Simple Summary
Pancreatic cancer (PC) continues to be characterized by high morbidity and mortality, owing to the fact, among others, that it is often diagnosed at late stages. Thus far, the search for reliable biomarkers has failed. A number of recent studies have found that there are differences in the microbiota between patients with PC and their healthy counterparts. These differences extend to specific anatomical locations such as the oral cavity, the gastrointestinal tract, and the pancreas itself. The purpose of this review is to outline some of the main differences in the bacterial and fungal populations between patients with PC and their healthy counterparts that have recently come to light. Additionally, the present review aims to highlight the mechanisms underlying the aforementioned microbial associations with PC.
Abstract
Pancreatic cancer (PC) remains a global health concern with high mortality and is expected to increase as a proportion of overall cancer cases in the coming years. Most patients are diagnosed at a late stage of disease progression, which contributes to the extremely low 5-year survival rates. Presently, screening for PC remains costly and time consuming, precluding the use of widespread testing. Biomarkers have been explored as an option by which to ameliorate this situation. The authors conducted a search of available literature on PubMed to present the current state of understanding as it pertains to the use of microbial biomarkers and their associations with PC. Carriage of certain bacteria in the oral cavity (e.g., Porphyromonas gingivalis, Aggregatibacter actinomycetemcomitans, Streptococcus sp.), gut (e.g., Helicobacter pylori, Synergistetes, Proteobacteria), and pancreas (e.g., Fusobacterium sp., Enterobacteriaceae, Pseudomonadaceae) has been associated with an increased risk of developing PC. Additionally, the fungal genus Malassezia has likewise been associated with PC development. This review further outlines potential oncogenic mechanisms involved in the microbial-associated development of PC.
Keywords:
pancreatic cancer; pancreas; cancer; cancer screening; microbiota; dysbiosis; Porphyromonas; oral cavity; Helicobacter 1. Introduction
Pancreatic cancer (PC) remains a major health concern across much of the globe. As of 2020, it was the 14th most common cancer (with an estimated 495,733 new cases and 466,003 new deaths reported in 2020) [1]. However, it is expected to become the third most common cancer in the EU by 2025 [2]. The incidence and mortality of PC is rising in many countries; however, the highest rates are observed in high-income countries [3]. Males are disproportionately affected globally; however, the highest discrepancies are likewise observed in high-income regions with an age-standardized rate per 100,000 of 7.2 compared with 5.0 for females [1]. At present, the 5-year survival rate associated with PC is low, with most studies placing it at, or below, 10% in high-income countries [4,5]. Interestingly, the survival rates have not improved since the 1960s [6]. It is believed that this is due in large part to low early detection rates. Pancreatic adenocarcinoma (PDAC) is a malignant epithelial neoplasm, accounting for approximately 85% of all pancreatic tumors [7]. Consistent with pancreatic cancers in general, PDAC screening is lacking, with only about one-fifth of patients presenting with surgically resectable forms of the disease [8].
It is held that the reason underpinning the low survival rates is the fact that the majority of patients are initially diagnosed only after the neoplasia has become metastatic [9]. A study by Canto et al. revealed that 42% (92/216) of asymptomatic individuals at high risk for developing PC had at least one pancreatic mass or a dilated pancreatic duct [10]. These findings underscore the insidious nature of PC; however, they do not account for individuals with population risk of developing the disease. The late presentation and difficulty associated with diagnosing PC can be at least partially explained by the nonspecific symptoms and close association with major blood vessels [11]. The latter is believed to play an important role in the high metastatic nature of PC; this is further enhanced by lymph node metastasis and neural invasion [12,13]. Additionally, the relatively low incidence has led to recommendations against population-based screening with only certain at-risk groups having well-established screening protocols [14]. Presently, the main diagnostic modalities utilized to screen those suspected of having PC include: multi-detector computed tomography, magnetic resonance imaging, endoscopic ultrasound (+/− fine needle aspirate), and positron emission tomography [9]. All four of the above require specialized facilities and prove to be costly [15]. This situation could be at least partially alleviated by the identification of biomarkers for PC. Unfortunately, no reliable and cost-effective biomarkers have been identified to date.
For many years, the association between microorganisms and certain neoplastic diseases has been well established. Recently, the role of other microorganisms in the pathogenesis of various cancers has come under increased scrutiny. It has been estimated that between 7–20% of all cancers globally may be attributed to infectious agents [16]. The microbiome has been implicated in a number of cancers, many of which can be classified as gastrointestinal in nature. The most well-known example of this phenomenon is that of Helicobacter pylori and the direct role these bacteria play in the pathobiology of gastric cancer [17,18]. Additionally, a number of oral microorganisms have been found to contribute to cancer development. Several of these bacteria appear to be associated with neoplastic diseases such as oral squamous cell carcinoma, colorectal cancer, and PC [19]. Periodontitis and tooth loss have been found to be associated with an increased risk of developing PC, although there are issues with the way in which several of these studies adjusted for risk factors [20,21]. Furthermore, infectious agents from beyond the oral cavity have likewise been associated with the development and progression of PC [22].
There has been an increase in the number of studies examining microbial associations with PC in recent years. However, to the best of our knowledge, the present review is the first to summarize the data from across anatomical locations and provide possible mechanistic explanations for the observed associations. Thus, the aim of this review is to highlight the main microorganisms which are associated with an increased risk of developing PC. The present investigation will likewise highlight the main mechanisms by which the microorganisms in question are believed to drive the development and progression of PC. The PubMed database was used to search for articles using the keywords “microbiome”, “pancreas”, “pancreatic cancer”, “oral bacteria”, “oncogenic mechanisms”, “pancreatic cancer screening”, “periodontitis”, “gut”, and “Helicobacter pylori”, with more recent literature being favored. Additionally, a manual review of references from the literature obtained from PubMed searches was performed.
2. Microbial Associations with Pancreatic Cancer
The following section aims to highlight the associations between carriage of certain microbial populations in specific anatomical locations and the development of PC (Table 1). It must be noted that at present, available data are mostly limited to associations rather than causal relationships between the presence of specific microorganisms and the development of PC. Section 3 of the present review outlines possible mechanisms underlying some of the associations presented below.

Table 1.
Microbial associations with pancreatic cancer.
2.1. Oral Cavity
The last several years have seen increased interest in the association between oral bacteria and PC. Several studies have found that the presence of Porphyromonas gingivalis in the oral cavity is associated with a higher incidence of PC [23,24]. Michaud et al. found that individuals with high levels of antibodies against P. gingivalis ATCC 53978 have a two-fold higher risk (odds ratio [OR] = 2.14) of developing PC than their counterparts with low levels of the same antibody [24]. In 2018, Fan et al., demonstrated that there is an increased risk of developing PC in patients with oral carriage of Aggregatibacter actinomycetemcomitans (OR = 2.20) and Alloprevotella (OR = 1.20) [23]. Furthermore, a recent prospective study by Wei et al. found that oral carriage of Streptococcus and Leptotrichia was associated with a higher risk of PDAC (OR = 5.344 and OR = 6.886, respectively) [25]. The same study demonstrated that Veillonella and Neisseria decreased the risk of PDAC (OR = 0.187 and OR = 0.309, respectively). The aforementioned relationships are further supported by an investigation from 2015 by Torres et al. which found that the Leptotrichia to Porphyromonas ratio was significantly higher in patients with PDAC [26].
The relative abundance of some bacteria may be decreased in patients with PDAC. Neisseria elongata [26,27] and Streptococcus mitis [27] were found to be less abundant in patients with PC when compared with healthy controls. By using a combination of both bacteria, Farrell et al. found that they were able to differentiate between chronic pancreatitis and PC with 85.7% sensitivity and 55.6% specificity. Farrell et al. likewise observed that levels of Granulicatella adiacens (increased in PC) and S. mitis (decreased in PC) differed significantly between patients with PC and those without cancer (including patients with chronic pancreatitis) [27]. By using both bacteria in combination as biomarkers, the authors were able to differentiate between PC patients and healthy controls with 96.4% sensitivity and 82.1% specificity. Lu et al. sequenced the tongue microbiota coating of patients with pancreatic head cancer (PHC), a PDAC found in the head of the pancreas, and healthy controls [28]. They found that significant differences exist between the two groups with respect to microbiota composition; specifically, PHC patients exhibited lower levels of Haemophilus and Porphyromonas and higher levels of Leptotrichia and Fusobacterium in their tongue microbiota coats. In contrast, Fan et al. found that oral carriage of Fusobacteria was associated with a decreased risk of developing PC (OR = 0.94) [23]. Carriage of the genus Leptotrichia was associated with an even greater decrease in the risk of developing PC (OR = 0.87). Bacterial associations related with pancreatic carcinogenesis are shown in Figure 1. Some of these bacteria and Malassezia fungus are presented as cultures in Figure 2.
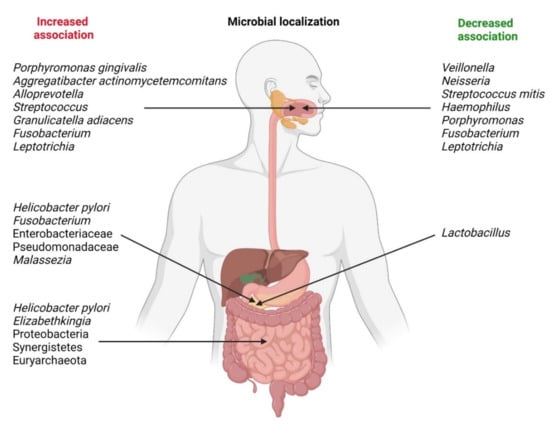
Figure 1.
Microbial localization and changes in bacterial associations related with pancreatic carcinogenesis.
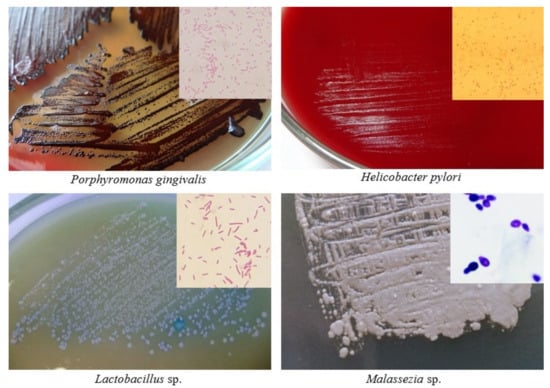
Figure 2.
Cultures and Gram staining (in corners) of some microorganisms associated with pancreatic carcinogenesis (author: Tomasz M. Karpiński).
The various aforementioned studies demonstrated that oral carriage of certain bacteria did not correlate with the development of PC. Fan et al. found that Tannerella forsythia and Prevotella intermedia were not associated with an increased risk of developing pancreatic cancer [23]. Additionally, Torres et al. found that the relative abundance of S. mitis and G. adiacens (both identified as potential biomarkers in other studies) did not differ between patients with PDAC and their healthy counterparts [26]. Furthermore, Lu et al. report they observed no difference in the relative abundance of Proteobacteria between patients with PHC and healthy controls [28]. Interestingly, the study likewise highlights that many of the differences in the relative abundance of various bacteria between the saliva of patients with PC and healthy controls do not extend to the tongue coat microbiome.
2.2. Gut
For some time, the association between gastric carriage of Helicobacter pylori and the development of PC has been known. A summary of meta-analyses conducted by Maisonneuve and Lowenfels calculated that H. pylori may be attributed to the development of 4% to 25% of all PC cases in Westernized countries [29]. Further highlighting the geographical differences between the association of H. pylori and PC is a study by Wang et al., which demonstrates that CagA+ subjects are more likely to develop the disease in Western, but not Eastern countries [45]. Furthermore, there are several studies and meta-analyses reporting positive associations between H. pylori infection and PC [30,31,32,33], as well as between CagA positivity and PC [34,35]. However, there are likewise several studies reporting no association between H. pylori infection and PC [35,36,37]. With the exception of these three studies, the majority of papers and meta-analyses examined during the present review found there to be an association between the presence of H. pylori and PC. Therefore, carriage of H. pylori may hold some value for potential screening protocols. Such screening measures would need to account for geography and the sensitivity and specificity of such tests would need to be determined in light of the limited associations.
Pushalkar et al., found that the relative abundance of Proteobacteria, Synergistetes, and Euryachaeota was significantly higher in the feces of patients with PDAC than in their healthy controls [38]. A small study conducted by Half et al., presented at a conference in Israel, highlights that the feces of patients with PC had higher levels of Sutterela, Veillonela, Bacteroides, Odoribacter, and Akkermansia than that of healthy controls [46]. Another study by Half et al. found that patients with PC had decreased levels of genera belonging to Firmicutes in their feces [47]. Studying overall gut microbial diversity, Ren et al. found that patients with PC had significantly reduced overall diversity [48]. Their study however failed to find any significant difference in microbial diversity between subtypes of PC (namely tumors located in the head of the pancreas vs. tumors located in the body and tail). Through the use of a murine model, Thomas et al. found that intestinal microbiota are important mediators of PC progression, with microbiota-depleted mice having decreased tumorigenicity [49]. Mendez et al. highlight that changes in the composition of fecal microbiota are evident early on in the course of tumor progression in a murine model of PDAC [50].
2.3. Pancreas
In addition to the potential association between gastric colonization by H. pylori and PC, several investigations have detected H. pylori in pancreatic tumors. A study of pancreatic tissue specimens by Nilsson et al. found that Helicobacter DNA was present in 48% of their tumor samples [39]. The authors also noted that only 5% of non-neoplastic tumors contained Helicobacter DNA when there was a nearby neuroendocrine or type 1 multiple endocrine neoplasia. There are several other intrapancreatic bacteria that have been associated with PC. According to Mitsuhashi et al., Fusobacterium species were found in 8.8% of their specimens, with Fusobacterium-positive patients having higher cancer-specific mortality rates [41]. On account of this, the authors suggest that screening for F. nucleatum in tumor samples may hold prognostic value, with patients positive for this species having a worse prognosis. More recently, Alkhaaran et al. demonstrated that patients with severe intraductal papillary mucinous neoplasm (IPMN) have higher levels of circulating IgG and salivary IgA reactive to F. nucleatum [42]. The positive correlation (r = 0.685, p < 0.0001) between F. nucleatum and Fap2 IgA antibodies is interesting on account of the fact that Fap2 is an important adhesin used by F. nucleatum to bind targets in the pancreatic TME [42,51,52]. A 2018 paper by del Castillo et al. likewise reported that a higher relative abundance of Fusobacterium spp. was observed in samples obtained from patients with PC [40]. Noncancer subjects had a higher relative abundance of Lactobacillus (µ = 0.06 vs. µ = 0.02 for PC patients, p < 0.0001).
An examination of human pancreatic tissue by Geller et al. revealed that the prevalence of bacterial DNA differed significantly between samples obtained from patients with PDAC and those of healthy controls (76% and 15%, respectively) [43]. Further 16S rDNA sequencing revealed that the most common bacterial class in the pancreatic tissue of PDAC patients is Gammaproteobacteria (specifically Enterobacteriaceae and Pseudomonadaceae). The above is supported by the work of Pushalkar et al., who noted that patients with PDAC have increased levels of gut Proteobacteria (specifically, Pseudomonas and Elizabethkingia) [38].
The role of the mycobiome as it relates to PC is only beginning to come under scrutiny. In the first study to examine the role of the mycobiome and its association with PC, Aykut et al. found that Malassezia is markedly increased in the pancreas of patients with PDAC [44]. The authors were able to further demonstrate that Malassezia promotes PDAC, with its ablation slowing tumor progression. Candida, Saccharomyces, and Aspergillus did not accelerate oncogenesis following pancreatic repopulation.
3. Oncogenic Mechanisms
3.1. Inflammatory Processes
It has long been known that inflammation plays a role in the development of cancer [53]. Certain oral pathogens such as Porphyromonas, Prevotella, and Fusobacterium may cause inflammation by contributing to the increase in inflammatory mediators such as IL-1β, IL-6, IL-17, IL-23, TNF-α, MMP-8, and MMP-9 [54].
Localized inflammatory processes may contribute to the development of neoplasia. Thus, the presence of bacteria in the pancreas may stimulate resident leukocytes to produce IL-1β upon contact with lipopolysaccharide (LPS) or other pathogen-associated molecular patterns (PAMPS). IL-1β is known to induce the production of proangiogenic factors associated with angiogenesis in the TME (e.g., VEGF, TNF) [55]. Furthermore, IL-1β is known to contribute to the metastasis [56,57] and aggressiveness [58,59] of neoplasms. Das et al. demonstrate that tumor-derived IL-1β is required for the establishment of the pancreatic TME, characterized by the activation of pancreatic stellate cells, and the induction of an immunosuppressive environment [60]. The authors report that the immunosuppressive milieu is specifically mediated by M2 macrophages, myeloid-derived suppressor cells, CD1dhiCD5+ regulatory B cells, and Th17 cells. This is in line with several other studies which have shown that IL-1β functions as an important modulator of TME immunosuppression [61,62]. In addition to the aforementioned leukocytes associated with immunosuppression, T lymphocytes likewise influence the progression of PC. In fact, Russano et al. found that through TCR sequencing it is possible to measure the heterogeneity of tumor-infiltrating T lymphocytes [63]. Moreover, this heterogeneity was found to reflect that of the mutational landscape within the tumor as well.
The proinflammatory cytokine IL-6 is likewise an important contributor to tumor growth and progression. Recent studies suggest that H. pylori may alter the expression levels of IL6 by means of miRNA regulation, particularly miR-195 and miR-488 [64,65]. IL-6 production may likewise be induced by contact between leukocytes and microorganisms such as bacteria or fungi [66,67]. By means of the JAK/STAT3 signaling cascade, IL-6 induces the expression of genes involved in cell proliferation and survival [68]. Moreover, the anti-apoptotic effects of IL-6 have been known for many years, and are thought to influence the development of various cancers [69,70]. Additionally, IL-6 has been implicated in the regulation of matrix metalloproteinases (MMPs) involved in the processes of tumor invasion and metastasis [71,72]. Interestingly, STAT3 induces the expression of IL6, thus creating a positive feedback loop, exacerbating tumorigenesis and metastasis [73].
TNF-α, another well-characterized inflammatory cytokine, is known to contribute to tumorigenesis when present at low concentrations [74]. The mechanism by which TNF-α is thought to induce tumorigenesis, relies on its induction of ROS and reactive nitrogen species (RNS), which in turn damage DNA [75]. Moreover, TNF-α has been found to play a role in metastasis by inducing lymphangiogenesis through VEGF-C and VEGF-D signaling in certain cancers [76,77]. In addition to the above, TNF-α likewise contributes to tumor invasiveness through NF-κB signaling [78]. The role of inflammatory mediators on pancreatic carcinogenesis is shown in Figure 3.
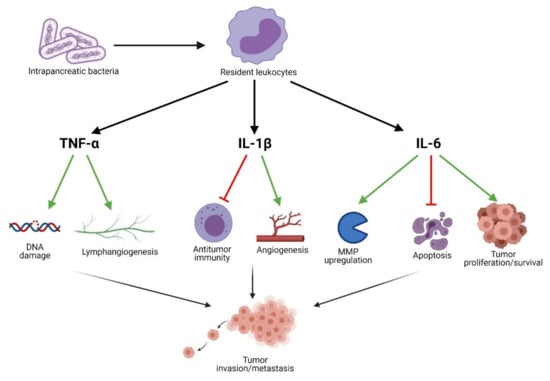
Figure 3.
Inflammatory mediators contributing to pancreatic carcinogenesis.
3.2. Translocation
Carcinogenesis may be enhanced by means of direct contact between bacteria and the tumor microenvironment (TME). Socransky and Haffajee calculated that individuals swallow approximately 1011 oral bacteria daily [79]. Furthermore, several studies have demonstrated that bacteria are able to translocate from the duodenum to the pancreas [50,51], offering a potential mechanism by which oral bacteria may come to colonize the pancreas. A review of pancreatic adenocarcinomas in the USA over a four-decade period revealed that most cases of the neoplasm were present in the head of the pancreas [80]. Thomas and Jobin suggest that a potential explanation for this may lie in the close association of the pancreatic head with the duodenum, thus being the first site exposed to translocating bacteria [81]. In addition to retrograde translocation through the GI tract, Mitsuhashi et al. outline a series of studies which suggest that some bacteria (namely F. nucleatum) may reach the pancreas via circulation [46].
For some years, F. nucleatum has been known to contribute to the modulation of the TME in various gastrointestinal neoplastic diseases. Fusobacterium adhesin A (FadA) enables F. nucleatum to bind host cells via interactions with cadherins [82]. Kostic et al. found that tumor-infiltrating myeloid cells are selectively recruited by F. nucleatum thus promoting tumor development by generating a pro-inflammatory microenvironment [83]. Specifically, this may include the generation of reactive oxygen species (ROS). These free radicals are directly responsible for DNA damage implicated in a number of characteristic features of cancer (e.g., cell growth, proliferation, genomic instability) [84]. In addition to DNA damage, ROS modulate the activity of tumor-infiltrating leukocytes (TILs), creating an immunosuppressive environment favorable for tumor progression [85]. Moreover, mast cells affect not only the development and progression of PDAC, but as noted by Porcelli et al., actively contribute to resistance against chemotherapeutics, notably gemcitabine/nabpaclitaxel [86]. Their study found that the mast cell mediated effects occur as a result of activation of TβRI signaling, promoting tumor cell regrowth. This suggests that tumor-infiltrating myeloid cells contribute to the development, progression, and resistance to treatment observed in patients with PDAC. Additionally, the virulence factor familial adenomatous polyposis 2 (Fap2) interacts with the receptor T cell immunoreceptor with Ig and ITIM domains (TIGIT) on NK cells and lymphocytes, suppressing their cytotoxic functions [87]. Chen et al. report that F. nucleatum upregulated the expression of long non-coding RNA Keratin7-antisense (KRT7-AS) via the NF-κB pathway both in vitro and in an in vivo murine model of colorectal cancer [88]. Such long non-coding RNAs have been found to play an essential role in metastasis.
The association between P. gingivalis and PC is well established, with a number of molecular mechanisms behind this association garnering increasingly more supporting evidence. P. gingivalis has been associated with a number of systemic diseases, with much of its pathogenic potential being attributed to the suppression of adaptive immune responses [89]. An in vitro model utilized by Gnanasekaran et al. demonstrates that P. gingivalis survives within pancreatic cancer cells and enhances their proliferation [90]. Additionally, Carvalho-Filho et al. demonstrated that PBMCs from patients with periodontitis more greatly downregulated genes associated with apoptosis than their healthy counterparts, when exposed to the P. gingivalis HmuY protein [91]. This study is in line with the author’s previous work which highlighted that HmuY increased the expression of Bcl-2, an anti-apoptotic protein, in CD3+ T cells [92]. Moreover, in a murine model, Hiraki et al. demonstrated that intraperitoneal administration of P. gingivalis LPS upregulated the expression of regenerating islet-derived 3G (Reg3G) in pancreatic tissue [93]. The overexpression of Reg3G has been found to accelerate tumor growth and induce an immunosuppressive microenvironment [94]. Mechanisms of bacterial-mediated carcinogenesis of the pancreas are presented in Figure 4.
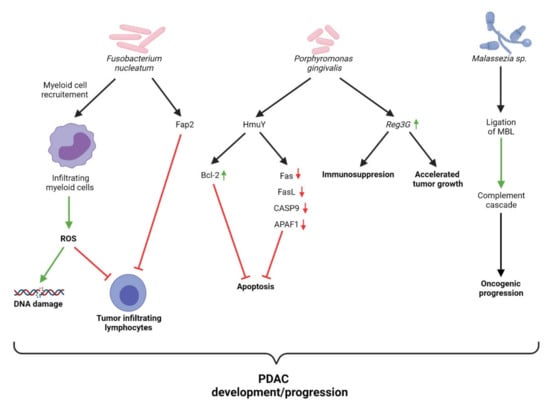
Figure 4.
Overview of mechanisms of bacterial-mediated carcinogenesis of pancreas.
The contribution of fungi to the development and progression of PC has only recently begun to be investigated. The seminal paper by Aykut et al. highlights several key important findings relating to Malassezia and PC [52]. The study reports that the relative abundance of Malassezia is markedly increased in the pancreas compared with the gut in both patients and mice with PC. Fungal ablation in the study’s murine model was found to decrease oncogenic progression, while repopulation with Malassezia globosa accelerated PDAC progression. Mannose-binding lectin (MBL) is responsible for the initiation of the lectin-pathway of complement activation [95]. Aykut et al. were able to demonstrate that the expression of MBL was associated with reduced survival in human PDAC patients, while knockdown of the pathway proved to mitigate tumor growth in their murine model. These results demonstrate that the MBL-mediated complement activation pathway is involved in pancreatic oncogenesis. With regards to other fungal species, Kaźmierczak-Siedlecka et al. outline several oncogenic mechanisms which have been identified; however, the results are confined to non-pancreatic cancers [96]. The scarcity of research pertaining to the role of fungi in relation to PC highlights the urgent need for more research.
4. Conclusions
There are a number of microorganisms which are associated with the development and progression of PC. Those with the most literature supporting such associations are Porphyromonas gingivalis, Fusobacterium nucleatum, and Helicobacter pylori. The mechanisms involved in this bacterial-mediated carcinogenesis include inflammatory mediators, immune cells, reactive oxygen species, and modulation of genes associated with apoptosis and growth/proliferation. The role of fungi in the development and progression of PC is only beginning to come under investigation and as such may reveal as yet unknown associations and mechanisms. The work of Aykut et al. with Malassezia highlights the important role fungi may play in carcinogenesis, calling attention to the lack of research connecting the mycobiome to cancer. The study of such microbial associations with PC may lead to the discovery of highly sensitive and specific changes which can serve as biomarkers for the purpose of screening larger proportions of the population. This in turn may help to alleviate the problems presented by low early detection rates in the general population. Furthermore, understanding the microbial basis for PC development and progression may prove to be beneficial for prophylactic measures and the establishment of new treatment guidelines. This may involve targeting specific microorganisms to abolish carrier status or attenuating their numbers in at-risk individuals.
Studies such as that by Guo et al., which found that the ratio of F. nucleatum to Bifidobacterium could detect colorectal cancer with 84.6% sensitivity and 92.3% specificity, show promise for the use of microorganisms as biomarkers in cancer screening [97]. Unfortunately, there is not yet enough information available to utilize microorganisms as biomarkers for the purpose of screening for PC. Even microbial populations exhibiting strong associations with cancer (e.g., H. pylori, P. gingivalis) fail to provide actionable diagnostic information for PC when examined in isolation. With the exception of the aforementioned 2012 paper by Farrell et al., research for the present review failed to find any subsequent work considering multiple microorganisms in tandem for diagnostic purposes relating to PC. Future work relating to biomarkers should take into account several bacterial and fungal species together to assess if they hold increased prognostic/diagnostic value when examined in tandem.
Author Contributions
Conceptualization, M.S. and T.M.K.; data analysis, M.S. and M.K.; writing—original draft preparation, M.S.; writing—review and editing, M.S. and T.M.K.; visualization, M.S. and T.M.K.; supervision, T.M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Ferlay, J.; Partensky, C.; Bray, F. More deaths from pancreatic cancer than breast cancer in the EU by 2017. Acta Oncol. Stockh. Swed. 2016, 55, 1158–1160. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.C.S.; Jiang, J.Y.; Liang, M.; Fang, Y.; Yeung, M.S.; Sung, J.J.Y. Global temporal patterns of pancreatic cancer and association with socioeconomic development. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ilic, M.; Ilic, I. Epidemiology of pancreatic cancer. World J. Gastroenterol. 2016, 22, 9694–9705. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, J.D.; Surana, R.; Valle, J.W.; Shroff, R.T. Pancreatic cancer. Lancet 2020, 395, 2008–2020. [Google Scholar] [CrossRef]
- Ansari, D.; Tingstedt, B.; Andersson, B.; Holmquist, F.; Sturesson, C.; Williamsson, C.; Sasor, A.; Borg, D.; Bauden, M.; Andersson, R. Pancreatic cancer: Yesterday, today and tomorrow. Futur. Oncol. 2016, 12, 1929–1946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schawkat, K.; Manning, M.A.; Glickman, J.N.; Mortele, K.J. Pancreatic Ductal Adenocarcinoma and Its Variants: Pearls and Perils. Radiogr. 2020, 40, e190184. [Google Scholar] [CrossRef]
- Singhi, A.D.; Koay, E.J.; Chari, S.T.; Maitra, A. Early Detection of Pancreatic Cancer: Opportunities and Challenges. Gastroenterol. 2019, 156, 2024–2040. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Sanagapalli, S.; Stoita, A. Challenges in diagnosis of pancreatic cancer. World J. Gastroenterol. 2018, 24, 2047–2060. [Google Scholar] [CrossRef]
- Canto, M.I.; Hruban, R.H.; Fishman, E.; Kamel, I.R.; Schulick, R.; Zhang, Z.; Topazian, M.; Takahashi, N.; Fletcher, J.; Petersen, G.; et al. Frequent Detection of Pancreatic Lesions in Asymptomatic High-Risk Individuals. Gastroenterol. 2012, 142, 796–804. [Google Scholar] [CrossRef] [Green Version]
- McGuigan, A.; Kelly, P.; Turkington, R.; Jones, C.; Coleman, H.G.; McCain, R.S. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J. Gastroenterol. 2018, 24, 4846–4861. [Google Scholar] [CrossRef]
- Lemberger, M.; Loewenstein, S.; Lubezky, N.; Nizri, E.; Pasmanik-Chor, M.; Barazovsky, E.; Klausner, J.M.; Lahat, G. MicroRNA profiling of pancreatic ductal adenocarcinoma (PDAC) reveals signature expression related to lymph node metastasis. Oncotarget 2019, 10, 2644–2656. [Google Scholar] [CrossRef] [PubMed]
- Liebl, F.; Demir, I.E.; Mayer, K.; Schuster, T.; D’Haese, J.G.; Becker, K.; Langer, R.; Bergmann, F.; Wang, K.; Rosenberg, R.; et al. The Impact of Neural Invasion Severity in Gastrointestinal Malignancies. Ann. Surg. 2014, 260, 900–908. [Google Scholar] [CrossRef] [Green Version]
- Goggins, M.; Overbeek, K.A.; Brand, R.; Syngal, S.; Del Chiaro, M.; Bartsch, D.K.; Bassi, C.; Carrato, A.; Farrell, J.; Fishman, E.K.; et al. Management of patients with increased risk for familial pancreatic cancer: Updated recommendations from the International Cancer of the Pancreas Screening (CAPS) Consortium. Gut 2020, 69, 7–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruenderman, E.; Martin, R.C. A cost analysis of a pancreatic cancer screening protocol in high-risk populations. Am. J. Surg. 2015, 210, 409–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Martel, C.; Ferlay, J.; Franceschi, S.; Vignat, J.; Bray, F.; Forman, D.; Plummer, M. Global burden of cancers attributable to infections in 2008: A review and synthetic analysis. Lancet Oncol. 2012, 13, 607–615. [Google Scholar] [CrossRef]
- Amieva, M.; Peek, R.M., Jr. Pathobiology of Helicobacter pylori–Induced Gastric Cancer. Gastroenterology 2016, 150, 64–78. [Google Scholar] [CrossRef] [Green Version]
- Park, J.Y.; Forman, D.; Waskito, L.A.; Yamaoka, Y.; Crabtree, J.E. Epidemiology of Helicobacter pylori and CagA-Positive Infections and Global Variations in Gastric Cancer. Toxins 2018, 10, 163. [Google Scholar] [CrossRef] [Green Version]
- Karpiński, T.M. Role of Oral Microbiota in Cancer Development. Microorganisms 2019, 7, 20. [Google Scholar] [CrossRef] [Green Version]
- Maisonneuve, P.; Amar, S.; Lowenfels, A.B. Periodontal disease, edentulism, and pancreatic cancer: A meta-analysis. Ann. Oncol. 2017, 28, 985–995. [Google Scholar] [CrossRef]
- Michaud, D.S.; Fu, Z.; Shi, J.; Chung, M. Periodontal Disease, Tooth Loss, and Cancer Risk. Epidemiologic Rev. 2017, 39, 49–58. [Google Scholar] [CrossRef] [Green Version]
- Karpiński, T.M. The Microbiota and Pancreatic Cancer. Gastroenterol. Clin. North Am. 2019, 48, 447–464. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Alekseyenko, A.V.; Wu, J.; Peters, B.A.; Jacobs, E.J.; Gapstur, S.M.; Purdue, M.P.; Abnet, C.C.; Stolzenberg-Solomon, R.; Miller, G.; et al. Human oral microbiome and prospective risk for pancreatic cancer: A population-based nested case-control study. Gut 2018, 67, 120–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michaud, D.S.; Izard, J.; Wilhelm-Benartzi, C.; You, D.-H.; A Grote, V.; Tjonneland, A.; Dahm, C.; Overvad, K.; Jenab, M.; Fedirko, V.; et al. Plasma antibodies to oral bacteria and risk of pancreatic cancer in a large European prospective cohort study. Gut 2013, 62, 1764–1770. [Google Scholar] [CrossRef]
- Wei, A.-L.; Li, M.; Li, G.-Q.; Wang, X.; Hu, W.-M.; Li, Z.-L.; Yuan, J.; Liu, H.-Y.; Zhou, L.-L.; Li, K.; et al. Oral microbiome and pancreatic cancer. World J. Gastroenterol. 2020, 26, 7679–7692. [Google Scholar] [CrossRef]
- Torres, P.J.; Fletcher, E.M.; Gibbons, S.; Bouvet, M.; Doran, K.S.; Kelley, S.T. Characterization of the salivary microbiome in patients with pancreatic cancer. PeerJ 2015, 3, e1373. [Google Scholar] [CrossRef]
- Farrell, J.J.; Zhang, L.; Zhou, H.; Chia, D.; Elashoff, D.; Akin, D.; Paster, B.J.; Joshipura, K.; Wong, D.T.W. Variations of oral microbiota are associated with pancreatic diseases including pancreatic cancer. Gut 2011, 61, 582–588. [Google Scholar] [CrossRef]
- Lu, H.; Ren, Z.; Li, A.; Li, J.; Xu, S.; Zhang, H.; Jiang, J.; Yang, J.; Luo, Q.; Zhou, K.; et al. Tongue coating microbiome data distinguish patients with pancreatic head cancer from healthy controls. J. Oral Microbiol. 2019, 11, 1563409. [Google Scholar] [CrossRef] [PubMed]
- Maisonneuve, P.; Lowenfels, A.B. Risk factors for pancreatic cancer: A summary review of meta-analytical studies. Int. J. Epidemiology 2015, 44, 186–198. [Google Scholar] [CrossRef]
- Raderer, M.; Wrba, F.; Kornek, G.; Maca, T.; Koller, D.Y.; Weinlaender, G.; Hejna, M.; Scheithauer, W. Association between Helicobacter pylori Infection and Pancreatic Cancer. Oncol. 1998, 55, 16–19. [Google Scholar] [CrossRef]
- Wu, J.; Guo, Y.; Liu, W. Helicobacter pylori infection and pancreatic cancer risk: A meta-analysis. J. Cancer Res. Ther. 2016, 12, 229. [Google Scholar] [CrossRef]
- Trikudanathan, G.; Philip, A.; A Dasanu, C.; Baker, W. Association between Helicobacter pylori Infection and Pancreatic Cancer. A Cumulative Meta-Analysis. JOP. J. Pancreas 2011, 12, 26–31. [Google Scholar] [CrossRef]
- Xiao, M.; Wang, Y.; Gao, Y. Association between Helicobacter pylori Infection and Pancreatic Cancer Development: A Meta-Analysis. PLoS ONE 2013, 8, e75559. [Google Scholar] [CrossRef] [Green Version]
- Ai, F.; Hua, X.; Liu, Y.; Lin, J.; Feng, Z. Preliminary Study of Pancreatic Cancer Associated with Helicobacter pylori Infection. Cell Biophys. 2015, 71, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-Z.; Wang, R.; Chen, H.-N.; Hu, J.-K. Cytotoxin-Associated Gene A-Negative Strains of Helicobacter pylori as a Potential Risk Factor of Pancreatic Cancer. Pancreas 2015, 44, 1340–1344. [Google Scholar] [CrossRef]
- Schulte, A.; Pandeya, N.; Fawcett, J.; Fritschi, L.; Risch, H.A.; Webb, P.M.; Whiteman, D.; Neale, R. Association between Helicobacter pylori and pancreatic cancer risk: A meta-analysis. Cancer Causes Control. 2015, 26, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, Y.-T.; Wang, R.; Chen, X.-Z. Helicobacter pylori infection, atrophic gastritis, and pancreatic cancer risk. Med. 2017, 96, e7811. [Google Scholar] [CrossRef]
- Pushalkar, S.; Hundeyin, M.; Daley, D.; Zambirinis, C.P.; Kurz, E.; Mishra, A.; Mohan, N.; Aykut, B.; Usyk, M.; Torres, L.E.; et al. The Pancreatic Cancer Microbiome Promotes Oncogenesis by Induction of Innate and Adaptive Immune Suppression. Cancer Discov. 2018, 8, 403–416. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, U.S.H.-O. Helicobacter species ribosomal DNA in the pancreas, stomach and duodenum of pancreatic cancer patients. World J. Gastroenterol. 2006, 12, 3038–3043. [Google Scholar] [CrossRef]
- Del Castillo, E.; Meier, R.; Chung, M.; Koestler, D.C.; Chen, T.; Paster, B.J.; Charpentier, K.P.; Kelsey, K.T.; Izard, J.; Michaud, D.S. The Microbiomes of Pancreatic and Duodenum Tissue Overlap and Are Highly Subject Specific but Differ between Pancreatic Cancer and Noncancer Subjects. Cancer Epidemiol. Biomarkers Prev. 2019, 28, 370–383. [Google Scholar] [CrossRef] [Green Version]
- Mitsuhashi, K.; Nosho, K.; Sukawa, Y.; Matsunaga, Y.; Ito, M.; Kurihara, H.; Kanno, S.; Igarashi, H.; Naito, T.; Adachi, Y.; et al. Association of Fusobacterium Species in Pancreatic Cancer Tissues with Molecular Features and Prognosis. Oncotarget 2015, 6, 7209–7220. [Google Scholar] [CrossRef] [Green Version]
- Alkharaan, H.; Lu, L.; Gabarrini, G.; Halimi, A.; Ateeb, Z.; Sobkowiak, M.J.; Davanian, H.; Moro, C.F.; Jansson, L.; Del Chiaro, M.; et al. Circulating and Salivary Antibodies to Fusobacterium nucleatum Are Associated With Cystic Pancreatic Neoplasm Malignancy. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Geller, L.T.; Barzily-Rokni, M.; Danino, T.; Jonas, O.H.; Shental, N.; Nejman, D.; Gavert, N.; Zwang, Y.; Cooper, Z.A.; Shee, K.; et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 2017, 357, 1156–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aykut, B.; Pushalkar, S.; Chen, R.; Li, Q.; Abengozar, R.; Kim, J.I.; Shadaloey, S.A.; Wu, D.; Preiss, P.; Verma, N.; et al. The fungal mycobiome promotes pancreatic oncogenesis via activation of MBL. Nature 2019, 574, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, F.-C.; Wang, Y.-J. Helicobacter pylori and pancreatic cancer risk: A meta-analysis based on 2,049 cases and 2,861 controls. Asian Pac. J. Cancer Prev. 2014, 15, 4449–4454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Half, E.; Keren, N.; Dorfman, T.; Reshef, L.; Lachter, I.; Kluger, Y.; Konikoff, F.M.; Gphna, U. P-165 Specific changes in fecal microbiota may differentiate Pancreatic Cancer patients from healthy individuals. Ann. Oncol. 2015, 26, iv48. [Google Scholar] [CrossRef] [Green Version]
- Half, E.; Keren, N.; Reshef, L.; Dorfman, T.; Lachter, I.; Kluger, Y.; Reshef, N.; Knobler, H.; Maor, Y.; Stein, A.; et al. Fecal microbiome signatures of pancreatic cancer patients. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Ren, Z.; Jiang, J.; Xie, H.; Li, A.; Lü, H.; Xu, S.; Zhou, L.; Zhang, H.; Cui, G.; Chen, X.; et al. Gut microbial profile analysis by MiSeq sequencing of pancreatic carcinoma patients in China. Oncotarget 2017, 8, 95176–95191. [Google Scholar] [CrossRef] [Green Version]
- Thomas, R.M.; Gharaibeh, R.Z.; Gauthier, J.; Beveridge, M.; Pope, J.L.; Guijarro, M.V.; Yu, Q.; He, Z.; Ohland, C.; Newsome, R.; et al. Intestinal microbiota enhances pancreatic carcinogenesis in preclinical models. Carcinogenesis 2018, 39, 1068–1078. [Google Scholar] [CrossRef]
- Mendez, R.; Kesh, K.; Arora, N.; Di Martino, L.; McAllister, F.; Merchant, N.; Banerjee, S.; Banerjee, S. Microbial dysbiosis and polyamine metabolism as predictive markers for early detection of pancreatic cancer. Carcinog. 2020, 41, 561–570. [Google Scholar] [CrossRef]
- Parhi, L.; Alon-Maimon, T.; Sol, A.; Nejman, D.; Shhadeh, A.; Fainsod-Levi, T.; Yajuk, O.; Isaacson, B.; Abed, J.; Maalouf, N.; et al. Breast cancer colonization by Fusobacterium nucleatum accelerates tumor growth and metastatic progression. Nat. Commun. 2020, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lopez, L.R.; Bleich, R.M.; Arthur, J.C. Microbiota Effects on Carcinogenesis: Initiation, Promotion, and Progression. Annu. Rev. Med. 2021, 72, 243–261. [Google Scholar] [CrossRef] [PubMed]
- Rakoff-Nahoum, S. Why Cancer and Inflammation? Yale J. Boil. Med. 2007, 79, 123–130. [Google Scholar]
- Szkaradkiewicz, A.K.; Karpiński, T.M. Microbiology of Chronic Periodontitis. J. Biol. Earth. Sci. 2013, 3, M14–M20. [Google Scholar]
- Carmi, Y.; Dotan, S.; Rider, P.; Kaplanov, I.; White, M.R.; Baron, R.; Abutbul, S.; Huszar, M.; Dinarello, C.A.; Apte, R.N.; et al. The Role of IL-1β in the Early Tumor Cell–Induced Angiogenic Response. J. Immunol. 2013, 190, 3500–3509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weichand, B.; Popp, R.; Dziumbla, S.; Mora, J.; Strack, E.; Elwakeel, E.; Frank, A.-C.; Scholich, K.; Pierre, S.; Syed, S.N.; et al. S1PR1 on tumor-associated macrophages promotes lymphangiogenesis and metastasis via NLRP3/IL-1β. J. Exp. Med. 2017, 214, 2695–2713. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Park, J.H.; Gi, S.H.; Hwang, Y.S. IL-1β Induces Fascin Expression and Increases Cancer Invasion. Anticancer. Res. 2018, 38, 6127–6132. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Chang, J.S.; Syu, S.; Wong, T.; Chan, J.Y.; Tang, Y.; Yang, Z.; Yang, W.; Chen, C.; Lu, S.; et al. IL-1β Promotes Malignant Transformation and Tumor Aggressiveness in Oral Cancer. J. Cell. Physiol. 2015, 230, 875–884. [Google Scholar] [CrossRef]
- Jiménez-Garduño, A.M.; Mendoza, M.; Urrutia-Cabrera, D.; Domínguez-Robles, M.C.; Pérez-Yépez, E.A.; Ayala-Sumuano, J.T.; Meza, I. IL-1β induced methylation of the estrogen receptor ERα gene correlates with EMT and chemoresistance in breast cancer cells. Biochem. Biophys. Res. Commun. 2017, 490, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Shapiro, B.; Vucic, E.A.; Vogt, S.; Bar-Sagi, D. Tumor Cell–Derived IL1β Promotes Desmoplasia and Immune Suppression in Pancreatic Cancer. Cancer Res. 2020, 80, 1088–1101. [Google Scholar] [CrossRef] [Green Version]
- Kaplanov, I.; Carmi, Y.; Kornetsky, R.; Shemesh, A.; Shurin, G.V.; Shurin, M.R.; Dinarello, C.A.; Voronov, E.; Apte, R.N. Blocking IL-1β reverses the immunosuppression in mouse breast cancer and synergizes with anti–PD-1 for tumor abrogation. Proc. Natl. Acad. Sci. 2019, 116, 1361–1369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daley, D.; Mani, V.R.; Mohan, N.; Akkad, N.; Pandian, G.S.B.; Savadkar, S.; Lee, K.B.; Torres-Hernandez, A.; Aykut, B.; Diskin, B.; et al. NLRP3 signaling drives macrophage-induced adaptive immune suppression in pancreatic carcinoma. J. Exp. Med. 2017, 214, 1711–1724. [Google Scholar] [CrossRef] [Green Version]
- Russano, M.; Napolitano, A.; Ribelli, G.; Iuliani, M.; Simonetti, S.; Citarella, F.; Pantano, F.; Dell’Aquila, E.; Anesi, C.; Silvestris, N.; et al. Liquid biopsy and tumor heterogeneity in metastatic solid tumors: The potentiality of blood samples. J. Exp. Clin. Cancer Res. 2020, 39, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Vainer, N.; Dehlendorff, C.; Johansen, J.S. Systematic literature review of IL-6 as a biomarker or treatment target in patients with gastric, bile duct, pancreatic and colorectal cancer. Oncotarget 2018, 9, 29820–29841. [Google Scholar] [CrossRef] [Green Version]
- Chung, J.-W.; Jeong, S.H.; Lee, S.M.; Pak, J.H.; Lee, G.H.; Jeong, J.-Y.; Kim, J.-H. Expression of MicroRNA in Host Cells Infected with Helicobacter pylori. Gut Liver 2017, 11, 392–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calabrese, L.H.; Rose-John, S. IL-6 biology: Implications for clinical targeting in rheumatic disease. Nat. Rev. Rheumatol. 2014, 10, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Uciechowski, P.; Dempke, W.C. Interleukin-6: A Masterplayer in the Cytokine Network. Oncology 2020, 98, 131–137. [Google Scholar] [CrossRef]
- Johnson, D.E.; O’Keefe, R.A.; Grandis, J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018, 15, 234–248. [Google Scholar] [CrossRef]
- Haura, E.B.; Turkson, J.; Jove, R. Mechanisms of Disease: Insights into the emerging role of signal transducers and activators of transcription in cancer. Nat. Clin. Pr. Oncol. 2005, 2, 315–324. [Google Scholar] [CrossRef]
- Cavarretta, I.T.; Neuwirt, H.; Untergasser, G.; Moser, P.L.; Zaki, M.H.; Steiner, H.; Rumpold, H.; Fuchs, D.; Hobisch, A.; A Nemeth, J.; et al. The antiapoptotic effect of IL-6 autocrine loop in a cellular model of advanced prostate cancer is mediated by Mcl-1. Oncogene 2006, 26, 2822–2832. [Google Scholar] [CrossRef] [Green Version]
- Zergoun, A.-A.; Zebboudj, A.; Sellam, S.L.; Kariche, N.; Djennaoui, D.; Ouraghi, S.; Kerboua, E.; Amir-Tidadini, Z.-C.; Chilla, D.; Asselah, F.; et al. IL-6/NOS2 inflammatory signals regulate MMP-9 and MMP-2 activity and disease outcome in nasopharyngeal carcinoma patients. Tumor Biol. 2016, 37, 3505–3514. [Google Scholar] [CrossRef] [Green Version]
- Tang, C.-H.; Chen, C.-F.; Chen, W.-M.; Fong, Y.-C. IL-6 Increases MMP-13 Expression and Motility in Human Chondrosarcoma Cells. J. Biol. Chem. 2011, 286, 11056–11066. [Google Scholar] [CrossRef] [Green Version]
- Chang, Q.; Bournazou, E.; Sansone, P.; Berishaj, M.; Gao, S.P.; Daly, L.; Wels, J.; Theilen, T.; Granitto, S.; Zhang, X.; et al. The IL-6/JAK/Stat3 Feed-Forward Loop Drives Tumorigenesis and Metastasis. Neoplasia 2013, 15, 848–862. [Google Scholar] [CrossRef] [Green Version]
- Balkwill, F. TNF-α in promotion and progression of cancer. Cancer Metastasis Rev. 2006, 25, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Landskron, G.; De La Fuente, M.; Thuwajit, P.; Thuwajit, C.; Hermoso, M.A. Chronic Inflammation and Cytokines in the Tumor Microenvironment. J. Immunol. Res. 2014, 2014, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, H.; Jiang, L.; Yanling, C.; He, C.; Zhu, G.; Du, Q.; Wang, X.; She, F.; Chen, Y. TNF-alpha promotes lymphangiogenesis and lymphatic metastasis of gallbladder cancer through the ERK1/2/AP-1/VEGF-D pathway. BMC Cancer 2016, 16, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, H.; Cao, R.; Yang, Y.; Zhang, Y.; Iwamoto, H.; Lim, S.; Nakamura, M.; Andersson, P.; Wang, J.; Sun, Y.; et al. TNFR1 mediates TNF-α-induced tumour lymphangiogenesis and metastasis by modulating VEGF-C-VEGFR3 signalling. Nat. Commun. 2014, 5, 4944. [Google Scholar] [CrossRef] [Green Version]
- Tang, D.; Tao, D.; Fang, Y.; Deng, C.; Xu, Q.; Zhou, J. TNF-Alpha Promotes Invasion and Metastasis via NF-Kappa B Pathway in Oral Squamous Cell Carcinoma. Med Sci. Monit. Basic Res. 2017, 23, 141–149. [Google Scholar] [CrossRef] [Green Version]
- Socransky, S.S.; Haffajee, A.D. Dental biofilms: Difficult therapeutic targets. Periodontol. 2000 2002, 28, 12–55. [Google Scholar] [CrossRef]
- Saad, A.M.; Turk, T.; Al-Husseini, M.J.; Abdel-Rahman, O. Trends in pancreatic adenocarcinoma incidence and mortality in the United States in the last four decades; a SEER-based study. BMC Cancer 2018, 18, 1–11. [Google Scholar] [CrossRef]
- Thomas, R.M.; Jobin, C. Microbiota in pancreatic health and disease: The next frontier in microbiome research. Nat. Rev. Gastroenterol. Hepatol. 2019, 17, 53–64. [Google Scholar] [CrossRef]
- Sun, Z.; Xiong, C.; Teh, S.W.; Lim, J.C.W.; Kumar, S.; Thilakavathy, K. Mechanisms of Oral Bacterial Virulence Factors in Pancreatic Cancer. Front. Cell. Infect. Microbiol. 2019, 9, 412. [Google Scholar] [CrossRef] [PubMed]
- Kostic, A.; Chun, E.; Robertson, L.; Glickman, J.N.; Gallini, C.A.; Michaud, M.; Clancy, T.E.; Chung, D.C.; Lochhead, P.; Hold, G.; et al. Fusobacterium nucleatum Potentiates Intestinal Tumorigenesis and Modulates the Tumor-Immune Microenvironment. Cell Host Microbe 2013, 14, 207–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srinivas, U.S.; Tan, B.W.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA damage response in cancer. Redox Biol. 2019, 25, 101084. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, F.; Ramnath, N.; Nagrath, D. Reactive Oxygen Species in the Tumor Microenvironment: An Overview. Cancers 2019, 11, 1191. [Google Scholar] [CrossRef] [Green Version]
- Porcelli, L.; Iacobazzi, R.M.; Di Fonte, R.; Serratì, S.; Intini, A.; Solimando, A.G.; Brunetti, O.; Calabrese, A.; Leonetti, F.; Azzariti, A.; et al. CAFs and TGF-β Signaling Activation by Mast Cells Contribute to Resistance to Gemcitabine/Nabpaclitaxel in Pancreatic Cancer. Cancers 2019, 11, 330. [Google Scholar] [CrossRef] [Green Version]
- Gur, C.; Ibrahim, Y.; Isaacson, B.; Yamin, R.; Abed, J.; Gamliel, M.; Enk, J.; Bar-On, Y.; Stanietsky-Kaynan, N.; Coppenhagen-Glazer, S.; et al. Binding of the Fap2 Protein of Fusobacterium nucleatum to Human Inhibitory Receptor TIGIT Protects Tumors from Immune Cell Attack. Immunity 2015, 42, 344–355. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Su, T.; Zhang, Y.; Lee, A.; He, J.; Ge, Q.; Wang, L.; Si, J.; Zhuo, W.; Wang, L. Fusobacterium nucleatum promotes colorectal cancer metastasis by modulating KRT7-AS/KRT7. Gut Microbes 2020, 11, 511–525. [Google Scholar] [CrossRef]
- Olsen, I.; Taubman, M.A.; Singhrao, S.K. Porphyromonas gingivalis suppresses adaptive immunity in periodontitis, atherosclerosis, and Alzheimer’s disease. J. Oral Microbiol. 2016, 8, 33029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gnanasekaran, J.; Gallimidi, A.B.; Saba, E.; Pandi, K.; Berchoer, L.E.; Hermano, E.; Angabo, S.; Makkawi, H.; Khashan, A.; Daoud, A.; et al. Intracellular Porphyromonas gingivalis Promotes the Tumorigenic Behavior of Pancreatic Carcinoma Cells. Cancers 2020, 12, 2331. [Google Scholar] [CrossRef]
- Carvalho-Filho, P.C.; Moura-Costa, L.F.; Pimentel, A.C.M.; Lopes, M.P.P.; Freitas, S.A.; Miranda, P.M.; Costa, R.S.; Figueiredo, C.; Meyer, R.; Gomes-Filho, I.; et al. Apoptosis Transcriptional Profile Induced byPorphyromonas gingivalisHmuY. Mediat. Inflamm. 2019, 2019, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Carvalho-Filho, P.C.; Trindade, S.C.; Olczak, T.; Sampaio, G.P.; Oliveira-Neto, M.G.; A Santos, H.; Pereira, B.F.P.; Moura-Costa, L.; Xavier, M.T.; Meyer, R. Porphyromonas gingivalis HmuY stimulates expression of Bcl-2 and Fas by human CD3+ T cells. BMC Microbiol. 2013, 13, 206. [Google Scholar] [CrossRef] [Green Version]
- Hiraki, D.; Uehara, O.; Kuramitsu, Y.; Morikawa, T.; Harada, F.; Yoshida, K.; Akino, K.; Chiba, I.; Asaka, M.; Abiko, Y.P. gingivalis Lipopolysaccharide Stimulates the Upregulated Expression of the Pancreatic Cancer-Related Genes Regenerating Islet-Derived 3 A/G in Mouse Pancreas. Int. J. Mol. Sci. 2020, 21, 7351. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhou, Z.; Cheng, Q.; Wang, H.; Cao, H.; Xu, Q.; Tuo, Y.; Jiang, L.; Zou, Y.; Ren, H.; et al. Acceleration of pancreatic tumorigenesis under immunosuppressive microenvironment induced by Reg3g overexpression. Cell Death Dis. 2017, 8, e3033. [Google Scholar] [CrossRef] [Green Version]
- Garred, P.; Genster, N.; Pilely, K.; Olmos, R.B.; Rosbjerg, A.; Ma, Y.J.; Skjødt, M.-O. A journey through the lectin pathway of complement-MBL and beyond. Immunol. Rev. 2016, 274, 74–97. [Google Scholar] [CrossRef] [PubMed]
- Kaźmierczak-Siedlecka, K.; Dvořák, A.; Folwarski, M.; Daca, A.; Przewłócka, K.; Makarewicz, W. Fungal Gut Microbiota Dysbiosis and Its Role in Colorectal, Oral, and Pancreatic Carcinogenesis. Cancers 2020, 12, 1326. [Google Scholar] [CrossRef]
- Guo, S.; Li, L.; Xu, B.; Li, M.; Zeng, Q.; Xiao, H.; Xue, Y.; Wu, Y.; Wang, Y.; Liu, W.; et al. A Simple and Novel Fecal Biomarker for Colorectal Cancer: Ratio of Fusobacterium Nucleatum to Probiotics Populations, Based on Their Antagonistic Effect. Clin. Chem. 2018, 64, 1327–1337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).