Association of the Lung Immune Prognostic Index with Immunotherapy Outcomes in Mismatch Repair Deficient Tumors
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Lung Immune Prognostic Index
2.3. MSI-H/dMMR Status
2.4. Statistical Analysis
3. Results
3.1. Study Population
3.2. LIPI in dMMR Tumors
3.3. LIPI Is Associated with ICI Survival Outcomes in MSI-H Tumors
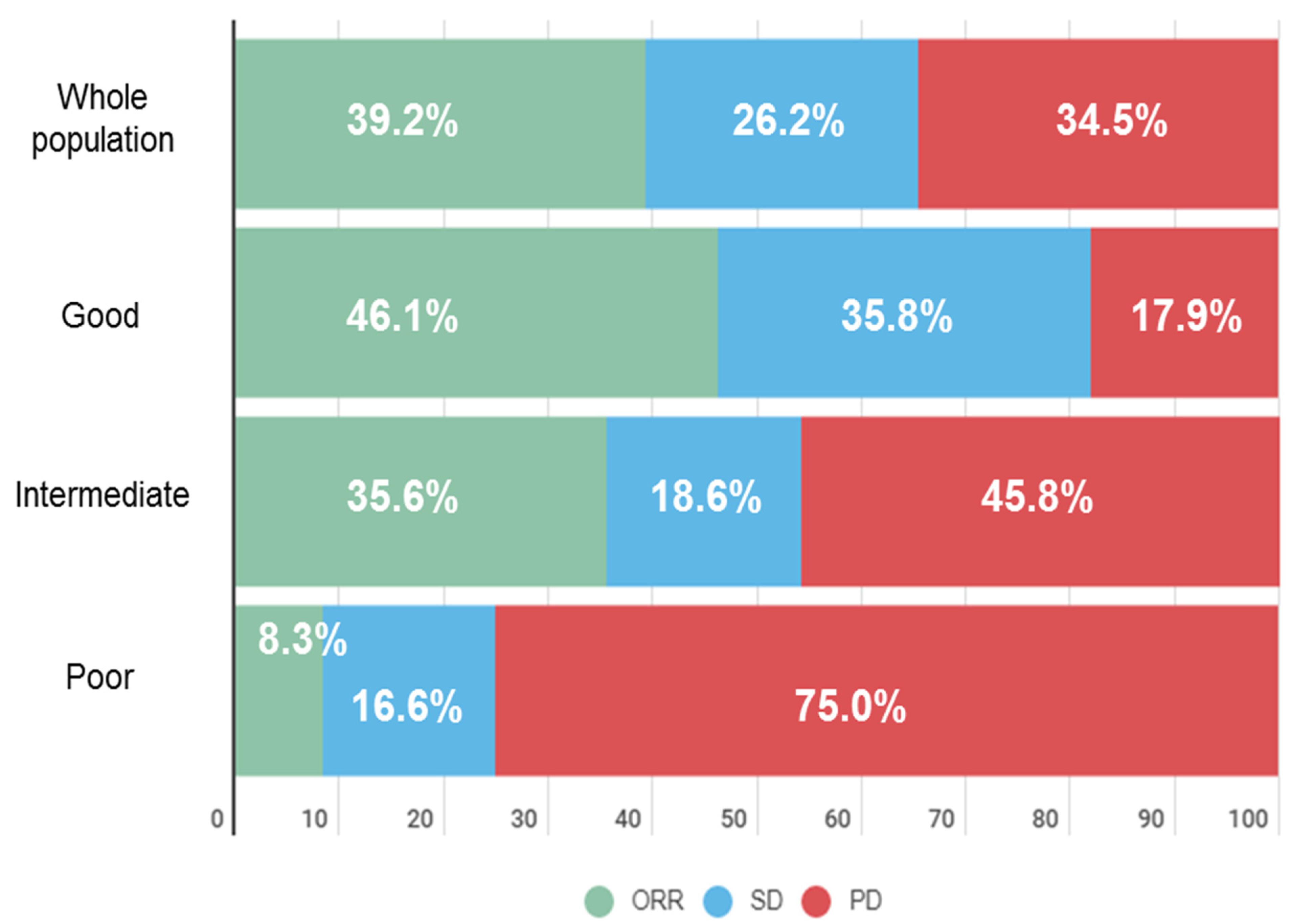
3.4. LIPI Is Associated with Tumor Response under ICI in dMMR Tumors
3.5. Fast Progressors Rate
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| dMMR | deficient mismatch repair |
| dNLR | derived neutrophils/leucocytes ratio |
| LIPI | lung immune prognostic index |
| MSI-H | microsatellites instable high |
| OS | overall survival |
| PFS | progression free survival |
References
- Cilona, M.; Locatello, L.G.; Novelli, L.; Gallo, O. The Mismatch Repair System (MMR) in Head and Neck Carcinogenesis and Its Role in Modulating the Response to Immunotherapy: A Critical Review. Cancers 2020, 12, 3006. [Google Scholar] [CrossRef] [PubMed]
- Luchini, C.; Bibeau, F.; Ligtenberg, M.; Singh, N.; Nottegar, A.; Bosse, T.; Miller, R.; Riaz, N.; Douillard, J.-Y.; Andre, F.; et al. ESMO recommendations on microsatellite instability testing for immunotherapy in cancer, and its relationship with PD-1/PD-L1 expression and tumour mutational burden: A systematic review-based approach. Ann. Oncol. 2019, 30, 1232–1243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deshpande, M.; Romanski, P.A.; Rosenwaks, Z.; Gerhardt, J. Gynecological Cancers Caused by Deficient Mismatch Repair and Microsatellite Instability. Cancers 2020, 12, 3319. [Google Scholar] [CrossRef] [PubMed]
- Eso, Y.; Seno, H. Current status of treatment with immune checkpoint inhibitors for gastrointestinal, hepatobiliary, and pancreatic cancers. Ther. Adv. Gastroenterol. 2020, 13. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, D.T.; Kim, T.W.; Van Cutsem, E.; Geva, R.; Jäger, D.; Hara, H.; Burge, M.; O’Neil, B.; Kavan, P.; Yoshino, T.; et al. Phase II Open-Label Study of Pembrolizumab in Treat-ment-Refractory, Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: KEYNOTE-164. J. Clin. Oncol. J. Am. Soc. Clin. Oncol. 2020, 38, 11–19. [Google Scholar] [CrossRef]
- André, T.; Shiu, K.-K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in Microsatellite-Instability–High Advanced Colorectal Cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef]
- Overman, M.J.; McDermott, R.; Leach, J.L.; Lonardi, S.; Lenz, H.-J.; Morse, M.A.; Desai, J.; Hill, A.; Axelson, M.; Moss, R.A.; et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): An open-label, multicentre, phase 2 study. Lancet Oncol. 2017, 18, 1182–1191. [Google Scholar] [CrossRef]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.-P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients with Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEY-NOTE-158 Study. J. Clin. Oncol. J. Am. Soc. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef]
- Mezquita, L.; Auclin, E.; Ferrara, R.; Charrier, M.; Remon, J.; Planchard, D.; Ponce, S.; Ares, L.P.; Leroy, L.; Audigier-Valette, C.; et al. Association of the Lung Immune Prognostic Index with Immune Checkpoint Inhibitor Outcomes in Patients With Advanced Non–Small Cell Lung Cancer. JAMA Oncol. 2018, 4, 351–357. [Google Scholar] [CrossRef]
- Varga, A.; Bernard-Tessier, A.; Auclin, E.; Pérez, L.M.; Baldini, C.; Planchard, D.; Marabelle, A.; Hollebecque, A.; Besse, B.; Massard, C. Applicability of the lung immune prognostic index (LIPI) in patients with metastatic solid tumors when treated with immune checkpoint inhibitors (ICI) in early clinical trials. Ann. Oncol. 2019, 30, i2. [Google Scholar] [CrossRef]
- Benitez, J.C.; Recondo, G.; Rassy, E.; Mezquita, L. The LIPI score and inflammatory biomarkers for selection of patients with solid tumors treated with checkpoint inhibitors. Q. J. Nucl. Med. Mol. Imaging 2020, 64. [Google Scholar] [CrossRef]
- Lavaud, P.; Dalban, C.; Negrier, S.; Chevreau, C.; Gravis, G.; Oudard, S.; Laguerre, B.; Barthelemy, P.; Borchiellini, D.; Goupil, M.G.; et al. Validation of the lung immune prognostic index (LIPI) in patients with metastatic renal cell carcinoma treated with nivolumab in the GETUG-AFU 26 NIVOREN trial. J. Clin. Oncol. 2020, 38, 735. [Google Scholar] [CrossRef]
- Chen, M.; You, R.; You-Ping, L.; Huang, P.-Y.; Zou, X.; Shen, G.-P.; Zhang, H.-D. Chemotherapy plus local-regional radiotherapy versus chemotherapy alone in primary metastatic nasopharyngeal carcinoma: A randomized, open-label, phase III trial. Ann. Oncol. 2019, 30, v449. [Google Scholar] [CrossRef]
- Meyers, D.E.; Stukalin, I.; Vallerand, I.A.; Lewinson, R.T.; Suo, A.; Dean, M.; North, S.; Pabani, A.; Cheng, T.; Heng, D.Y.; et al. The Lung Immune Prognostic Index Discriminates Survival Outcomes in Patients with Solid Tumors Treated with Immune Checkpoint Inhibitors. Cancers 2019, 11, 1713. [Google Scholar] [CrossRef] [Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diakos, C.; Charles, K.A.; McMillan, D.; Clarke, S.J. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014, 15, e493–e503. [Google Scholar] [CrossRef]
- Nebot-Bral, L.; Brandao, D.; Verlingue, L.; Rouleau, E.; Caron, O.; Despras, E.; El-Dakdoukibe, Y.; Champiat, S.; Aoufouchi, S.; Leary, A.; et al. Hypermutated tumours in the era of immuno-therapy: The paradigm of personalised medicine. Eur. J. Cancer Oxf. Engl. 1990 2017, 84, 290–303. [Google Scholar]
- Atretkhany, K.-S.; Drutskaya, M.; Nedospasov, S.; Grivennikov, S.; Kuprash, D. Chemokines, cytokines and exosomes help tumors to shape inflammatory microenvironment. Pharmacol. Ther. 2016, 168, 98–112. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.W.; Hajjar, J.; Hwu, P.; Naing, A. Targeting the indoleamine 2,3-dioxygenase pathway in cancer. J. Immunother. Cancer 2015, 3, 51. [Google Scholar] [CrossRef] [Green Version]
- Tseng-Rogenski, S.S.; Hamaya, Y.; Choi, D.Y.; Carethers, J.M. Interleukin 6 Alters Localization of hMSH3, Leading to DNA Mismatch Repair Defects in Colorectal Cancer Cells. Gastroenterology 2015, 148, 579–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanc-Durand, F.; Rubio, X.M.; Auclin, E.; Ponce-Aix, S.; Castro, R.L.; Nadal, E.; Planchard, D.; Routy, B.; Hendriks, L.; Sullivan, I.; et al. FP07.06 Lung Immune Prognostic Index (LIPI) in Advanced NSCLC Patients Treated with Immunotherapy, Chemotherapy and both Combined Upfront. J. Thorac. Oncol. 2021, 16, S205–S206. [Google Scholar] [CrossRef]
- Sorich, M.J.; Rowland, A.; Karapetis, C.; Hopkins, A. Evaluation of the Lung Immune Prognostic Index for Prediction of Survival and Response in Patients Treated With Atezolizumab for NSCLC: Pooled Analysis of Clinical Trials. J. Thorac. Oncol. 2019, 14, 1440–1446. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, A.; Kichenadasse, G.; Abuhelwa, A.; McKinnon, R.; Rowland, A.; Sorich, M. Value of the Lung Immune Prognostic Index in Patients with Non-Small Cell Lung Cancer Initiating First-Line Atezolizumab Combination Therapy: Subgroup Analysis of the IMPOWER150 Trial. Cancers 2021, 13, 1176. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, R.; Mezquita, L.; Texier, M.; Lahmar, J.; Audigier-Valette, C.; Tessonnier, L.; Mazieres, J.; Zalcman, G.; Brosseau, S.; Le Moulec, S.; et al. Hyperprogressive Disease in Patients With Advanced Non–Small Cell Lung Cancer Treated With PD-1/PD-L1 Inhibitors or With Single-Agent Chemotherapy. JAMA Oncol. 2018, 4, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, R.; Mezquita, L.; Texier, M.; Lahmar, J.; Audigier-Valette, C.; Tessonnier, L.; Mazieres, J.; Zalcman, G.; Brosseau, S.; Le Moulec, S.; et al. Comparison of Fast-Progression, Hyperprogressive Disease, and Early Deaths in Advanced Non–Small-Cell Lung Cancer Treated With PD-1/PD-L1 Inhibitors or Chemotherapy. JCO Precis. Oncol. 2020, 829–840. [Google Scholar] [CrossRef]

| Variable | All Patients (N = 151) | |
|---|---|---|
| Age | Median (IQR) | 64 (51.5-70.5) |
| >65 | 64 (43.0%) | |
| Missing | 2 | |
| Gender | female | 89 (58.9%) |
| male | 62 (41.1%) | |
| Primary tumor site | gastrointestinal | 99 (65.6%) |
| gynecologic | 33 (21.8%) | |
| other | 19 (12.6%) | |
| Lynch syndrome | yes | 40 (32.0%) |
| missing | 26 | |
| Line of ICI start | median (IQR) | 2 (2–3) |
| >2 | 62 (41.3%) | |
| missing | 1 | |
| Number of metastasis at ICI start | >2 | 34 (23.6%) |
| missing | 7 | |
| Metastasis sites | lung metastasis | 31 (20.5%) |
| bone metastasis | 13 (8.6%) | |
| liver metastasis | 47 (31.1%) | |
| brain metastasis | 7 (4.6%) | |
| Type of ICI antibody | PD-1 | 109 (72.2%) |
| PD-L1 | 42 (27.8%) | |
| Monotherapy or combination | combination | 20 (13.3%) |
| monotherapy | 131 (86.7%) | |
| Performance status at ICI start | 0 | 58 (42.3%) |
| ≥1 | 79 (57.7%) | |
| missing | 14 | |
| dNLR | >3 | 39 (25.8%) |
| LDH | high | 55 (38.5%) |
| missing | 8 | |
| Albumin (g/L) | ≤35 | 64 (43.5%) |
| missing | 4 |
| Variable | All Patients (N = 151) | LIPI Good Prognostic Group (N = 67) | LIPI Intermediate Prognostic Group (N = 62) | LIPI Poor Prognostic Group (N = 14) | p | |
|---|---|---|---|---|---|---|
| Median (95%CI) | OS | NR (23.4 to NR) | NR (36.5 to NR) | NR (16.2 to NR) | 3.3 (2.6 to NR) | <0.001 |
| PFS | 10.5 (7.1 to 35.1) | 20.9 (8.4 to NR) | 9.9 (2.8 to NR) | 2.3 (1.8 to NR) | <0.001 | |
| Fast progressors rate | yes | 24 (16.0%) | 5 (7.5%) | 11 (18.0%) | 5 (35.7%) | 0.02 |
| no | 126 (84.0%) | 62 (92.5%) | 50 (82.0%) | 9 (64.3%) | ||
| missing | 1 | 0 | 1 | 0 | ||
| ORR | no | 87 (60.8%) | 35 (53.8%) | 38 (64.4%) | 11 (91.7%) | 0.03 |
| yes | 56 (39.2%) | 30 (46.2%) | 21 (35.6%) | 1 (8.3%) | ||
| missing | 8 | 2 | 3 | 2 | ||
| DCR | no | 47 (32.6%) | 10 (15.2%) | 26 (44.1%) | 9 (75.0%) | <0.001 |
| yes | 97 (67.4%) | 56 (84.8%) | 33 (55.9%) | 3 (25.0%) | ||
| missing | 7 | 1 | 3 | 2 |
| Variable | OS N = 125, n Events = 49 | PFS N = 124, n Events = 71 | |||||
|---|---|---|---|---|---|---|---|
| HR | 95%CI | p | HR | 95%CI | p | ||
| Tumor site | gastrointestinal | 1 | 1 | ||||
| gynecologic | 1.65 | 0.82 to 3.29 | 0.03 | 1.53 | 0.84 to 2.81 | 0.0002 | |
| other | 2.59 | 1.25 to 5.35 | 4.08 | 2.08 to 8.01 | |||
| N metastatic sites at ICI start | >2 | 1.99 | 1.06 to 3.70 | 0.03 | 1.06 | 0.61 to 1.85 | 0.84 |
| Performance status | 0 | ||||||
| ≥1 | 2.11 | 1.05 to 4.24 | 0.04 | 1.91 | 1.10 to 3.31 | 0.02 | |
| Albumin (g/L) | >35 | 0.82 | 0.45 to 1.50 | 0.51 | 0.96 | 0.58 to 1.59 | 0.87 |
| LIPI | good | 1 | 1 | ||||
| tntermediate | 1.43 | 0.75 to 2.74 | 0.02 | 1.09 | 0.65 to 1.82 | 0.07 | |
| poor | 3.50 | 1.46 to 8.40 | 2.41 | 1.12 to 5.19 | |||
| Variable | ORR | DCR | Fast Progressors | |||
|---|---|---|---|---|---|---|
| OR (95%CI) | p | OR (95%CI) | p | OR (95%CI) | p | |
| LIPI | ||||||
| Good | Ref | Ref | Ref | |||
| Intermediate | 1.55 (0.75–3.19) | 0.23 | 4.41 (1.89–10.29) | 0.01 | 2.73 (0.89–8.37) | 0.08 |
| Poor | 9.43 (1.15–77.27) | 0.04 | 16.8 (3.86–73.05) | <0.0001 | 6.89 (1.66–28.59) | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Auclin, E.; Vuagnat, P.; Smolenschi, C.; Taieb, J.; Adeva, J.; Nebot-Bral, L.; Garcia de Herreros, M.; Vidal Tocino, R.; Longo-Muñoz, F.; El Dakdouki, Y.; et al. Association of the Lung Immune Prognostic Index with Immunotherapy Outcomes in Mismatch Repair Deficient Tumors. Cancers 2021, 13, 3776. https://doi.org/10.3390/cancers13153776
Auclin E, Vuagnat P, Smolenschi C, Taieb J, Adeva J, Nebot-Bral L, Garcia de Herreros M, Vidal Tocino R, Longo-Muñoz F, El Dakdouki Y, et al. Association of the Lung Immune Prognostic Index with Immunotherapy Outcomes in Mismatch Repair Deficient Tumors. Cancers. 2021; 13(15):3776. https://doi.org/10.3390/cancers13153776
Chicago/Turabian StyleAuclin, Edouard, Perrine Vuagnat, Cristina Smolenschi, Julien Taieb, Jorge Adeva, Laetitia Nebot-Bral, Marta Garcia de Herreros, Rosario Vidal Tocino, Federico Longo-Muñoz, Yola El Dakdouki, and et al. 2021. "Association of the Lung Immune Prognostic Index with Immunotherapy Outcomes in Mismatch Repair Deficient Tumors" Cancers 13, no. 15: 3776. https://doi.org/10.3390/cancers13153776
APA StyleAuclin, E., Vuagnat, P., Smolenschi, C., Taieb, J., Adeva, J., Nebot-Bral, L., Garcia de Herreros, M., Vidal Tocino, R., Longo-Muñoz, F., El Dakdouki, Y., Martín-Romano, P., Gaba, L., Saurí, T., Oliveres, H., Castañón, E., Garcia-Carbonero, R., Besse, B., Massard, C., Mezquita, L., & Hollebecque, A. (2021). Association of the Lung Immune Prognostic Index with Immunotherapy Outcomes in Mismatch Repair Deficient Tumors. Cancers, 13(15), 3776. https://doi.org/10.3390/cancers13153776







