Preferences of Treatment Strategies among Women with Low-Risk DCIS and Oncologists
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population: Patients and Oncologists
2.2. Questionnaire Design for Patients and Health Care Professionals
2.3. Intolerance of Uncertainty
2.4. Design of the Discrete Choice Experiment
2.5. Conditional Logit Model and Comparing Patient and Oncologist Preferences
3. Results
3.1. Respondents
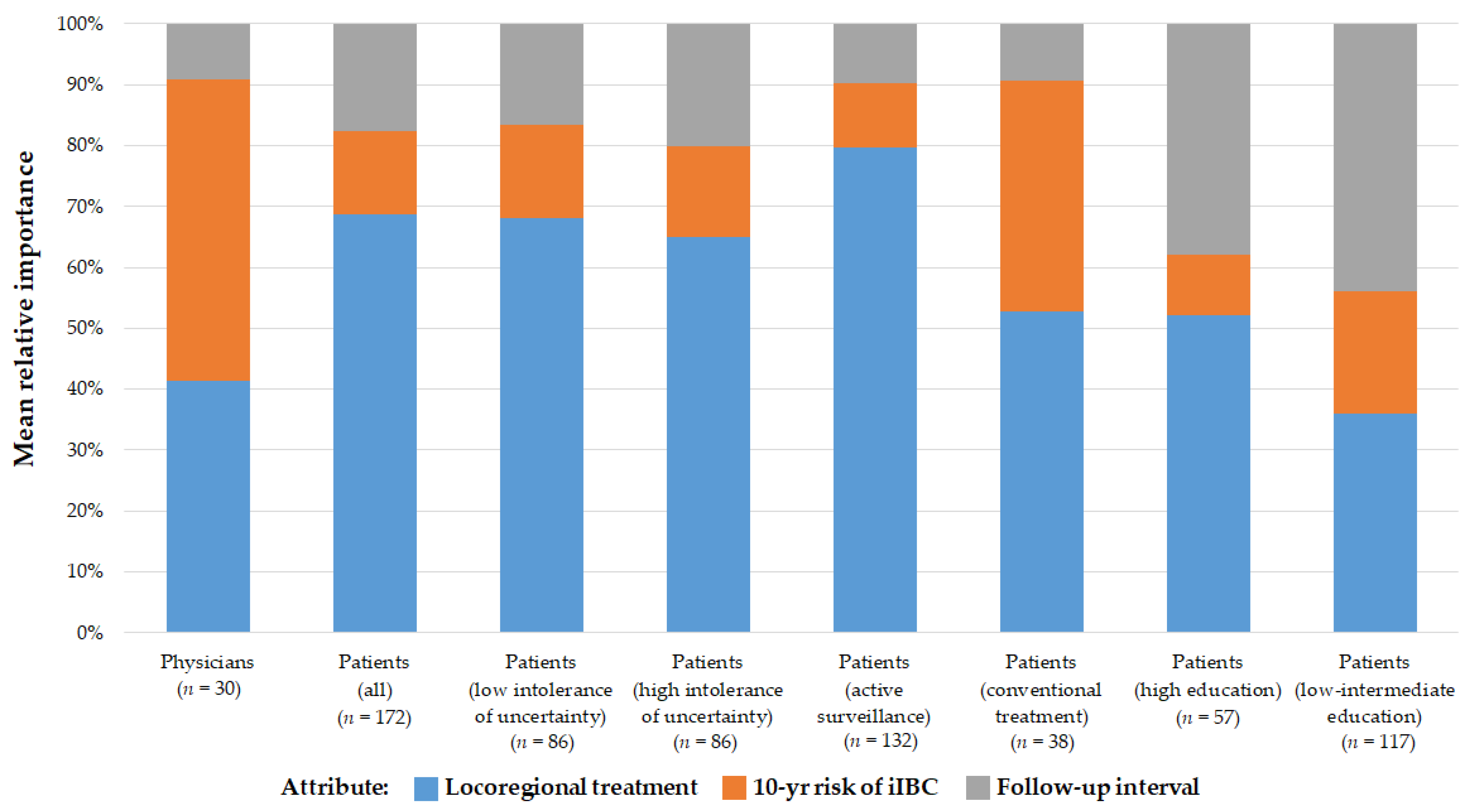
3.2. Importance of Treatment Characteristics
3.3. Influence of the Attributes on Patients’ and Oncologists’ Preference
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elshof, L.E.; Tryfonidis, K.; Slaets, L.; van Leeuwen-Stok, A.E.; Skinner, V.P.; Dif, N.; Pijnappel, R.M.; Bijker, N.; Rutgers, E.J.; Wesseling, J. Feasibility of a prospective, randomised, open-label, international multicentre, phase III, non-inferiority trial to assess the safety of active surveillance for low risk ductal carcinoma in situ—The LORD study. Eur. J. Cancer 2015, 51, 1497–1510. [Google Scholar] [CrossRef] [Green Version]
- Francis, A.; Thomas, J.; Fallowfield, L.; Wallis, M.; Bartlett, J.M.; Brookes, C.; Roberts, T.; Pirrie, S.; Gaunt, C.; Young, J.; et al. Addressing overtreatment of screen detected DCIS; the LORIS trial. Eur. J. Cancer 2015, 51, 2296–2303. [Google Scholar] [CrossRef] [Green Version]
- Wallis, M.; Bartlett, J.; Billingham, L.; Bowden, S.; Brookes, C.; Dodwell, D.; Evans, A.; Fairbrother, P.; Fallowfield, L.; Gaunt, C.; et al. The LORIS trial: Randomising patients with lowor low intermediate-grade ductal carcinoma in situ (DCIS) to surgery or active monitoring. Breast Cancer Res. Treat. 2018, 167, 325–326. [Google Scholar]
- Hwang, E.S.; Hyslop, T.; Lynch, T.; Frank, E.; Pinto, D.; Basila, D.; Collyar, D.; Bennett, A.; Kaplan, C.; Rosenberg, S.; et al. The COMET (comparison of operative versus monitoring and endocrine therapy) trial: A phase III randomised controlled clinical trial for low-risk ductal carcinoma in situ (DCIS). BMJ Open 2019, 9, e026797. [Google Scholar] [CrossRef] [Green Version]
- Youngwirth, L.M.; Boughey, J.C.; Hwang, E.S. Surgery versus monitoring and endocrine therapy for low-risk DCIS: The COMET trial. Bull. Am. Coll. Surg. 2017, 102, 62–63. [Google Scholar] [PubMed]
- Cuzick, J.; Sestak, I.; Forbes, J.F.; Dowsett, M.; Cawthorn, S.; Mansel, R.E.; Loibl, S.; Bonanni, B.; Howell, A. UK Interdisciplinary Breast Cancer Symposium 2020. Breast Cancer Res. Treat. 2020, 180, 527–596. [Google Scholar]
- Wasmann, K.A.; Wijsman, P.; van Dieren, S.; Bemelman, W.; Buskens, C. Partially randomised patient preference trials as an alternative design to randomised controlled trials: Systematic review and meta-analyses. BMJ Open 2019, 9, e031151. [Google Scholar] [CrossRef]
- Piccart, M.J.; Hilbers, F.S.; Bliss, J.M.; Caballero, C.; Frank, E.S.; Renault, P.; Naït Kaoudjt, R.; Schumacher, E.; Spears, P.A.; Regan, M.M. Road map to safe and well-designed de-escalation trials of systemic adjuvant therapy for solid tumors. J. Clin. Oncol. 2020, 38, 4120–4129. [Google Scholar] [CrossRef] [PubMed]
- Correa, C.; McGale, P.; Taylor, C.; Wang, Y.; Clarke, M.; Davies, C.; Peto, R.; Bijker, N.; Solin, L.; Darby, S. Overview of the randomized trials of radiotherapy in ductal carcinoma in situ of the breast. J. Natl. Cancer Inst. Monogr. 2010, 2010, 162–177. [Google Scholar] [PubMed]
- McCormick, B.; Winter, K.; Hudis, C.; Kuerer, H.M.; Rakovitch, E.; Smith, B.L.; Sneige, N.; Moughan, J.; Shah, A.; Germain, I.; et al. Rtog 9804: A prospective randomized trial for good-risk ductal carcinoma in situ comparing radiotherapy with observation. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 709–715. [Google Scholar] [CrossRef] [Green Version]
- Wapnir, I.L.; Dignam, J.J.; Fisher, B.; Mamounas, E.P.; Anderson, S.J.; Julian, T.B.; Land, S.R.; Margolese, R.G.; Swain, S.M.; Costantino, J.P.; et al. Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS. J. Natl. Cancer Inst. 2011, 103, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Wärnberg, F.; Garmo, H.; Emdin, S.; Hedberg, V.; Adwall, L.; Sandelin, K.; Ringberg, A.; Karlsson, P.; Arnesson, L.G.; Anderson, H.; et al. Effect of radiotherapy after breast-conserving surgery for ductal carcinoma in situ: 20 years follow-up in the randomized swedcis trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2014, 32, 3613–3618. [Google Scholar] [CrossRef] [PubMed]
- Allred, D.C.; Anderson, S.J.; Paik, S.; Wickerham, D.L.; Nagtegaal, I.D.; Swain, S.M.; Mamounas, E.P.; Julian, T.B.; Geyer, C.E., Jr.; Costantino, J.P.; et al. Adjuvant tamoxifen reduces subsequent breast cancer in women with estrogen receptor-positive ductal carcinoma in situ: A study based on nsabp protocol b-24. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012, 30, 1268–1273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forbes, J.F.; Sestak, I.; Howell, A.; Bonanni, B.; Bundred, N.; Levy, C.; von Minckwitz, G.; Eiermann, W.; Neven, P.; Stierer, M.; et al. Anastrozole versus tamoxifen for the prevention of locoregional and contralateral breast cancer in postmenopausal women with locally excised ductal carcinoma in situ (ibis-ii dcis): A double-blind, randomised controlled trial. Lancet 2016, 387, 866–873. [Google Scholar] [CrossRef] [Green Version]
- Margolese, R.G.; Cecchini, R.S.; Julian, T.B.; Ganz, P.A.; Costantino, J.P.; Vallow, L.A.; Albain, K.S.; Whitworth, P.W.; Cianfrocca, M.E.; Brufsky, A.M.; et al. Anastrozole versus tamoxifen in postmenopausal women with ductal carcinoma in situ undergoing lumpectomy plus radiotherapy (NSABP B-35): A randomised, double-blind, phase 3 clinical trial. Lancet 2016, 387, 849–856. [Google Scholar] [CrossRef] [Green Version]
- McCormick, B. Randomized trial evaluating radiation following surgical excision for “good risk” DCIS: 12-year report from NRG/RTOG 9804. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 1603. [Google Scholar] [CrossRef]
- Carleton, R.N.; Norton, M.A.; Asmundson, G.J. Fearing the unknown: A short version of the intolerance of uncertainty scale. J. Anxiety Disord. 2007, 21, 105–117. [Google Scholar] [CrossRef]
- Helsen, K.; Van den Bussche, E.; Vlaeyen, J.W.; Goubert, L. Confirmatory factor analysis of the Dutch intolerance of uncertainty scale: Comparison of the full and short version. J. Behav. Ther. Exp. Psychiatry 2013, 44, 21–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boswell, J.F.; Thompson-Hollands, J.; Farchione, T.J.; Barlow, D.H. Intolerance of uncertainty: A common factor in the treatment of emotional disorders. J. Clin. Psychol. 2013, 69, 630–645. [Google Scholar] [CrossRef] [PubMed]
- Hauber, A.B.; González, J.M.; Groothuis-Oudshoorn, C.G.; Prior, T.; Marshall, D.A.; Cunningham, C.; MJ, I.J.; Bridges, J.F. Statistical methods for the analysis of discrete choice experiments: A report of the ISPOR conjoint analysis good research practices task force. Value Health J. Int. Soc. Pharm. Outcomes Res. 2016, 19, 300–315. [Google Scholar] [CrossRef] [Green Version]
- Engelhardt, E.; Byng, D.; Klaver, K.; Gerritsma, M.; Verschuur, E.; van Oirsouw, M.; Wesseling, J.; van Duijnhoven, F.; Retèl, V.; Bleiker, E. Women diagnosed with ductal carcinoma in situ (DCIS) and healthcare providers’ views on active surveillance for DCIS. Results from focus groups and in-depth interviews. Eur. J. Cancer 2020, 138, S36. [Google Scholar] [CrossRef]
- Johnson, R.; Orme, B. Getting the Most from CBC; Sawtooth Software Research Paper Series; Sawtooth Software: Sequim, WA, USA, 2003. [Google Scholar]
- Orme, B. Sample Size Issues for Conjoint Analysis Studies; Sawtooth Software Technical Paper; Sawtooth Software: Sequim, WA, USA, 1998. [Google Scholar]
- McFadden, D. Conditional Logit Analysis of Qualitative Choice Behavior. In Frontiers in Econometrics; Zarembka, P., Ed.; Academic Press: New York, NY, USA, 1973; pp. 105–142. [Google Scholar]
- Jonczyk, M.M.; Fisher, C.S.; Babbitt, R.; Paulus, J.K.; Freund, K.M.; Czerniecki, B.; Margenthaler, J.A.; Losken, A.; Chatterjee, A. Surgical predictive model for breast cancer patients assessing acute postoperative complications: The breast cancer surgery risk calculator. Ann. Surg. Oncol. 2021, 1–11. [Google Scholar] [CrossRef]
- Rose, M.; Manjer, J.; Ringberg, A.; Svensson, H. Surgical strategy, methods of reconstruction, surgical margins and postoperative complications in oncoplastic breast surgery. Eur. J. Plast. Surg. 2014, 37, 205–214. [Google Scholar] [CrossRef] [Green Version]
- Kahneman, D.; Tversky, A. Prospect theory: An analysis of decision under risk. In Handbook of the Fundamentals of Financial Decision Making: Part I; World Scientific: Singapore, 2013; pp. 99–127. [Google Scholar]
- Lloyd, A.J. Threats to the estimation of benefit: Are preference elicitation methods accurate? Health Econ. 2003, 12, 393–402. [Google Scholar] [CrossRef]
- Tan, H.-J.; Marks, L.S.; Hoyt, M.A.; Kwan, L.; Filson, C.P.; Macairan, M.; Lieu, P.; Litwin, M.S.; Stanton, A.L. The relationship between intolerance of uncertainty and anxiety in men on active surveillance for prostate cancer. J. Urol. 2016, 195, 1724–1730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimm, L.J.; Ryser, M.D.; Partridge, A.H.; Thompson, A.M.; Thomas, J.S.; Wesseling, J.; Hwang, E.S. Surgical Upstaging Rates for Vacuum Assisted Biopsy Proven DCIS: Implications for Active Surveillance Trials. Ann. Surg. Oncol. 2017, 12, 3534–3540. [Google Scholar] [CrossRef] [PubMed]
- Nicosia, L.; di Giulio, G.; Bozzini, A.C.; Fanizza, M.; Ballati, F.; Rotili, A.; Lazzeroni, M.; Latronico, A.; Abbate, F.; Renne, G.; et al. Complete Removal of the Lesion as a Guidance in the Management of Patients with Breast Ductal Carcinoma In Situ. Cancers 2021, 13, 868. [Google Scholar] [CrossRef] [PubMed]
- Mannu, G.S.; Groen, E.J.; Wang, Z.; Schaapveld, M.; Lips, E.H.; Chung, M.; Joore, I.; van Leeuwen, F.E.; Teertstra, H.J.; Winter-Warnars, G.A.O.; et al. Reliability of preoperative breast biopsies showing ductal carcinoma in situ and implications for non-operative treatment: A cohort study. Breast Cancer Res. Treat. 2019, 178, 409–418. [Google Scholar] [CrossRef] [Green Version]
- Bridges, J.F.; Hauber, A.B.; Marshall, D.; Lloyd, A.; Prosser, L.A.; Regier, D.A.; Johnson, F.R.; Mauskopf, J. Conjoint analysis applications in health—A checklist: A report of the ISPOR good research practices for conjoint analysis task force. Value Health 2011, 14, 403–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E. Early breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up†. Ann. Oncol. Off. J. Eur. Soc. Med Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://clinicaltrials.gov/ct2/show/NCT02492607 (accessed on 14 February 2021).
- Chapman, B.M.; Yang, J.C.; Gonzalez, J.M.; Havrilesky, L.; Reed, S.D.; Hwang, E.S. Patient preferences for outcomes following DCIS management strategies: A discrete choice experiment. JCO Oncol. Pract. 2021, OP2000614. [Google Scholar] [CrossRef] [PubMed]
- Vass, C.M.; Wright, S.; Burton, M.; Payne, K. Scale heterogeneity in healthcare discrete choice experiments: A primer. Patient 2018, 11, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Raue, M.; Streicher, B.; Lermer, E.; Frey, D. How far does it feel? Construal level and decisions under risk. J. Appl. Res. Mem. Cogn. 2015, 4, 256–264. [Google Scholar] [CrossRef] [Green Version]
| Attributes | Levels |
|---|---|
| Locoregional treatment strategy | No surgery; breast conserving surgery; breast conserving surgery followed by radiotherapy; mastectomy |
| 10-year risk of ipsilateral invasive breast cancer (iIBC) | 5%; 10%; 15% |
| Surveillance mammography follow-up interval | 6 months; 1 year; 2 years |
| Characteristics | Patients (n = 172) N (%) | Oncologists (n = 30) N (%) |
|---|---|---|
| Age, years (median, range) | 59 (45–77) | N.A. |
| Sex | ||
| Female | 172 (100%) | 21 (70.0%) |
| Male | 0 | 9 (30.0%) |
| Actual treatment selected | ||
| Active surveillance | 132 (76.7%) | N.A. |
| Conventional treatment | 38 (22.1%) | N.A. |
| Unknown | 2 (1.2%) | N.A. |
| Educational level | ||
| Low | 37 (21.5%) | 0 |
| Intermediate | 78 (45.3%) | 0 |
| High | 57 (33.1%) | 30 (100%) |
| Employment status | ||
| Employed (part-time or full-time) | 95 (55.2%) | 30 (100%) |
| Unemployed/pension | 77 (44.8%) | 0 |
| Hospital type | ||
| Academic medical center | 3 (1.7%) | 8 (26.7%) |
| General teaching hospital | 105 (61.0%) | 14 (46.7%) |
| Specialized oncology hospital | 25 (14.5%) | 5 (16.7%) |
| General hospital | 39 (22.7%) | 3 (10.0%) |
| Region of the Netherlands | ||
| North | 3 (1.7%) | 3 (10.0%) |
| East | 60 (34.9%) | 5 (16.7%) |
| West | 98 (57.0%) | 17 (56.7%) |
| South | 11 (6.4%) | 5 (16.7%) |
| Subspecialty | ||
| Surgical oncology | N.A. | 20 (66.7%) |
| Radiation oncology | N.A. | 10 (33.3%) |
| Number of patients with DCIS treated per year | ||
| 2–5 patients | N.A. | 1 (3.3%) |
| 6–10 patients | N.A. | 7 (23.3%) |
| 11–15 patients | N.A. | 3 (10.0%) |
| 16–20 patients | N.A. | 11 (36.7%) |
| >20 patients | N.A. | 8 (26.7%) |
| Years’ experience treating patients with DCIS | ||
| 2–5 years | N.A. | 1 (3.3%) |
| 6–10 years | N.A. | 9 (30.0%) |
| >10 years | N.A. | 20 (66.7%) |
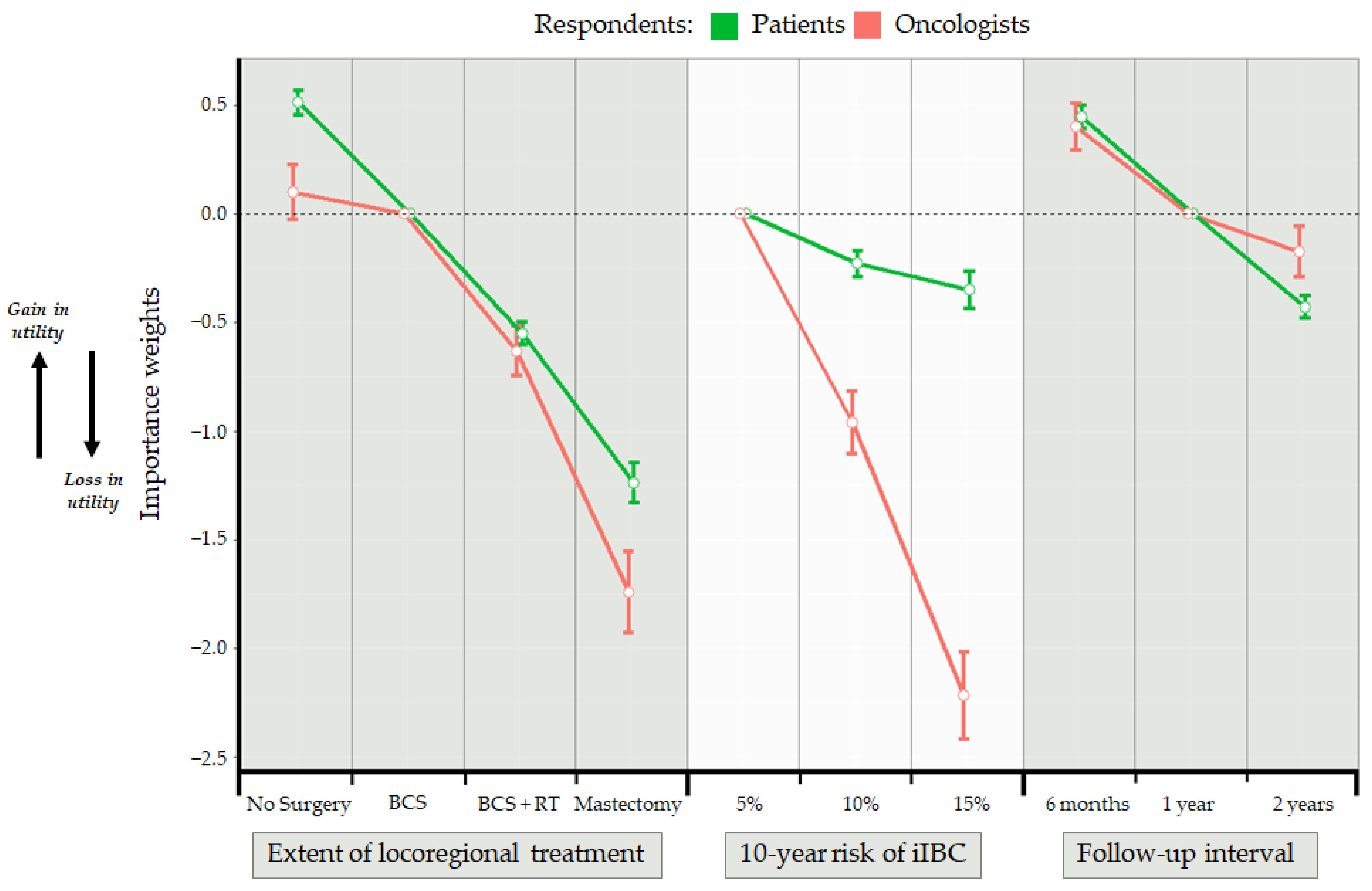
| Attribute Levels | Patients | Oncologists | ||||
|---|---|---|---|---|---|---|
| Coefficient (β) | SE | Exp (β) | Coefficient (β) | SE | Exp (β) | |
| Locoregional treatment | ||||||
| Breast conserving surgery | (ref.) | (ref.) | ||||
| No surgery | 0.513 * | 0.111 | 1.67 | 0.100 | 0.251 | 1.11 |
| Breast conserving surgery + radiotherapy | −0.551 * | 0.102 | 0.58 | −0.632 * | 0.229 | 0.53 |
| Mastectomy | −1.239 * | 0.185 | 0.29 | −1.743 * | 0.371 | 0.18 |
| 10-year risk of ipsilateral invasive breast cancer | ||||||
| 5% | (ref.) | (ref.) | ||||
| 10% | −0.229 | 0.122 | 0.79 | −0.962 * | 0.290 | 0.38 |
| 15% | −0.350 * | 0.174 | 0.70 | −2.219 * | 0.399 | 0.11 |
| Interval surveillance follow-up | ||||||
| 1 year | (ref.) | (ref.) | ||||
| 6 months | 0.448 * | 0.110 | 1.56 | 0.403 | 0.218 | 1.50 |
| 2 years | −0.429 * | 0.103 | 0.65 | −0.175 | 0.235 | 0.84 |
| Interaction Terms a | Coefficient (β) | SE | Exp (β) | p-Value | ||
| Attribute: Locoregional treatment * respondent type | <0.001 b | |||||
| Level: No surgery | ||||||
| Patient | (ref.) | |||||
| Oncologist | −0.413 | 0.275 | 0.66 | 0.13 | ||
| Level: Breast conserving surgery + radiotherapy | ||||||
| Patient | (ref.) | |||||
| Oncologist | −0.082 | 0.251 | 0.92 | 0.75 | ||
| Level: Mastectomy | ||||||
| Patient | (ref.) | |||||
| Oncologist | −0.504 | 0.414 | 0.60 | 0.22 | ||
| Attribute: 10-year risk of ipsilateral invasive breast cancer * respondent type | <0.001 b | |||||
| Level: 10% risk of iIBC | ||||||
| Patient | (ref.) | |||||
| Oncologist | −0.734 | 0.314 | 0.48 | 0.02 | ||
| Level: 15% risk of iIBC | ||||||
| Patient | (ref.) | |||||
| Oncologist | −1.870 | 0.435 | 0.15 | <0.001 | ||
| Attribute: follow-up interval * respondent type | <0.001 b | |||||
| Level: 6mo follow-up interval | ||||||
| Patient | (ref.) | |||||
| Physician | −0.045 | 0.245 | 0.96 | 0.85 | ||
| Level: 2 year follow-up interval | ||||||
| Patient | (ref.) | |||||
| Oncologist | 0.254 | 0.258 | 1.29 | 0.32 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Byng, D.; Retèl, V.P.; Engelhardt, E.G.; Groothuis-Oudshoorn, C.G.M.; van Til, J.A.; Schmitz, R.S.J.M.; van Duijnhoven, F.; Wesseling, J.; Bleiker, E.; van Harten, W.H.; et al. Preferences of Treatment Strategies among Women with Low-Risk DCIS and Oncologists. Cancers 2021, 13, 3962. https://doi.org/10.3390/cancers13163962
Byng D, Retèl VP, Engelhardt EG, Groothuis-Oudshoorn CGM, van Til JA, Schmitz RSJM, van Duijnhoven F, Wesseling J, Bleiker E, van Harten WH, et al. Preferences of Treatment Strategies among Women with Low-Risk DCIS and Oncologists. Cancers. 2021; 13(16):3962. https://doi.org/10.3390/cancers13163962
Chicago/Turabian StyleByng, Danalyn, Valesca P. Retèl, Ellen G. Engelhardt, Catharina G. M. Groothuis-Oudshoorn, Janine A. van Til, Renée S. J. M. Schmitz, Frederieke van Duijnhoven, Jelle Wesseling, Eveline Bleiker, Wim H. van Harten, and et al. 2021. "Preferences of Treatment Strategies among Women with Low-Risk DCIS and Oncologists" Cancers 13, no. 16: 3962. https://doi.org/10.3390/cancers13163962
APA StyleByng, D., Retèl, V. P., Engelhardt, E. G., Groothuis-Oudshoorn, C. G. M., van Til, J. A., Schmitz, R. S. J. M., van Duijnhoven, F., Wesseling, J., Bleiker, E., van Harten, W. H., & on behalf of the Grand Challenge Precision Consortium. (2021). Preferences of Treatment Strategies among Women with Low-Risk DCIS and Oncologists. Cancers, 13(16), 3962. https://doi.org/10.3390/cancers13163962






