.; Beuschlein, F.; et al. Sino-European Differences in the Genetic Landscape and Clinical Presentation of Pheochromocytoma and Paraganglioma. J. Clin. Endocrinol. Metab. 2020, 105, 3295–3307. [Google Scholar] [CrossRef] [PubMed]
- Albattal, S.; Alswailem, M.; Moria, Y.; Al-Hindi, H.; Dasouki, M.; Abouelhoda, M.; Alkhail, H.A.; Alsuhaibani, E.; Alzahrani, A.S. Mutational profile and genotype/phenotype correlation of non-familial pheochromocytoma and paraganglioma. Oncotarget 2019, 10, 5919–5931. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ma, X.; Li, M.; Tong, A.; Wang, F.; Cui, Y.; Zhang, X.; Zhang, Y.; Chen, S.; Li, Y. Genetic and Clinical Profiles of Pheochromocytoma and Paraganglioma: A Single Center Study. Front. Endocrinol. 2020, 11, 574662. [Google Scholar] [CrossRef]
- Pandit, R.; Khadilkar, K.; Sarathi, V.; Kasaliwal, R.; Goroshi, M.; Khare, S.; Nair, S.; Raghavan, V.; Dalvi, A.; Hira, P.; et al. Germline mutations and genotype-phenotype correlation in Asian Indian patients with pheochromocytoma and paraganglioma. Eur. J. Endocrinol. 2016, 175, X3. [Google Scholar] [CrossRef]
- Kimura, N.; Takekoshi, K.; Horii, A.; Morimoto, R.; Imai, T.; Oki, Y.; Saito, T.; Midorikawa, S.; Arao, T.; Sugisawa, C.; et al. Clinicopathological study of SDHB mutation-related pheochromocytoma and sympathetic paraganglioma. Endocr.-Relat. Cancer 2014, 21, L13–L16. [Google Scholar] [CrossRef] [PubMed]
- Shibata, M.; Inaishi, T.; Miyajima, N.; Adachi, Y.; Takano, Y.; Nakanishi, K.; Takeuchi, D.; Noda, S.; Aita, Y.; Takekoshi, K.; et al. Synchronous bilateral pheochromocytomas and paraganglioma with novel germline mutation in MAX: A case report. Surg. Case Rep. 2017, 3, 131. [Google Scholar] [CrossRef] [PubMed]
- Takeichi, N.; Midorikawa, S.; Watanabe, A.; Naing, B.T.; Tamura, H.; Wakakuri-Kano, T.; Ishizaki, A.; Sugihara, H.; Nissato, S.; Saito, Y.; et al. Identical germline mutations in the TMEM127 gene in two unrelated Japanese patients with bilateral pheochromocytoma. Clin. Endocrinol. 2012, 77, 707–714. [Google Scholar] [CrossRef]
- Takekoshi, K.; Isobe, K.; Suzuki, H.; Nissato, S.; Kawakami, Y.; Kawai, K.; Yamada, N. R46Q mutation in the succinate dehydrogenase B gene (SDHB) in a Japanese family with both abdominal and thoracic paraganglioma following metastasis. Endocr. J. 2008, 55, 299–303. [Google Scholar] [CrossRef]
- Saito, T.; Saito, Y.; Matsumura, K.; Tsubota, Y.; Maniwa, T.; Kaneda, H.; Minami, K.; Sakaida, N.; Uemura, Y.; Kawa, G.; et al. Novel mutation (L157X) in the succinate dehydrogenase B gene (SDHB) in a Japanese family with abdominal paraganglioma following lung metastasis. Endocr. J. 2009, 56, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Li, M.M.; Datto, M.; Duncavage, E.J.; Kulkarni, S.; Lindeman, N.I.; Roy, S.; Tsimberidou, A.M.; Vnencak-Jones, C.L.; Wolff, D.J.; Younes, A.; et al. Standards and Guidelines for the Interpretation and Reporting of Sequence Variants in Cancer: A Joint Consensus Recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J. Mol. Diagn. JMD 2017, 19, 4–23. [Google Scholar] [CrossRef]
- Hirose, R.; Tsurutani, Y.; Sugisawa, C.; Inoue, K.; Suematsu, S.; Nagata, M.; Hasegawa, N.; Kakuta, Y.; Yonamine, M.; Takekoshi, K.; et al. Hereditary pheochromocytoma/paraganglioma syndrome with a novel mutation in the succinate dehydrogenase subunit B gene in a Japanese family: Two case reports. J. Med. Case Rep. 2021, 15, 282. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Kim, K.J.; Hong, N.; Shin, S.; Choi, J.R.; Kang, S.W.; Lee, S.T.; Rhee, Y. Genetic Analysis and Clinical Characteristics of Hereditary Pheochromocytoma and Paraganglioma Syndrome in Korean Population. Endocrinol. Metab. (Seoul Korea) 2020, 35, 858–872. [Google Scholar] [CrossRef]
- Flores, S.K.; Cheng, Z.; Jasper, A.M.; Natori, K.; Okamoto, T.; Tanabe, A.; Gotoh, K.; Shibata, H.; Sakurai, A.; Nakai, T.; et al. A synonymous VHL variant in exon 2 confers susceptibility to familial pheochromocytoma and von Hippel-Lindau disease. J. Clin. Endocrinol. Metab. 2019, 104, 3826–3834. [Google Scholar] [CrossRef]
- Benn, D.E.; Robinson, B.G.; Clifton-Bligh, R.J. 15 YEARS OF PARAGANGLIOMA: Clinical manifestations of paraganglioma syndromes types 1–5. Endocr.-Relat. Cancer 2015, 22, T91–T103. [Google Scholar] [CrossRef]
- Baysal, B.E. Genomic imprinting and environment in hereditary paraganglioma. Am. J. Med. Genet. Part C Semin. Med. Genet. 2004, 129c, 85–90. [Google Scholar] [CrossRef]
- Buffet, A.; Ben Aim, L.; Leboulleux, S.; Drui, D.; Vezzosi, D.; Libé, R.; Ajzenberg, C.; Bernardeschi, D.; Cariou, B.; Chabolle, F.; et al. Positive Impact of Genetic Test on the Management and Outcome of Patients With Paraganglioma and/or Pheochromocytoma. J. Clin. Endocrinol. Metab. 2019, 104, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Sbardella, E.; Cranston, T.; Isidori, A.M.; Shine, B.; Pal, A.; Jafar-Mohammadi, B.; Sadler, G.; Mihai, R.; Grossman, A.B. Routine genetic screening with a multi-gene panel in patients with pheochromocytomas. Endocrine 2018, 59, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Piccini, V.; Rapizzi, E.; Bacca, A.; Di Trapani, G.; Pulli, R.; Giachè, V.; Zampetti, B.; Lucci-Cordisco, E.; Canu, L.; Corsini, E.; et al. Head and neck paragangliomas: Genetic spectrum and clinical variability in 79 consecutive patients. Endocr.-Relat. Cancer 2012, 19, 149–155. [Google Scholar] [CrossRef]
- Williams, M.D. Paragangliomas of the Head and Neck: An Overview from Diagnosis to Genetics. Head Neck Pathol. 2017, 11, 278–287. [Google Scholar] [CrossRef]
- Benn, D.E.; Gimenez-Roqueplo, A.P.; Reilly, J.R.; Bertherat, J.; Burgess, J.; Byth, K.; Croxson, M.; Dahia, P.L.; Elston, M.; Gimm, O.; et al. Clinical presentation and penetrance of pheochromocytoma/paraganglioma syndromes. J. Clin. Endocrinol. Metab. 2006, 91, 827–836. [Google Scholar] [CrossRef]
- King, K.S.; Prodanov, T.; Kantorovich, V.; Fojo, T.; Hewitt, J.K.; Zacharin, M.; Wesley, R.; Lodish, M.; Raygada, M.; Gimenez-Roqueplo, A.P.; et al. Metastatic pheochromocytoma/paraganglioma related to primary tumor development in childhood or adolescence: Significant link to SDHB mutations. J. Clin. Oncol. 2011, 29, 4137–4142. [Google Scholar] [CrossRef] [PubMed]
- Turkova, H.; Prodanov, T.; Maly, M.; Martucci, V.; Adams, K.; Widimsky, J., Jr.; Chen, C.C.; Ling, A.; Kebebew, E.; Stratakis, C.A.; et al. Characteristics and outcomes of metastatic sdhb and sporadic pheochromocytoma/paraganglioma: An national institutes of health study. Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 2016, 22, 302–314. [Google Scholar] [CrossRef]
- Accetturo, M.; Bartolomeo, N.; Stella, A. In-silico Analysis of NF1 Missense Variants in ClinVar: Translating Variant Predictions into Variant Interpretation and Classification. Int. J. Mol. Sci. 2020, 21, 721. [Google Scholar] [CrossRef]
- Accetturo, M.; D’Uggento, A.M.; Portincasa, P.; Stella, A. Improvement of MEFV gene variants classification to aid treatment decision making in familial Mediterranean fever. Rheumatology (Oxf. Engl.) 2020, 59, 754–761. [Google Scholar] [CrossRef]
- Ioannidis, N.M.; Rothstein, J.H.; Pejaver, V.; Middha, S.; McDonnell, S.K.; Baheti, S.; Musolf, A.; Li, Q.; Holzinger, E.; Karyadi, D.; et al. REVEL: An Ensemble Method for Predicting the Pathogenicity of Rare Missense Variants. Am. J. Hum. Genet. 2016, 99, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Toledo, R.A.; Burnichon, N.; Cascon, A.; Benn, D.E.; Bayley, J.P.; Welander, J.; Tops, C.M.; Firth, H.; Dwight, T.; Ercolino, T.; et al. Consensus Statement on next-generation-sequencing-based diagnostic testing of hereditary phaeochromocytomas and paragangliomas. Nat. Rev. Endocrinol. 2017, 13, 233–247. [Google Scholar] [CrossRef]
- Miller, D.T.; Lee, K.; Chung, W.K.; Gordon, A.S.; Herman, G.E.; Klein, T.E.; Stewart, D.R.; Amendola, L.M.; Adelman, K.; Bale, S.J.; et al. ACMG SF v3.0 list for reporting of secondary findings in clinical exome and genome sequencing: A policy statement of the American College of Medical Genetics and Genomics (ACMG). Genet. Med. Off. J. Am. Coll. Med. Genet. 2021. [Google Scholar] [CrossRef]
- Alirezaie, N.; Kernohan, K.D.; Hartley, T.; Majewski, J.; Hocking, T.D. ClinPred: Prediction Tool to Identify Disease-Relevant Nonsynonymous Single-Nucleotide Variants. Am. J. Hum. Genet. 2018, 103, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Desmet, F.O.; Hamroun, D.; Lalande, M.; Collod-Béroud, G.; Claustres, M.; Béroud, C. Human Splicing Finder: An online bioinformatics tool to predict splicing signals. Nucleic Acids Res. 2009, 37, e67. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).