Three-Dimensional Vascularized Lung Cancer-on-a-Chip with Lung Extracellular Matrix Hydrogels for In Vitro Screening
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Cell Culture
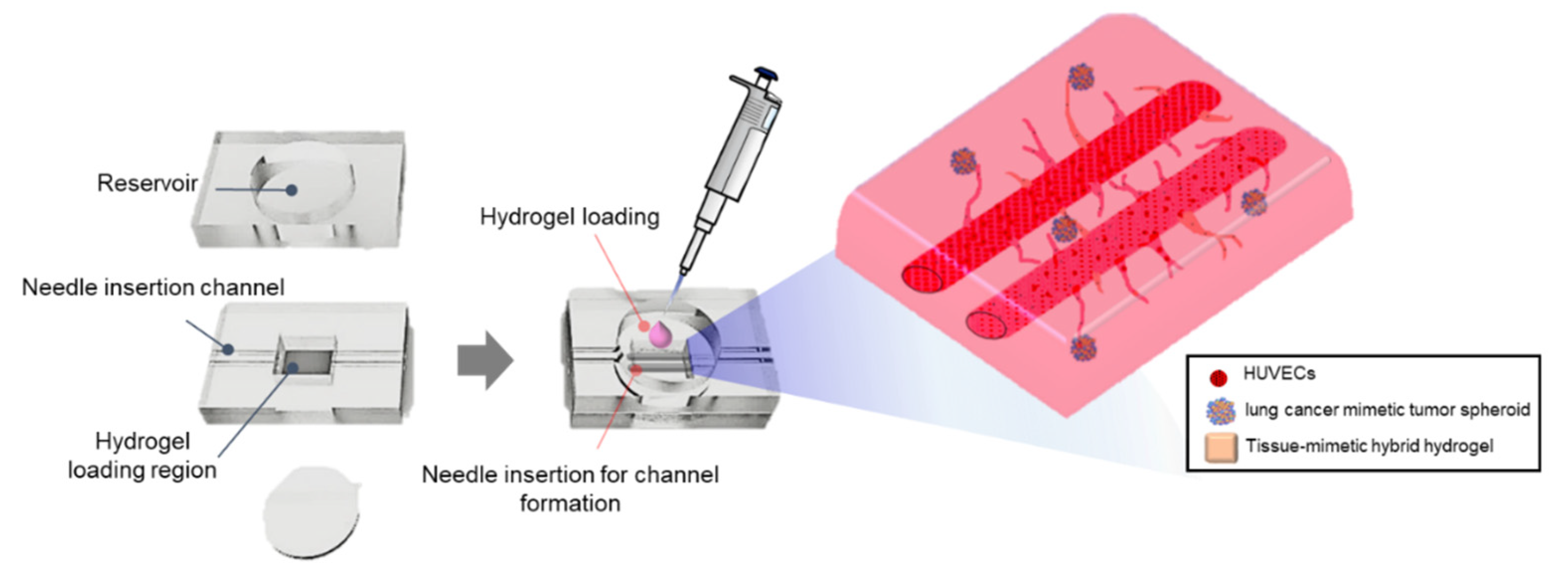
2.2. VLCC Design and Fabrication
2.3. Decellularization of Porcine Lung Tissue
2.4. Biochemical Characterization of ldECM
2.5. Generation of ldECM Hydrogel and Optimization of TMH Hydrogel
2.6. Evaluation of Compressive Modulus
2.7. Scanning Electron Microscopy and Quantification of Fibers Diameter
2.8. Evaluation of Cell Viability in TMH Hydrogel
2.9. Characterization of Angiogenic Activity
2.10. Optimization of Tumor Spheroid Formation
2.11. Characterization of the VLCC
2.12. Drug Administration for Drug Screening in 2D Culture, Sph-H, and VLCC
2.13. The Assessment of Drug Efficacy in 2D Culture, Sph-H, and VLCC
2.14. Statistical Analysis
3. Results
3.1. Components of ldECM Shown to Be Effective in Mimicking the Native Lung Tissue
3.2. The Optimized Concentration of ldECM Showed A Rigid Morphology and High Cellular Activity
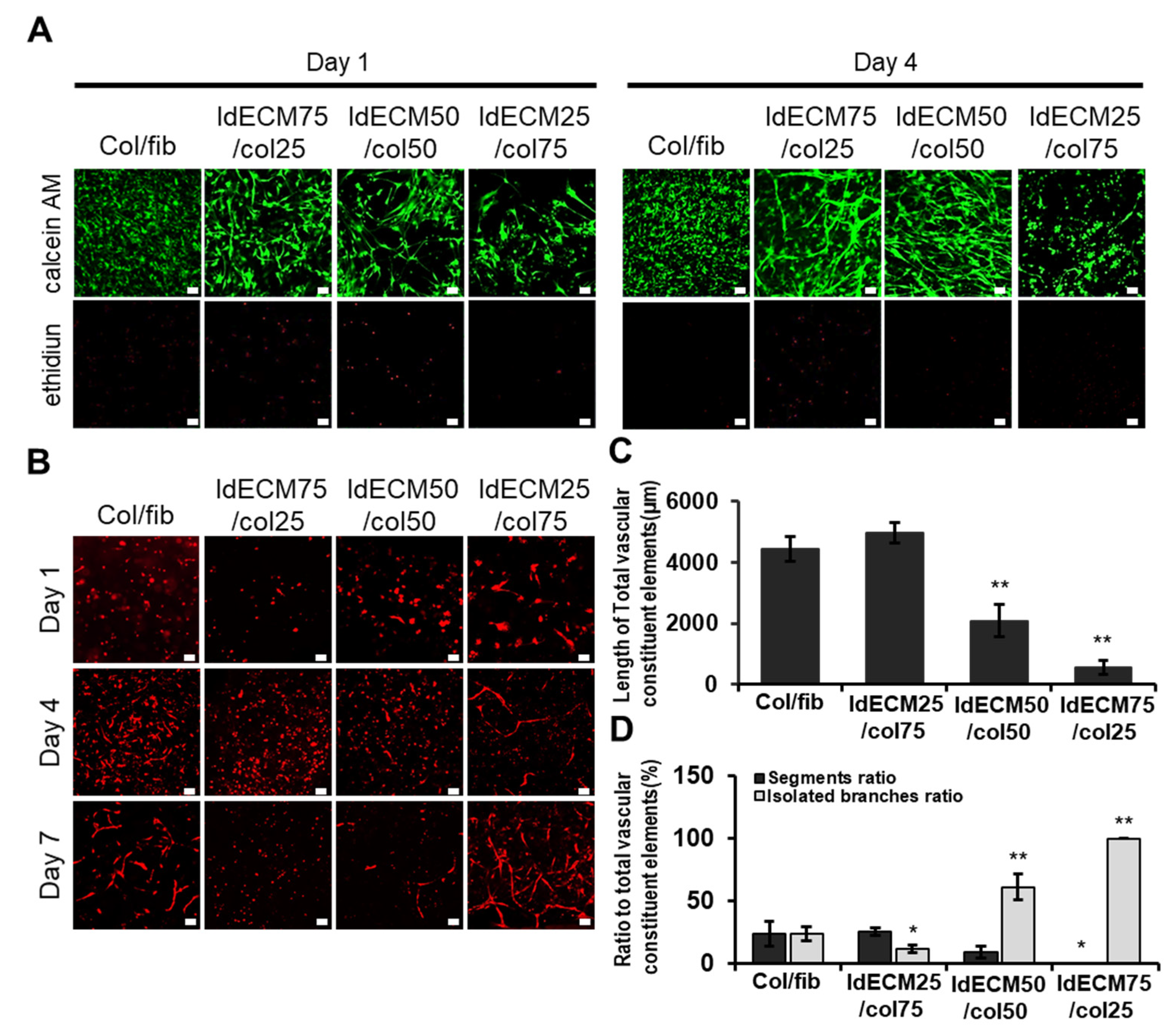
3.3. The Ratio of ldECM/Collagen Composition Having Good Performances Was Optimized
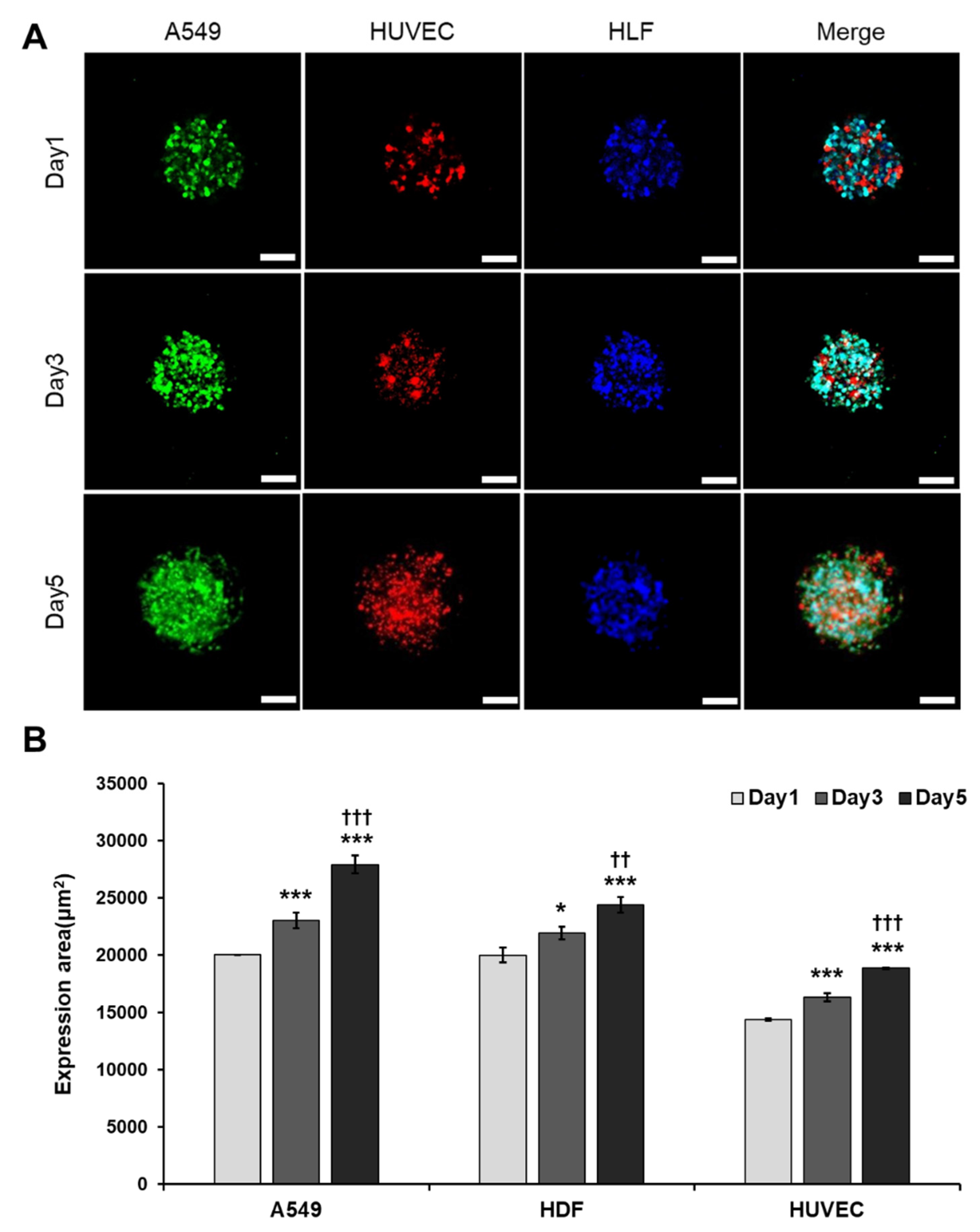
3.4. A More Compact and Angiogenic Spheroid Was Produced through Tri-Cellular Culture
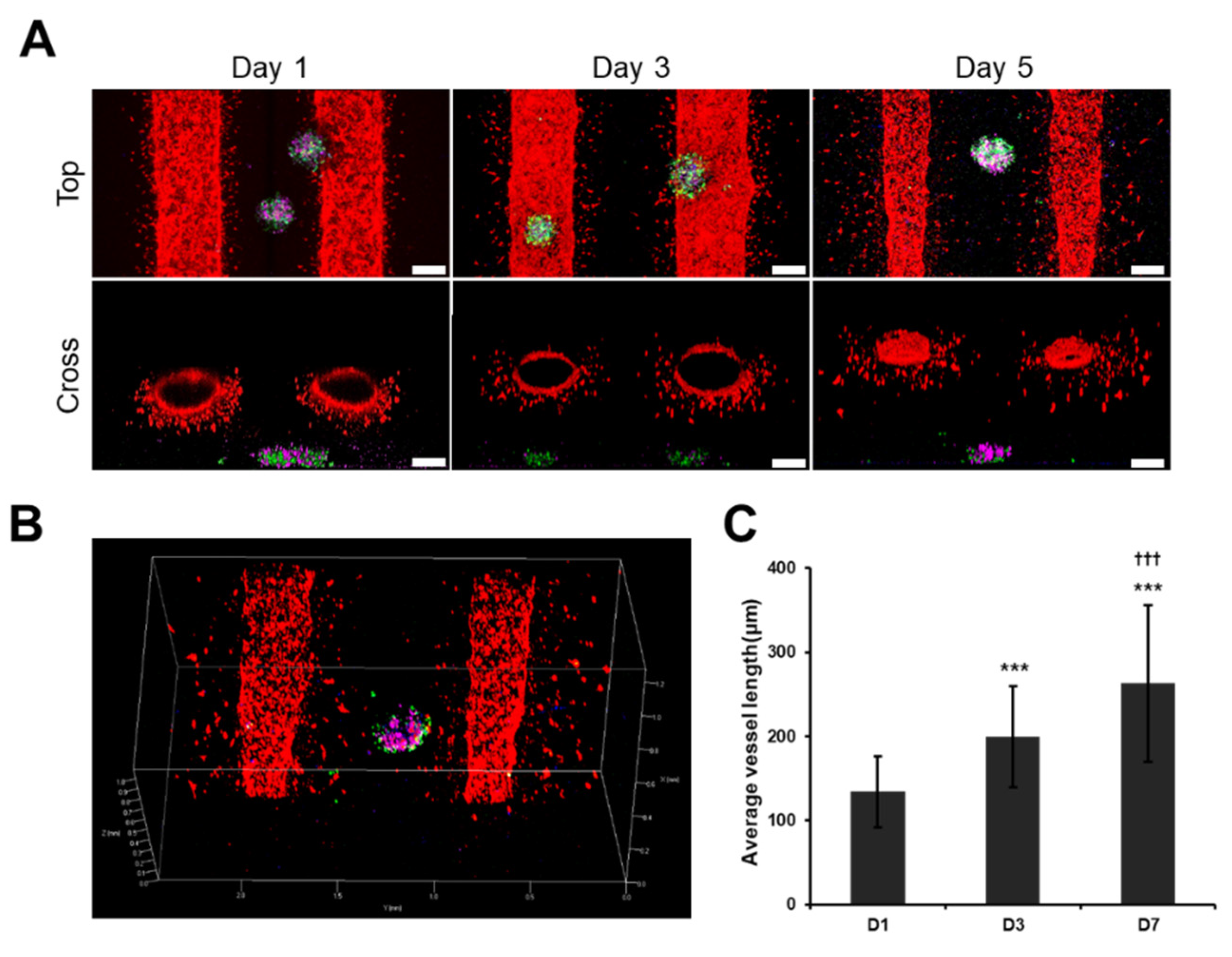
3.5. Angiogenic Sprouting Was Confirmed in VLCC under Optimized Conditions
3.6. VLCC Responded MORE Efficiently for Drug Efficacy Evaluation and Mechanism Estimation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Esch, E.W.; Bahinski, A.; Huh, D. Organs-on-chips at the frontiers of drug discovery. Nat. Rev. Drug Discov. 2015, 14, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.-H.; Lee, S.; Park, J.; Jeon, J.; Cho, Y.-J.; Kim, S. Potential of drug efficacy evaluation in lung and kidney cancer models using organ-on-a-chip technology. Micromachines 2021, 12, 215. [Google Scholar] [CrossRef]
- Katt, M.E.; Placone, A.L.; Wong, A.D.; Xu, Z.S.; Searson, P.C. In vitro tumor models: Advantages, disadvantages, variables, and selecting the right platform. Front. Bioeng. Biotechnol. 2016, 4, 12. [Google Scholar] [CrossRef]
- Antoni, D.; Burckel, H.; Josset, E.; Noel, G. Three-dimensional cell culture: A breakthrough in vivo. Int. J. Mol. Sci. 2015, 16, 5517–5527. [Google Scholar] [CrossRef]
- Marusyk, A.; Polyak, K. Tumor heterogeneity: Causes and consequences. Biochim. Biophys Acta 2010, 1805, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Duzagac, F.; Saorin, G.; Memeo, L.; Canzonieri, V.; Rizzolio, F. Microfluidic organoids-on-a-chip: Quantum leap in cancer research. Cancers 2021, 13, 737. [Google Scholar] [CrossRef]
- Morgan, J.P.; Delnero, P.F.; Zheng, Y.; Verbridge, S.S.; Chen, J.; Craven, M.; Choi, N.W.; Diaz-Santana, A.; Kermani, P.; Hempstead, B.; et al. Formation of microvascular networks in vitro. Nat. Protoc. 2013, 8, 1820–1836. [Google Scholar] [CrossRef]
- Vailhé, B.; Vittet, D.; Feige, J.-J. In vitro models of vasculogenesis and angiogenesis. Lab. Investig. 2001, 81, 439–452. [Google Scholar] [CrossRef]
- Risau, W. Mechanisms of angiogenesis. Nat. Cell Biol. 1997, 386, 671–674. [Google Scholar] [CrossRef]
- Li, Q.; Hickman, M.; Wei, P. Therapeutic and toxicological inhibition of vasculogenesis and angiogenesis mediated by artesunate, a compound with both antimalarial and anticancer efficacy. Vasc. Angiogenesis Embryonic Dev. Regen. Med. 2011. [Google Scholar] [CrossRef][Green Version]
- Yeon, J.H.; Ryu, H.R.; Chung, M.; Hu, Q.P.; Jeon, N.L. In vitro formation and characterization of a perfusable three-dimensional tubular capillary network in microfluidic devices. Lab Chip 2012, 12, 2815–2822. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Ahn, J.; Kim, S.; Lee, Y.; Lee, J.; Park, D.; Jeon, N.L. Tumor spheroid-on-a-chip: A standardized microfluidic culture platform for investigating tumor angiogenesis. Lab Chip 2019, 19, 2822–2833. [Google Scholar] [CrossRef]
- Nashimoto, Y.; Okada, R.; Hanada, S.; Arima, Y.; Nishiyama, K.; Miura, T.; Yokokawa, R. Vascularized cancer on a chip: The effect of perfusion on growth and drug delivery of tumor spheroid. Biomaterials 2020, 229, 119547. [Google Scholar] [CrossRef]
- Hay, E.D. Cell Biology of Extracellular Matrix; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Gilbert, T.; Sellaro, T.L.; Badylak, S.F. Decellularization of tissues and organs. Biomaterials 2006, 27, 3675–3683. [Google Scholar] [CrossRef]
- Hong, Y.J.; Bae, S.E.; Do, S.H.; Kim, I.H.; Han, D.K.; Park, K. Decellularized PLGA-based scaffolds and their osteogenic potential with bone marrow stromal cells. Macromol. Res. 2011, 19, 1090–1096. [Google Scholar] [CrossRef]
- Choi, J.S.; Choi, Y.C.; Kim, J.D.; Kim, E.J.; Lee, H.Y.; Kwon, I.C.; Cho, Y.W. Adipose tissue: A valuable resource of biomaterials for soft tissue engineering. Macromol. Res. 2014, 22, 932–947. [Google Scholar] [CrossRef]
- Park, G.R.; Lee, J.G.; Chun, H.J.; Han, D.K.; Park, K. Characterization of naturally derived macromolecular matrix and its osteogenic activity with preosteoblasts. Macromol. Res. 2012, 20, 868–874. [Google Scholar] [CrossRef]
- Lu, T.-Y.; Lin, B.; Kim, J.; Sullivan, M.; Tobita, K.; Salama, G.; Yang, L. Repopulation of decellularized mouse heart with human induced pluripotent stem cell-derived cardiovascular progenitor cells. Nat. Commun. 2013, 4, 2307. [Google Scholar] [CrossRef] [PubMed]
- Pati, F.; Jang, J.; Ha, D.-H.; Kim, S.W.; Rhie, J.-W.; Shim, J.-H.; Kim, D.-H.; Cho, D.-W. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. 2014, 5, 3935. [Google Scholar] [CrossRef] [PubMed]
- Mishra, D.K.; Thrall, M.J.; Baird, B.N.; Ott, H.C.; Blackmon, S.H.; Kurie, J.M.; Kim, M.P. Human lung cancer cells grown on acellular rat lung matrix create perfusable tumor nodules. Ann. Thorac. Surg. 2012, 93, 1075–1081. [Google Scholar] [CrossRef]
- Dunne, L.W.; Huang, Z.; Meng, W.; Fan, X.; Zhang, N.; Zhang, Q.; An, Z. Human decellularized adipose tissue scaffold as a model for breast cancer cell growth and drug treatments. Biomaterials 2014, 35, 4940–4949. [Google Scholar] [CrossRef]
- Xiong, G.; Flynn, T.J.; Chen, J.; Trinkle, C.A.; Xu, R. Development of an ex vivo breast cancer lung colonization model utilizing a decellularized lung matrix. Integr. Biol. 2015, 7, 1518–1525. [Google Scholar] [CrossRef]
- Allen, P.; Melero-Martin, J.; Bischoff, J. Type I collagen, fibrin and PuraMatrix matrices provide permissive environments for human endothelial and mesenchymal progenitor cells to form neovascular networks. J. Tissue Eng. Regen. Med. 2011, 5, e74–e86. [Google Scholar] [CrossRef]
- Raninga, R.; Page, K.; Parkin, I. Polydimethylsiloxane coated glass frits for low-cost and reusable water-organic solvent separation. Chem. Commun. 2014, 50, 12656–12658. [Google Scholar] [CrossRef]
- Buschmann, M.D.; Gluzband, Y.A.; Grodzinsky, A.J.; Hunziker, E.B. Mechanical compression modulates matrix biosynthesis in chondrocyte/agarose culture. J. Cell Sci. 1995, 108, 1497–1508. [Google Scholar] [CrossRef]
- Kim, T.H.; Kim, S.H.; Leong, K.W.; Jung, Y.; Kim, M.T.H. Nanografted substrata and triculture of human pericytes, fibroblasts, and endothelial cells for studying the effects on angiogenesis. Tissue Eng. Part A 2016, 22, 698–706. [Google Scholar] [CrossRef]
- Kim, T.H.; Jung, Y.; Kim, S.H. Nanofibrous electrospun heart decellularized extracellular matrix-based hybrid scaffold as wound dressing for reducing scarring in wound healing. Tissue Eng. Part A 2018, 24, 830–848. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Hirao, A.; Kong, Y.-Y.; Matsuoka, S.; Wakeham, A.; Ruland, J.; Yoshida, H.; Liu, D.; Elledge, S.J.; Mak, T.W. DNA Damage-induced activation of p53 by the checkpoint kinase Chk2. Science 2000, 287, 1824–1827. [Google Scholar] [CrossRef]
- Lee, E.; Song, H.-H.G.; Chen, C.S. Biomimetic on-a-chip platforms for studying cancer metastasis. Curr. Opin. Chem. Eng. 2016, 11, 20–27. [Google Scholar] [CrossRef]
- Hayashi, T.; Takigawa-Imamura, H.; Nishiyama, K.; Shintaku, H.; Kotera, H.; Miura, T.; Yokokawa, R. Vascular network formation for a long-term spheroid culture by co-culturing endothelial cells and fibroblasts. In Proceedings of the 2015 28th IEEE International Conference on Micro Electro Mechanical Systems (MEMS), Estoril, Portugal, 18–22 January 2015; Institute of Electrical and Electronics Engineers (IEEE): Piscataway, NJ, USA, 2015; pp. 476–479. [Google Scholar]
- Lee, S.-I.; Yeo, S.-I.; Kim, B.-B.; Ko, Y.; Park, J.-B. Formation of size-controllable spheroids using gingiva-derived stem cells and concave microwells: Morphology and viability tests. Biomed. Rep. 2016, 4, 97–101. [Google Scholar] [CrossRef]
- Tacar, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 2013, 65, 157–170. [Google Scholar] [CrossRef]
- Ozaki, T.; Nakagawara, A. Role of p53 in cell death and human cancers. Cancers 2011, 3, 994–1013. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.M.; Kobayashi, H.; Rafii, S. Instructive role of the vascular niche in promoting tumour growth and tissue repair by angiocrine factors. Nat. Rev. Cancer 2010, 10, 138–146. [Google Scholar] [CrossRef]
- Bergers, G.; Brekken, R.; McMahon, G.; Vu, T.H.; Itoh, T.; Tamaki, K.; Tanzawa, K.; Thorpe, P.; Itohara, S.; Werb, Z.; et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat. Cell Biol. 2000, 2, 737–744. [Google Scholar] [CrossRef]
- Fagiani, E.; Christofori, G. Angiopoietins in angiogenesis. Cancer Lett. 2013, 328, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Tang, Z.; Hou, X.; Lennartsson, J.; Li, Y.; Koch, A.W.; Scotney, P.; Lee, C.; Arjunan, P.; Dong, L.; et al. VEGF-B is dispensable for blood vessel growth but critical for their survival, and VEGF-B targeting inhibits pathological angiogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 6152–6157. [Google Scholar] [CrossRef] [PubMed]
- Hanatani, Y.; Kobayashi, H.; Kodaira, S.; Takami, H.; Asagoe, T.; Kaneshiro, E. An in vitro chemosensitivity test for gastric cancer using collagen gel droplet embedded culture. Oncol. Rep. 2000, 7, 1027–1060. [Google Scholar] [CrossRef]
- Kniazeva, E.; Kachgal, S.; Putnam, A.J. Effects of extracellular matrix density and mesenchymal stem cells on neovascularization in vivo. Tissue Eng. Part A 2011, 17, 905–914. [Google Scholar] [CrossRef]
- Rao, R.R.; Peterson, A.W.; Ceccarelli, J.; Putnam, A.; Stegemann, J.P. Matrix composition regulates three-dimensional network formation by endothelial cells and mesenchymal stem cells in collagen/fibrin materials. Angiogenesis 2012, 15, 253–264. [Google Scholar] [CrossRef]
- Roeder, S.M.A.B.A.; Kokini, M.A.K.; Sturgis, J.E.; Robinson, J.P.; Voytik-Harbin, S.L. Tensile mechanical properties of three-dimensional type i collagen extracellular matrices with varied microstructure. J. Biomech. Eng. 2002, 124, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Raub, C.B.; Suresh, V.; Krasieva, T.; Lyubovitsky, J.; Mih, J.D.; Putnam, A.; Tromberg, B.J.; George, S.C. Noninvasive assessment of collagen gel microstructure and mechanics using multiphoton microscopy. Biophys. J. 2007, 92, 2212–2222. [Google Scholar] [CrossRef]
- Liu, Y.; Sakolish, C.; Chen, Z.; Phan, D.T.; Bender, R.H.F.; Hughes, C.C.; Rusyn, I. Human in vitro vascularized micro-organ and micro-tumor models are reproducible organ-on-a-chip platforms for studies of anticancer drugs. Toxicology 2020, 445, 152601. [Google Scholar] [CrossRef]
- Mizia-Malarz, A.; Sobol, G.; Woś, H. Proangiogenic factors: Vascular-endothelial growth factor (VEGF) and basic fibro-blast growth factor—The characteristics and function. Przeg. Lek. 2008, 65, 353–357. [Google Scholar]
- Papapetropoulos, A.; Fulton, D.; Mahboubi, K.; Kalb, R.G.; O’Connor, D.S.; Li, F.; Altieri, D.C.; Sessa, W.C. Angiopoietin-1 inhibits endothelial cell apoptosis via the akt/survivin pathway. J. Biol. Chem. 2000, 275, 9102–9105. [Google Scholar] [CrossRef]
- Augustin, H.G.; Koh, G.Y.; Thurston, G.; Alitalo, K. Control of vascular morphogenesis and homeostasis through the angiopoietintie system. Nat. Rev. Mol. Cell Biol. 2009, 10, 165–177. [Google Scholar] [CrossRef]
- Ziyad, S.; Iruela-Arispe, M.L. Molecular mechanisms of tumor angiogenesis. Genes Cancer 2011, 2, 1085–1096. [Google Scholar] [CrossRef]
- Hanahan, D.; Folkman, J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996, 86, 353–364. [Google Scholar] [CrossRef]
- Khalid, M.A.U.; Kim, Y.S.; Ali, M.; Lee, B.G.; Cho, Y.-J.; Choi, K.H. A lung cancer-on-chip platform with integrated biosensors for physiological monitoring and toxicity assessment. Biochem. Eng. J. 2020, 155, 107469. [Google Scholar] [CrossRef]
- Ozcelikkale, A.; Shin, K.; Noe-Kim, V.; Elzey, B.D.; Dong, Z.; Zhang, J.-T.; Kim, K.; Kwon, I.C.; Park, K.; Han, B. Differential response to doxorubicin in breast cancer subtypes simulated by a microfluidic tumor model. J. Control. Release 2017, 266, 129–139. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, S.; Chen, C.; Huang, H.; Tao, L.; Qian, Z.; Li, W. Microfluidic-enabled self-organized tumor model for in vitro cytotoxicity assessment of doxorubicin. Biomed. Microdevices 2020, 22, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yu, D.; Hicklin, D.J.; Hannay, J.A.F.; Ellis, L.M.; Pollock, R.E. Combined anti-fetal liver kinase 1 monoclonal antibody and continuous low-dose doxorubicin inhibits angiogenesis and growth of human soft tissue sarcoma xenografts by induction of endothelial cell apoptosis. Cancer Res. 2002, 62, 2034–2042. [Google Scholar] [PubMed]
- Loges, S.; Schmidt, T.; Carmeliet, P. Mechanisms of resistance to anti-angiogenic therapy and development of third-generation anti-angiogenic drug candidates. Genes Cancer 2010, 1, 12–25. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Kim, T.H.; Kim, S.H.; You, S.; Jung, Y. Three-Dimensional Vascularized Lung Cancer-on-a-Chip with Lung Extracellular Matrix Hydrogels for In Vitro Screening. Cancers 2021, 13, 3930. https://doi.org/10.3390/cancers13163930
Park S, Kim TH, Kim SH, You S, Jung Y. Three-Dimensional Vascularized Lung Cancer-on-a-Chip with Lung Extracellular Matrix Hydrogels for In Vitro Screening. Cancers. 2021; 13(16):3930. https://doi.org/10.3390/cancers13163930
Chicago/Turabian StylePark, Sangun, Tae Hee Kim, Soo Hyun Kim, Seungkwon You, and Youngmee Jung. 2021. "Three-Dimensional Vascularized Lung Cancer-on-a-Chip with Lung Extracellular Matrix Hydrogels for In Vitro Screening" Cancers 13, no. 16: 3930. https://doi.org/10.3390/cancers13163930
APA StylePark, S., Kim, T. H., Kim, S. H., You, S., & Jung, Y. (2021). Three-Dimensional Vascularized Lung Cancer-on-a-Chip with Lung Extracellular Matrix Hydrogels for In Vitro Screening. Cancers, 13(16), 3930. https://doi.org/10.3390/cancers13163930






