Family History and Gastric Cancer Risk: A Pooled Investigation in the Stomach Cancer Pooling (STOP) Project Consortium
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
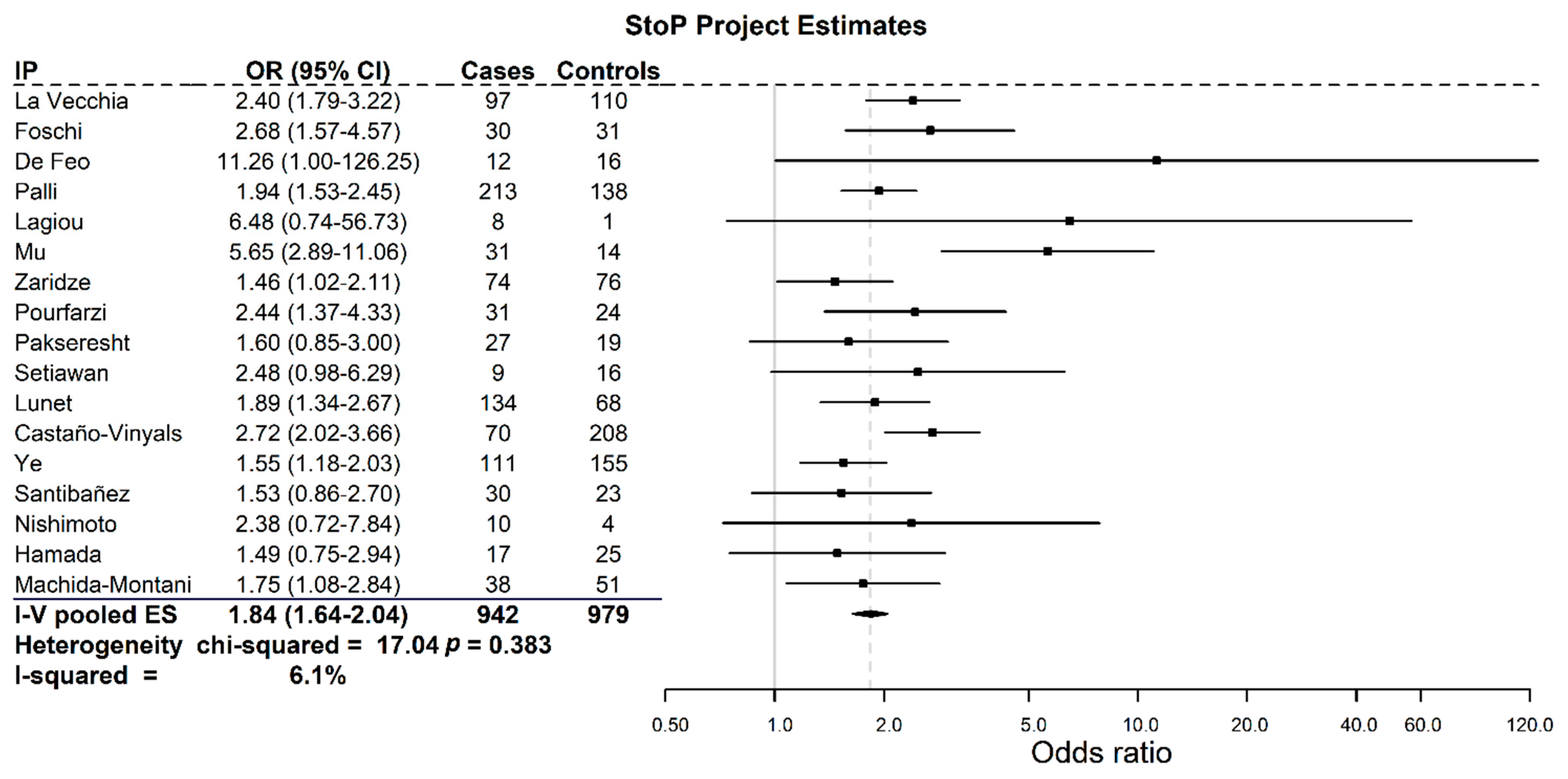
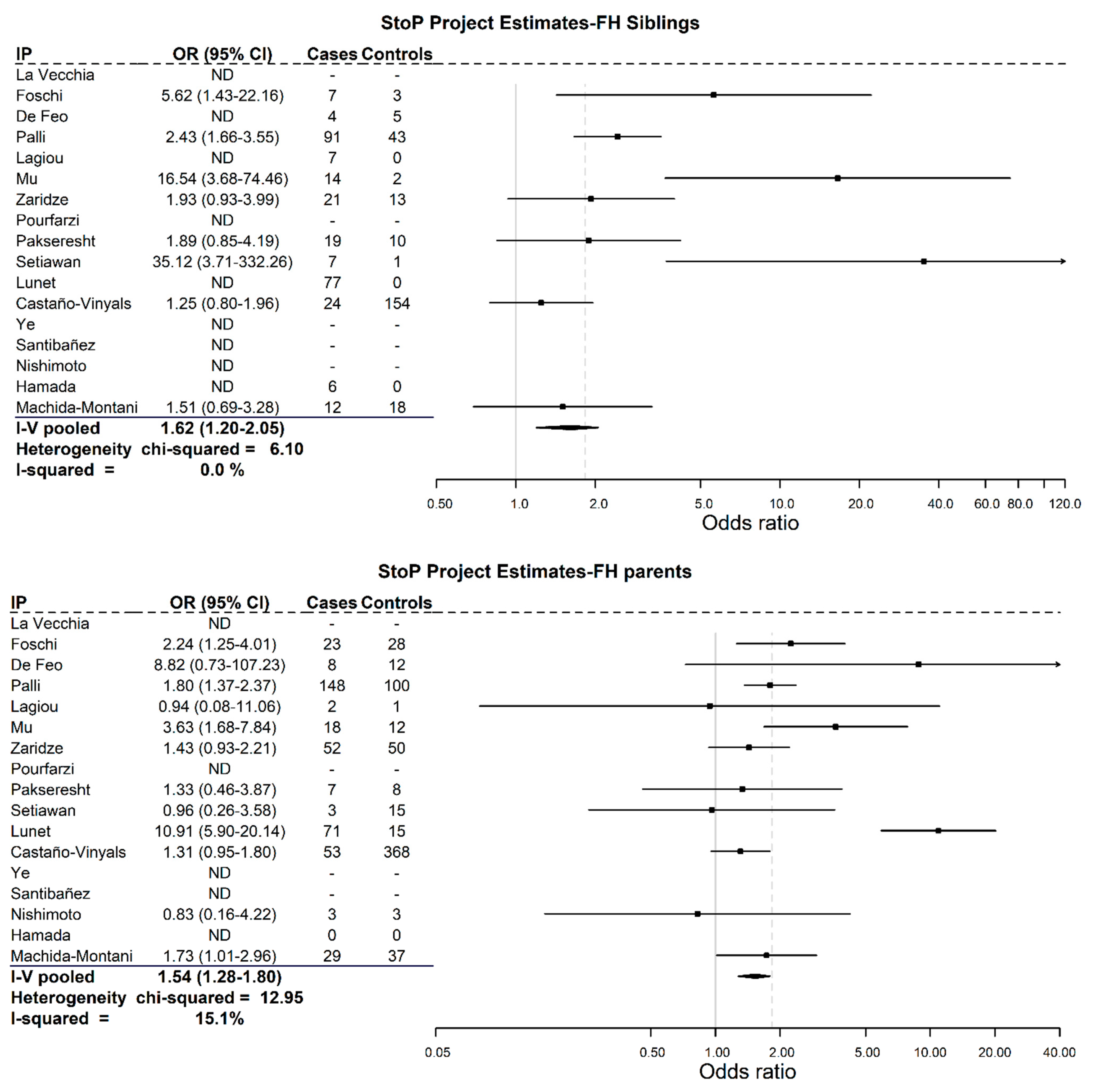
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Yusefi, A.R.; Bagheri Lankarani, K.; Bastani, P.; Radinmanesh, M.; Kavosi, Z. Risk Factors for Gastric Cancer: A Systematic Review. Asian Pac. J. Cancer Prev. APJCP 2018, 19, 591–603. [Google Scholar] [CrossRef]
- Corso, G.; Roncalli, F.; Marrelli, D.; Carneiro, F.; Roviello, F. History, Pathogenesis, and Management of Familial Gastric Cancer: Original Study of John XXIII’s Family. BioMed Res. Int. 2013, 2013, 385132. [Google Scholar] [CrossRef] [PubMed]
- Yaghoobi, M.; Bijarchi, R.; Narod, S.A. Family History and the Risk of Gastric Cancer. Br. J. Cancer 2010, 102, 237–242. [Google Scholar] [CrossRef]
- Choi, Y.J.; Kim, N. Gastric Cancer and Family History. Korean J. Intern. Med. 2016, 31, 1042–1053. [Google Scholar] [CrossRef] [PubMed]
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Niksic, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Esteve, J.; et al. Global Surveillance of Trends in Cancer Survival 2000-14 (CONCORD-3): Analysis of Individual Records for 37 513 025 Patients Diagnosed with One of 18 Cancers from 322 Population-Based Registries in 71 Countries. Lancet 2018, 39, 1023–1075. [Google Scholar] [CrossRef] [Green Version]
- Pelucchi, C.; Lunet, N.; Boccia, S.; Zhang, Z.F.; Praud, D.; Boffetta, P.; Levi, F.; Matsuo, K.; Ito, H.; Hu, J.; et al. The Stomach Cancer Pooling (StoP) Project: Study Design and Presentation. Eur. J. Cancer Prev. Off. J. Eur. Cancer Prev. Organ. 2015, 24, 16–23. [Google Scholar] [CrossRef]
- La Vecchia, C.; D’Avanzo, B.; Negri, E.; Decarli, A.; Benichou, J. Attributable Risks for Stomach Cancer in Northern Italy. Int. J. Cancer 1995, 60, 748–752. [Google Scholar] [CrossRef]
- Foschi, R.; Lucenteforte, E.; Bosetti, C.; Bertuccio, P.; Tavani, A.; La Vecchia, C.; Negri, E. Family History of Cancer and Stomach Cancer Risk. Int. J. Cancer 2008, 123, 1429–1432. [Google Scholar] [CrossRef]
- De Feo, E.; Simone, B.; Persiani, R.; Cananzi, F.; Biondi, A.; Arzani, D.; Amore, R.; D’Ugo, D.; Ricciardi, G.; Boccia, S. A Case-Control Study on the Effect of Apolipoprotein E Genotypes on Gastric Cancer Risk and Progression. BMC Cancer 2012, 12, 494. [Google Scholar] [CrossRef] [Green Version]
- Palli, D.; Galli, M.; Caporaso, N.E.; Cipriani, F.; Decarli, A.; Saieva, C.; Fraumeni, J.F.; Buiatti, E. Family History and Risk of Stomach Cancer in Italy. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 1994, 3, 15–18. [Google Scholar]
- Lagiou, P.; Samoli, E.; Lagiou, A.; Peterson, J.; Tzonou, A.; Dwyer, J.; Trichopoulos, D. Flavonoids, Vitamin C and Adenocarcinoma of the Stomach. Cancer Causes Control CCC 2004, 15, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.N.; Lu, Q.Y.; Yu, S.Z.; Jiang, Q.W.; Cao, W.; You, N.C.; Setiawan, V.W.; Zhou, X.-F.; Ding, B.-G.; Wang, R.-H.; et al. Green Tea Drinking and Multigenetic Index on the Risk of Stomach Cancer in a Chinese Population. Int. J. Cancer 2005, 116, 972–983. [Google Scholar] [CrossRef] [Green Version]
- Zaridze, D.; Borisova, E.; Maximovitch, D.; Chkhikvadze, V. Aspirin Protects against Gastric Cancer: Results of a Case-Control Study from Moscow, Russia. Int. J. Cancer 1999, 82, 473–476. [Google Scholar] [CrossRef]
- Pourfarzi, F.; Whelan, A.; Kaldor, J.; Malekzadeh, R. The Role of Diet and Other Environmental Factors in the Causation of Gastric Cancer in Iran--a Population Based Study. Int. J. Cancer 2009, 125, 1953–1960. [Google Scholar] [CrossRef] [Green Version]
- Pakseresht, M.; Forman, D.; Malekzadeh, R.; Yazdanbod, A.; West, R.M.; Greenwood, D.C.; Crabtree, J.E.; Cade, J.E. Dietary Habits and Gastric Cancer Risk in North-West Iran. Cancer Causes Control CCC 2011, 22, 725–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Setiawan, V.W.; Zhang, Z.F.; Yu, G.P.; Li, Y.L.; Lu, M.L.; Tsai, C.J.; Cordova, D.; Wang, M.-R.; Guo, C.H.; Yu, S.-Z.; et al. GSTT1 and GSTM1 Null Genotypes and the Risk of Gastric Cancer: A Case-Control Study in a Chinese Population. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2000, 9, 73–80. [Google Scholar]
- Castano-Vinyals, G.; Aragones, N.; Perez-Gomez, B.; Martin, V.; Llorca, J.; Moreno, V.; Altzibar, J.M.; Ardanaz, E.; Sanjose, S.D.; Jimenez-Moleon, J.J.; et al. Population-Based Multicase-Control Study in Common Tumors in Spain (MCC-Spain): Rationale and Study Design. Gac. Sanit. 2015, 29, 308–315. [Google Scholar] [CrossRef] [Green Version]
- Ye, W.; Chow, W.H.; Lagergren, J.; Yin, L.; Nyren, O. Risk of Adenocarcinomas of the Esophagus and Gastric Cardia in Patients with Gastroesophageal Reflux Diseases and after Antireflux Surgery. Gastroenterology 2001, 121, 1286–1293. [Google Scholar] [CrossRef]
- Santibanez, M.; Alguacil, J.; de La Hera, M.G.; Navarrete-Munoz, E.M.; Llorca, J.; Aragones, N.; Kauppinen, T.; Vioque, J. Occupational Exposures and Risk of Stomach Cancer by Histological Type. Occup. Environ. Med. 2012, 69, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, N.; Fahey, M.T.; Hamada, G.S.; Nishimoto, I.N.; Kowalski, L.P.; Iriya, K.; Rodrigues, J.J.G.; Tajiri, H.; Tsugane, S. Serological Immunoglobulin G Antibody Titers to Helicobacter Pylori in Japanese Brazilian and Non-Japanese Brazilian Gastric Cancer Patients and Controls in Sao Paulo. Jpn. J. Cancer Res. Gann 2001, 92, 829–835. [Google Scholar] [CrossRef]
- Nishimoto, I.N.; Hamada, G.S.; Kowalski, L.P.; Rodrigues, J.G.; Iriya, K.; Sasazuki, S.; Hanaoka, T.; Tsugane, S. São Paulo--Japan Cancer Project Gastric Cancer Study Group. Risk Factors for Stomach Cancer in Brazil (I): A Case-Control Study among Non-Japanese Brazilians in São Paulo. Jpn. J. Clin. Oncol. 2002, 32, 277–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamada, G.S.; Kowalski, L.P.; Nishimoto, I.N.; Rodrigues, J.J.; Iriya, K.; Sasazuki, S.; Hanaoka, T.; Tsugane, S. São Paulo--Japan Cancer Project Gastric Cancer Study Group. Risk Factors for Stomach Cancer in Brazil (II): A Case-Control Study among Japanese Brazilians in São Paulo. Jpn. J. Clin. Oncol. 2002, 32, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Machida-Montani, A.; Sasazuki, S.; Inoue, M.; Natsukawa, S.; Shaura, K.; Koizumi, Y.; Kasuga, Y.; Hanaoka, T.; Tsugane, S. Association of Helicobacter Pylori Infection and Environmental Factors in Non-Cardia Gastric Cancer in Japan. Gastric Cancer Off. J. Int. Gastric Cancer Assoc. Jpn. Gastric Cancer Assoc. 2004, 7, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Lunet, N.; Valbuena, C.; Vieira, A.L.; Lopes, C.; Lopes, C.; David, L.; Carneiro, F.; Barros, H. Fruit and Vegetable Consumption and Gastric Cancer by Location and Histological Type: Case-Control and Meta-Analysis. Eur. J. Cancer Prev. 2007, 16, 312–327. [Google Scholar] [CrossRef]
- Smith-Warner, S.A.; Spiegelman, D.; Ritz, J.; Albanes, D.; Beeson, W.L.; Bernstein, L.; Berrino, F.; Van Den Brandt, P.A.; Buring, J.E.; Cho, E.; et al. Methods for Pooling Results of Epidemiologic Studies: The Pooling Project of Prospective Studies of Diet and Cancer. Am. J. Epidemiol. 2006, 163, 1053–1064. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring Inconsistency in Meta-Analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [Green Version]
- Song, M.; Camargo, M.C.; Weinstein, S.J.; Best, A.F.; Mannisto, S.; Albanes, D.; Rabkin, C.S. Family History of Cancer in First-Degree Relatives and Risk of Gastric Cancer and Its Precursors in a Western Population. Gastric Cancer Off. J. Int. Gastric Cancer Assoc. Jpn. Gastric Cancer Assoc. 2018, 21, 729–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lott, P.C.; Carvajal-Carmona, L.G. Resolving Gastric Cancer Aetiology: An Update in Genetic Predisposition. Lancet Gastroenterol. Hepatol. 2018, 3, 874–8838. [Google Scholar] [CrossRef]
- Butterworth, A. Family History as a Risk Factor for Common, Complex Disease; Public Health Genetics Unit PHG Found: Cambridge, UK, 2007; pp. 1–2. [Google Scholar]
- Lichtenstein, P.; Holm, N.V.; Verkasalo, P.K.; Iliadou, A.; Kaprio, J.; Koskenvuo, M.; Pukkala, E.; Skytthe, A.; Hemminki, K. Environmental and Heritable Factors in the Causation of Cancer—Analyses of Cohorts of Twins from Sweden, Denmark, and Finland. N. Engl. J. Med. 2000, 343, 78–85. [Google Scholar] [CrossRef]
- Yatsuya, H.; Toyoshima, H.; Mizoue, T.; Kondo, T.; Tamakoshi, K.; Hori, Y.; Tokui, N.; Hoshiyama, Y.; Kikuchi, S.; Sakata, K.; et al. Family History and the Risk of Stomach Cancer Death in Japan: Differences by Age and Gender. Int. J. Cancer 2002, 97, 688–694. [Google Scholar] [CrossRef]
- Kharazmi, E.; Babaei, M.; Fallah, M.; Chen, T.; Sundquist, K.; Hemminki, K. Importance of Tumor Location and Histology in Familial Risk of Upper Gastrointestinal Cancers: A Nationwide Cohort Study. Clin. Epidemiol. 2018, 10, 1169–1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Martel, C.; Forman, D.; Plummer, M. Gastric Cancer: Epidemiology and Risk Factors. Gastroenterol. Clin. N. Am. 2013, 42, 219–240. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, L.; Wang, X.; Wang, J.; Yan, Z.; Cheng, J.; Gong, G.; Li, G. Body Mass Index and Risk of Gastric Cancer: A Meta-Analysis of a Population with More than Ten Million from 24 Prospective Studies. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2013, 22, 1395–1408. [Google Scholar] [CrossRef] [Green Version]
- Yang, P.; Zhou, Y.; Chen, B.; Wan, H.W.; Jia, G.Q.; Bai, H.L.; Wu, X.T. Overweight, Obesity and Gastric Cancer Risk: Results from a Meta-Analysis of Cohort Studies. Eur. J. Cancer 2009, 45, 2867–2873. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, J.D.; Terry, M.B.; Gammon, M.D.; Vaughan, T.L.; Risch, H.A.; Zhang, F.F.; Kleiner, D.E.; Bennett, W.P.; Howe, C.L.; Dubrow, R.; et al. Cigarette Smoking, Body Mass Index, Gastro-Esophageal Reflux Disease, and Non-Steroidal Anti-Inflammatory Drug Use and Risk of Subtypes of Esophageal and Gastric Cancers by P53 Overexpression. Cancer Causes Control CCC 2009, 20, 361–368. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, C.A.; Pera, G.; Agudo, A.; Palli, D.; Krogh, V.; Vineis, P.; Tumino, R.; Panico, S.; Berglund, G.; Siman, H.; et al. Smoking and the Risk of Gastric Cancer in the European Prospective Investigation Into Cancer and Nutrition (EPIC). Int. J. Cancer 2003, 107, 629–634. [Google Scholar] [CrossRef]
- Bakir, T.; Can, G.; Erkul, S.; Siviloglu, C. Stomach Cancer History in the Siblings of Patients with Gastric Carcinoma. Eur. J. Cancer Prev. 2000, 9, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Bakir, T.; Can, G.; Siviloglu, C.; Erkul, S. Gastric Cancer and Other Organ Cancer History in the Parents of Patients with Gastric Cancer. Eur. J. Cancer Prev. 2003, 12, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Chung, S.J.; Choi, J.M.; Han, Y.M.; Kim, J.S. Clinicopathologic Characteristics and Long-Term Outcome of Gastric Cancer Patients with Family History: Seven-Year Follow-Up Study for Korean Health Check-Up Subjects. Gastroenterol. Res. Pract. 2020, 2020, 4028136. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Esquinas, E.; Perez-Gomez, B.; Pollan, M.; Boldo, E.; Fernandez-Navarro, P.; Lope, V.; Vidal, E.; Lopez-Abente, G.; Aragones, N. Gastric Cancer Mortality Trends in Spain, 1976-2005, Differences by Autonomous Region and Sex. BMC Cancer 2009, 9, 346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stadtlander, C.T.; Waterbor, J.W. Molecular Epidemiology, Pathogenesis and Prevention of Gastric Cancer. Carcinogenesis 1999, 20, 2195–2208. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| StoP Study ID | Study Area(s) | Period | Study Type | Cases | Controls | References | ||
|---|---|---|---|---|---|---|---|---|
| With FH | Total | With FH | Total | |||||
| 1 | Milan, Italy | 1985–1997 | CC, hospital-based | 97 | 768 | 110 | 2081 | La Vecchia et al. [8] |
| 3 | Milan, Italy | 1997–2007 | CC, hospital-based | 30 | 230 | 31 | 547 | Foschi et al. [9] |
| 4 | Rome, Italy | 2006–2010 | CC, hospital-based | 12 | 152 | 16 | 411 | De Feo et al. [10] |
| 5 | Four areas, Italy | 1985–1987 | CC, population-based | 213 | 1016 | 138 | 1159 | Palli et al. [11] |
| 6 | Athens, Greece | 1981–1984 | CC, hospital-based | 8 | 86 | 1 | 97 | Lagiou et al. [12] |
| 8 | Taixing, Jiangsu, China | 2000 | CC, population-based | 31 | 206 | 14 | 415 | Mu et al. [13] |
| 9 | Moscow, Russia | 1996–1997 | CC, hospital-based | 74 | 433 | 76 | 593 | Zaridze et al. [14] |
| 10 | Ardabil, Iran | 2004–2005 | CC, population-based | 31 | 217 | 24 | 394 | Pourfarzi et al. [15] |
| 11 | Ardabil, Iran | 2005–2007 | CC, population-based | 27 | 286 | 19 | 304 | Pakseresht et al. [16] |
| 13 | Yangzhong, China | 1995 | CC, population-based | 9 | 133 | 16 | 433 | Setiawan et al. [17] |
| 17 | North of Portugal | 2001–2006 | CC, population-based | 134 | 584 | 68 | 612 | Lunet et al. [25] |
| 21 | Ten provinces, Spain | 2008–2013 | CC, population-based | 70 | 435 | 208 | 3418 | Castaño-Vinyals et al. [18] |
| 22 | Five counties, Sweden | 1989–1995 | CC, population-based | 111 | 561 | 155 | 1164 | Ye et al. [19] |
| 23 | Two provinces, Spain | 1995–1999 | CC, hospital-based | 30 | 367 | 23 | 433 | Santibañez et al. [20] |
| 28 | Brazil-Brazilian origin | 1991–1994 | CC, hospital-based | 10 | 226 | 4 | 226 | Nishimoto et al. [22] |
| 29 | Brazil-Japanese origin | 1991–1994 | CC, hospital-based | 17 | 93 | 25 | 186 | Hamada et al. [23] |
| 30 | Japan | 1998–2002 | CC, hospital-based | 38 | 153 | 51 | 303 | Machida-Montani et al. [24] |
| Variables | Cases | Controls | ||
|---|---|---|---|---|
| n | % | n | % | |
| Sex | ||||
| Male | 3790 | 63.7 | 7454 | 58.3 |
| Female | 2156 | 36.3 | 5322 | 41.7 |
| Age | ||||
| 18–49 | 759 | 12.8 | 2553 | 20.0 |
| 50–65 | 2184 | 36.7 | 4724 | 37.0 |
| 65–74 | 2171 | 36.5 | 3940 | 30.8 |
| 75–106 | 832 | 14.0 | 1557 | 12.2 |
| Missing | 0 | 0.0 | 2 | 0.0 |
| Socioeconomic status | ||||
| Low | 3799 | 63.9 | 6488 | 50.8 |
| Medium | 1543 | 26.0 | 3760 | 29.4 |
| High | 501 | 8.4 | 2198 | 17.2 |
| Missing | 103 | 1.7 | 330 | 2.6 |
| Smoking habit (1) | ||||
| Current | 1720 | 28.9 | 3255 | 25.5 |
| No | 4132 | 69.5 | 9386 | 73.4 |
| Missing | 94 | 1.6 | 135 | 1.1 |
| Alcohol intake g of ethanol/day | ||||
| Never | 1526 | 25.7 | 3375 | 26.4 |
| Low (≤12) | 1190 | 20.0 | 3468 | 27.1 |
| Intermediate (>12–47) | 1745 | 29.4 | 3047 | 23.9 |
| High (>47) | 860 | 14.5 | 1353 | 10.6 |
| Missing | 625 | 10.5 | 1533 | 12.0 |
| Fruit intake (2) | ||||
| Low | 2157 | 36.3 | 3701 | 29.0 |
| Medium | 1872 | 31.5 | 4109 | 32.2 |
| High | 1685 | 28.3 | 4293 | 33.6 |
| Missing | 232 | 3.9 | 673 | 5.3 |
| H. pylori infection | ||||
| Positive | 688 | 11.6 | 3662 | 28.7 |
| Negative | 1661 | 27.9 | 1252 | 9.8 |
| Missing | 3597 | 60.5 | 7862 | 61.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitelli-Storelli, F.; Rubín-García, M.; Pelucchi, C.; Benavente, Y.; Bonzi, R.; Rota, M.; Palli, D.; Ferraroni, M.; Lunet, N.; Morais, S.; et al. Family History and Gastric Cancer Risk: A Pooled Investigation in the Stomach Cancer Pooling (STOP) Project Consortium. Cancers 2021, 13, 3844. https://doi.org/10.3390/cancers13153844
Vitelli-Storelli F, Rubín-García M, Pelucchi C, Benavente Y, Bonzi R, Rota M, Palli D, Ferraroni M, Lunet N, Morais S, et al. Family History and Gastric Cancer Risk: A Pooled Investigation in the Stomach Cancer Pooling (STOP) Project Consortium. Cancers. 2021; 13(15):3844. https://doi.org/10.3390/cancers13153844
Chicago/Turabian StyleVitelli-Storelli, Facundo, María Rubín-García, Claudio Pelucchi, Yolanda Benavente, Rossella Bonzi, Matteo Rota, Domenico Palli, Monica Ferraroni, Nuno Lunet, Samantha Morais, and et al. 2021. "Family History and Gastric Cancer Risk: A Pooled Investigation in the Stomach Cancer Pooling (STOP) Project Consortium" Cancers 13, no. 15: 3844. https://doi.org/10.3390/cancers13153844
APA StyleVitelli-Storelli, F., Rubín-García, M., Pelucchi, C., Benavente, Y., Bonzi, R., Rota, M., Palli, D., Ferraroni, M., Lunet, N., Morais, S., Ye, W., Plymoth, A., Malekzadeh, R., Tsugane, S., Hidaka, A., Aragonés, N., Castaño-Vinyals, G., Zaridze, D. G., Maximovich, D., ... Martín, V. (2021). Family History and Gastric Cancer Risk: A Pooled Investigation in the Stomach Cancer Pooling (STOP) Project Consortium. Cancers, 13(15), 3844. https://doi.org/10.3390/cancers13153844












