Triple Negative Breast Cancer: A Mountain Yet to Be Scaled Despite the Triumphs
Abstract
Simple Summary
Abstract
1. Introduction
2. Therapeutic Approaches for Triple-Negative Breast Cancer
2.1. Surgery
2.2. Radiation Treatment
2.3. Chemotherapy
2.3.1. Taxanes
2.3.2. Anthracyclines
2.3.3. Platinum Agents
2.3.4. Cyclophosphamide
3. Key ‘Targetable’ Mechanisms Underlying the Development of Chemoresistance in Triple-Negative Breast Cancer
3.1. ABC Transporters and Drug Efflux
3.2. DNA Damage and Repair
3.3. Metabolic Reprogramming
3.4. EMT and Cancer Stem Cells
3.5. Exosome and TNBC Metastasis
4. Therapeutic Approaches to Target Specific Pathways in Triple-Negative Breast Cancer
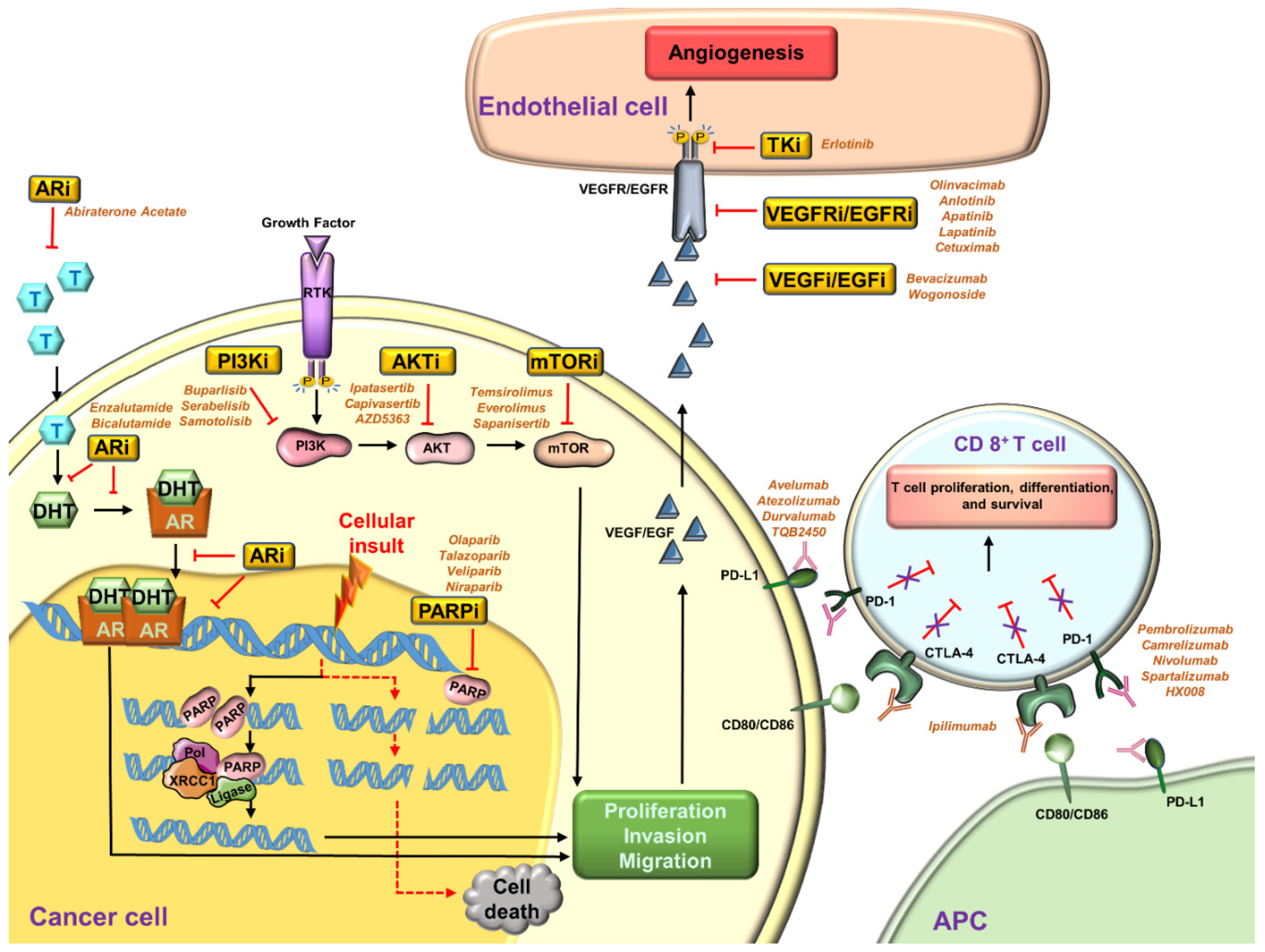
4.1. PARP Inhibitors
4.2. Angiogenesis Inhibitors
4.3. Inhibitors for the PI3K/AKT/mTOR Pathway
4.4. Inhibitors for Androgen Receptor (AR)
4.5. Immunotherapy
5. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Elias, A.D. Triple-negative breast cancer: A short review. Am. J. Clin. Oncol. 2010, 33, 637–645. [Google Scholar] [CrossRef]
- Haffty, B.G.; Yang, Q.; Reiss, M.; Kearney, T.; Higgins, S.A.; Weidhaas, J.; Harris, L.; Hait, W.; Toppmeyer, D. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J. Clin. Oncol. 2006, 24, 5652–5657. [Google Scholar] [CrossRef]
- Rakha, E.A.; El-Sayed, M.E.; Green, A.R.; Lee, A.H.; Robertson, J.F.; Ellis, I.O. Prognostic markers in triple-negative breast cancer. Cancer 2007, 109, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Bauer, K.R.; Brown, M.; Cress, R.D.; Parise, C.A.; Caggiano, V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: A population-based study from the California cancer Registry. Cancer 2007, 109, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Urru, S.A.M.; Gallus, S.; Bosetti, C.; Moi, T.; Medda, R.; Sollai, E.; Murgia, A.; Sanges, F.; Pira, G.; Manca, A.; et al. Clinical and pathological factors influencing survival in a large cohort of triple-negative breast cancer patients. BMC Cancer. 2018, 18, 56. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Investig. 2011, 121, 2750–2767. [Google Scholar] [CrossRef] [PubMed]
- Kalimutho, M.; Parsons, K.; Mittal, D.; López, J.A.; Srihari, S.; Khanna, K.K. Targeted Therapies for Triple-Negative Breast Cancer: Combating a Stubborn Disease. Trends Pharmacol. Sci. 2015, 36, 822–846. [Google Scholar] [CrossRef]
- Marotti, J.D.; de Abreu, F.B.; Wells, W.A.; Tsongalis, G.J. Triple-Negative Breast Cancer: Next-Generation Sequencing for Target Identification. Am. J. Pathol. 2017, 187, 2133–2138. [Google Scholar] [CrossRef]
- Doval, D.C.; Sharma, A.; Sinha, R.; Kumar, K.; Dewan, A.K.; Chaturvedi, H.; Batra, U.; Talwar, V.; Gupta, S.K.; Singh, S.; et al. Immunohistochemical Profile of Breast Cancer Patients at a Tertiary Care Hospital in New Delhi, India. Asian Pac. J. Cancer Prev. 2015, 16, 4959–4964. [Google Scholar] [CrossRef]
- Kassam, F.; Enright, K.; Dent, R.; Dranitsaris, G.; Myers, J.; Flynn, C.; Fralick, M.; Kumar, R.; Clemons, M. Survival outcomes for patients with metastatic triple-negative breast cancer: Implications for clinical practice and trial design. Clin. Breast Cancer 2009, 9, 29–33. [Google Scholar] [CrossRef]
- Plasilova, M.L.; Hayse, B.; Killelea, B.K.; Horowitz, N.R.; Chagpar, A.B.; Lannin, D.R. Features of triple-negative breast cancer: Analysis of 38,813 cases from the national cancer database. Medicine 2016, 95, e4614. [Google Scholar] [CrossRef]
- Thakur, K.K.; Bordoloi, D.; Kunnumakkara, A.B. Alarming Burden of Triple-Negative Breast Cancer in India. Clin. Breast Cancer 2018, 18, e393–e399. [Google Scholar] [CrossRef]
- Brewster, A.M.; Chavez-MacGregor, M.; Brown, P. Epidemiology, biology, and treatment of triple-negative breast cancer in women of African ancestry. Lancet Oncol. 2014, 15, e625–e634. [Google Scholar] [CrossRef]
- Kurian, A.W.; Fish, K.; Shema, S.J.; Clarke, C.A. Lifetime risks of specific breast cancer subtypes among women in four racial/ethnic groups. Breast Cancer Res. 2010, 12, R99. [Google Scholar] [CrossRef] [PubMed]
- Amirikia, K.C.; Mills, P.; Bush, J.; Newman, L.A. Higher population-based incidence rates of triple-negative breast cancer among young African-American women: Implications for breast cancer screening recommendations. Cancer 2011, 117, 2747–2753. [Google Scholar] [CrossRef]
- Parise, C.A.; Bauer, K.R.; Caggiano, V. Variation in breast cancer subtypes with age and race/ethnicity. Rit. Rev. Oncol. Hematol. 2010, 76, 44–52. [Google Scholar] [CrossRef]
- Trivers, K.F.; Lund, M.J.; Porter, P.L.; Liff, J.M.; Flagg, E.W.; Coates, R.J.; Eley, J.W. The epidemiology of triple-negative breast cancer, including race. Cancer Causes Control. 2009, 20, 1071–1082. [Google Scholar] [CrossRef] [PubMed]
- Bowen, R.L.; Duffy, S.W.; Ryan, D.A.; Hart, I.R.; Jones, J.L. Early onset of breast cancer in a group of British black women. Br. J. Cancer 2008, 98, 277–281. [Google Scholar] [CrossRef]
- Carey, L.A.; Perou, C.M.; Livasy, C.A.; Dressler, L.G.; Cowan, D.; Conway, K.; Karaca, G.; Troester, M.A.; Tse, C.K.; Edmiston, S.; et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 2006, 295, 2492–2502. [Google Scholar] [CrossRef]
- Anderson, W.F.; Jatoi, I.; Devesa, S.S. Distinct breast cancer incidence and prognostic patterns in the NCI’s SEER program: Suggesting a possible link between etiology and outcome. Breast Cancer Res. Treat. 2005, 90, 127–137. [Google Scholar] [CrossRef]
- Gierach, G.L.; Burke, A.; Anderson, W.F. Epidemiology of triple negative breast cancers. Breast Dis. 2010, 32, 5–24. [Google Scholar] [CrossRef] [PubMed]
- Dolle, J.M.; Daling, J.R.; White, E.; Brinton, L.A.; Doody, D.R.; Porter, P.L.; Malone, K.E. Risk factors for triple-negative breast cancer in women under the age of 45 years. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Beaber, E.F.; Malone, K.E.; Tang, M.T.; Barlow, W.E.; Porter, P.L.; Daling, J.R.; Li, C.I. Oral contraceptives and breast cancer risk overall and by molecular subtype among young women. Cancer Epidemiol. Biomark. Prev. 2014, 23, 755–764. [Google Scholar] [CrossRef]
- Kabat, G.C.; Kim, M.; Phipps, A.I.; Li, C.I.; Messina, C.R.; Wactawski-Wende, J.; Kuller, L.; Simon, M.S.; Yasmeen, S.; Wassertheil-Smoller, S.; et al. Smoking and alcohol consumption in relation to risk of triple-negative breast cancer in a cohort of postmenopausal women. Cancer Causes Control. 2011, 22, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Marchbanks, P.A.; Curtis, K.M.; Mandel, M.G.; Wilson, H.G.; Jeng, G.; Folger, S.G.; McDonald, J.A.; Daling, J.R.; Bernstein, L.; Malone, K.E.; et al. Oral contraceptive formulation and risk of breast cancer. Contraception 2012, 85, 342–350. [Google Scholar] [CrossRef]
- Kawai, M.; Malone, K.E.; Tang, M.T.; Li, C.I. Active smoking and the risk of estrogen receptor-positive and triple-negative breast cancer among women ages 20 to 44 years. Cancer 2014, 120, 1026–1034. [Google Scholar] [CrossRef]
- Kwan, M.L.; Kushi, L.H.; Weltzien, E.; Maring, B.; Kutner, S.E.; Fulton, R.S.; Lee, M.M.; Ambrosone, C.B.; Caan, B.J. Epidemiology of breast cancer subtypes in two prospective cohort studies of breast cancer survivors. Breast Cancer Res. 2009, 11, R31. [Google Scholar] [CrossRef]
- Li, L.; Zhong, Y.; Zhang, H.; Yu, H.; Huang, Y.; Li, Z.; Chen, G.; Hua, X. Association between oral contraceptive use as a risk factor and triple-negative breast cancer: A systematic review and meta-analysis. Mol. Clin. Oncol. 2017, 7, 76–80. [Google Scholar] [CrossRef]
- Dent, R.; Trudeau, M.; Pritchard, K.I.; Hanna, W.M.; Kahn, H.K.; Sawka, C.A.; Lickley, L.A.; Rawlinson, E.; Sun, P.; Narod, S.A. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin. Cancer Res. 2007, 13, 4429–4434. [Google Scholar] [CrossRef]
- De-la-Cruz-Ku, G.; Valcarcel, B.; Morante, Z.; Möller, M.G.; Lizandro, S.; Rebaza, L.P.; Enriquez, D.; Luque, R.; Luján-Peche, M.G.; Eyzaguirre-Sandoval, M.E.; et al. Breast-conserving surgery vs. total mastectomy in patients with triple negative breast cancer in early stages: A propensity score analysis. Breast Dis. 2020, 39, 29–35. [Google Scholar] [CrossRef]
- Uematsu, T.; Kasami, M.; Yuen, S. Triple-negative breast cancer: Correlation between MR imaging and pathologic findings. Radiology 2009, 250, 638–647. [Google Scholar] [CrossRef]
- Yagata, H.; Kajiura, Y.; Yamauchi, H. Current strategy for triple-negative breast cancer: Appropriate combination of surgery, radiation, and chemotherapy. Breast Cancer 2011, 18, 165–173. [Google Scholar] [CrossRef]
- Cho, S.W.; Kim, S.; Kim, J.M.; Kim, J.-S. Targeted genome engineering in human cells with the Cas9 RNA-guided endonuclease. Nat. Biotechnol. 2013, 31, 230–232. [Google Scholar] [CrossRef]
- Black, D.M.; Mittendorf, E.A. Landmark trials affecting the surgical management of invasive breast cancer. Surg. Clin. N. Am. 2013, 93, 501–518. [Google Scholar] [CrossRef]
- El Gammal, E.R.; Abdulmohaymen, A.M.; Abdulmutaleb, B.A.M.M. Comparative Study between Conservative Breast Surgery and Modified Radical Mastectomy in Triple Negative Cases. J. Hosp. Med. 2019, 76, 4269–4273. [Google Scholar] [CrossRef]
- Dawood, S. Triple-negative breast cancer: Epidemiology and management options. Drugs 2010, 70, 2247–2258. [Google Scholar] [CrossRef] [PubMed]
- Dixit, A.; Frampton, C.; Davey, V.; Robinson, B.; James, M. Radiation treatment in early stage triple-negative breast cancer in New Zealand: A national database study. J. Med. Imaging Radiat. Oncol. 2019, 63, 698–706. [Google Scholar] [CrossRef]
- McGale, P.; Taylor, C.; Correa, C.; Cutter, D.; Duane, F.; Ewertz, M.; Gray, R.; Mannu, G.; Peto, R.; Whelan, T.; et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: Meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 2014, 383, 2127–2135. [Google Scholar] [CrossRef] [PubMed]
- Her, S.; Cui, L.; Bristow, R.G.; Allen, C. Dual Action Enhancement of Gold Nanoparticle Radiosensitization by Pentamidine in Triple Negative Breast Cancer. Radiat. Res. 2016, 185, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Foulkes, W.D.; Smith, I.E.; Reis-Filho, J.S. Triple-negative breast cancer. N. Engl. J. Med. 2010, 363, 1938–1948. [Google Scholar] [CrossRef]
- Bianchini, G.; Balko, J.M.; Mayer, I.A.; Sanders, M.E.; Gianni, L. Triple-negative breast cancer: Challenges and opportunities of a heterogeneous disease. Nat. Rev. Clin. Oncol. 2016, 13, 674–690. [Google Scholar] [CrossRef] [PubMed]
- Eastman, A.; Tammaro, Y.; Moldrem, A.; Andrews, V.; Huth, J.; Euhus, D.; Leitch, M.; Rao, R. Outcomes of delays in time to treatment in triple negative breast cancer. Ann. Surg. Oncol. 2013, 20, 1880–1885. [Google Scholar] [CrossRef] [PubMed]
- Marra, A.; Viale, G.; Curigliano, G. Recent advances in triple negative breast cancer: The immunotherapy era. BMC Med. 2019, 17, 90. [Google Scholar] [CrossRef] [PubMed]
- Pandy, J.G.P.; Balolong-Garcia, J.C.; Cruz-Ordinario, M.V.B.; Que, F.V.F. Triple negative breast cancer and platinum-based systemic treatment: A meta-analysis and systematic review. BMC Cancer 2019, 19, 1065. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.K.; Collier, A.L.; Lee, D.; Hoefer, R.A.; Zheleva, V.; Siewertsz van Reesema, L.L.; Tang-Tan, A.M.; Guye, M.L.; Chang, D.Z.; Winston, J.S.; et al. Perspectives on Triple-Negative Breast Cancer: Current Treatment Strategies, Unmet Needs, and Potential Targets for Future Therapies. Cancers 2020, 12, 2392. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.Y.; Hu, Q.L.; Zhang, J.; Xu, W.Y.; Li, X.S. Survival outcomes of neoadjuvant versus adjuvant chemotherapy in triple-negative breast cancer: A me-ta-analysis of 36,480 cases. World J. Surg. Oncol. 2020, 18, 129. [Google Scholar] [CrossRef]
- Haddad, T.C.; Goetz, M.P. Landscape of neoadjuvant therapy for breast cancer. Ann. Surg. Oncol. 2015, 22, 1408–1415. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Ahn, J.H.; Kim, S.B. How shall we treat early triple-negative breast cancer (TNBC): From the current standard to upcoming immuno-molecular strategies. ESMO Open 2018, 3, e000357. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.J.; Rivory, L.P. Clinical pharmacokinetics of docetaxel. Clin. Pharm. 1999, 36, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Ghersi, D.; Wilcken, N.; Simes, R.J. A systematic review of taxane-containing regimens for metastatic breast cancer. Br. J. Cancer 2005, 93, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Duan, J.-J.; Bian, X.-W.; Yu, S.-c. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020, 22, 61. [Google Scholar] [CrossRef]
- Martín, M.; Rodríguez-Lescure, Á.; Ruiz, A.; Alba, E.; Calvo, L.; Ruiz-Borrego, M.; Santaballa, A.; Rodríguez, C.A.; Crespo, C.; Abad, M.; et al. Molecular predictors of efficacy of adjuvant weekly paclitaxel in early breast cancer. Breast Cancer Res. Treat. 2010, 123, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.A.; Chakravarthy, A.B.; Rosenbluth, J.M.; Mi, D.; Seeley, E.H.; De Matos Granja-Ingram, N.; Olivares, M.G.; Kelley, M.C.; Mayer, I.A.; Meszoely, I.M.; et al. Identification of markers of taxane sensitivity using proteomic and genomic analyses of breast tumors from patients receiving neoadjuvant paclitaxel and radiation. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2010, 16, 681–690. [Google Scholar] [CrossRef]
- Juul, N.; Szallasi, Z.; Eklund, A.C.; Li, Q.; Burrell, R.A.; Gerlinger, M.; Valero, V.; Andreopoulou, E.; Esteva, F.J.; Symmans, W.F.; et al. Assessment of an RNA interference screen-derived mitotic and ceramide pathway metagene as a predictor of response to neoadjuvant paclitaxel for primary triple-negative breast cancer: A retrospective analysis of five clinical trials. Lancet Oncol. 2010, 11, 358–365. [Google Scholar] [CrossRef]
- Escalante, L.; Ramos, I.; Imriskova, I.; Langley, E.; Sanchez, S. Glucose repression of anthracycline formation in Streptomyces peucetius var. caesius. Appl. Microbiol. Biotechnol. 1999, 52, 572–578. [Google Scholar] [CrossRef]
- Tsuji, W.; Plock, J.A. Chapter 2-Breast Cancer Metastasis. In Introduction to Cancer Metastasis; Ahmad, A., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 13–31. [Google Scholar]
- Levine, M.N.; Pritchard, K.I.; Bramwell, V.H.; Shepherd, L.E.; Tu, D.; Paul, N. Randomized trial comparing cyclophosphamide, epirubicin, and fluorouracil with cyclophosphamide, methotrexate, and fluorouracil in premenopausal women with node-positive breast cancer: Update of National Cancer Institute of Canada Clinical Trials Group Trial MA5. J. Clin. Oncol. 2005, 23, 5166–5170. [Google Scholar] [CrossRef]
- Bonneterre, J.; Roché, H.; Kerbrat, P.; Brémond, A.; Fumoleau, P.; Namer, M.; Goudier, M.J.; Schraub, S.; Fargeot, P.; Chapelle-Marcillac, I. Epirubicin increases long-term survival in adjuvant chemotherapy of patients with poor-prognosis, node-positive, early breast cancer: 10-year follow-up results of the French Adjuvant Study Group 05 randomized trial. J. Clin. Oncol. 2005, 23, 2686–2693. [Google Scholar] [CrossRef]
- Trudeau, M.; Charbonneau, F.; Gelmon, K.; Laing, K.; Latreille, J.; Mackey, J.; McLeod, D.; Pritchard, K.; Provencher, L.; Verma, S. Selection of adjuvant chemotherapy for treatment of node-positive breast cancer. Lancet Oncol. 2005, 6, 886–898. [Google Scholar] [CrossRef]
- Keam, B.; Im, S.A.; Kim, H.J.; Oh, D.Y.; Kim, J.H.; Lee, S.H.; Chie, E.K.; Han, W.; Kim, D.W.; Moon, W.K.; et al. Prognostic impact of clinicopathologic parameters in stage II/III breast cancer treated with neoadjuvant docetaxel and doxorubicin chemotherapy: Paradoxical features of the triple negative breast cancer. BMC Cancer 2007, 7, 203. [Google Scholar] [CrossRef] [PubMed]
- Decatris, M.P.; Sundar, S.; O’Byrne, K.J. Platinum-based chemotherapy in metastatic breast cancer: Current status. Cancer Treat. Rev. 2004, 30, 53–81. [Google Scholar] [CrossRef]
- Isakoff, S.J. Triple-negative breast cancer: Role of specific chemotherapy agents. Cancer J. 2010, 16, 53–61. [Google Scholar] [CrossRef]
- Tanida, S.; Mizoshita, T.; Ozeki, K.; Tsukamoto, H.; Kamiya, T.; Kataoka, H.; Sakamuro, D.; Joh, T. Mechanisms of Cisplatin-Induced Apoptosis and of Cisplatin Sensitivity: Potential of BIN1 to Act as a Potent Predictor of Cisplatin Sensitivity in Gastric Cancer Treatment. Int. J. Surg. Oncol. 2012, 2012, 862879. [Google Scholar] [CrossRef]
- Jamieson, E.R.; Lippard, S.J. Structure, Recognition, and Processing of Cisplatin-DNA Adducts. Chem. Rev. 1999, 99, 2467–2498. [Google Scholar] [CrossRef]
- Basourakos, S.P.; Li, L.; Aparicio, A.M.; Corn, P.G.; Kim, J.; Thompson, T.C. Combination Platinum-based and DNA Damage Response-targeting Cancer Therapy: Evolution and Future Directions. Curr. Med. Chem. 2017, 24, 1586–1606. [Google Scholar] [CrossRef]
- Lafarge, S.; Sylvain, V.; Ferrara, M.; Bignon, Y.J. Inhibition of BRCA1 leads to increased chemoresistance to microtubule-interfering agents, an effect that involves the JNK pathway. Oncogene 2001, 20, 6597–6606. [Google Scholar] [CrossRef]
- Leong, C.O.; Vidnovic, N.; DeYoung, M.P.; Sgroi, D.; Ellisen, L.W. The p63/p73 network mediates chemosensitivity to cisplatin in a biologically defined subset of primary breast cancers. J. Clin. Investig. 2007, 117, 1370–1380. [Google Scholar] [CrossRef]
- DeYoung, M.P.; Johannessen, C.M.; Leong, C.O.; Faquin, W.; Rocco, J.W.; Ellisen, L.W. Tumor-specific p73 up-regulation mediates p63 dependence in squamous cell carcinoma. Cancer Res. 2006, 66, 9362–9368. [Google Scholar] [CrossRef]
- Rocco, J.W.; Leong, C.O.; Kuperwasser, N.; DeYoung, M.P.; Ellisen, L.W. p63 mediates survival in squamous cell carcinoma by suppression of p73-dependent apoptosis. Cancer Cell 2006, 9, 45–56. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z.; Hu, X.; Wang, B.; Wang, L.; Yang, W.; Liu, Y.; Liu, G.; Di, G.; Hu, Z.; et al. Cisplatin and gemcitabine as the first line therapy in metastatic triple negative breast cancer. Int. J. Cancer. 2015, 136, 204–211. [Google Scholar] [CrossRef]
- von Minckwitz, G.; Schneeweiss, A.; Loibl, S.; Salat, C.; Denkert, C.; Rezai, M.; Blohmer, J.U.; Jackisch, C.; Paepke, S.; Gerber, B.; et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): A randomised phase 2 trial. Lancet Oncol. 2014, 15, 747–756. [Google Scholar] [CrossRef]
- Jovanović, B.; Mayer, I.A.; Mayer, E.L.; Abramson, V.G.; Bardia, A.; Sanders, M.E.; Kuba, M.G.; Estrada, M.V.; Beeler, J.S.; Shaver, T.M.; et al. A Randomized Phase II Neoadjuvant Study of Cisplatin, Paclitaxel With or Without Everolimus in Patients with Stage II/III Triple-Negative Breast Cancer (TNBC): Responses and Long-term Outcome Correlated with Increased Frequency of DNA Damage Response Gene Mutations, TNBC Subtype, AR Status, and Ki67. Clin. Cancer Res. 2017, 23, 4035–4045. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.; Bishop, J.B.; Garner, R.C.; Ostrosky-Wegman, P.; Selby, P.B. Cyclophosphamide: Review of its mutagenicity for an assessment of potential germ cell risks. Mutat. Res. 1995, 330, 115–181. [Google Scholar] [CrossRef]
- Thomas, S.S.; Clements, P.J.; Furst, D.E. 58-Immunosuppressives: Cyclosporine, cyclophosphamide, azathioprine, mycophenolate mofetil. In Rheumatology (Sixth Edition); Hochberg, M.C., Silman, A.J., Smolen, J.S., Weinblatt, M.E., Weisman, M.H., Eds.; Mosby: Philadelphia, PA, USA, 2015; pp. 459–467. [Google Scholar]
- Delay, E.R.; Socia, S.H.; Girardin, J.L.; Jewkes, B.C.; King, J.H.; Delay, R.J. Cyclophosphamide and the taste system: Effects of dose fractionation and amifostine on taste cell renewal. PLoS ONE 2019, 14, e0214890. [Google Scholar] [CrossRef] [PubMed]
- Nakatsukasa, K.; Koyama, H.; Oouchi, Y.; Imanishi, S.; Mizuta, N.; Sakaguchi, K.; Fujita, Y.; Fujiwara, I.; Kotani, T.; Matsuda, T.; et al. Docetaxel and cyclophosphamide as neoadjuvant chemotherapy in HER2-negative primary breast cancer. Breast Cancer 2017, 24, 63–68. [Google Scholar] [CrossRef]
- Masuda, H.; Baggerly, K.A.; Wang, Y.; Zhang, Y.; Gonzalez-Angulo, A.M.; Meric-Bernstam, F.; Valero, V.; Lehmann, B.D.; Pietenpol, J.A.; Hortobagyi, G.N.; et al. Differential response to neoadjuvant chemotherapy among 7 triple-negative breast cancer molecular subtypes. Clin. Cancer Res. 2013, 19, 5533–5540. [Google Scholar] [CrossRef] [PubMed]
- Longley, D.B.; Johnston, P.G. Molecular mechanisms of drug resistance. J. Pathol. 2005, 205, 275–292. [Google Scholar] [CrossRef] [PubMed]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef]
- von Minckwitz, G.; Untch, M.; Blohmer, J.U.; Costa, S.D.; Eidtmann, H.; Fasching, P.A.; Gerber, B.; Eiermann, W.; Hilfrich, J.; Huober, J.; et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J. Clin. Oncol. 2012, 30, 1796–1804. [Google Scholar] [CrossRef]
- Liedtke, C.; Mazouni, C.; Hess, K.R.; André, F.; Tordai, A.; Mejia, J.A.; Symmans, W.F.; Gonzalez-Angulo, A.M.; Hennessy, B.; Green, M.; et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J. Clin. Oncol. 2008, 26, 1275–1281. [Google Scholar] [CrossRef]
- Bai, X.; Ni, J.; Beretov, J.; Graham, P.; Li, Y. Triple-negative breast cancer therapeutic resistance: Where is the Achilles’ heel? Cancer Lett. 2021, 497, 100–111. [Google Scholar] [CrossRef]
- Hudis, C.A.; Gianni, L. Triple-negative breast cancer: An unmet medical need. Oncologist 2011, 16 (Suppl. 1), 1–11. [Google Scholar] [CrossRef]
- Cheang, M.C.; Voduc, D.; Bajdik, C.; Leung, S.; McKinney, S.; Chia, S.K.; Perou, C.M.; Nielsen, T.O. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin. Cancer Res. 2008, 14, 1368–1376. [Google Scholar] [CrossRef]
- Howlader, N.; Cronin, K.A.; Kurian, A.W.; Andridge, R. Differences in Breast Cancer Survival by Molecular Subtypes in the United States. Cancer Epidemiol. Biomark. Prev. 2018, 27, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Tseng, L.M.; Hsu, N.C.; Chen, S.C.; Lu, Y.S.; Lin, C.H.; Chang, D.Y.; Li, H.; Lin, Y.C.; Chang, H.K.; Chao, T.C.; et al. Distant metastasis in triple-negative breast cancer. Neoplasma 2013, 60, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B.; Dignam, J.; Bryant, J.; DeCillis, A.; Wickerham, D.L.; Wolmark, N.; Costantino, J.; Redmond, C.; Fisher, E.R.; Bowman, D.M.; et al. Five versus more than five years of tamoxifen therapy for breast cancer patients with negative lymph nodes and estrogen receptor-positive tumors. J. Natl. Cancer Inst. 1996, 88, 1529–1542. [Google Scholar] [CrossRef] [PubMed]
- Dahabreh, I.J.; Linardou, H.; Siannis, F.; Fountzilas, G.; Murray, S. Trastuzumab in the adjuvant treatment of early-stage breast cancer: A systematic review and meta-analysis of randomized controlled trials. Oncologist 2008, 13, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Kiess, A.P.; McArthur, H.L.; Mahoney, K.; Patil, S.; Morris, P.G.; Ho, A.; Hudis, C.A.; McCormick, B. Adjuvant trastuzumab reduces locoregional recurrence in women who receive breast-conservation therapy for lymph node-negative, human epidermal growth factor receptor 2-positive breast cancer. Cancer 2012, 118, 1982–1988. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, S.; Israel, H.P.; Yan, S.X.; Horowitz, D.P.; Crockford, S.; Gidea-Addeo, D.; Clifford Chao, K.S.; Kalinsky, K.; Connolly, E.P. Higher locoregional recurrence rate for triple-negative breast cancer following neoadjuvant chemotherapy, surgery and radiotherapy. SpringerPlus 2015, 4, 386. [Google Scholar] [CrossRef]
- Sissung, T.M.; Baum, C.E.; Kirkland, C.T.; Gao, R.; Gardner, E.R.; Figg, W.D. Pharmacogenetics of membrane transporters: An update on current approaches. Mol. Biotechnol. 2010, 44, 152–167. [Google Scholar] [CrossRef]
- Yamada, A.; Ishikawa, T.; Ota, I.; Kimura, M.; Shimizu, D.; Tanabe, M.; Chishima, T.; Sasaki, T.; Ichikawa, Y.; Morita, S.; et al. High expression of ATP-binding cassette transporter ABCC11 in breast tumors is associated with aggressive subtypes and low disease-free survival. Breast Cancer Res. Treat. 2013, 137, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhao, Z.; Wang, K.; Zhou, H.; Xing, C. Expression of aldehyde dehydrogenase 1 and ATP-binding cassette superfamily G member 2 is enhanced in primary foci and metastatic lymph node from patients with triple-negative breast cancer. Biomed. Res. 2017, 28, 5078–5083. [Google Scholar]
- Das, S.; Samant, R.S.; Shevde, L.A. Nonclassical activation of Hedgehog signaling enhances multidrug resistance and makes cancer cells refractory to Smoothened-targeting Hedgehog inhibition. J. Biol. Chem. 2013, 288, 11824–11833. [Google Scholar] [CrossRef]
- Britton, K.M.; Eyre, R.; Harvey, I.J.; Stemke-Hale, K.; Browell, D.; Lennard, T.W.J.; Meeson, A.P. Breast cancer, side population cells and ABCG2 expression. Cancer Lett. 2012, 323, 97–105. [Google Scholar] [CrossRef]
- Arumugam, A.; Subramani, R.; Nandy, S.B.; Terreros, D.; Dwivedi, A.K.; Saltzstein, E.; Lakshmanaswamy, R. Silencing growth hormone receptor inhibits estrogen receptor negative breast cancer through ATP-binding cassette sub-family G member 2. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Guestini, F.; Ono, K.; Miyashita, M.; Ishida, T.; Ohuchi, N.; Nakagawa, S.; Hirakawa, H.; Tamaki, K.; Ohi, Y.; Rai, Y.; et al. Impact of Topoisomerase IIα, PTEN, ABCC1/MRP1, and KI67 on triple-negative breast cancer patients treated with neoadjuvant chemotherapy. Breast Cancer Res. Treat. 2019, 173, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Westover, D.; Ling, X.; Lam, H.; Welch, J.; Jin, C.; Gongora, C.; Del Rio, M.; Wani, M.; Li, F. FL118, a novel camptothecin derivative, is insensitive to ABCG2 expression and shows improved efficacy in comparison with irinotecan in colon and lung cancer models with ABCG2-induced resistance. Mol. Cancer 2015, 14, 92. [Google Scholar] [CrossRef]
- Ling, X.; Liu, X.; Zhong, K.; Smith, N.; Prey, J.; Li, F. FL118, a novel camptothecin analogue, overcomes irinotecan and topotecan resistance in human tumor xenograft models. Am. J. Transl. Res. 2015, 7, 1765–1781. [Google Scholar]
- Amiri-Kordestani, L.; Basseville, A.; Kurdziel, K.; Fojo, A.T.; Bates, S.E. Targeting MDR in breast and lung cancer: Discriminating its potential importance from the failure of drug resistance reversal studies. Drug Resist. Updates 2012, 15, 50–61. [Google Scholar] [CrossRef]
- Maréchal, A.; Zou, L. DNA damage sensing by the ATM and ATR kinases. Cold Spring Harb. Perspect. Biol. 2013, 5, a012716. [Google Scholar] [CrossRef]
- Sørensen, C.S.; Hansen, L.T.; Dziegielewski, J.; Syljuåsen, R.G.; Lundin, C.; Bartek, J.; Helleday, T. The cell-cycle checkpoint kinase Chk1 is required for mammalian homologous recombination repair. Nat. Cell Biol. 2005, 7, 195–201. [Google Scholar] [CrossRef]
- Loibl, S.; Weber, K.E.; Timms, K.M.; Elkin, E.P.; Hahnen, E.; Fasching, P.A.; Lederer, B.; Denkert, C.; Schneeweiss, A.; Braun, S.; et al. Survival analysis of carboplatin added to an anthracycline/taxane-based neoadjuvant chemotherapy and HRD score as predictor of response—final results from GeparSixto. Ann. Oncol. 2018, 29, 2341–2347. [Google Scholar] [CrossRef]
- Meyer, F.; Becker, S.; Classen, S.; Parplys, A.C.; Mansour, W.Y.; Riepen, B.; Timm, S.; Ruebe, C.; Jasin, M.; Wikman, H.; et al. Prevention of DNA Replication Stress by CHK1 Leads to Chemoresistance Despite a DNA Repair Defect in Homologous Recombination in Breast Cancer. Cells 2020, 9, 238. [Google Scholar] [CrossRef]
- Tu, X.; Kahila, M.M.; Zhou, Q.; Yu, J.; Kalari, K.R.; Wang, L.; Harmsen, W.S.; Yuan, J.; Boughey, J.C.; Goetz, M.P.; et al. ATR Inhibition Is a Promising Radiosensitizing Strategy for Triple-Negative Breast Cancer. Mol. Cancer Ther. 2018, 17, 2462–2472. [Google Scholar] [CrossRef]
- Kunkel, T.A.; Erie, D.A. DNA MISMATCH REPAIR. Annu. Rev. Biochem. 2005, 74, 681–710. [Google Scholar] [CrossRef]
- Marcus, L.; Lemery, S.J.; Keegan, P.; Pazdur, R. FDA Approval Summary: Pembrolizumab for the Treatment of Microsatellite Instability-High Solid Tumors. Clin. Cancer Res. 2019, 25, 3753. [Google Scholar] [CrossRef]
- Hou, Y.; Nitta, H.; Parwani, A.V.; Li, Z. PD-L1 and CD8 are associated with deficient mismatch repair status in triple-negative and HER2-positive breast cancers. Hum. Pathol. 2019, 86, 108–114. [Google Scholar] [CrossRef]
- Daniels, D.S.; Woo, T.T.; Luu, K.X.; Noll, D.M.; Clarke, N.D.; Pegg, A.E.; Tainer, J.A. DNA binding and nucleotide flipping by the human DNA repair protein AGT. Nat. Struct. Mol. Biol. 2004, 11, 714–720. [Google Scholar] [CrossRef]
- Herman, J.G.; Baylin, S.B. Gene silencing in cancer in association with promoter hypermethylation. N. Engl. J. Med. 2003, 349, 2042–2054. [Google Scholar] [CrossRef]
- Hou, P.; Ji, M.; Yang, B.; Chen, Z.; Qiu, J.; Shi, X.; Lu, Z. Quantitative analysis of promoter hypermethylation in multiple genes in osteosarcoma. Cancer 2006, 106, 1602–1609. [Google Scholar] [CrossRef]
- Kim, J.I.; Suh, J.T.; Choi, K.U.; Kang, H.J.; Shin, D.H.; Lee, I.S.; Moon, T.Y.; Kim, W.T. Inactivation of O6-methylguanine-DNA methyltransferase in soft tissue sarcomas: Association with K-ras mutations. Hum. Pathol. 2009, 40, 934–941. [Google Scholar] [CrossRef]
- Busch, C.; Geisler, J.; Lillehaug, J.R.; Lønning, P.E. MGMT expression levels predict disease stabilisation, progression-free and overall survival in patients with advanced melanomas treated with DTIC. Eur. J. Cancer 2010, 46, 2127–2133. [Google Scholar] [CrossRef]
- Glas, M.; Happold, C.; Rieger, J.; Wiewrodt, D.; Bähr, O.; Steinbach, J.P.; Wick, W.; Kortmann, R.-D.; Reifenberger, G.; Weller, M.; et al. Long-Term Survival of Patients With Glioblastoma Treated With Radiotherapy and Lomustine Plus Temozolomide. Biochem. Pharmacol. 2009, 27, 1257–1261. [Google Scholar] [CrossRef]
- Dunn, J.; Baborie, A.; Alam, F.; Joyce, K.; Moxham, M.; Sibson, R.; Crooks, D.; Husband, D.; Shenoy, A.; Brodbelt, A.; et al. Extent of MGMT promoter methylation correlates with outcome in glioblastomas given temozolomide and radiotherapy. Br. J. Cancer. 2009, 101, 124–131. [Google Scholar] [CrossRef]
- Kulke, M.H.; Hornick, J.L.; Frauenhoffer, C.; Hooshmand, S.; Ryan, D.P.; Enzinger, P.C.; Meyerhardt, J.A.; Clark, J.W.; Stuart, K.; Fuchs, C.S.; et al. O6-methylguanine DNA methyltransferase deficiency and response to temozolomide-based therapy in patients with neuroendocrine tumors. Clin. Cancer Res. 2009, 15, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, C.; Pruneri, G.; Possanzini, P.; Manzotti, M.; Barile, M.; Feroce, I.; Colleoni, M.; Bonanni, B.; Maisonneuve, P.; Radice, P.; et al. Methylation of O 6-methylguanine-DNA methyltransferase (MGMT) promoter gene in triple-negative breast cancer patients. Breast Cancer Res. Treat. 2012, 134, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Raguz, S.; Adams, C.; Masrour, N.; Rasul, S.; Papoutsoglou, P.; Hu, Y.; Cazzanelli, G.; Zhou, Y.; Patel, N.; Coombes, C.; et al. Loss of O⁶-methylguanine-DNA methyltransferase confers collateral sensitivity to carmustine in topoisomerase II-mediated doxorubicin resistant triple negative breast cancer cells. Biochem. Pharmacol. 2013, 85, 186–196. [Google Scholar] [CrossRef][Green Version]
- Lee, K.M.; Giltnane, J.M.; Balko, J.M.; Schwarz, L.J.; Guerrero-Zotano, A.L.; Hutchinson, K.E.; Nixon, M.J.; Estrada, M.V.; Sánchez, V.; Sanders, M.E.; et al. MYC and MCL1 Cooperatively Promote Chemotherapy-Resistant Breast Cancer Stem Cells via Regulation of Mitochondrial Oxidative Phosphorylation. Cell Metab. 2017, 26, 633–647.e637. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Fahrmann, J.F.; Lee, H.; Li, Y.-J.; Tripathi, S.C.; Yue, C.; Zhang, C.; Lifshitz, V.; Song, J.; Yuan, Y.; et al. JAK/STAT3-Regulated Fatty Acid β-Oxidation Is Critical for Breast Cancer Stem Cell Self-Renewal and Chemoresistance. Cell Metab. 2018, 27, 136–150.e135. [Google Scholar] [CrossRef]
- Carracedo, A.; Weiss, D.; Leliaert, A.K.; Bhasin, M.; de Boer, V.C.; Laurent, G.; Adams, A.C.; Sundvall, M.; Song, S.J.; Ito, K.; et al. A metabolic prosurvival role for PML in breast cancer. J. Clin. Investig. 2012, 122, 3088–3100. [Google Scholar] [CrossRef]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef]
- Lim, S.O.; Li, C.W.; Xia, W.; Lee, H.H.; Chang, S.S.; Shen, J.; Hsu, J.L.; Raftery, D.; Djukovic, D.; Gu, H.; et al. EGFR Signaling Enhances Aerobic Glycolysis in Triple-Negative Breast Cancer Cells to Promote Tumor Growth and Immune Escape. Cancer Res. 2016, 76, 1284–1296. [Google Scholar] [CrossRef]
- Vera-Ramirez, L.; Sanchez-Rovira, P.; Ramirez-Tortosa, M.C.; Ramirez-Tortosa, C.L.; Granados-Principal, S.; Lorente, J.A.; Quiles, J.L. Free radicals in breast carcinogenesis, breast cancer progression and cancer stem cells. Biological bases to develop oxidative-based therapies. Crit. Rev. Oncol. Hematol. 2011, 80, 347–368. [Google Scholar] [CrossRef]
- Koo, J.S.; Jung, W. Alteration of REDD1-Mediated Mammalian Target of Rapamycin Pathway and Hypoxia-Inducible Factor-1α Regulation in Human Breast Cancer. Pathobiology 2010, 77, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Kim, H.P.; Jin, Y.; Choi, A.M.; Ryter, S.W. Beclin 1 deficiency is associated with increased hypoxia-induced angiogenesis. Autophagy 2011, 7, 829–839. [Google Scholar] [CrossRef]
- O’Reilly, E.A.; Gubbins, L.; Sharma, S.; Tully, R.; Guang, M.H.; Weiner-Gorzel, K.; McCaffrey, J.; Harrison, M.; Furlong, F.; Kell, M.; et al. The fate of chemoresistance in triple negative breast cancer (TNBC). BBA Clin. 2015, 3, 257–275. [Google Scholar] [CrossRef]
- Notte, A.; Ninane, N.; Arnould, T.; Michiels, C. Hypoxia counteracts taxol-induced apoptosis in MDA-MB-231 breast cancer cells: Role of autophagy and JNK activation. Cell Death Dis. 2013, 4, e638. [Google Scholar] [CrossRef]
- Liu, M.-q.; Chen, Z.; Chen, L.-x. Endoplasmic reticulum stress: A novel mechanism and therapeutic target for cardiovascular diseases. Acta Pharmacol. Sin. 2016, 37, 425–443. [Google Scholar] [CrossRef]
- Bernardi, R.; Gianni, L. Hallmarks of triple negative breast cancer emerging at last? Cell Res. 2014, 24, 904–905. [Google Scholar] [CrossRef]
- Martinez-Outschoorn, U.E.; Pavlides, S.; Howell, A.; Pestell, R.G.; Tanowitz, H.B.; Sotgia, F.; Lisanti, M.P. Stromal-epithelial metabolic coupling in cancer: Integrating autophagy and metabolism in the tumor microenvironment. Int. J. Biochem. Cell Biol. 2011, 43, 1045–1051. [Google Scholar] [CrossRef]
- Gluz, O.; Liedtke, C.; Gottschalk, N.; Pusztai, L.; Nitz, U.; Harbeck, N. Triple-negative breast cancer--current status and future directions. Ann. Oncol. 2009, 20, 1913–1927. [Google Scholar] [CrossRef]
- Wu, X.; Baig, A.; Kasymjanova, G.; Kafi, K.; Holcroft, C.; Mekouar, H.; Carbonneau, A.; Bahoric, B.; Sultanem, K.; Muanza, T. Pattern of Local Recurrence and Distant Metastasis in Breast Cancer By Molecular Subtype. Cureus 2016, 8, e924. [Google Scholar] [CrossRef]
- Zhang, F.L.; Cao, J.L.; Xie, H.Y.; Sun, R.; Yang, L.F.; Shao, Z.M.; Li, D.Q. Cancer-Associated MORC2-Mutant M276I Regulates an hnRNPM-Mediated CD44 Splicing Switch to Promote Invasion and Metastasis in Triple-Negative Breast Cancer. Cancer Res. 2018, 78, 5780–5792. [Google Scholar] [CrossRef]
- Neuzillet, C.; Tijeras-Raballand, A.; Cohen, R.; Cros, J.; Faivre, S.; Raymond, E.; de Gramont, A. Targeting the TGFβ pathway for cancer therapy. Pharmacol. Ther. 2015, 147, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Asiedu, M.K.; Ingle, J.N.; Behrens, M.D.; Radisky, D.C.; Knutson, K.L. TGFbeta/TNF(alpha)-mediated epithelial-mesenchymal transition generates breast cancer stem cells with a claudin-low phenotype. Cancer Res. 2011, 71, 4707–4719. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Samanta, D.; Xiang, L.; Zhang, H.; Hu, H.; Chen, I.; Bullen, J.W.; Semenza, G.L. Chemotherapy triggers HIF-1-dependent glutathione synthesis and copper chelation that induces the breast cancer stem cell phenotype. Proc. Natl. Acad. Sci. USA 2015, 112, E4600–E4609. [Google Scholar] [CrossRef] [PubMed]
- Cazet, A.S.; Hui, M.N.; Elsworth, B.L.; Wu, S.Z.; Roden, D.; Chan, C.-L.; Skhinas, J.N.; Collot, R.; Yang, J.; Harvey, K.; et al. Targeting stromal remodeling and cancer stem cell plasticity overcomes chemoresistance in triple negative breast cancer. Nat. Commun. 2018, 9, 2897. [Google Scholar] [CrossRef]
- Bai, X.; Ni, J.; Beretov, J.; Graham, P.; Li, Y. Cancer stem cell in breast cancer therapeutic resistance. Cancer Treat. Rev. 2018, 69, 152–163. [Google Scholar] [CrossRef]
- Tsai, C.H.; Chiu, J.H.; Yang, C.W.; Wang, J.Y.; Tsai, Y.F.; Tseng, L.M.; Chen, W.S.; Shyr, Y.M. Molecular characteristics of recurrent triple-negative breast cancer. Mol. Med. Rep. 2015, 12, 7326–7334. [Google Scholar] [CrossRef]
- Patel, S.; Alam, A.; Pant, R.; Chattopadhyay, S. Wnt Signaling and Its Significance Within the Tumor Microenvironment: Novel Therapeutic Insights. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- MacDonagh, L.; Gray, S.G.; Breen, E.; Cuffe, S.; Finn, S.P.; O’Byrne, K.J.; Barr, M.P. Lung cancer stem cells: The root of resistance. Cancer Lett. 2016, 372, 147–156. [Google Scholar] [CrossRef]
- Qu, H.; Li, R.; Liu, Z.; Zhang, J.; Luo, R. Prognostic value of cancer stem cell marker CD133 expression in non-small cell lung cancer: A systematic review. Int. J. Clin. Exp. Pathol. 2013, 6, 2644–2650. [Google Scholar]
- Singh, A.; Settleman, J. EMT, cancer stem cells and drug resistance: An emerging axis of evil in the war on cancer. Oncogene 2010, 29, 4741–4751. [Google Scholar] [CrossRef]
- Chen, Z.; Fillmore, C.M.; Hammerman, P.S.; Kim, C.F.; Wong, K.K. Non-small-cell lung cancers: A heterogeneous set of diseases. Nat. Rev. Cancer 2014, 14, 535–546. [Google Scholar] [CrossRef]
- Testa, U.; Castelli, G.; Pelosi, E. Lung Cancers: Molecular Characterization, Clonal Heterogeneity and Evolution, and Cancer Stem Cells. Cancers 2018, 10, 248. [Google Scholar] [CrossRef]
- Desai, A.; Yan, Y.; Gerson, S.L. Concise Reviews: Cancer Stem Cell Targeted Therapies: Toward Clinical Success. Stem Cells Transl. Med. 2019, 8, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Creighton, C.J.; Li, X.; Landis, M.; Dixon, J.M.; Neumeister, V.M.; Sjolund, A.; Rimm, D.L.; Wong, H.; Rodriguez, A.; Herschkowitz, J.I.; et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc. Natl. Acad. Sci. USA 2009, 106, 13820–13825. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, N.; Satar, N.A.; Abu Halim, N.H.; Ngalim, S.H.; Yusoff, N.M.; Lin, J.; Yahaya, B.H. Targeting Lung Cancer Stem Cells: Research and Clinical Impacts. Front. Oncol. 2017, 7, 80. [Google Scholar] [CrossRef] [PubMed]
- Leon, G.; MacDonagh, L.; Finn, S.P.; Cuffe, S.; Barr, M.P. Cancer stem cells in drug resistant lung cancer: Targeting cell surface markers and signaling pathways. Pharmacol. Ther. 2016, 158, 71–90. [Google Scholar] [CrossRef] [PubMed]
- Suresh, R.; Ali, S.; Ahmad, A.; Philip, P.A.; Sarkar, F.H. The Role of Cancer Stem Cells in Recurrent and Drug-Resistant Lung Cancer. Adv. Exp. Med. Biol. 2016, 890, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Chellappan, S. Lung cancer stem cells: Molecular features and therapeutic targets. Mol. Asp. Med. 2014, 39, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Gottschling, S.; Schnabel, P.A.; Herth, F.J.; Herpel, E. Are we missing the target? Cancer stem cells and drug resistance in non-small cell lung cancer. Cancer Genom. Proteom. 2012, 9, 275–286. [Google Scholar]
- Shibue, T.; Weinberg, R.A. EMT, CSCs, and drug resistance: The mechanistic link and clinical implications. Nat. Rev. Clin. Oncol. 2017, 14, 611–629. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Chen, I.; Shimoda, L.A.; Park, Y.; Zhang, C.; Tran, L.; Zhang, H.; Semenza, G.L. Chemotherapy-Induced Ca(2+) Release Stimulates Breast Cancer Stem Cell Enrichment. Cell Rep. 2017, 18, 1946–1957. [Google Scholar] [CrossRef] [PubMed]
- Kurtova, A.V.; Xiao, J.; Mo, Q.; Pazhanisamy, S.; Krasnow, R.; Lerner, S.P.; Chen, F.; Roh, T.T.; Lay, E.; Ho, P.L.; et al. Blocking PGE2-induced tumour repopulation abrogates bladder cancer chemoresistance. Nature 2015, 517, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Lee, H.E.; Li, H.; Shipitsin, M.; Gelman, R.; Polyak, K. Heterogeneity for stem cell-related markers according to tumor subtype and histologic stage in breast cancer. Clin. Cancer Res. 2010, 16, 876–887. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Li, H.; Wang, H.; Shi, X.; Fan, Y.; Ding, X.; Lin, C.; Zhan, Q.; Qian, H.; Xu, B. Enriched CD44(+)/CD24(-) population drives the aggressive phenotypes presented in triple-negative breast cancer (TNBC). Cancer Lett. 2014, 353, 153–159. [Google Scholar] [CrossRef]
- Bhola, N.E.; Balko, J.M.; Dugger, T.C.; Kuba, M.G.; Sánchez, V.; Sanders, M.; Stanford, J.; Cook, R.S.; Arteaga, C.L. TGF-β inhibition enhances chemotherapy action against triple-negative breast cancer. J. Clin. Investig. 2013, 123, 1348–1358. [Google Scholar] [CrossRef]
- Samanta, D.; Gilkes, D.M.; Chaturvedi, P.; Xiang, L.; Semenza, G.L. Hypoxia-inducible factors are required for chemotherapy resistance of breast cancer stem cells. Proc. Natl. Acad. Sci. USA 2014, 111, E5429–E5438. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Lee, H.J.; Saha, S.; Ruan, D.; Guo, H.; Chan, C.H. Inhibition of USP2 eliminates cancer stem cells and enhances TNBC responsiveness to chemotherapy. Cell Death Dis. 2019, 10, 285. [Google Scholar] [CrossRef]
- Maas, S.L.N.; Breakefield, X.O.; Weaver, A.M. Extracellular Vesicles: Unique Intercellular Delivery Vehicles. Trends Cell Biol. 2017, 27, 172–188. [Google Scholar] [CrossRef]
- Maji, S.; Chaudhary, P.; Akopova, I.; Nguyen, P.M.; Hare, R.J.; Gryczynski, I.; Vishwanatha, J.K. Exosomal Annexin II Promotes Angiogenesis and Breast Cancer Metastasis. Mol. Cancer Res. MCR 2017, 15, 93–105. [Google Scholar] [CrossRef]
- Ozawa, P.M.M.; Alkhilaiwi, F.; Cavalli, I.J.; Malheiros, D.; de Souza Fonseca Ribeiro, E.M.; Cavalli, L.R. Extracellular vesicles from triple-negative breast cancer cells promote proliferation and drug resistance in non-tumorigenic breast cells. Breast Cancer Res. Treat. 2018, 172, 713–723. [Google Scholar] [CrossRef]
- Kavanagh, E.L.; Lindsay, S.; Halasz, M.; Gubbins, L.C.; Weiner-Gorzel, K.; Guang, M.H.Z.; McGoldrick, A.; Collins, E.; Henry, M.; Blanco-Fernández, A.; et al. Protein and chemotherapy profiling of extracellular vesicles harvested from therapeutic induced senescent triple negative breast cancer cells. Oncogenesis 2017, 6, e388. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.-m.; Zhu, X.-y.; Chen, W.-x.; Zhong, S.-l.; Hu, Q.; Ma, T.-f.; Zhang, J.; Chen, L.; Tang, J.-h.; Zhao, J.-h. Exosomes mediate drug resistance transfer in MCF-7 breast cancer cells and a probable mechanism is delivery of P-glycoprotein. Tumor Biol. 2014, 35, 10773–10779. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, C.; Rani, S.; O’Brien, K.; O’Neill, A.; Prencipe, M.; Sheikh, R.; Webb, G.; McDermott, R.; Watson, W.; Crown, J.; et al. Docetaxel-resistance in prostate cancer: Evaluating associated phenotypic changes and potential for resistance transfer via exosomes. PLoS ONE 2012, 7, e50999. [Google Scholar] [CrossRef]
- Peinado, H.; Lavotshkin, S.; Lyden, D. The secreted factors responsible for pre-metastatic niche formation: Old sayings and new thoughts. Semin. Cancer Biol. 2011, 21, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Grange, C.; Tapparo, M.; Collino, F.; Vitillo, L.; Damasco, C.; Deregibus, M.C.; Tetta, C.; Bussolati, B.; Camussi, G. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res. 2011, 71, 5346–5356. [Google Scholar] [CrossRef] [PubMed]
- Castellana, D.; Zobairi, F.; Martinez, M.C.; Panaro, M.A.; Mitolo, V.; Freyssinet, J.M.; Kunzelmann, C. Membrane microvesicles as actors in the establishment of a favorable prostatic tumoral niche: A role for activated fibroblasts and CX3CL1-CX3CR1 axis. Cancer Res. 2009, 69, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Wysoczynski, M.; Ratajczak, M.Z. Lung cancer secreted microvesicles: Underappreciated modulators of microenvironment in expanding tumors. Int. J. Cancer 2009, 125, 1595–1603. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, S.; Guo, C.; Zong, J.; Sun, M.Z. The association of annexin A2 and cancers. Clin. Transl. Oncol. 2012, 14, 634–640. [Google Scholar] [CrossRef]
- Lokman, N.A.; Ween, M.P.; Oehler, M.K.; Ricciardelli, C. The role of annexin A2 in tumorigenesis and cancer progression. Cancer Microenviron. 2011, 4, 199–208. [Google Scholar] [CrossRef]
- Jeon, Y.R.; Kim, S.Y.; Lee, E.J.; Kim, Y.N.; Noh, D.-Y.; Park, S.Y.; Moon, A. Identification of annexin II as a novel secretory biomarker for breast cancer. Proteomics 2013, 13, 3145–3156. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.J.; Kalra, H.; Mathivanan, S. ExoCarta as a resource for exosomal research. J. Extracell Vesicles 2012, 1. [Google Scholar] [CrossRef]
- Yuan, X.; Qian, N.; Ling, S.; Li, Y.; Sun, W.; Li, J.; Du, R.; Zhong, G.; Liu, C.; Yu, G.; et al. Breast cancer exosomes contribute to pre-metastatic niche formation and promote bone metastasis of tumor cells. Theranostics 2021, 11, 1429–1445. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Xie, J.; Zhang, M.; Zhao, Z.; Wan, Y.; Yao, Y. miRNA-21 promotes proliferation and invasion of triple-negative breast cancer cells through targeting PTEN. Am. J. Transl. Res. 2017, 9, 953–961. [Google Scholar] [PubMed]
- Dong, G.; Liang, X.; Wang, D.; Gao, H.; Wang, L.; Wang, L.; Liu, J.; Du, Z. High expression of miR-21 in triple-negative breast cancers was correlated with a poor prognosis and promoted tumor cell in vitro proliferation. Med. Oncol. 2014, 31, 57. [Google Scholar] [CrossRef] [PubMed]
- Krichevsky, A.M.; Gabriely, G. miR-21: A small multi-faceted RNA. J. Cell Mol. Med. 2009, 13, 39–53. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, C.; Xie, Y.; Tang, M.; Luo, G.; Chen, X.; Tian, L.; Yu, X. Lung Cancer Cells Derived Circulating miR-21 Promotes Differentiation of Monocytes into Osteoclasts. OncoTargets Ther. 2020, 13, 2643–2656. [Google Scholar] [CrossRef]
- Sugatani, T.; Vacher, J.; Hruska, K.A. A microRNA expression signature of osteoclastogenesis. Blood 2011, 117, 3648–3657. [Google Scholar] [CrossRef]
- Kagiya, T. MicroRNAs: Potential Biomarkers and Therapeutic Targets for Alveolar Bone Loss in Periodontal Disease. Int. J. Mol. Sci. 2016, 17, 1317. [Google Scholar] [CrossRef]
- Chen, Y.; Knösel, T.; Kristiansen, G.; Pietas, A.; Garber, M.E.; Matsuhashi, S.; Ozaki, I.; Petersen, I. Loss of PDCD4 expression in human lung cancer correlates with tumour progression and prognosis. J. Pathol. 2003, 200, 640–646. [Google Scholar] [CrossRef]
- Wen, Y.H.; Shi, X.; Chiriboga, L.; Matsahashi, S.; Yee, H.; Afonja, O. Alterations in the expression of PDCD4 in ductal carcinoma of the breast. Oncol. Rep. 2007, 18, 1387–1393. [Google Scholar] [CrossRef][Green Version]
- Wei, N.; Liu, S.S.; Leung, T.H.Y.; Tam, K.F.; Liao, X.Y.; Cheung, A.N.Y.; Chan, K.K.L.; Ngan, H.Y.S. Loss of Programmed cell death 4 (Pdcd4) associates with the progression of ovarian cancer. Mol. Cancer 2009, 8, 70. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Wang, Q.; Lei, Z.; Feng, M.; Zhao, Z.; Wang, Y.; Wei, G. DTL promotes cancer progression by PDCD4 ubiquitin-dependent degradation. J. Exp. Clin. Cancer Res. 2019, 38, 350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, J.; Jiang, Y.; Xu, Y.; Qin, C. Programmed cell death 4 (PDCD4) suppresses metastastic potential of human hepatocellular carcinoma cells. J. Exp. Clin. Cancer Res. 2009, 28, 71. [Google Scholar] [CrossRef] [PubMed]
- Nieves-Alicea, R.; Colburn, N.H.; Simeone, A.-M.; Tari, A.M. Programmed cell death 4 inhibits breast cancer cell invasion by increasing tissue inhibitor of metalloproteinases-2 expression. Breast Cancer Res. Treat. 2009, 114, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Nieves-Alicea, R.; McMurtry, V.; Simeone, A.-M.; Colburn, N.; Tari, A. Programmed Cell Death 4, a potential novel suppressor of breast cancer bone metastasis. Cancer Res. 2008, 68, 3676. [Google Scholar]
- Santhanam, A.N.; Baker, A.R.; Hegamyer, G.; Kirschmann, D.; Colburn, N.H. PDCD4 repression of lysyl oxidase inhibits hypoxia-induced breast cancer cell invasion. Oncogene 2010, 29, 3921–3932. [Google Scholar] [CrossRef]
- Wu, H.; Wang, Q.; Zhong, H.; Li, L.; Zhang, Q.; Huang, Q.; Yu, Z. Differentially expressed microRNAs in exosomes of patients with breast cancer revealed by next-generation sequencing. Oncol. Rep. 2020, 43, 240–250. [Google Scholar] [CrossRef]
- Atchley, D.P.; Albarracin, C.T.; Lopez, A.; Valero, V.; Amos, C.I.; Gonzalez-Angulo, A.M.; Hortobagyi, G.N.; Arun, B.K. Clinical and pathologic characteristics of patients with BRCA-positive and BRCA-negative breast cancer. J. Clin. Oncol. 2008, 26, 4282–4288. [Google Scholar] [CrossRef]
- Moynahan, M.E.; Chiu, J.W.; Koller, B.H.; Jasin, M. Brca1 controls homology-directed DNA repair. Mol. Cell. 1999, 4, 511–518. [Google Scholar] [CrossRef]
- Anwar, M.; Aslam, H.M.; Anwar, S. PARP inhibitors. Hered. Cancer Clin. Pract. 2015, 13, 4. [Google Scholar] [CrossRef]
- Ashworth, A.; Lord, C.J. Synthetic lethal therapies for cancer: What’s next after PARP inhibitors? Nat. Rev. Clin. Oncol. 2018, 15, 564–576. [Google Scholar] [CrossRef] [PubMed]
- Bryant, H.E.; Schultz, N.; Thomas, H.D.; Parker, K.M.; Flower, D.; Lopez, E.; Kyle, S.; Meuth, M.; Curtin, N.J.; Helleday, T. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 2005, 434, 913–917. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Liu, F.; Song, Y. Progress: Targeted Therapy, Immunotherapy, and New Chemotherapy Strategies in Advanced Triple-Negative Breast Cancer. Cancer Manag. Res. 2020, 12, 9375–9387. [Google Scholar] [CrossRef]
- Robson, M.; Im, S.A.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; Delaloge, S.; Li, W.; Tung, N.; Armstrong, A.; et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N. Engl. J. Med. 2017, 377, 523–533. [Google Scholar] [CrossRef]
- Rodler, E.T.; Kurland, B.F.; Griffin, M.; Gralow, J.R.; Porter, P.; Yeh, R.F.; Gadi, V.K.; Guenthoer, J.; Beumer, J.H.; Korde, L.; et al. Phase I Study of Veliparib (ABT-888) Combined with Cisplatin and Vinorelbine in Advanced Triple-Negative Breast Cancer and/or BRCA Mutation–Associated Breast Cancer. Clin. Cancer Res. 2016, 22, 2855. [Google Scholar] [CrossRef]
- Bergers, G.; Hanahan, D. Modes of resistance to anti-angiogenic therapy. Nat. Rev. Cancer 2008, 8, 592–603. [Google Scholar] [CrossRef]
- Mohammed, R.A.A.; Ellis, I.O.; Mahmmod, A.M.; Hawkes, E.C.; Green, A.R.; Rakha, E.A.; Martin, S.G. Lymphatic and blood vessels in basal and triple-negative breast cancers: Characteristics and prognostic significance. Mod. Pathol. 2011, 24, 774–785. [Google Scholar] [CrossRef]
- Linderholm, B.K.; Hellborg, H.; Johansson, U.; Elmberger, G.; Skoog, L.; Lehtiö, J.; Lewensohn, R. Significantly higher levels of vascular endothelial growth factor (VEGF) and shorter survival times for patients with primary operable triple-negative breast cancer. Ann. Oncol. 2009, 20, 1639–1646. [Google Scholar] [CrossRef]
- Hutchinson, L. Breast cancer: BEATRICE bevacizumab trial--every cloud has a silver lining. Nat. Rev. Clin. Oncol. 2013, 10, 548. [Google Scholar] [CrossRef]
- Miles, D.W.; Diéras, V.; Cortés, J.; Duenne, A.A.; Yi, J.; O’Shaughnessy, J. First-line bevacizumab in combination with chemotherapy for HER2-negative metastatic breast cancer: Pooled and subgroup analyses of data from 2447 patients. Ann. Oncol. 2013, 24, 2773–2780. [Google Scholar] [CrossRef]
- Miller, K.; Wang, M.; Gralow, J.; Dickler, M.; Cobleigh, M.; Perez, E.A.; Shenkier, T.; Cella, D.; Davidson, N.E. Paclitaxel plus Bevacizumab versus Paclitaxel Alone for Metastatic Breast Cancer. N. Engl. J. Med. 2007, 357, 2666–2676. [Google Scholar] [CrossRef]
- Saloustros, E.; Nikolaou, M.; Kalbakis, K.; Polyzos, A.; Christofillakis, C.; Kentepozidis, N.; Pistamaltzian, N.; Kourousis, C.; Vamvakas, L.; Georgoulias, V.; et al. Weekly Paclitaxel and Carboplatin Plus Bevacizumab as First-Line Treatment of Metastatic Triple-Negative Breast Cancer. A Multicenter Phase II Trial by the Hellenic Oncology Research Group. Clin. Breast Cancer 2018, 18, 88–94. [Google Scholar] [CrossRef]
- Symonds, L.; Linden, H.; Gadi, V.; Korde, L.; Rodler, E.; Gralow, J.; Redman, M.; Baker, K.; Wu, Q.V.; Jenkins, I.; et al. Combined Targeted Therapies for First-line Treatment of Metastatic Triple Negative Breast Cancer-A Phase II Trial of Weekly Nab-Paclitaxel and Bevacizumab Followed by Maintenance Targeted Therapy With Bevacizumab and Erlotinib. Clin. Breast Cancer 2019, 19, e283–e296. [Google Scholar] [CrossRef] [PubMed]
- Koboldt, D.C.; Fulton, R.S.; McLellan, M.D.; Schmidt, H.; Kalicki-Veizer, J.; McMichael, J.F.; Fulton, L.L.; Dooling, D.J.; Ding, L.; Mardis, E.R.; et al. Comprehensive molecular portraits of human breast tumours. Nature 2012, 490, 61–70. [Google Scholar] [CrossRef]
- Costa, R.L.B.; Han, H.S.; Gradishar, W.J. Targeting the PI3K/AKT/mTOR pathway in triple-negative breast cancer: A review. Breast Cancer Res. Treat. 2018, 169, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Castro, A.C.; Saura, C.; Barroso-Sousa, R.; Guo, H.; Ciruelos, E.; Bermejo, B.; Gavilá, J.; Serra, V.; Prat, A.; Paré, L.; et al. Phase 2 study of buparlisib (BKM120), a pan-class I PI3K inhibitor, in patients with metastatic triple-negative breast cancer. Breast Cancer Res. BCR 2020, 22, 120. [Google Scholar] [CrossRef] [PubMed]
- de Gooijer, M.C.; Zhang, P.; Buil, L.C.M.; Çitirikkaya, C.H.; Thota, N.; Beijnen, J.H.; van Tellingen, O. Buparlisib is a brain penetrable pan-PI3K inhibitor. Sci. Rep. 2018, 8, 10784. [Google Scholar] [CrossRef]
- Rodon, J.; Braña, I.; Siu, L.L.; De Jonge, M.J.; Homji, N.; Mills, D.; Di Tomaso, E.; Sarr, C.; Trandafir, L.; Massacesi, C.; et al. Phase I dose-escalation and -expansion study of buparlisib (BKM120), an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. Investig. New Drugs 2014, 32, 670–681. [Google Scholar] [CrossRef]
- Ibrahim, Y.H.; García-García, C.; Serra, V.; He, L.; Torres-Lockhart, K.; Prat, A.; Anton, P.; Cozar, P.; Guzmán, M.; Grueso, J.; et al. PI3K inhibition impairs BRCA1/2 expression and sensitizes BRCA-proficient triple-negative breast cancer to PARP inhibition. Cancer Discov. 2012, 2, 1036–1047. [Google Scholar] [CrossRef]
- Juvekar, A.; Burga, L.N.; Hu, H.; Lunsford, E.P.; Ibrahim, Y.H.; Balmañà, J.; Rajendran, A.; Papa, A.; Spencer, K.; Lyssiotis, C.A.; et al. Combining a PI3K inhibitor with a PARP inhibitor provides an effective therapy for BRCA1-related breast cancer. Cancer Discov. 2012, 2, 1048–1063. [Google Scholar] [CrossRef]
- Basho, R.K.; Gilcrease, M.; Murthy, R.K.; Helgason, T.; Karp, D.D.; Meric-Bernstam, F.; Hess, K.R.; Herbrich, S.M.; Valero, V.; Albarracin, C.; et al. Targeting the PI3K/AKT/mTOR Pathway for the Treatment of Mesenchymal Triple-Negative Breast Cancer: Evidence From a Phase 1 Trial of mTOR Inhibition in Combination With Liposomal Doxorubicin and Bevacizumab. JAMA Oncol. 2017, 3, 509–515. [Google Scholar] [CrossRef]
- Flax, H.; Newton, K.A.; Salih, H.; Hobbs, J.R. ARE SOME WOMEN’S BREAST CANCERS ANDROGEN DEPENDENT? Lancet 1973, 301, 1204–1207. [Google Scholar] [CrossRef]
- Won, K.-A.; Spruck, C. Triple-negative breast cancer therapy: Current and future perspectives (Review). Int. J. Oncol. 2020, 57, 1245–1261. [Google Scholar] [CrossRef]
- Bhattarai, S.; Klimov, S.; Mittal, K.; Krishnamurti, U.; Li, X.B.; Oprea-Ilies, G.; Wetherilt, C.S.; Riaz, A.; Aleskandarany, M.A.; Green, A.R.; et al. Prognostic Role of Androgen Receptor in Triple Negative Breast Cancer: A Multi-Institutional Study. Cancers 2019, 11, 995. [Google Scholar] [CrossRef]
- Chan, J.J.; Tan, T.J.Y.; Dent, R.A. Novel therapeutic avenues in triple-negative breast cancer: PI3K/AKT inhibition, androgen receptor blockade, and beyond. Ther. Adv. Med. Oncol. 2019, 11, 1758835919880429. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, G.R.; Adamo, B.; Ieni, A.; Licata, L.; Cardia, R.; Ferraro, G.; Franchina, T.; Tuccari, G.; Adamo, V. Androgen Receptor (AR), E-Cadherin, and Ki-67 as Emerging Targets and Novel Prognostic Markers in Triple-Negative Breast Cancer (TNBC) Patients. PLoS ONE 2015, 10, e0128368. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Bauer, J.A.; Schafer, J.M.; Pendleton, C.S.; Tang, L.; Johnson, K.C.; Chen, X.; Balko, J.M.; Gómez, H.; Arteaga, C.L.; et al. PIK3CA mutations in androgen receptor-positive triple negative breast cancer confer sensitivity to the combination of PI3K and androgen receptor inhibitors. Breast Cancer Res. 2014, 16, 406. [Google Scholar] [CrossRef] [PubMed]
- Gucalp, A.; Tolaney, S.; Isakoff, S.J.; Ingle, J.N.; Liu, M.C.; Carey, L.A.; Blackwell, K.; Rugo, H.; Nabell, L.; Forero, A.; et al. Phase II trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic Breast Cancer. Clin. Cancer Res. 2013, 19, 5505–5512. [Google Scholar] [CrossRef] [PubMed]
- Traina, T.A.; Miller, K.; Yardley, D.A.; Eakle, J.; Schwartzberg, L.S.; O’Shaughnessy, J.; Gradishar, W.; Schmid, P.; Winer, E.; Kelly, C.; et al. Enzalutamide for the Treatment of Androgen Receptor-Expressing Triple-Negative Breast Cancer. J. Clin. Oncol. 2018, 36, 884–890. [Google Scholar] [CrossRef]
- Taplin, M.E.; Montgomery, B.; Logothetis, C.J.; Bubley, G.J.; Richie, J.P.; Dalkin, B.L.; Sanda, M.G.; Davis, J.W.; Loda, M.; True, L.D.; et al. Intense androgen-deprivation therapy with abiraterone acetate plus leuprolide acetate in patients with localized high-risk prostate cancer: Results of a randomized phase II neoadjuvant study. J. Clin. Oncol. 2014, 32, 3705–3715. [Google Scholar] [CrossRef]
- Bonnefoi, H.; Grellety, T.; Tredan, O.; Saghatchian, M.; Dalenc, F.; Mailliez, A.; L’Haridon, T.; Cottu, P.; Abadie-Lacourtoisie, S.; You, B.; et al. A phase II trial of abiraterone acetate plus prednisone in patients with triple-negative androgen receptor positive locally advanced or metastatic breast cancer (UCBG 12-1). Ann. Oncol. 2016, 27, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Carey, A.M.; Pramanik, R.; Nicholson, L.J.; Dew, T.K.; Martin, F.L.; Muir, G.H.; Morris, J.D. Ras-MEK-ERK signaling cascade regulates androgen receptor element-inducible gene transcription and DNA synthesis in prostate cancer cells. Int. J. Cancer 2007, 121, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Kono, M.; Fujii, T.; Lim, B.; Karuturi, M.S.; Tripathy, D.; Ueno, N.T. Androgen Receptor Function and Androgen Receptor-Targeted Therapies in Breast Cancer: A Review. JAMA Oncol. 2017, 3, 1266–1273. [Google Scholar] [CrossRef] [PubMed]
- Pascual, J.; Turner, N.C. Targeting the PI3-kinase pathway in triple-negative breast cancer. Ann. Oncol. 2019, 30, 1051–1060. [Google Scholar] [CrossRef]
- Topalian, S.L.; Drake, C.G.; Pardoll, D.M. Immune checkpoint blockade: A common denominator approach to cancer therapy. Cancer Cell 2015, 27, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Luen, S.; Virassamy, B.; Savas, P.; Salgado, R.; Loi, S. The genomic landscape of breast cancer and its interaction with host immunity. Breast 2016, 29, 241–250. [Google Scholar] [CrossRef]
- Sabatier, R.; Finetti, P.; Mamessier, E.; Adelaide, J.; Chaffanet, M.; Ali, H.R.; Viens, P.; Caldas, C.; Birnbaum, D.; Bertucci, F. Prognostic and predictive value of PDL1 expression in breast cancer. Oncotarget 2015, 6, 5449–5464. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Philips, A.V.; Meric-Bernstam, F.; Qiao, N.; Wu, Y.; Harrington, S.; Su, X.; Wang, Y.; Gonzalez-Angulo, A.M.; Akcakanat, A.; et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol. Res. 2014, 2, 361–370. [Google Scholar] [CrossRef]
- Dill, E.A.; Gru, A.A.; Atkins, K.A.; Friedman, L.A.; Moore, M.E.; Bullock, T.N.; Cross, J.V.; Dillon, P.M.; Mills, A.M. PD-L1 Expression and Intratumoral Heterogeneity Across Breast Cancer Subtypes and Stages: An Assessment of 245 Primary and 40 Metastatic Tumors. Am. J. Surg. Pathol. 2017, 41, 334–342. [Google Scholar] [CrossRef]
- Zhu, H.; Du, C.; Yuan, M.; Fu, P.; He, Q.; Yang, B.; Cao, J. PD-1/PD-L1 counterattack alliance: Multiple strategies for treating triple-negative breast cancer. Drug Discov. Today 2020, 25, 1762–1771. [Google Scholar] [CrossRef]
- Shukla, A.A.; Thömmes, J. Recent advances in large-scale production of monoclonal antibodies and related proteins. Trends Biotechnol. 2010, 28, 253–261. [Google Scholar] [CrossRef]
- Dirix, L.Y.; Takacs, I.; Jerusalem, G.; Nikolinakos, P.; Arkenau, H.-T.; Forero-Torres, A.; Boccia, R.; Lippman, M.E.; Somer, R.; Smakal, M.; et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: A phase 1b JAVELIN Solid Tumor study. Breast Cancer Res. Treat. 2018, 167, 671–686. [Google Scholar] [CrossRef] [PubMed]
- Miles, D.; Gligorov, J.; André, F.; Cameron, D.; Schneeweiss, A.; Barrios, C.; Xu, B.; Wardley, A.; Kaen, D.; Andrade, L.; et al. Primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/metastatic triple-negative breast cancer. Ann. Oncol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Rugo, H.S.; Adams, S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Henschel, V.; Molinero, L.; Chui, S.Y.; et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): Updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020, 21, 44–59. [Google Scholar] [CrossRef]
- Adams, S.; Loi, S.; Toppmeyer, D.; Cescon, D.W.; De Laurentiis, M.; Nanda, R.; Winer, E.P.; Mukai, H.; Tamura, K.; Armstrong, A.; et al. Pembrolizumab monotherapy for previously untreated, PD-L1-positive, metastatic triple-negative breast cancer: Cohort B of the phase II KEYNOTE-086 study. Ann. Oncol. 2019, 30, 405–411. [Google Scholar] [CrossRef]
- Adams, S.; Schmid, P.; Rugo, H.S.; Winer, E.P.; Loirat, D.; Awada, A.; Cescon, D.W.; Iwata, H.; Campone, M.; Nanda, R.; et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: Cohort A of the phase II KEYNOTE-086 study. Ann. Oncol. 2019, 30, 397–404. [Google Scholar] [CrossRef]
- Cortés, J.; Lipatov, O.; Im, S.A.; Gonçalves, A.; Lee, K.S.; Schmid, P.; Tamura, K.; Testa, L.; Witzel, I.; Ohtani, S.; et al. LBA21 - KEYNOTE-119: Phase III study of pembrolizumab (pembro) versus single-agent chemotherapy (chemo) for metastatic triple negative breast cancer (mTNBC). Ann. Oncol. 2019, 30, v859–v860. [Google Scholar] [CrossRef]
- Slavik, J.M.; Hutchcroft, J.E.; Bierer, B.E. CD28/CTLA-4 and CD80/CD86 families: Signaling and function. Immunol. Res. 1999, 19, 1–24. [Google Scholar] [CrossRef]
- Navarrete-Bernal, M.G.C.; Cervantes-Badillo, M.G.; Martínez-Herrera, J.F.; Lara-Torres, C.O.; Gerson-Cwilich, R.; Zentella-Dehesa, A.; Ibarra-Sánchez, M.J.; Esparza-López, J.; Montesinos, J.J.; Cortés-Morales, V.A.; et al. Biological Landscape of Triple Negative Breast Cancers Expressing CTLA-4. Front. Oncol. 2020, 10, 1206. [Google Scholar] [CrossRef]
- Intlekofer, A.M.; Thompson, C.B. At the bench: Preclinical rationale for CTLA-4 and PD-1 blockade as cancer immunotherapy. J. Leukoc. Biol. 2013, 94, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Bernier, C.; Soliman, A.; Gravel, M.; Dankner, M.; Savage, P.; Petrecca, K.; Park, M.; Siegel, P.M.; Shore, G.C.; Roulston, A. DZ-2384 has a superior preclinical profile to taxanes for the treatment of triple-negative breast cancer and is synergistic with anti-CTLA-4 immunotherapy. Anti Cancer Drugs 2018, 29, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Vasilatos, S.N.; Chen, L.; Wu, H.; Cao, Z.; Fu, Y.; Huang, M.; Vlad, A.M.; Lu, B.; Oesterreich, S.; et al. Inhibition of histone lysine-specific demethylase 1 elicits breast tumor immunity and enhances antitumor efficacy of immune checkpoint blockade. Oncogene 2019, 38, 390–405. [Google Scholar] [CrossRef]
- Zheng, H.; Zhao, W.; Yan, C.; Watson, C.C.; Massengill, M.; Xie, M.; Massengill, C.; Noyes, D.R.; Martinez, G.V.; Afzal, R.; et al. HDAC Inhibitors Enhance T-Cell Chemokine Expression and Augment Response to PD-1 Immunotherapy in Lung Adenocarcinoma. Clin. Cancer Res. 2016, 22, 4119. [Google Scholar] [CrossRef] [PubMed]
- Zaman, S.; Jadid, H.; Denson, A.C.; Gray, J.E. Targeting Trop-2 in solid tumors: Future prospects. Onco Targets Ther. 2019, 12, 1781–1790. [Google Scholar] [CrossRef]
- Goldenberg, D.M.; Cardillo, T.M.; Govindan, S.V.; Rossi, E.A.; Sharkey, R.M. Trop-2 is a novel target for solid cancer therapy with sacituzumab govitecan (IMMU-132), an antibody-drug conjugate (ADC). Oncotarget 2015, 6, 22496–22512. [Google Scholar] [CrossRef]
- Seligson, J.M.; Patron, A.M.; Berger, M.J.; Harvey, R.D.; Seligson, N.D. Sacituzumab Govitecan-hziy: An Antibody-Drug Conjugate for the Treatment of Refractory, Metastatic, Triple-Negative Breast Cancer. Ann. Pharmacother. 2020, 1060028020966548. [Google Scholar] [CrossRef] [PubMed]
- Khoury, K.; Feldman, R.; Pohlmann, P.R.; Heeke, A.L.; Gatalica, Z.; Veloso, Y.; Vidal, G.A.; Schwartzberg, L.S.; Swain, S.M.; Isaacs, C.; et al. Molecular characterization of trophoblast cell surface antigen 2 (Trop-2) positive triple negative breast cancer (TNBC). J. Clin. Oncol. 2019, 37, e14651. [Google Scholar] [CrossRef]
- Bardia, A.; Mayer, I.A.; Diamond, J.R.; Moroose, R.L.; Isakoff, S.J.; Starodub, A.N.; Shah, N.C.; O’Shaughnessy, J.; Kalinsky, K.; Guarino, M.; et al. Efficacy and Safety of Anti-Trop-2 Antibody Drug Conjugate Sacituzumab Govitecan (IMMU-132) in Heavily Pretreated Patients With Metastatic Triple-Negative Breast Cancer. J. Clin. Oncol. 2017, 35, 2141–2148. [Google Scholar] [CrossRef]
- Rothenberg, M.L. Topoisomerase I inhibitors: Review and update. Ann. Oncol. 1997, 8, 837–855. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Mayer, I.A.; Vahdat, L.T.; Tolaney, S.M.; Isakoff, S.J.; Diamond, J.R.; O’Shaughnessy, J.; Moroose, R.L.; Santin, A.D.; Abramson, V.G.; et al. Sacituzumab Govitecan-hziy in Refractory Metastatic Triple-Negative Breast Cancer. N. Engl. J. Med. 2019, 380, 741–751. [Google Scholar] [CrossRef] [PubMed]
| Taxanes | |||
|---|---|---|---|
| Phase | Clinical Trial | Treatment | Identifier |
| 3 | A Randomized Controlled Trial of Neoadjuvant Weekly Paclitaxel Versus Weekly Paclitaxel Plus Weekly Carboplatin In Women With Large Operable or Locally Advanced, Triple-Negative Breast Cancer | Paclitaxel with or without Carboplatin as neoadjuvant following cyclophosphamide with doxorubicin or epirubicin | NCT03168880 |
| 2 | A Randomized Phase II Study of Preoperative Cisplatin Versus Paclitaxel in Patients With Triple-Negative Breast Cancer: Evaluating the Homologous Recombination Deficiency (HRD) Biomarker | Either Paclitaxel or cisplatin | NCT01982448 |
| 2 | Phase II Clinical Trial of Treatment With TAK-228 and TAK-117 to Inhibit Homologous Recombination (HR) Followed by Cisplatin and Nab Paclitaxel in Patients With Chemotherapy-pretreated Metastatic Triple-Negative Breast Cancer | Sapanisertib and Serabelisib with or without Cisplatin and Nab-Paclitaxel | NCT03193853 |
| 3 | A Phase III, Multicenter, Randomized, Placebo-Controlled Study of Atezolizumab (Anti-PD-L1 Antibody) in Combination With Nab-Paclitaxel Compared With Placebo With Nab-Paclitaxel for Patients With Previously Untreated Metastatic Triple-Negative Breast Cancer | Nab-Paclitaxel plus placebo or Atezolizumab | NCT02425891 |
| 2 | Triple-Negative First-Line Study: Neoadjuvant Trial of Nab-Paclitaxel and MPDL3280A, a PDL-1 Inhibitor in Patients With Triple-Negative Breast Cancer | Nab-paclitaxel and Atezolizumab | NCT02530489 |
| 2 | A Phase II, Double-blind, Randomised, Placebo-controlled Study of the AKT Inhibitor AZD5363 in Combination With Paclitaxel in Triple-NegativeAdvanced or Metastatic Breast Cancer (PAKT). | Paclitaxel with placebo or AZD5363 | NCT02423603 |
| 2 | A Phase II Study of Neoadjuvant Carboplatin/Paclitaxel Followed by Dose-Dense Doxorubicin/Cyclophosphamide in Patients With Hormone Receptor Negative, HER2 Receptor Negative Breast Cancer | Carboplatin/Paclitaxel followed by Doxorubicin or Cyclophosphamide | NCT03301350 |
| 2 | CADENCE: Carboplatin and Docetaxel in Neoadjuvant Treatment of ER-Negative, HER2-Negative Breast Cancer: A Co-Clinical Trial With Genoproteomic Discovery | Carboplatin plus Docetaxel | NCT02547987 |
| 1 | A Phase Ib, Open-Label, Multicenter Study Evaluating the Safety and Efficacy of Ipatasertib in Combination With Atezolizumab and Paclitaxel or Nab-Paclitaxel in Patients With Locally Advanced or Metastatic Triple-Negative Breast Cancer | Ipatasertib and Atezolizumab with or without Paclitaxel or Nab-Paclitaxel; or Ipatasertib and Atezolizumab with Doxorubicin and Cyclophosphamide followed by Ipatasertib and Atezolizumab with Paclitaxe | NCT03800836 |
| Anthracyclines | |||
| 2 | Anti-EGFR-immunoliposomes Loaded With Doxorubicin in Patients With Advanced Triple-Negative EGFR Positive Breast Cancer—A Multicenter Single Arm Phase II Trial | Doxorubicin-loaded anti-EGFR immunoliposomes (anti-EGFR-IL-dox) | NCT02833766 |
| 2 | Randomized Phase II 2 × 2 Factorial Trial of the Addition of Carboplatin +/- Bevacizumab to Neoadjuvant Weekly Paclitaxel Followed by Dose-Dense AC in Hormone Receptor-Poor/HER2-Negative Resectable Breast Cancer | Paclitaxel with or without Carboplatin and/or Bevacizumab followed by Doxorubicin and Cyclophosphamide | NCT00861705 |
| Cyclophosphamide | |||
| 2 | Phase II Study Of Single-dose Cyclophosphamide + Pembrolizumab In Patients With Metastatic Triple-Negative Breast Cancer | Cyclophosphamide followed by Pembrolizumab | NCT02768701 |
| 2 | A Phase II Study of Neoadjuvant Carboplatin/Paclitaxel Followed by Dose-Dense Doxorubicin/Cyclophosphamide in Patients With Hormone Receptor Negative, HER2 Receptor Negative Breast Cancer | Low dose weekly Carboplatin/Paclitaxel followed by dose-dense Doxorubicin/Cyclophosphamide | NCT03301350 |
| 2/3 | Randomized Phase II/III Study of Individualized Neoadjuvant Chemotherapy in ‘Triple-Negative’ Breast Tumors | Doxorubicin plus Cyclophosphamide or Carboplatin plus Thiotepa, or Doxorubicin plus Cyclophosphamide with Carboplatin and Thiotepa | NCT01057069 |
| Platinum agents | |||
| 2 | Phase II Trial of Neoadjuvant Chemotherapy With Carboplatin and NAB-Paclitaxel in Patients With Locally Advanced and Inflammatory Triple-Negative Breast Cancer | Carboplatin once, followed by weekly Nab-Paclitaxel | NCT01525966 |
| 2 | A Randomized Phase II Trial of Carboplatin With or Without Nivolumab in First-line Metastatic Triple-negative Breast Cancer | Carboplatin with or without Nivolumab | NCT03414684 |
| 1/2 | A Single-armed Multicenter Phase Ib/II Study of HX008 (a Recombinant Humanized Anti-PD-1 Monoclonal Antibody) Combined With GP Regimen as the First-line Treatment in Patients With Metastatic Triple-Negative Breast Cancer | HX008 with Cisplatin and Gemcitabine | NCT04750382 |
| 2 | Pilot Study of Carboplatin, Nab-Paclitaxel and Pembrolizumab for Metastatic Triple-Negative Breast Cancer | Carboplatin, Nab-paclitaxel, and Pembrolizumab | NCT03121352 |
| Phase | Clinical Trial | Treatment | Identifier |
|---|---|---|---|
| 1 | A Phase I, Open-Label Study to Assess the Safety and Tolerability of KU-0059436 in Combination With Carboplatin, KU-0059436 in Combination With a Paclitaxel/Carboplatin T/C Doublet and KU-0059436 in Combination With Paclitaxel in the Treatment of Patients With Advanced Solid Tumours | Olaparib with Carboplatin and/or Paclitaxel | NCT00516724 |
| 2 | Phase 1/2 Clinical Study of Niraparib in Combination With Pembrolizumab (MK-3475) in Patients With Advanced or Metastatic Triple-Negative Breast Cancer and in Patients With Recurrent Ovarian Cancer | Niraparib plus Pembrolizumab | NCT02657889 |
| 2 | Phase II Multicenter Study of Durvalumab and Olaparib in Platinum tReated Advanced Triple-Negative Breast Cancer | Olaparib with or without Durvalumab | NCT03167619 |
| 2 | A Phase II, Open Label, Randomised, Multi-centre Study to Assess the Safety and Efficacy of Agents Targeting DNA Damage Repair in Combination With Olaparib Versus Olaparib Monotherapy in the Treatment of Metastatic Triple-Negative Breast Cancer Patients Stratified by Alterations in Homologous Recombinant Repair (HRR)-Related Genes (Including BRCA1/2) (VIOLETTE). | Olaparib alone or with Ceralasertib or Adavosertib | NCT03330847 |
| 1 | A Phase I, Open-Label Study to Assess the Safety and Tolerability of KU-0059436 in Combination With Carboplatin, KU-0059436 in Combination With a Paclitaxel/Carboplatin T/C Doublet and KU-0059436 in Combination With Paclitaxel in the Treatment of Patients With Advanced Solid Tumours | Olaparib with Carboplatin or Paclitaxel, or Olaparib with Carboplatin and Paclitaxel | NCT00516724 |
| N/A | An Open Label, Pilot Study of Veliparib (ABT-888) and Lapatinib (Tykerb) in Patients With Metastatic, Triple-Negative (ER, PR, and HER-2 Negative) Breast Cancer | Veliparib and Lapatinib | NCT02158507 |
| 2 | Phase II Randomized Placebo-Controlled Trial of Cisplatin With or Without ABT-888 (Veliparib) in Metastatic Triple-Negative Breast Cancer and/or BRCA Mutation-Associated Breast Cancer, With or Without Brain Metastases | Cisplatin with or without Veliparib | NCT02595905 |
| 1 | An Open, Non-randomised, Multi-centre Phase I Study to Assess the Safety and Efficacy of Fluzoparib Given in Combination With Apatinib in Patients With Recurrent Ovarian Cancer or Triple-Negative Breast Cancer | Fluzoparib and Apatinib | NCT03075462 |
| Phase | Clinical Trial | Treatment | Identifier |
|---|---|---|---|
| 2 | Women’s Triple-Negative First-Line Study: A Phase II Trial of Liposomal Doxorubicin, Bevacizumab and Everolimus (DAE) in Patients With Localized Triple-Negative Breast Cancer (TNBC) With Tumors Predicted Insensitive to Standard Neoadjuvant Chemotherapy | Doxorubicin, Bevacizumab, and Everolimus | NCT02456857 |
| 1 | A Phase 1b, Open-Label, Safety and Tolerability Study of TTAC-0001 in Combination With Pembrolizumab in Patients With Metastatic Triple-Negative Breast Cancer | Olinvacimab and Pembrolizumab | NCT03720431 |
| Phase | Clinical Trial | Treatment | Identifier |
|---|---|---|---|
| 3 | A Phase III, Double-blind, Placebo-controlled, Randomized Study of Ipatasertib in Combination With Atezolizumab and Paclitaxel as a Treatment for Participants With Locally Advanced Unresectable or Metastatic Triple-Negative Breast Cancer. | Paclitaxel with Atezolizumab and placebo, or Paclitaxel with Ipatasertib and placebo, or combination of Paclitaxel, Atezolizumab, and Ipatasertib, or Paclitaxel with two placebos. | NCT04177108 |
| 3 | A Double-Blind, Placebo-Controlled, Randomized Phase III Study of Ipatasertib in Combination with Paclitaxel as a Treatment for Patients with PIK3CA/AKT1/PTEN-Altered, Locally Advanced or Metastatic, Triple-Negative Breast Cancer or Hormone Receptor-Positive, HER2-Negative Breast Cancer | Paclitaxel with Ipatasertib or placebo | NCT03337724 |
| Phase | Clinical Trial | Treatment | Identifier |
|---|---|---|---|
| 3 | Adjuvant Treatment for High-risk Triple-Negative Breast Cancer Patients with the Anti-PD-l1 Antibody Avelumab: A Phase III Randomized Trial. Sponsor: Dipartimento di Scienze Chirurgiche, Oncologiche e Gastroenterologiche, Università di Padova | Surgery with or without Avelumab as adjuvant | NCT02926196 |
| 1/2 | A Pilot and Phase II Study to Assess the Safety, Tolerability and Efficacy of Pembrolizumab Plus Chemotherapy in Metastatic Triple-Negative Breast Cancer Patients | Pembrolizumab with either Paclitaxel or Capecitabine | NCT02734290 |
| 3 | A Phase III, Randomized, Double-blind Study to Evaluate Pembrolizumab Plus Chemotherapy vs. Placebo Plus Chemotherapy as Neoadjuvant Therapy and Pembrolizumab vs. Placebo as Adjuvant Therapy for Triple-Negative Breast Cancer (TNBC) | Pembrolizumab or placebo before and after Carboplatin and Paclitaxel | NCT03036488 |
| 1 | A Phase 1b Trial of the Cyclin-dependent Kinase Inhibitor Dinaciclib in Combination With Pembrolizumab in Patients With Advanced Breast Cancer and Assessment of MYC Oncogene Overexpression | Dinacicilib and Pembrolizumab | NCT01676753 |
| 3 | A Phase III Randomized Study to Investigate the Efficacy and Safety of Atezolizumab (Anti-PD-L1 Antibody) in Combination With Neoadjuvant Anthracycline/Nab-Paclitaxel-Based Chemotherapy Compared With Placebo and Chemotherapy in Patients With Primary Invasive Triple-Negative Breast Cancer | Atezolizumab plus Nab-Paclitaxel followed by Atezolizumab plus Doxorubicin and Cyclophosphamide or placebo plus Nab-Paclitaxel followed by placebo plus Doxorubicin and Cyclophosphamide | NCT03197935 |
| 2 | Randomized Phase 2 Study of Neoadjuvant Chemotherapy, Carboplatin and Paclitaxel, With or Without Atezolizumab in Triple-Negative Breast Cancer (TNBC) | Carboplatin and Paclitaxel with or without Atezolizumab before surgery | NCT02883062 |
| 2 | A Phase II Trial of Atezolizumab (Anti-PD-L1) With Carboplatin in Patients With Metastatic Triple-Negative Breast Cancer | Carboplatin with or without Atezolizumab | NCT03206203 |
| 3 | Neo-Adjuvant Study With the PD-L1-directed Antibody in Triple-Negative Locally Advanced Breast Cancer Undergoing Treatment With Nab-paclitaxel and Carboplatin | Carboplatin and Nab-Paclitaxel with or without Atezolizumab as neoadjuvant | NCT02620280 |
| 1/2 | Single Arm Neoadjuvant Phase I/II Study of MEDI4736 (Anti-PD-L1 Antibody) Concomitant With Weekly Nab-paclitaxel and Dose-dense Doxorubicin/Cyclophosphamide (ddAC) Chemotherapy for Clinical Stage I-III Triple-Negative Breast Cancer | Durvalumab followed by Nab-Paclitaxel, Docetaxel, and Cyclophosphamide | NCT02489448 |
| 2 | Adaptive Phase II Randomized Non-comparative Trial of Nivolumab After Induction Treatment in Triple-negative Breast Cancer (TNBC) Patients: TONIC-trial | Nivolumab alone, or Nivolumab with Doxorubincin or Cyclophosphamide, or Cisplatin, or Radiation therapy | NCT02499367 |
| 2 | A Randomized Phase II Trial of Carboplatin With or Without Nivolumab in First-line Metastatic Triple-negative Breast Cancer | Carboplatin with or without Nivolumab | NCT03414684 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Q.; Siddharth, S.; Sharma, D. Triple Negative Breast Cancer: A Mountain Yet to Be Scaled Despite the Triumphs. Cancers 2021, 13, 3697. https://doi.org/10.3390/cancers13153697
Wu Q, Siddharth S, Sharma D. Triple Negative Breast Cancer: A Mountain Yet to Be Scaled Despite the Triumphs. Cancers. 2021; 13(15):3697. https://doi.org/10.3390/cancers13153697
Chicago/Turabian StyleWu, Qitong, Sumit Siddharth, and Dipali Sharma. 2021. "Triple Negative Breast Cancer: A Mountain Yet to Be Scaled Despite the Triumphs" Cancers 13, no. 15: 3697. https://doi.org/10.3390/cancers13153697
APA StyleWu, Q., Siddharth, S., & Sharma, D. (2021). Triple Negative Breast Cancer: A Mountain Yet to Be Scaled Despite the Triumphs. Cancers, 13(15), 3697. https://doi.org/10.3390/cancers13153697







