Regulation and Functions of Protumoral Unconventional T Cells in Solid Tumors
Abstract
Simple Summary
Abstract
1. Introduction
2. Generalities on UT Cells
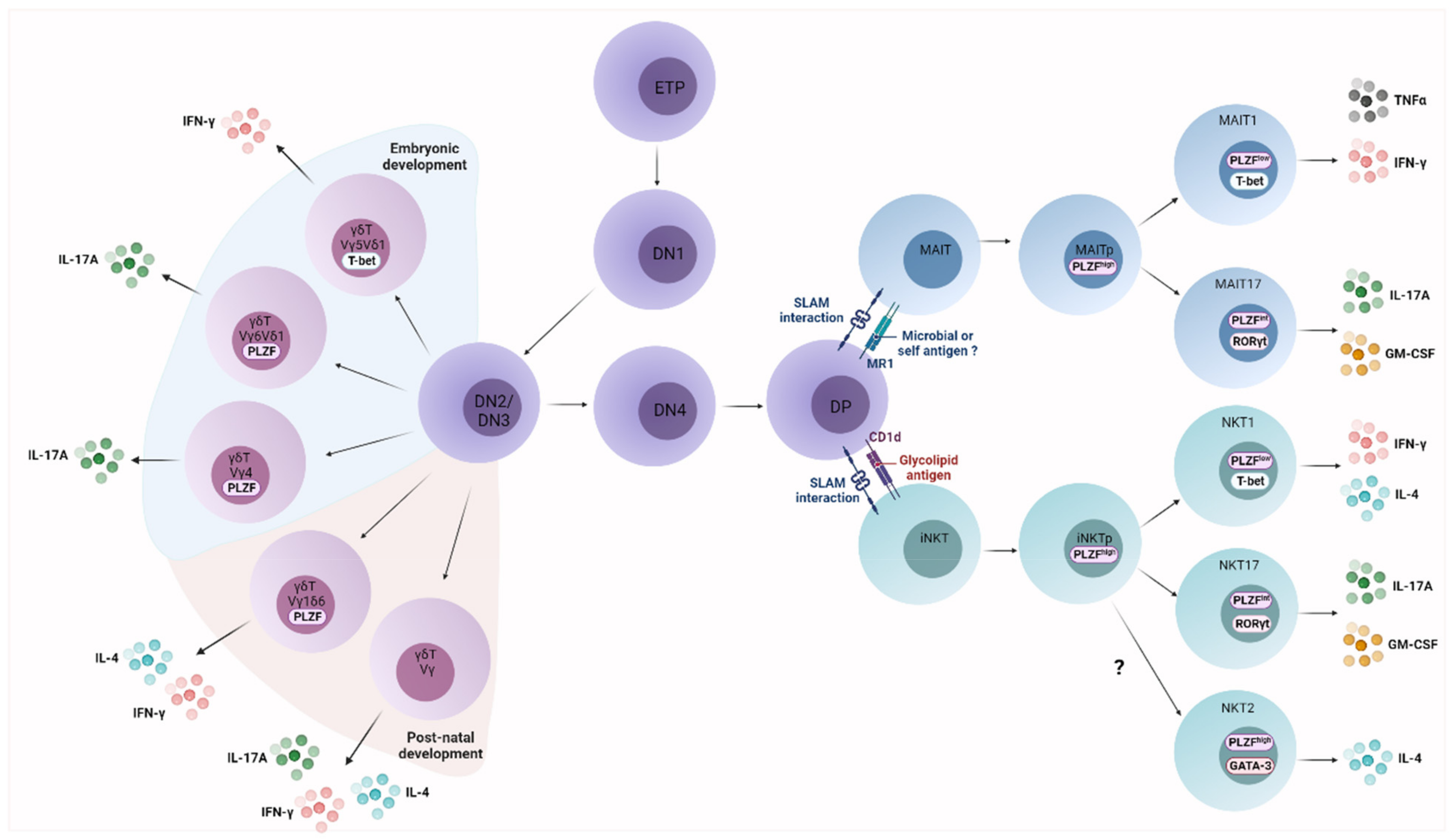
2.1. UT Cell Ontogeny
2.2. UT Cell Effector Differentiation
2.3. Activation Mechanisms of UT Cells
2.3.1. TCR-Dependent Signals
2.3.2. TCR-Independent Signals
2.4. Functional Diversity of UT Cells
2.4.1. Cytotoxicity
2.4.2. Release of Immunoregulatory Factors
2.5. UT Cell Populations: Redundant Functions for Specific Roles?
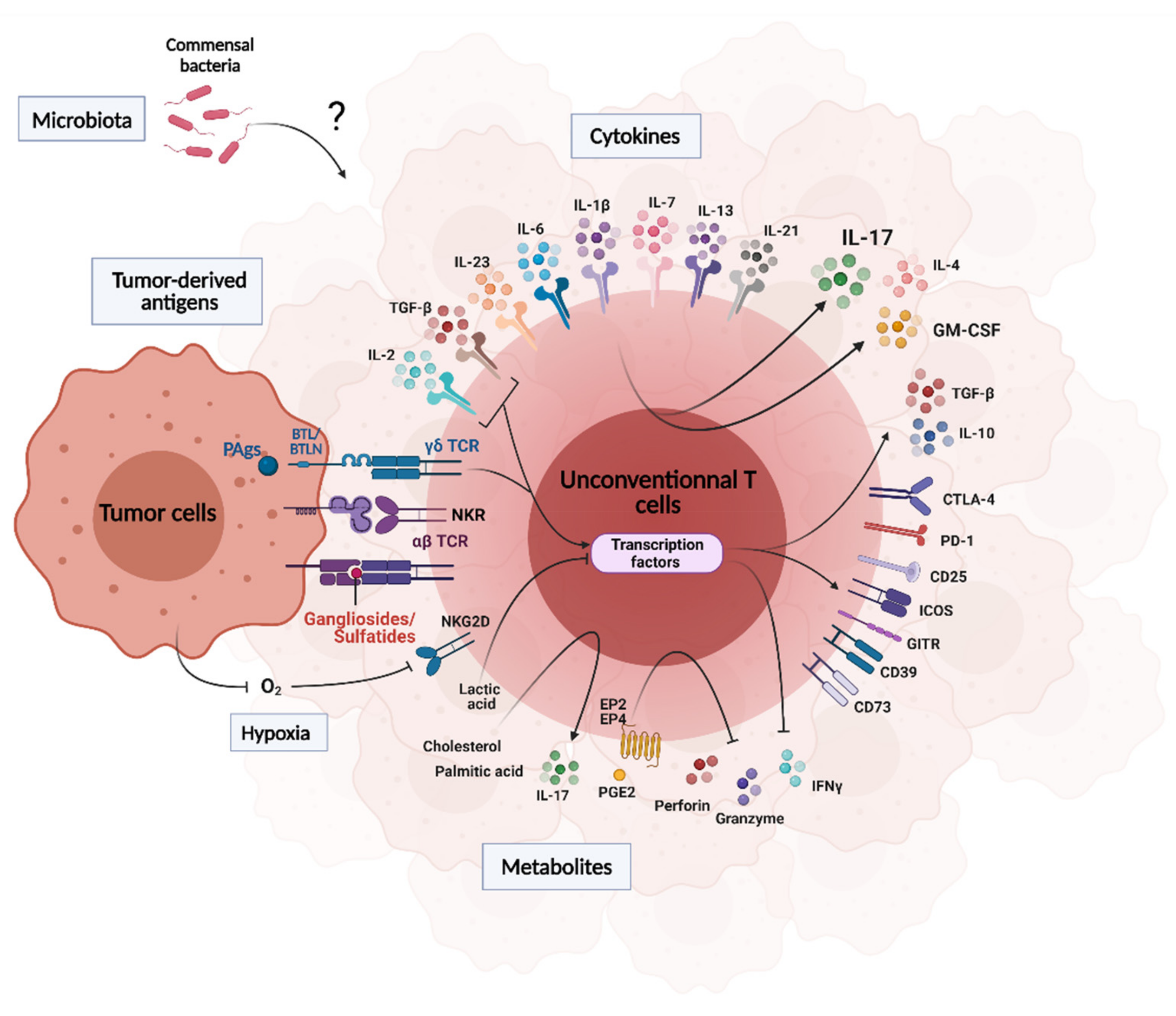
3. Influence of the Tumor Microenvironment on UT Cell Functions
3.1. Tumor-Derived Antigens
3.2. Cytokines, Metabolites, pH and Hypoxia
3.3. Microbiota
4. Emerging Protumoral Functions of Intratumoral Unconventional T Cells
4.1. Angiogenesis and Tumor Cell Proliferation
4.2. Shaping the Immunosuppressive TME
4.3. Inhibition of Antitumoral Functions
5. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Leone, R.D.; Powell, J.D. Metabolism of Immune Cells in Cancer. Nat. Rev. Cancer 2020, 20, 516–531. [Google Scholar] [CrossRef]
- Bergers, G.; Fendt, S.-M. The Metabolism of Cancer Cells during Metastasis. Nat. Rev. Cancer 2021, 21, 162–180. [Google Scholar] [CrossRef]
- Gentles, A.J.; Newman, A.M.; Liu, C.L.; Bratman, S.V.; Feng, W.; Kim, D.; Nair, V.S.; Xu, Y.; Khuong, A.; Hoang, C.D.; et al. The Prognostic Landscape of Genes and Infiltrating Immune Cells across Human Cancers. Nat. Med. 2015, 21, 938–945. [Google Scholar] [CrossRef]
- Robinette, M.L.; Colonna, M. Immune Modules Shared by Innate Lymphoid Cells and T Cells. J. Allergy Clin. Immunol. 2016, 138, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Pellicci, D.G.; Koay, H.-F.; Berzins, S.P. Thymic Development of Unconventional T Cells: How NKT Cells, MAIT Cells and Γδ T Cells Emerge. Nat. Rev. Immunol. 2020, 20, 756–770. [Google Scholar] [CrossRef] [PubMed]
- Tilloy, F.; Treiner, E.; Park, S.H.; Garcia, C.; Lemonnier, F.; de la Salle, H.; Bendelac, A.; Bonneville, M.; Lantz, O. An Invariant T Cell Receptor Alpha Chain Defines a Novel TAP-Independent Major Histocompatibility Complex Class Ib-Restricted Alpha/Beta T Cell Subpopulation in Mammals. J. Exp. Med. 1999, 189, 1907–1921. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, D.I.; Koay, H.-F.; McCluskey, J.; Gherardin, N.A. The Biology and Functional Importance of MAIT Cells. Nat. Immunol. 2019, 20, 1110–1128. [Google Scholar] [CrossRef]
- Gapin, L.; Matsuda, J.L.; Surh, C.D.; Kronenberg, M. NKT Cells Derive from Double-Positive Thymocytes That Are Positively Selected by CD1d. Nat. Immunol. 2001, 2, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Macho-Fernandez, E.; Brigl, M. The Extended Family of CD1d-Restricted NKT Cells: Sifting through a Mixed Bag of TCRs, Antigens, and Functions. Front. Immunol. 2015, 6, 362. [Google Scholar] [CrossRef]
- Vermijlen, D.; Prinz, I. Ontogeny of Innate T Lymphocytes—Some Innate Lymphocytes Are More Innate than Others. Front. Immunol. 2014, 5, 486. [Google Scholar] [CrossRef] [PubMed]
- Uldrich, A.P.; Rigau, M.; Godfrey, D.I. Immune Recognition of Phosphoantigen-Butyrophilin Molecular Complexes by Γδ T Cells. Immunol. Rev. 2020, 298, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Treiner, E.; Duban, L.; Bahram, S.; Radosavljevic, M.; Wanner, V.; Tilloy, F.; Affaticati, P.; Gilfillan, S.; Lantz, O. Selection of Evolutionarily Conserved Mucosal-Associated Invariant T Cells by MR1. Nature 2003, 422, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Bendelac, A.; Savage, P.B.; Teyton, L. The Biology of NKT Cells. Annu. Rev. Immunol. 2007, 25, 297–336. [Google Scholar] [CrossRef] [PubMed]
- Prinz, I.; Silva-Santos, B.; Pennington, D.J. Functional Development of Γδ T Cells. Eur. J. Immunol. 2013, 43, 1988–1994. [Google Scholar] [CrossRef] [PubMed]
- Castro, C.D.; Boughter, C.T.; Broughton, A.E.; Ramesh, A.; Adams, E.J. Diversity in Recognition and Function of Human Γδ T Cells. Immunol. Rev. 2020, 298, 134–152. [Google Scholar] [CrossRef]
- Corbett, A.J.; Eckle, S.B.G.; Birkinshaw, R.W.; Liu, L.; Patel, O.; Mahony, J.; Chen, Z.; Reantragoon, R.; Meehan, B.; Cao, H.; et al. T-Cell Activation by Transitory Neo-Antigens Derived from Distinct Microbial Pathways. Nature 2014, 509, 361–365. [Google Scholar] [CrossRef]
- Kawano, T.; Cui, J.; Koezuka, Y.; Toura, I.; Kaneko, Y.; Motoki, K.; Ueno, H.; Nakagawa, R.; Sato, H.; Kondo, E.; et al. CD1d-Restricted and TCR-Mediated Activation of Valpha14 NKT Cells by Glycosylceramides. Science 1997, 278, 1626–1629. [Google Scholar] [CrossRef]
- Kinjo, Y.; Illarionov, P.; Vela, J.L.; Pei, B.; Girardi, E.; Li, X.; Li, Y.; Imamura, M.; Kaneko, Y.; Okawara, A.; et al. Invariant Natural Killer T Cells Recognize Glycolipids from Pathogenic Gram-Positive Bacteria. Nat. Immunol. 2011, 12, 966–974. [Google Scholar] [CrossRef]
- Paget, C.; Mallevaey, T.; Speak, A.O.; Torres, D.; Fontaine, J.; Sheehan, K.C.F.; Capron, M.; Ryffel, B.; Faveeuw, C.; Leite de Moraes, M.; et al. Activation of Invariant NKT Cells by Toll-like Receptor 9-Stimulated Dendritic Cells Requires Type I Interferon and Charged Glycosphingolipids. Immunity 2007, 27, 597–609. [Google Scholar] [CrossRef]
- Terabe, M.; Berzofsky, J.A. Tissue-Specific Roles of NKT Cells in Tumor Immunity. Front. Immunol. 2018, 9, 1838. [Google Scholar] [CrossRef]
- Kunzmann, V.; Bauer, E.; Wilhelm, M. Gamma/Delta T-Cell Stimulation by Pamidronate. N. Engl. J. Med. 1999, 340, 737–738. [Google Scholar] [CrossRef] [PubMed]
- Mak, J.Y.W.; Xu, W.; Reid, R.C.; Corbett, A.J.; Meehan, B.S.; Wang, H.; Chen, Z.; Rossjohn, J.; McCluskey, J.; Liu, L.; et al. Stabilizing Short-Lived Schiff Base Derivatives of 5-Aminouracils That Activate Mucosal-Associated Invariant T Cells. Nat. Commun. 2017, 8, 14599. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, D.I.; Le Nours, J.; Andrews, D.M.; Uldrich, A.P.; Rossjohn, J. Unconventional T Cell Targets for Cancer Immunotherapy. Immunity 2018, 48, 453–473. [Google Scholar] [CrossRef] [PubMed]
- Paget, C.; Deng, S.; Soulard, D.; Priestman, D.A.; Speca, S.; von Gerichten, J.; Speak, A.O.; Saroha, A.; Pewzner-Jung, Y.; Futerman, A.H.; et al. TLR9-Mediated Dendritic Cell Activation Uncovers Mammalian Ganglioside Species with Specific Ceramide Backbones That Activate Invariant Natural Killer T Cells. PLoS Biol. 2019, 17, e3000169. [Google Scholar] [CrossRef] [PubMed]
- Birkholz, A.M.; Kronenberg, M. Antigen Specificity of Invariant Natural Killer T-Cells. Biomed. J. 2015, 38, 470–483. [Google Scholar] [CrossRef]
- Livák, F.; Tourigny, M.; Schatz, D.G.; Petrie, H.T. Characterization of TCR Gene Rearrangements During Adult Murine T Cell Development. J. Immunol. 1999, 162, 2575–2580. [Google Scholar]
- Ciofani, M.; Knowles, G.C.; Wiest, D.L.; von Boehmer, H.; Zúñiga-Pflücker, J.C. Stage-Specific and Differential Notch Dependency at the Aβ and Γδ T Lineage Bifurcation. Immunity 2006, 25, 105–116. [Google Scholar] [CrossRef]
- Prinz, I.; Sansoni, A.; Kissenpfennig, A.; Ardouin, L.; Malissen, M.; Malissen, B. Visualization of the Earliest Steps of Γδ T Cell Development in the Adult Thymus. Nat. Immunol. 2006, 7, 995–1003. [Google Scholar] [CrossRef]
- Lantz, O.; Legoux, F. MAIT Cells: Programmed in the Thymus to Mediate Immunity within Tissues. Curr. Opin. Immunol. 2019, 58, 75–82. [Google Scholar] [CrossRef]
- Gapin, L. Development of Invariant Natural Killer T Cells. Curr. Opin. Immunol. 2016, 39, 68–74. [Google Scholar] [CrossRef]
- Martin, E.; Treiner, E.; Duban, L.; Guerri, L.; Laude, H.; Toly, C.; Premel, V.; Devys, A.; Moura, I.C.; Tilloy, F.; et al. Stepwise Development of MAIT Cells in Mouse and Human. PLoS Biol. 2009, 7, e1000054. [Google Scholar] [CrossRef]
- Coles, M.C.; Raulet, D.H. NK1.1+ T Cells in the Liver Arise in the Thymus and Are Selected by Interactions with Class I Molecules on CD4+CD8+ Cells. J. Immunol. 2000, 164, 2412–2418. [Google Scholar] [CrossRef]
- Seach, N.; Guerri, L.; Bourhis, L.L.; Mburu, Y.; Cui, Y.; Bessoles, S.; Soudais, C.; Lantz, O. Double Positive Thymocytes Select Mucosal-Associated Invariant T Cells. J. Immunol. 2013, 191, 6002–6009. [Google Scholar] [CrossRef]
- Koay, H.-F.; Su, S.; Amann-Zalcenstein, D.; Daley, S.R.; Comerford, I.; Miosge, L.; Whyte, C.E.; Konstantinov, I.E.; d’Udekem, Y.; Baldwin, T.; et al. A Divergent Transcriptional Landscape Underpins the Development and Functional Branching of MAIT Cells. Sci. Immunol. 2019, 4. [Google Scholar] [CrossRef] [PubMed]
- Legoux, F.; Gilet, J.; Procopio, E.; Echasserieau, K.; Bernardeau, K.; Lantz, O. Molecular Mechanisms of Lineage Decisions in Metabolite-Specific T Cells. Nat. Immunol. 2019, 20, 1244–1255. [Google Scholar] [CrossRef]
- Griewank, K.; Borowski, C.; Rietdijk, S.; Wang, N.; Julien, A.; Wei, D.G.; Mamchak, A.A.; Terhorst, C.; Bendelac, A. Homotypic Interactions Mediated by Slamf1 and Slamf6 Receptors Control NKT Cell Lineage Development. Immunity 2007, 27, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Kovalovsky, D.; Uche, O.U.; Eladad, S.; Hobbs, R.M.; Yi, W.; Alonzo, E.; Chua, K.; Eidson, M.; Kim, H.-J.; Im, J.S.; et al. The BTB–Zinc Finger Transcriptional Regulator PLZF Controls the Development of Invariant Natural Killer T Cell Effector Functions. Nat. Immunol. 2008, 9, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Savage, A.K.; Constantinides, M.G.; Han, J.; Picard, D.; Martin, E.; Li, B.; Lantz, O.; Bendelac, A. The Transcription Factor PLZF Directs the Effector Program of the NKT Cell Lineage. Immunity 2008, 29, 391–403. [Google Scholar] [CrossRef]
- Seiler, M.P.; Mathew, R.; Liszewski, M.K.; Spooner, C.J.; Barr, K.; Meng, F.; Singh, H.; Bendelac, A. Elevated and Sustained Expression of the Transcription Factors Egr1 and Egr2 Controls NKT Lineage Differentiation in Response to TCR Signaling. Nat. Immunol. 2012, 13, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Rahimpour, A.; Koay, H.F.; Enders, A.; Clanchy, R.; Eckle, S.B.G.; Meehan, B.; Chen, Z.; Whittle, B.; Liu, L.; Fairlie, D.P.; et al. Identification of Phenotypically and Functionally Heterogeneous Mouse Mucosal-Associated Invariant T Cells Using MR1 Tetramers. J. Exp. Med. 2015, 212, 1095–1108. [Google Scholar] [CrossRef] [PubMed]
- Koay, H.-F.; Gherardin, N.A.; Enders, A.; Loh, L.; Mackay, L.K.; Almeida, C.F.; Russ, B.E.; Nold-Petry, C.A.; Nold, M.F.; Bedoui, S.; et al. A Three-Stage Intrathymic Development Pathway for the Mucosal-Associated Invariant T Cell Lineage. Nat. Immunol. 2016, 17, 1300–1311. [Google Scholar] [CrossRef]
- Winter, S.J.; Kunze-Schumacher, H.; Imelmann, E.; Grewers, Z.; Osthues, T.; Krueger, A. MicroRNA MiR-181a/b-1 Controls MAIT Cell Development. Immunol. Cell Biol. 2019, 97, 190–202. [Google Scholar] [CrossRef]
- Dhodapkar, M.V.; Kumar, V. Type II NKT Cells and Their Emerging Role in Health and Disease. J. Immunol. 2017, 198, 1015–1021. [Google Scholar] [CrossRef]
- Harsha Krovi, S.; Zhang, J.; Michaels-Foster, M.J.; Brunetti, T.; Loh, L.; Scott-Browne, J.; Gapin, L. Thymic INKT Single Cell Analyses Unmask the Common Developmental Program of Mouse Innate T Cells. Nat. Commun. 2020, 11, 6238. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Adrianto, I.; Wang, J.; Wu, X.; Datta, I.; Mi, Q.-S. Single-Cell RNA-Seq Analysis Uncovers Distinct Functional Human NKT Cell Sub-Populations in Peripheral Blood. Front. Cell Dev. Biol. 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Ben Youssef, G.; Tourret, M.; Salou, M.; Ghazarian, L.; Houdouin, V.; Mondot, S.; Mburu, Y.; Lambert, M.; Azarnoush, S.; Diana, J.-S.; et al. Ontogeny of Human Mucosal-Associated Invariant T Cells and Related T Cell Subsets. J. Exp. Med. 2018, 215, 459–479. [Google Scholar] [CrossRef] [PubMed]
- Venken, K.; Jacques, P.; Mortier, C.; Labadia, M.E.; Decruy, T.; Coudenys, J.; Hoyt, K.; Wayne, A.L.; Hughes, R.; Turner, M.; et al. RORγt Inhibition Selectively Targets IL-17 Producing INKT and Γδ-T Cells Enriched in Spondyloarthritis Patients. Nat. Commun. 2019, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Baranek, T.; Lebrigand, K.; de Amat Herbozo, C.; Gonzalez, L.; Bogard, G.; Dietrich, C.; Magnone, V.; Boisseau, C.; Jouan, Y.; Trottein, F.; et al. High Dimensional Single-Cell Analysis Reveals INKT Cell Developmental Trajectories and Effector Fate Decision. Cell Rep. 2020, 32, 108116. [Google Scholar] [CrossRef] [PubMed]
- Gapin, L.; Godfrey, D.I.; Rossjohn, J. Natural Killer T Cell Obsession with Self-Antigens. Curr. Opin. Immunol. 2013, 25, 168–173. [Google Scholar] [CrossRef]
- Oh, J.; Unutmaz, D. Immune Cells for Microbiota Surveillance. Science 2019, 366, 419–420. [Google Scholar] [CrossRef]
- Paget, C.; Chow, M.; Gherardin, N.; Beavis, P.; Uldrich, A.; Duret, H.; Hassane, M.; Souza-Fonseca-Guimaraes, F.; Mogilenko, D.; Staumont-Sallé, D.; et al. CD3bright Signals on Γδ T Cells Identify IL-17A-Producing Vγ6Vδ1+ T Cells. Immunol. Cell Biol. 2015, 93, 198–212. [Google Scholar] [CrossRef]
- Dieudé, M.; Striegl, H.; Tyznik, A.J.; Wang, J.; Behar, S.M.; Piccirillo, C.A.; Levine, J.S.; Zajonc, D.M.; Rauch, J. Cardiolipin Binds to CD1d and Stimulates CD1d-Restricted Γδ T Cells in the Normal Murine Repertoire. J. Immunol. 2011, 186, 4771–4781. [Google Scholar] [CrossRef]
- Luoma, A.M.; Castro, C.D.; Mayassi, T.; Bembinster, L.A.; Bai, L.; Picard, D.; Anderson, B.; Scharf, L.; Kung, J.E.; Sibener, L.V.; et al. Crystal Structure of Vδ1 T Cell Receptor in Complex with CD1d-Sulfatide Shows MHC-like Recognition of a Self-Lipid by Human Γδ T Cells. Immunity 2013, 39, 1032–1042. [Google Scholar] [CrossRef]
- Kjer-Nielsen, L.; Patel, O.; Corbett, A.J.; Le Nours, J.; Meehan, B.; Liu, L.; Bhati, M.; Chen, Z.; Kostenko, L.; Reantragoon, R.; et al. MR1 Presents Microbial Vitamin B Metabolites to MAIT Cells. Nature 2012, 491, 717–723. [Google Scholar] [CrossRef]
- Harly, C.; Guillaume, Y.; Nedellec, S.; Peigné, C.-M.; Mönkkönen, H.; Mönkkönen, J.; Li, J.; Kuball, J.; Adams, E.J.; Netzer, S.; et al. Key Implication of CD277/Butyrophilin-3 (BTN3A) in Cellular Stress Sensing by a Major Human Γδ T-Cell Subset. Blood 2012, 120, 2269–2279. [Google Scholar] [CrossRef]
- Sandstrom, A.; Peigné, C.-M.; Léger, A.; Crooks, J.E.; Konczak, F.; Gesnel, M.-C.; Breathnach, R.; Bonneville, M.; Scotet, E.; Adams, E.J. The Intracellular B30.2 Domain of Butyrophilin 3A1 Binds Phosphoantigens to Mediate Activation of Human Vγ9Vδ2 T Cells. Immunity 2014, 40, 490–500. [Google Scholar] [CrossRef]
- Rigau, M.; Ostrouska, S.; Fulford, T.S.; Johnson, D.N.; Woods, K.; Ruan, Z.; McWilliam, H.E.G.; Hudson, C.; Tutuka, C.; Wheatley, A.K.; et al. Butyrophilin 2A1 Is Essential for Phosphoantigen Reactivity by Γδ T Cells. Science 2020, 367. [Google Scholar] [CrossRef] [PubMed]
- Russano, A.M.; Agea, E.; Corazzi, L.; Postle, A.D.; De Libero, G.; Porcelli, S.; de Benedictis, F.M.; Spinozzi, F. Recognition of Pollen-Derived Phosphatidyl-Ethanolamine by Human CD1d-Restricted Gamma Delta T Cells. J. Allergy Clin. Immunol. 2006, 117, 1178–1184. [Google Scholar] [CrossRef]
- Uldrich, A.P.; Le Nours, J.; Pellicci, D.G.; Gherardin, N.A.; McPherson, K.G.; Lim, R.T.; Patel, O.; Beddoe, T.; Gras, S.; Rossjohn, J.; et al. CD1d-Lipid Antigen Recognition by the Γδ TCR. Nat. Immunol. 2013, 14, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- McEwen-Smith, R.M.; Salio, M.; Cerundolo, V. The Regulatory Role of Invariant NKT Cells in Tumor Immunity. Cancer Immunol. Res. 2015, 3, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Silva-Santos, B.; Serre, K.; Norell, H. Γδ T Cells in Cancer. Nat. Rev. Immunol. 2015, 15, 683–691. [Google Scholar] [CrossRef]
- Seyda, M.; Elkhal, A.; Quante, M.; Falk, C.S.; Tullius, S.G. T Cells Going Innate. Trends Immunol. 2016, 37, 546–556. [Google Scholar] [CrossRef]
- De Araújo, N.D.; Gama, F.M.; de Souza Barros, M.; Ribeiro, T.L.P.; Alves, F.S.; Xabregas, L.A.; Tarragô, A.M.; Malheiro, A.; Costa, A.G. Translating Unconventional T Cells and Their Roles in Leukemia Antitumor Immunity. J. Immunol. Res. 2021, 2021, 6633824. [Google Scholar] [CrossRef]
- Chiossone, L.; Dumas, P.-Y.; Vienne, M.; Vivier, E. Natural Killer Cells and Other Innate Lymphoid Cells in Cancer. Nat. Rev. Immunol. 2018, 18, 671–688. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xu, Q.; Peng, H.; Cheng, R.; Sun, Z.; Ye, Z. IFN-γ Enhances HOS and U2OS Cell Lines Susceptibility to Γδ T Cell-Mediated Killing through the Fas/Fas Ligand Pathway. Int. Immunopharmacol. 2011, 11, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Rodin, W.; Sundström, P.; Ahlmanner, F.; Szeponik, L.; Zajt, K.K.; Wettergren, Y.; Bexe Lindskog, E.; Quiding Järbrink, M. Exhaustion in Tumor-Infiltrating Mucosal-Associated Invariant T (MAIT) Cells from Colon Cancer Patients. Cancer Immunol. Immunother. 2021. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, J.L.; Mallevaey, T.; Scott-Browne, J.; Gapin, L. CD1d-Restricted INKT Cells, the “Swiss-Army Knife” of the Immune System. Curr Opin Immunol. 2008, 20, 358–368. [Google Scholar] [CrossRef]
- Bonneville, M.; O’Brien, R.L.; Born, W.K. Gammadelta T Cell Effector Functions: A Blend of Innate Programming and Acquired Plasticity. Nat. Rev. Immunol. 2010, 10, 467–478. [Google Scholar] [CrossRef]
- Kinjo, Y.; Kronenberg, M. V Alpha14 i NKT Cells Are Innate Lymphocytes That Participate in the Immune Response to Diverse Microbes. J. Clin. Immunol. 2005, 25, 522–533. [Google Scholar] [CrossRef]
- Toubal, A.; Nel, I.; Lotersztajn, S.; Lehuen, A. Mucosal-Associated Invariant T Cells and Disease. Nat. Rev. Immunol. 2019, 19, 643–657. [Google Scholar] [CrossRef]
- Gold, M.C.; Lewinsohn, D.M. Co-Dependents: MR1-Restricted MAIT Cells and Their Antimicrobial Function. Nat. Rev. Microbiol. 2013, 11, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Fuss, I.J.; Joshi, B.; Yang, Z.; Degheidy, H.; Fichtner-Feigl, S.; de Souza, H.; Rieder, F.; Scaldaferri, F.; Schirbel, A.; Scarpa, M.; et al. IL-13Rα2-Bearing, Type II NKT Cells Reactive to Sulfatide Self-Antigen Populate the Mucosa of Ulcerative Colitis. Gut 2014, 63, 1728–1736. [Google Scholar] [CrossRef]
- Dokouhaki, P.; Schuh, N.W.; Joe, B.; Allen, C.A.D.; Der, S.D.; Tsao, M.-S.; Zhang, L. NKG2D Regulates Production of Soluble TRAIL by Ex Vivo Expanded Human Γδ T Cells. Eur. J. Immunol. 2013, 43, 3175–3182. [Google Scholar] [CrossRef] [PubMed]
- Nieda, M.; Nicol, A.; Koezuka, Y.; Kikuchi, A.; Lapteva, N.; Tanaka, Y.; Tokunaga, K.; Suzuki, K.; Kayagaki, N.; Yagita, H.; et al. TRAIL Expression by Activated Human CD4(+)V Alpha 24NKT Cells Induces in Vitro and in Vivo Apoptosis of Human Acute Myeloid Leukemia Cells. Blood 2001, 97, 2067–2074. [Google Scholar] [CrossRef]
- Wingender, G.; Krebs, P.; Beutler, B.; Kronenberg, M. Antigen-Specific Cytotoxicity by Invariant NKT Cells in Vivo Is CD95/CD178-Dependent and Is Correlated with Antigenic Potency. J. Immunol. 2010, 185, 2721–2729. [Google Scholar] [CrossRef] [PubMed]
- De Biasi, S.; Gibellini, L.; Lo Tartaro, D.; Puccio, S.; Rabacchi, C.; Mazza, E.M.C.; Brummelman, J.; Williams, B.; Kaihara, K.; Forcato, M.; et al. Circulating Mucosal-Associated Invariant T Cells Identify Patients Responding to Anti-PD-1 Therapy. Nat. Commun. 2021, 12, 1669. [Google Scholar] [CrossRef] [PubMed]
- Halder, R.C.; Aguilera, C.; Maricic, I.; Kumar, V. Type II NKT Cell-Mediated Anergy Induction in Type I NKT Cells Prevents Inflammatory Liver Disease. J. Clin. Investig. 2007, 117, 2302–2312. [Google Scholar] [CrossRef] [PubMed]
- Imataki, O.; Arai, T.; Yamaoka, G.; Matsuoka, A.; Uemura, M. NKT Cell-Infiltrating Aseptic Meningitis on the Central Nervous System in Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia Treated with Dasatinib. Ann. Hematol. 2014, 93, 1935–1936. [Google Scholar] [CrossRef] [PubMed]
- Ambrosino, E.; Terabe, M.; Halder, R.C.; Peng, J.; Takaku, S.; Miyake, S.; Yamamura, T.; Kumar, V.; Berzofsky, J.A. Cross-Regulation between Type I and Type II NKT Cells in Regulating Tumor Immunity: A New Immunoregulatory Axis. J. Immunol. 2007, 179, 5126–5136. [Google Scholar] [CrossRef]
- Paget, C.; Chow, M.T.; Duret, H.; Mattarollo, S.R.; Smyth, M.J. Role of Γδ T Cells in α-Galactosylceramide-Mediated Immunity. J. Immunol. 2012, 188, 3928–3939. [Google Scholar] [CrossRef]
- Jin, N.; Miyahara, N.; Roark, C.L.; French, J.D.; Aydintug, M.K.; Matsuda, J.L.; Gapin, L.; O’Brien, R.L.; Gelfand, E.W.; Born, W.K. Airway Hyperresponsiveness through Synergy of Gammadelta} T Cells and NKT Cells. J. Immunol. 2007, 179, 2961–2968. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Rausell, A. Primary Immunodeficiencies Suggest Redundancy within the Human Immune System. Sci. Immunol. 2016, 1, eaah5861. [Google Scholar] [CrossRef]
- Cooper, M.D.; Alder, M.N. The Evolution of Adaptive Immune Systems. Cell 2006, 124, 815–822. [Google Scholar] [CrossRef]
- Salou, M.; Legoux, F.; Gilet, J.; Darbois, A.; du Halgouet, A.; Alonso, R.; Richer, W.; Goubet, A.-G.; Daviaud, C.; Menger, L.; et al. A Common Transcriptomic Program Acquired in the Thymus Defines Tissue Residency of MAIT and NKT Subsets. J. Exp. Med. 2018, 216, 133–151. [Google Scholar] [CrossRef]
- Jameson, J.; Ugarte, K.; Chen, N.; Yachi, P.; Fuchs, E.; Boismenu, R.; Havran, W.L. A Role for Skin Gammadelta T Cells in Wound Repair. Science 2002, 296, 747–749. [Google Scholar] [CrossRef]
- Simonian, P.L.; Wehrmann, F.; Roark, C.L.; Born, W.K.; O’Brien, R.L.; Fontenot, A.P. Γδ T Cells Protect against Lung Fibrosis via IL-22. J. Exp. Med. 2010, 207, 2239–2253. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.-G.; Gong, J.; Yeung, W.S.B.; Haidl, G.; Allam, J.-P. Natural Killer and NKT Cells in the Male Reproductive Tract. J. Reprod. Immunol. 2020, 142, 103178. [Google Scholar] [CrossRef] [PubMed]
- Wilharm, A.; Brigas, H.C.; Sandrock, I.; Ribeiro, M.; Amado, T.; Reinhardt, A.; Demera, A.; Hoenicke, L.; Strowig, T.; Carvalho, T.; et al. Microbiota-Dependent Expansion of Testicular IL-17-Producing Vγ6+ Γδ T Cells upon Puberty Promotes Local Tissue Immune Surveillance. Mucosal Immunol. 2021, 14, 242–252. [Google Scholar] [CrossRef]
- Ribeiro, M.; Brigas, H.C.; Temido-Ferreira, M.; Pousinha, P.A.; Regen, T.; Santa, C.; Coelho, J.E.; Marques-Morgado, I.; Valente, C.A.; Omenetti, S.; et al. Meningeal Γδ T Cell-Derived IL-17 Controls Synaptic Plasticity and Short-Term Memory. Sci. Immunol. 2019, 4. [Google Scholar] [CrossRef]
- Blankenstein, T.; Coulie, P.G.; Gilboa, E.; Jaffee, E.M. The Determinants of Tumour Immunogenicity. Nat. Rev. Cancer 2012, 12, 307–313. [Google Scholar] [CrossRef]
- Griffin, J.L.; Shockcor, J.P. Metabolic Profiles of Cancer Cells. Nat. Rev. Cancer 2004, 4, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Mullen, P.J.; Yu, R.; Longo, J.; Archer, M.C.; Penn, L.Z. The Interplay between Cell Signalling and the Mevalonate Pathway in Cancer. Nat. Rev. Cancer 2016, 16, 718–731. [Google Scholar] [CrossRef]
- Metelitsa, L.S. Anti-Tumor Potential of Type-I NKT Cells against CD1d-Positive and CD1d-Negative Tumors in Humans. Clin. Immunol. 2011, 140, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Asgharzadeh, S.; Salo, J.; Engell, K.; Wu, H.; Sposto, R.; Ara, T.; Silverman, A.M.; DeClerck, Y.A.; Seeger, R.C.; et al. Valpha24-Invariant NKT Cells Mediate Antitumor Activity via Killing of Tumor-Associated Macrophages. J. Clin. Investig. 2009, 119, 1524–1536. [Google Scholar] [CrossRef] [PubMed]
- Ritter, G.; Livingston, P.O. Ganglioside Antigens Expressed by Human Cancer Cells. Semin. Cancer Biol. 1991, 2, 401–409. [Google Scholar] [PubMed]
- Gentilini, M.V.; Pérez, M.E.; Fernández, P.M.; Fainboim, L.; Arana, E. The Tumor Antigen N-Glycolyl-GM3 Is a Human CD1d Ligand Capable of Mediating B Cell and Natural Killer T Cell Interaction. Cancer Immunol. Immunother. 2016, 65, 551–562. [Google Scholar] [CrossRef]
- Wu, D.Y.; Segal, N.H.; Sidobre, S.; Kronenberg, M.; Chapman, P.B. Cross-Presentation of Disialoganglioside GD3 to Natural Killer T Cells. J. Exp. Med. 2003, 198, 173–181. [Google Scholar] [CrossRef]
- Park, J.-E.; Wu, D.Y.; Prendes, M.; Lu, S.X.; Ragupathi, G.; Schrantz, N.; Chapman, P.B. Fine Specificity of Natural Killer T Cells against GD3 Ganglioside and Identification of GM3 as an Inhibitory Natural Killer T-Cell Ligand. Immunology 2008, 123, 145–155. [Google Scholar] [CrossRef]
- Mallevaey, T.; Clarke, A.J.; Scott-Browne, J.P.; Young, M.H.; Roisman, L.C.; Pellicci, D.G.; Patel, O.; Vivian, J.P.; Matsuda, J.L.; McCluskey, J.; et al. A Molecular Basis for NKT Cell Recognition of CD1d-Self-Antigen. Immunity 2011, 34, 315–326. [Google Scholar] [CrossRef]
- Tiper, I.V.; Temkin, S.M.; Spiegel, S.; Goldblum, S.E.; Giuntoli, R.L.; Oelke, M.; Schneck, J.P.; Webb, T.J. VEGF Potentiates GD3-Mediated Immunosuppression by Human Ovarian Cancer Cells. Clin. Cancer Res. 2016, 22, 4249–4258. [Google Scholar] [CrossRef]
- Webb, T.J.; Li, X.; Giuntoli, R.L.; Lopez, P.H.H.; Heuser, C.; Schnaar, R.L.; Tsuji, M.; Kurts, C.; Oelke, M.; Schneck, J.P. Molecular Identification of GD3 as a Suppressor of the Innate Immune Response in Ovarian Cancer. Cancer Res. 2012, 72, 3744–3752. [Google Scholar] [CrossRef]
- Heczey, A.; Courtney, A.N.; Montalbano, A.; Robinson, S.; Liu, K.; Li, M.; Ghatwai, N.; Dakhova, O.; Liu, B.; Raveh-Sadka, T.; et al. Anti-GD2 CAR-NKT Cells in Patients with Relapsed or Refractory Neuroblastoma: An Interim Analysis. Nat. Med. 2020, 26, 1686–1690. [Google Scholar] [CrossRef]
- Xu, X.; Huang, W.; Heczey, A.; Liu, D.; Guo, L.; Wood, M.; Jin, J.; Courtney, A.N.; Liu, B.; Di Pierro, E.J.; et al. NKT Cells Coexpressing a GD2-Specific Chimeric Antigen Receptor and IL15 Show Enhanced In Vivo Persistence and Antitumor Activity against Neuroblastoma. Clin. Cancer Res. 2019, 25, 7126–7138. [Google Scholar] [CrossRef]
- Heczey, A.; Liu, D.; Tian, G.; Courtney, A.N.; Wei, J.; Marinova, E.; Gao, X.; Guo, L.; Yvon, E.; Hicks, J.; et al. Invariant NKT Cells with Chimeric Antigen Receptor Provide a Novel Platform for Safe and Effective Cancer Immunotherapy. Blood 2014, 124, 2824–2833. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Weissman, A.M. The Unfolded Protein Response, Degradation from Endoplasmic Reticulum and Cancer. Genes Cancer 2010, 1, 764–778. [Google Scholar] [CrossRef]
- Govindarajan, S.; Verheugen, E.; Venken, K.; Gaublomme, D.; Maelegheer, M.; Cloots, E.; Gysens, F.; De Geest, B.G.; Cheng, T.-Y.; Moody, D.B.; et al. ER Stress in Antigen-Presenting Cells Promotes NKT Cell Activation through Endogenous Neutral Lipids. EMBO Rep. 2020, 21, e48927. [Google Scholar] [CrossRef]
- Singh, A.K.; Tripathi, P.; Cardell, S.L. Type II NKT Cells: An Elusive Population with Immunoregulatory Properties. Front. Immunol. 2018, 9, 1969. [Google Scholar] [CrossRef] [PubMed]
- Van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane Lipids: Where They Are and How They Behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef]
- Ward, P.S.; Thompson, C.B. Metabolic Reprogramming: A Cancer Hallmark Even Warburg Did Not Anticipate. Cancer Cell 2012, 21, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Blomqvist, M.; Rhost, S.; Teneberg, S.; Löfbom, L.; Osterbye, T.; Brigl, M.; Månsson, J.-E.; Cardell, S.L. Multiple Tissue-Specific Isoforms of Sulfatide Activate CD1d-Restricted Type II NKT Cells. Eur. J. Immunol. 2009, 39, 1726–1735. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Suzuki, T. Role of Sulfatide in Normal and Pathological Cells and Tissues. J. Lipid Res. 2012, 53, 1437–1450. [Google Scholar] [CrossRef]
- Gober, H.-J.; Kistowska, M.; Angman, L.; Jenö, P.; Mori, L.; De Libero, G. Human T Cell Receptor Gammadelta Cells Recognize Endogenous Mevalonate Metabolites in Tumor Cells. J. Exp. Med. 2003, 197, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Scotet, E.; Martinez, L.O.; Grant, E.; Barbaras, R.; Jenö, P.; Guiraud, M.; Monsarrat, B.; Saulquin, X.; Maillet, S.; Estève, J.-P.; et al. Tumor Recognition Following Vgamma9Vdelta2 T Cell Receptor Interactions with a Surface F1-ATPase-Related Structure and Apolipoprotein A-I. Immunity 2005, 22, 71–80. [Google Scholar] [CrossRef]
- Yan, J.; Allen, S.; McDonald, E.; Das, I.; Mak, J.Y.W.; Liu, L.; Fairlie, D.P.; Meehan, B.S.; Chen, Z.; Corbett, A.J.; et al. MAIT Cells Promote Tumor Initiation, Growth, and Metastases via Tumor MR1. Cancer Discov. 2020, 10, 124–141. [Google Scholar] [CrossRef] [PubMed]
- Lepore, M.; Kalinichenko, A.; Calogero, S.; Kumar, P.; Paleja, B.; Schmaler, M.; Narang, V.; Zolezzi, F.; Poidinger, M.; Mori, L.; et al. Functionally Diverse Human T Cells Recognize Non-Microbial Antigens Presented by MR1. eLife 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Coffelt, S.B.; Kersten, K.; Doornebal, C.W.; Weiden, J.; Vrijland, K.; Hau, C.-S.; Verstegen, N.J.M.; Ciampricotti, M.; Hawinkels, L.J.A.C.; Jonkers, J.; et al. IL-17-Producing Γδ T Cells and Neutrophils Conspire to Promote Breast Cancer Metastasis. Nature 2015, 522, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Carmi, Y.; Rinott, G.; Dotan, S.; Elkabets, M.; Rider, P.; Voronov, E.; Apte, R.N. Microenvironment-Derived IL-1 and IL-17 Interact in the Control of Lung Metastasis. J. Immunol. 2011, 186, 3462–3471. [Google Scholar] [CrossRef] [PubMed]
- Wakita, D.; Sumida, K.; Iwakura, Y.; Nishikawa, H.; Ohkuri, T.; Chamoto, K.; Kitamura, H.; Nishimura, T. Tumor-Infiltrating IL-17-Producing Gammadelta T Cells Support the Progression of Tumor by Promoting Angiogenesis. Eur. J. Immunol. 2010, 40, 1927–1937. [Google Scholar] [CrossRef] [PubMed]
- Al-Rawi, M.A.A.; Rmali, K.; Watkins, G.; Mansel, R.E.; Jiang, W.G. Aberrant Expression of Interleukin-7 (IL-7) and Its Signalling Complex in Human Breast Cancer. Eur. J. Cancer 2004, 40, 494–502. [Google Scholar] [CrossRef]
- Silva, A.; Laranjeira, A.B.A.; Martins, L.R.; Cardoso, B.A.; Demengeot, J.; Yunes, J.A.; Seddon, B.; Barata, J.T. IL-7 Contributes to the Progression of Human T-Cell Acute Lymphoblastic Leukemias. Cancer Res. 2011, 71, 4780–4789. [Google Scholar] [CrossRef]
- Schroten, C.; Dits, N.F.; Steyerberg, E.W.; Kranse, R.; van Leenders, A.G.J.L.H.; Bangma, C.H.; Kraaij, R. The Additional Value of TGFβ1 and IL-7 to Predict the Course of Prostate Cancer Progression. Cancer Immunol. Immunother. 2012, 61, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Patin, E.C.; Soulard, D.; Fleury, S.; Hassane, M.; Dombrowicz, D.; Faveeuw, C.; Trottein, F.; Paget, C. Type I IFN Receptor Signaling Controls IL7-Dependent Accumulation and Activity of Protumoral IL17A-Producing ΓδT Cells in Breast Cancer. Cancer Res. 2018, 78, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Rei, M.; Gonçalves-Sousa, N.; Lança, T.; Thompson, R.G.; Mensurado, S.; Balkwill, F.R.; Kulbe, H.; Pennington, D.J.; Silva-Santos, B. Murine CD27(-) Vγ6(+) Γδ T Cells Producing IL-17A Promote Ovarian Cancer Growth via Mobilization of Protumor Small Peritoneal Macrophages. Proc. Natl. Acad. Sci. USA 2014, 111, E3562–E3570. [Google Scholar] [CrossRef]
- Hassane, M.; Jouan, Y.; Creusat, F.; Soulard, D.; Boisseau, C.; Gonzalez, L.; Patin, E.C.; Heuzé-Vourc’h, N.; Sirard, J.-C.; Faveeuw, C.; et al. Interleukin-7 Protects against Bacterial Respiratory Infection by Promoting IL-17A-Producing Innate T-Cell Response. Mucosal Immunol. 2020, 13, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Michel, M.-L.; Pang, D.J.; Haque, S.F.Y.; Potocnik, A.J.; Pennington, D.J.; Hayday, A.C. Interleukin 7 (IL-7) Selectively Promotes Mouse and Human IL-17-Producing Γδ Cells. Proc. Natl. Acad. Sci. USA 2012, 109, 17549–17554. [Google Scholar] [CrossRef] [PubMed]
- Webster, K.E.; Kim, H.-O.; Kyparissoudis, K.; Corpuz, T.M.; Pinget, G.V.; Uldrich, A.P.; Brink, R.; Belz, G.T.; Cho, J.-H.; Godfrey, D.I.; et al. IL-17-Producing NKT Cells Depend Exclusively on IL-7 for Homeostasis and Survival. Mucosal Immunol. 2014, 7, 1058–1067. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.-Z.; Jo, J.; Tan, A.T.; Sandalova, E.; Chia, A.; Tan, K.C.; Lee, K.H.; Gehring, A.J.; De Libero, G.; Bertoletti, A. IL-7 Licenses Activation of Human Liver Intrasinusoidal Mucosal-Associated Invariant T Cells. J. Immunol. 2013, 190, 3142–3152. [Google Scholar] [CrossRef]
- Casetti, R.; Agrati, C.; Wallace, M.; Sacchi, A.; Martini, F.; Martino, A.; Rinaldi, A.; Malkovsky, M. Cutting Edge: TGF-Beta1 and IL-15 Induce FOXP3+ Gammadelta Regulatory T Cells in the Presence of Antigen Stimulation. J. Immunol. 2009, 183, 3574–3577. [Google Scholar] [CrossRef]
- Hu, Y.; Cui, Q.; Gu, Y.; Sheng, L.; Wu, K.; Shi, J.; Tan, Y.; Fu, H.; Liu, L.; Fu, S.; et al. Decitabine Facilitates the Generation and Immunosuppressive Function of Regulatory ΓδT Cells Derived from Human Peripheral Blood Mononuclear Cells. Leukemia 2013, 27, 1580–1585. [Google Scholar] [CrossRef]
- Monteiro, M.; Almeida, C.F.; Caridade, M.; Ribot, J.C.; Duarte, J.; Agua-Doce, A.; Wollenberg, I.; Silva-Santos, B.; Graca, L. Identification of Regulatory Foxp3+ Invariant NKT Cells Induced by TGF-Beta. J. Immunol. 2010, 185, 2157–2163. [Google Scholar] [CrossRef]
- Moreira-Teixeira, L.; Resende, M.; Devergne, O.; Herbeuval, J.-P.; Hermine, O.; Schneider, E.; Dy, M.; Cordeiro-da-Silva, A.; Leite-de-Moraes, M.C. Rapamycin Combined with TGF-β Converts Human Invariant NKT Cells into Suppressive Foxp3+ Regulatory Cells. J. Immunol. 2012, 188, 624–631. [Google Scholar] [CrossRef]
- Hu, G.; Wu, P.; Cheng, P.; Zhang, Z.; Wang, Z.; Yu, X.; Shao, X.; Wu, D.; Ye, J.; Zhang, T.; et al. Tumor-Infiltrating CD39+γδTregs Are Novel Immunosuppressive T Cells in Human Colorectal Cancer. Oncoimmunology 2017, 6, e1277305. [Google Scholar] [CrossRef] [PubMed]
- Itatani, Y.; Kawada, K.; Sakai, Y. Transforming Growth Factor-β Signaling Pathway in Colorectal Cancer and Its Tumor Microenvironment. Int. J. Mol. Sci. 2019, 20, 5822. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Yin, S.; Zhang, J.; Hu, Y.; Huang, B.; Cui, L.; Kang, N.; He, W. A New Effect of IL-4 on Human Γδ T Cells: Promoting Regulatory Vδ1 T Cells via IL-10 Production and Inhibiting Function of Vδ2 T Cells. Cell Mol. Immunol. 2016, 13, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Dong, S.; Xia, S.; He, W.; Jia, H.; Zhang, S.; Wei, J.; O’Brien, R.L.; Born, W.K.; Wu, Z.; et al. Regulatory Role of Vγ1 Γδ T Cells in Tumor Immunity through IL-4 Production. J. Immunol. 2011, 187, 4979–4986. [Google Scholar] [CrossRef]
- Terabe, M.; Matsui, S.; Noben-Trauth, N.; Chen, H.; Watson, C.; Donaldson, D.D.; Carbone, D.P.; Paul, W.E.; Berzofsky, J.A. NKT Cell-Mediated Repression of Tumor Immunosurveillance by IL-13 and the IL-4R-STAT6 Pathway. Nat. Immunol. 2000, 1, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Spolski, R.; Leonard, W.J. Interleukin-21: A Double-Edged Sword with Therapeutic Potential. Nat. Rev. Drug Discov. 2014, 13, 379–395. [Google Scholar] [CrossRef]
- Chabab, G.; Bonnefoy, N.; Lafont, V. IL-21 Signaling in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1240, 73–82. [Google Scholar] [CrossRef]
- Chabab, G.; Barjon, C.; Abdellaoui, N.; Salvador-Prince, L.; Dejou, C.; Michaud, H.-A.; Boissière-Michot, F.; Lopez-Crapez, E.; Jacot, W.; Pourquier, D.; et al. Identification of a Regulatory Vδ1 Gamma Delta T Cell Subpopulation Expressing CD73 in Human Breast Cancer. J. Leukoc. Biol. 2020, 107, 1057–1067. [Google Scholar] [CrossRef]
- Barjon, C.; Michaud, H.-A.; Fages, A.; Dejou, C.; Zampieri, A.; They, L.; Gennetier, A.; Sanchez, F.; Gros, L.; Eliaou, J.-F.; et al. IL-21 Promotes the Development of a CD73-Positive Vγ9Vδ2 T Cell Regulatory Population. Oncoimmunology 2017, 7, e1379642. [Google Scholar] [CrossRef]
- Coquet, J.M.; Skak, K.; Davis, I.D.; Smyth, M.J.; Godfrey, D.I. IL-21 Modulates Activation of NKT Cells in Patients with Stage IV Malignant Melanoma. Clin. Transl. Immunol. 2013, 2, e6. [Google Scholar] [CrossRef]
- Neri, D.; Supuran, C.T. Interfering with PH Regulation in Tumours as a Therapeutic Strategy. Nat. Rev. Drug Discov. 2011, 10, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.R.; Hay, M.P. Targeting Hypoxia in Cancer Therapy. Nat. Rev. Cancer 2011, 11, 393–410. [Google Scholar] [CrossRef] [PubMed]
- DePeaux, K.; Delgoffe, G.M. Metabolic Barriers to Cancer Immunotherapy. Nat. Rev. Immunol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Zhu, S.; Bai, L. Lactic Acid in Tumor Microenvironments Causes Dysfunction of NKT Cells by Interfering with MTOR Signaling. Sci. China Life Sci. 2016, 59, 1290–1296. [Google Scholar] [CrossRef]
- Fu, S.; He, K.; Tian, C.; Sun, H.; Zhu, C.; Bai, S.; Liu, J.; Wu, Q.; Xie, D.; Yue, T.; et al. Impaired Lipid Biosynthesis Hinders Anti-Tumor Efficacy of Intratumoral INKT Cells. Nat. Commun. 2020, 11, 438. [Google Scholar] [CrossRef]
- O’Brien, A.; Loftus, R.M.; Pisarska, M.M.; Tobin, L.M.; Bergin, R.; Wood, N.A.W.; Foley, C.; Mat, A.; Tinley, F.C.; Bannan, C.; et al. Obesity Reduces MTORC1 Activity in Mucosal-Associated Invariant T Cells, Driving Defective Metabolic and Functional Responses. J. Immunol. 2019, 202, 3404–3411. [Google Scholar] [CrossRef]
- O’Neill, C.; Cassidy, F.C.; O’Shea, D.; Hogan, A.E. Mucosal Associated Invariant T Cells in Cancer-Friend or Foe? Cancers 2021, 13, 1582. [Google Scholar] [CrossRef]
- Martinet, L.; Fleury-Cappellesso, S.; Gadelorge, M.; Dietrich, G.; Bourin, P.; Fournié, J.-J.; Poupot, R. A Regulatory Cross-Talk between Vgamma9Vdelta2 T Lymphocytes and Mesenchymal Stem Cells. Eur. J. Immunol. 2009, 39, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Martinet, L.; Jean, C.; Dietrich, G.; Fournié, J.-J.; Poupot, R. PGE2 Inhibits Natural Killer and Gamma Delta T Cell Cytotoxicity Triggered by NKR and TCR through a CAMP-Mediated PKA Type I-Dependent Signaling. Biochem. Pharmacol. 2010, 80, 838–845. [Google Scholar] [CrossRef]
- Gonnermann, D.; Oberg, H.-H.; Kellner, C.; Peipp, M.; Sebens, S.; Kabelitz, D.; Wesch, D. Resistance of Cyclooxygenase-2 Expressing Pancreatic Ductal Adenocarcinoma Cells against Γδ T Cell Cytotoxicity. Oncoimmunology 2015, 4, e988460. [Google Scholar] [CrossRef] [PubMed]
- Hodge, G.; Barnawi, J.; Jurisevic, C.; Moffat, D.; Holmes, M.; Reynolds, P.N.; Jersmann, H.; Hodge, S. Lung Cancer Is Associated with Decreased Expression of Perforin, Granzyme B and Interferon (IFN)-γ by Infiltrating Lung Tissue T Cells, Natural Killer (NK) T-like and NK Cells. Clin. Exp. Immunol. 2014, 178, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Klatka, J.; Grywalska, E.; Hymos, A.; Guz, M.; Polberg, K.; Roliński, J.; Stepulak, A. Cyclooxygenase-2 Inhibition Enhances Proliferation of NKT Cells Derived from Patients with Laryngeal Cancer. Anticancer Res. 2017, 37, 4059–4066. [Google Scholar] [CrossRef][Green Version]
- Lopes, N.; McIntyre, C.; Martin, S.; Raverdeau, M.; Sumaria, N.; Kohlgruber, A.C.; Fiala, G.J.; Agudelo, L.Z.; Dyck, L.; Kane, H.; et al. Distinct Metabolic Programs Established in the Thymus Control Effector Functions of Γδ T Cell Subsets in Tumor Microenvironments. Nat. Immunol. 2021, 22, 179–192. [Google Scholar] [CrossRef]
- Ko, J.S.; Koh, J.M.; So, J.-S.; Jeon, Y.K.; Kim, H.Y.; Chung, D.H. Palmitate Inhibits Arthritis by Inducing T-Bet and Gata-3 MRNA Degradation in INKT Cells via IRE1α-Dependent Decay. Sci. Rep. 2017, 7, 14940. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, H.-J.; Kim, C.W.; Kim, H.C.; Jung, Y.; Lee, H.-S.; Lee, Y.; Ju, Y.S.; Oh, J.E.; Park, S.-H.; et al. Tumor Hypoxia Represses Γδ T Cell-Mediated Antitumor Immunity against Brain Tumors. Nat. Immunol. 2021, 22, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Ayyoub, M.; Routy, B.; Kroemer, G. Microbiome and Anticancer Immunosurveillance. Cell 2016, 165, 276–287. [Google Scholar] [CrossRef]
- Routy, B.; Gopalakrishnan, V.; Daillère, R.; Zitvogel, L.; Wargo, J.A.; Kroemer, G. The Gut Microbiota Influences Anticancer Immunosurveillance and General Health. Nat. Rev. Clin. Oncol. 2018, 15, 382–396. [Google Scholar] [CrossRef]
- Wu, X.; Sun, R.; Chen, Y.; Zheng, X.; Bai, L.; Lian, Z.; Wei, H.; Tian, Z. Oral Ampicillin Inhibits Liver Regeneration by Breaking Hepatic Innate Immune Tolerance Normally Maintained by Gut Commensal Bacteria. Hepatology 2015, 62, 253–264. [Google Scholar] [CrossRef]
- Qian, X.; Chen, H.; Wu, X.; Hu, L.; Huang, Q.; Jin, Y. Interleukin-17 Acts as Double-Edged Sword in Anti-Tumor Immunity and Tumorigenesis. Cytokine 2017, 89, 34–44. [Google Scholar] [CrossRef]
- Lefrancois, L.; Goodman, T. In Vivo Modulation of Cytolytic Activity and Thy-1 Expression in TCR-Gamma Delta+ Intraepithelial Lymphocytes. Science 1989, 243, 1716–1718. [Google Scholar] [CrossRef] [PubMed]
- McAllister, F.; Bailey, J.M.; Alsina, J.; Nirschl, C.J.; Sharma, R.; Fan, H.; Rattigan, Y.; Roeser, J.C.; Lankapalli, R.H.; Zhang, H.; et al. Oncogenic Kras Activates a Hematopoietic-to-Epithelial IL-17 Signaling Axis in Preinvasive Pancreatic Neoplasia. Cancer Cell 2014, 25, 621–637. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yi, T.; Kortylewski, M.; Pardoll, D.M.; Zeng, D.; Yu, H. IL-17 Can Promote Tumor Growth through an IL-6-Stat3 Signaling Pathway. J. Exp. Med. 2009, 206, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Geddes, K.; Rubino, S.J.; Magalhaes, J.G.; Streutker, C.; Le Bourhis, L.; Cho, J.H.; Robertson, S.J.; Kim, C.J.; Kaul, R.; Philpott, D.J.; et al. Identification of an Innate T Helper Type 17 Response to Intestinal Bacterial Pathogens. Nat. Med. 2011, 17, 837–844. [Google Scholar] [CrossRef]
- Wingender, G.; Stepniak, D.; Krebs, P.; Lin, L.; McBride, S.; Wei, B.; Braun, J.; Mazmanian, S.K.; Kronenberg, M. Intestinal Microbes Affect Phenotypes and Functions of Invariant Natural Killer T Cells in Mice. Gastroenterology 2012, 143, 418–428. [Google Scholar] [CrossRef]
- Olszak, T.; An, D.; Zeissig, S.; Vera, M.P.; Richter, J.; Franke, A.; Glickman, J.N.; Siebert, R.; Baron, R.M.; Kasper, D.L.; et al. Microbial Exposure during Early Life Has Persistent Effects on Natural Killer T Cell Function. Science 2012, 336, 489–493. [Google Scholar] [CrossRef]
- Sundström, P.; Ahlmanner, F.; Akéus, P.; Sundquist, M.; Alsén, S.; Yrlid, U.; Börjesson, L.; Sjöling, Å.; Gustavsson, B.; Wong, S.B.J.; et al. Human Mucosa-Associated Invariant T Cells Accumulate in Colon Adenocarcinomas but Produce Reduced Amounts of IFN-γ. J. Immunol. 2015, 195, 3472–3481. [Google Scholar] [CrossRef]
- De Palma, M.; Biziato, D.; Petrova, T.V. Microenvironmental Regulation of Tumour Angiogenesis. Nat. Rev. Cancer 2017, 17, 457–474. [Google Scholar] [CrossRef]
- Duan, M.; Goswami, S.; Shi, J.-Y.; Wu, L.-J.; Wang, X.-Y.; Ma, J.-Q.; Zhang, Z.; Shi, Y.; Ma, L.-J.; Zhang, S.; et al. Activated and Exhausted MAIT Cells Foster Disease Progression and Indicate Poor Outcome in Hepatocellular Carcinoma. Clin. Cancer Res. 2019, 25, 3304–3316. [Google Scholar] [CrossRef]
- An, D.; Oh, S.F.; Olszak, T.; Neves, J.F.; Avci, F.Y.; Erturk-Hasdemir, D.; Lu, X.; Zeissig, S.; Blumberg, R.S.; Kasper, D.L. Sphingolipids from a Symbiotic Microbe Regulate Homeostasis of Host Intestinal Natural Killer T Cells. Cell 2014, 156, 123–133. [Google Scholar] [CrossRef]
- Ridaura, V.K.; Bouladoux, N.; Claesen, J.; Chen, Y.E.; Byrd, A.L.; Constantinides, M.G.; Merrill, E.D.; Tamoutounour, S.; Fischbach, M.A.; Belkaid, Y. Contextual Control of Skin Immunity and Inflammation by Corynebacterium. J. Exp. Med. 2018, 215, 785–799. [Google Scholar] [CrossRef]
- Jin, C.; Lagoudas, G.K.; Zhao, C.; Bullman, S.; Bhutkar, A.; Hu, B.; Ameh, S.; Sandel, D.; Liang, X.S.; Mazzilli, S.; et al. Commensal Microbiota Promote Lung Cancer Development via Γδ T Cells. Cell 2019, 176, 998.e16–1013.e16. [Google Scholar] [CrossRef]
- Selvanantham, T.; Lin, Q.; Guo, C.X.; Surendra, A.; Fieve, S.; Escalante, N.K.; Guttman, D.S.; Streutker, C.J.; Robertson, S.J.; Philpott, D.J.; et al. NKT Cell-Deficient Mice Harbor an Altered Microbiota That Fuels Intestinal Inflammation during Chemically Induced Colitis. J. Immunol. 2016, 197, 4464–4472. [Google Scholar] [CrossRef]
- Ma, C.; Han, M.; Heinrich, B.; Fu, Q.; Zhang, Q.; Sandhu, M.; Agdashian, D.; Terabe, M.; Berzofsky, J.A.; Fako, V.; et al. Gut Microbiome-Mediated Bile Acid Metabolism Regulates Liver Cancer via NKT Cells. Science 2018, 360. [Google Scholar] [CrossRef] [PubMed]
- Aliper, A.M.; Frieden-Korovkina, V.P.; Buzdin, A.; Roumiantsev, S.A.; Zhavoronkov, A. A Role for G-CSF and GM-CSF in Nonmyeloid Cancers. Cancer Med. 2014, 3, 737–746. [Google Scholar] [CrossRef]
- Van Hede, D.; Polese, B.; Humblet, C.; Wilharm, A.; Renoux, V.; Dortu, E.; de Leval, L.; Delvenne, P.; Desmet, C.J.; Bureau, F.; et al. Human Papillomavirus Oncoproteins Induce a Reorganization of Epithelial-Associated Γδ T Cells Promoting Tumor Formation. Proc. Natl. Acad. Sci. USA 2017, 114, E9056–E9065. [Google Scholar] [CrossRef] [PubMed]
- Kulig, P.; Burkhard, S.; Mikita-Geoffroy, J.; Croxford, A.L.; Hövelmeyer, N.; Gyülvészi, G.; Gorzelanny, C.; Waisman, A.; Borsig, L.; Becher, B. IL17A-Mediated Endothelial Breach Promotes Metastasis Formation. Cancer Immunol. Res. 2016, 4, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Leng, T.; Akther, H.D.; Hackstein, C.-P.; Powell, K.; King, T.; Friedrich, M.; Christoforidou, Z.; McCuaig, S.; Neyazi, M.; Arancibia-Cárcamo, C.V.; et al. TCR and Inflammatory Signals Tune Human MAIT Cells to Exert Specific Tissue Repair and Effector Functions. Cell Rep. 2019, 28, 3077.e5–3091.e5. [Google Scholar] [CrossRef]
- Hinks, T.S.C.; Marchi, E.; Jabeen, M.; Olshansky, M.; Kurioka, A.; Pediongco, T.J.; Meehan, B.S.; Kostenko, L.; Turner, S.J.; Corbett, A.J.; et al. Activation and In Vivo Evolution of the MAIT Cell Transcriptome in Mice and Humans Reveals Tissue Repair Functionality. Cell Rep. 2019, 28, 3249.e5–3262.e5. [Google Scholar] [CrossRef]
- Xie, K. Interleukin-8 and Human Cancer Biology. Cytokine Growth Factor Rev. 2001, 12, 375–391. [Google Scholar] [CrossRef]
- Khosravi, N.; Caetano, M.S.; Cumpian, A.M.; Unver, N.; De la Garza Ramos, C.; Noble, O.; Daliri, S.; Hernandez, B.J.; Gutierrez, B.A.; Evans, S.E.; et al. IL22 Promotes Kras-Mutant Lung Cancer by Induction of a Protumor Immune Response and Protection of Stemness Properties. Cancer Immunol. Res. 2018, 6, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Wang, H.Y.; Peng, W.; Kiniwa, Y.; Seo, K.H.; Wang, R.-F. Tumor-Infiltrating Gammadelta T Cells Suppress T and Dendritic Cell Function via Mechanisms Controlled by a Unique Toll-like Receptor Signaling Pathway. Immunity 2007, 27, 334–348. [Google Scholar] [CrossRef]
- Rutkowski, M.R.; Stephen, T.L.; Svoronos, N.; Allegrezza, M.J.; Tesone, A.J.; Perales-Puchalt, A.; Brencicova, E.; Escovar-Fadul, X.; Nguyen, J.M.; Cadungog, M.G.; et al. Microbially Driven TLR5-Dependent Signaling Governs Distal Malignant Progression through Tumor-Promoting Inflammation. Cancer Cell 2015, 27, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Anderson, A.C.; Schubart, A.; Xiong, H.; Imitola, J.; Khoury, S.J.; Zheng, X.X.; Strom, T.B.; Kuchroo, V.K. The Tim-3 Ligand Galectin-9 Negatively Regulates T Helper Type 1 Immunity. Nat. Immunol. 2005, 6, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer Immunoediting: Integrating Immunity’s Roles in Cancer Suppression and Promotion. Science 2011, 331, 1565–1570. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Bronte, V. Coordinated Regulation of Myeloid Cells by Tumours. Nat. Rev. Immunol. 2012, 12, 253–268. [Google Scholar] [CrossRef] [PubMed]
- Jaillon, S.; Ponzetta, A.; Di Mitri, D.; Santoni, A.; Bonecchi, R.; Mantovani, A. Neutrophil Diversity and Plasticity in Tumour Progression and Therapy. Nat. Rev. Cancer 2020, 20, 485–503. [Google Scholar] [CrossRef]
- Togashi, Y.; Shitara, K.; Nishikawa, H. Regulatory T Cells in Cancer Immunosuppression—Implications for Anticancer Therapy. Nat. Rev. Clin. Oncol. 2019, 16, 356–371. [Google Scholar] [CrossRef]
- Wculek, S.K.; Cueto, F.J.; Mujal, A.M.; Melero, I.; Krummel, M.F.; Sancho, D. Dendritic Cells in Cancer Immunology and Immunotherapy. Nat. Rev. Immunol. 2020, 20, 7–24. [Google Scholar] [CrossRef]
- Wu, P.; Wu, D.; Ni, C.; Ye, J.; Chen, W.; Hu, G.; Wang, Z.; Wang, C.; Zhang, Z.; Xia, W.; et al. ΓδT17 Cells Promote the Accumulation and Expansion of Myeloid-Derived Suppressor Cells in Human Colorectal Cancer. Immunity 2014, 40, 785–800. [Google Scholar] [CrossRef]
- Lu, Z.; Zhu, M.; Marley, J.L.; Bi, K.; Wang, K.; Zhai, M.; Hu, H.; Guo, P.; Li, C.; Xu, Y.; et al. The Combined Action of Monocytic Myeloid-Derived Suppressor Cells and Mucosal-Associated Invariant T Cells Promotes the Progression of Cervical Cancer. Int. J. Cancer 2021, 148, 1499–1507. [Google Scholar] [CrossRef]
- Terabe, M.; Matsui, S.; Park, J.-M.; Mamura, M.; Noben-Trauth, N.; Donaldson, D.D.; Chen, W.; Wahl, S.M.; Ledbetter, S.; Pratt, B.; et al. Transforming Growth Factor-Beta Production and Myeloid Cells Are an Effector Mechanism through Which CD1d-Restricted T Cells Block Cytotoxic T Lymphocyte-Mediated Tumor Immunosurveillance: Abrogation Prevents Tumor Recurrence. J. Exp. Med. 2003, 198, 1741–1752. [Google Scholar] [CrossRef]
- Kelly, J.; Minoda, Y.; Meredith, T.; Cameron, G.; Philipp, M.-S.; Pellicci, D.G.; Corbett, A.J.; Kurts, C.; Gray, D.H.; Godfrey, D.I.; et al. Chronically Stimulated Human MAIT Cells Are Unexpectedly Potent IL-13 Producers. Immunol. Cell Biol. 2019, 97, 689–699. [Google Scholar] [CrossRef]
- Wang, Y.; Sedimbi, S.; Löfbom, L.; Singh, A.K.; Porcelli, S.A.; Cardell, S.L. Unique Invariant Natural Killer T Cells Promote Intestinal Polyps by Suppressing TH1 Immunity and Promoting Regulatory T Cells. Mucosal Immunol. 2018, 11, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Venken, K.; Decruy, T.; Aspeslagh, S.; Van Calenbergh, S.; Lambrecht, B.N.; Elewaut, D. Bacterial CD1d-Restricted Glycolipids Induce IL-10 Production by Human Regulatory T Cells upon Cross-Talk with Invariant NKT Cells. J. Immunol. 2013, 191, 2174–2183. [Google Scholar] [CrossRef] [PubMed]
- Beavis, P.A.; Stagg, J.; Darcy, P.K.; Smyth, M.J. CD73: A Potent Suppressor of Antitumor Immune Responses. Trends Immunol. 2012, 33, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Perillo, N.L.; Pace, K.E.; Seilhamer, J.J.; Baum, L.G. Apoptosis of T Cells Mediated by Galectin-1. Nature 1995, 378, 736–739. [Google Scholar] [CrossRef] [PubMed]
- Daley, D.; Zambirinis, C.P.; Seifert, L.; Akkad, N.; Mohan, N.; Werba, G.; Barilla, R.; Torres-Hernandez, A.; Hundeyin, M.; Mani, V.R.K.; et al. Γδ T Cells Support Pancreatic Oncogenesis by Restraining Aβ T Cell Activation. Cell 2016, 166, 1485.e15–1499.e15. [Google Scholar] [CrossRef]
- Banach, M.; Robert, J. Evolutionary Underpinnings of Innate-Like T Cell Interactions with Cancer. Immunol. Investig. 2019, 48, 737–758. [Google Scholar] [CrossRef]
- Kabelitz, D.; Serrano, R.; Kouakanou, L.; Peters, C.; Kalyan, S. Cancer Immunotherapy with Γδ T Cells: Many Paths Ahead of Us. Cell Mol. Immunol. 2020, 17, 925–939. [Google Scholar] [CrossRef]
- Cogswell, D.T.; Gapin, L.; Tobin, H.M.; McCarter, M.D.; Tobin, R.P. MAIT Cells: Partners or Enemies in Cancer Immunotherapy? Cancers 2021, 13, 1502. [Google Scholar] [CrossRef]
- Aehnlich, P.; Carnaz Simões, A.M.; Skadborg, S.K.; Holmen Olofsson, G.; Thor Straten, P. Expansion with IL-15 Increases Cytotoxicity of Vγ9Vδ2 T Cells and Is Associated with Higher Levels of Cytotoxic Molecules and T-Bet. Front. Immunol. 2020, 11, 1868. [Google Scholar] [CrossRef] [PubMed]
- Yamada, D.; Iyoda, T.; Vizcardo, R.; Shimizu, K.; Sato, Y.; Endo, T.A.; Kitahara, G.; Okoshi, M.; Kobayashi, M.; Sakurai, M.; et al. Efficient Regeneration of Human Vα24+ Invariant Natural Killer T Cells and Their Anti-Tumor Activity In Vivo. Stem Cells 2016, 34, 2852–2860. [Google Scholar] [CrossRef] [PubMed]
- Parrot, T.; Healy, K.; Boulouis, C.; Sobkowiak, M.J.; Leeansyah, E.; Aleman, S.; Bertoletti, A.; Sällberg Chen, M.; Sandberg, J.K. Expansion of Donor-Unrestricted MAIT Cells with Enhanced Cytolytic Function Suitable for TCR Redirection. JCI Insight 2021, 6, 140074. [Google Scholar] [CrossRef] [PubMed]
- Akkapeddi, P.; Fragoso, R.; Hixon, J.A.; Ramalho, A.S.; Oliveira, M.L.; Carvalho, T.; Gloger, A.; Matasci, M.; Corzana, F.; Durum, S.K.; et al. A Fully Human Anti-IL-7Rα Antibody Promotes Antitumor Activity against T-Cell Acute Lymphoblastic Leukemia. Leukemia 2019, 33, 2155–2168. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; MacFadyen, J.G.; Thuren, T.; Everett, B.M.; Libby, P.; Glynn, R.J.; CANTOS Trial Group. Effect of Interleukin-1β Inhibition with Canakinumab on Incident Lung Cancer in Patients with Atherosclerosis: Exploratory Results from a Randomised, Double-Blind, Placebo-Controlled Trial. Lancet 2017, 390, 1833–1842. [Google Scholar] [CrossRef]
- Dodagatta-Marri, E.; Meyer, D.S.; Reeves, M.Q.; Paniagua, R.; To, M.D.; Binnewies, M.; Broz, M.L.; Mori, H.; Wu, D.; Adoumie, M.; et al. α-PD-1 Therapy Elevates Treg/Th Balance and Increases Tumor Cell PSmad3 That Are Both Targeted by α-TGFβ Antibody to Promote Durable Rejection and Immunity in Squamous Cell Carcinomas. J. Immunother. Cancer 2019, 7, 62. [Google Scholar] [CrossRef]
- Michelet, X.; Dyck, L.; Hogan, A.; Loftus, R.M.; Duquette, D.; Wei, K.; Beyaz, S.; Tavakkoli, A.; Foley, C.; Donnelly, R.; et al. Metabolic Reprogramming of Natural Killer Cells in Obesity Limits Antitumor Responses. Nat. Immunol. 2018, 19, 1330–1340. [Google Scholar] [CrossRef]
- Wang, T.; Gnanaprakasam, J.N.R.; Chen, X.; Kang, S.; Xu, X.; Sun, H.; Liu, L.; Rodgers, H.; Miller, E.; Cassel, T.A.; et al. Inosine Is an Alternative Carbon Source for CD8+-T-Cell Function under Glucose Restriction. Nat. Metab. 2020, 2, 635–647. [Google Scholar] [CrossRef]
- Ma, E.H.; Bantug, G.; Griss, T.; Condotta, S.; Johnson, R.M.; Samborska, B.; Mainolfi, N.; Suri, V.; Guak, H.; Balmer, M.L.; et al. Serine Is an Essential Metabolite for Effector T Cell Expansion. Cell Metab. 2017, 25, 345–357. [Google Scholar] [CrossRef]
- Poznanski, S.M.; Singh, K.; Ritchie, T.M.; Aguiar, J.A.; Fan, I.Y.; Portillo, A.L.; Rojas, E.A.; Vahedi, F.; El-Sayes, A.; Xing, S.; et al. Metabolic Flexibility Determines Human NK Cell Functional Fate in the Tumor Microenvironment. Cell Metab. 2021, 33, 1205.e5–1220.e5. [Google Scholar] [CrossRef]
- Qiu, J.; Villa, M.; Sanin, D.E.; Buck, M.D.; O’Sullivan, D.; Ching, R.; Matsushita, M.; Grzes, K.M.; Winkler, F.; Chang, C.-H.; et al. Acetate Promotes T Cell Effector Function during Glucose Restriction. Cell Rep. 2019, 27, 2063.e5–2074.e5. [Google Scholar] [CrossRef] [PubMed]
- Verykokakis, M.; Kee, B.L. Transcriptional and Epigenetic Regulation of Innate-like T Lymphocyte Development. Curr. Opin Immunol. 2018, 51, 39–45. [Google Scholar] [CrossRef]
- Inácio, D.P.; Amado, T.; Silva-Santos, B.; Gomes, A.Q. Control of T Cell Effector Functions by MiRNAs. Cancer Lett. 2018, 427, 63–73. [Google Scholar] [CrossRef]
- Liu, T.; Wang, J.; Subedi, K.; Yi, Q.; Zhou, L.; Mi, Q.-S. MicroRNA-155 Regulates MAIT1 and MAIT17 Cell Differentiation. Front. Cell Dev. Biol. 2021, 9, 670531. [Google Scholar] [CrossRef] [PubMed]
- Schmolka, N.; Papotto, P.H.; Romero, P.V.; Amado, T.; Enguita, F.J.; Amorim, A.; Rodrigues, A.F.; Gordon, K.E.; Coroadinha, A.S.; Boldin, M.; et al. MicroRNA-146a Controls Functional Plasticity in Γδ T Cells by Targeting NOD1. Sci. Immunol. 2018, 3. [Google Scholar] [CrossRef]
- Yu, X.; Rollins, D.; Ruhn, K.A.; Stubblefield, J.J.; Green, C.B.; Kashiwada, M.; Rothman, P.B.; Takahashi, J.S.; Hooper, L.V. TH17 Cell Differentiation Is Regulated by the Circadian Clock. Science 2013, 342, 727–730. [Google Scholar] [CrossRef]
- Gomes, A.L.; Teijeiro, A.; Burén, S.; Tummala, K.S.; Yilmaz, M.; Waisman, A.; Theurillat, J.-P.; Perna, C.; Djouder, N. Metabolic Inflammation-Associated IL-17A Causes Non-Alcoholic Steatohepatitis and Hepatocellular Carcinoma. Cancer Cell 2016, 30, 161–175. [Google Scholar] [CrossRef] [PubMed]

| Lineages | γδT Cells | MAIT Cells | iNKT Cells | vNKT Cells | |||||
|---|---|---|---|---|---|---|---|---|---|
| Species | Mouse | Human | Mouse | Human | Mouse | Human | Mouse | Human | |
| TCR repertoire | Restricted including germline-encoded TCRs (e.g., Vγ1Vδ6.3, Vγ5Vδ1, Vγ6Vδ1) [5] | Semi-invariant or variant Restricted number of γ and δ chains [5] | Semi-invariant Vα19-Jα33 Restricted number of β chains [6,7] | Semi-invariant Vα7.2-Jα33 Restricted number of β chains [7] | Semi-invariant Vα14-Jα18 Restricted number of β chains [8] | Semi-invariant Vα24-Jα18 Restricted number of β chains [8] | Diverse, including oligoclonal Vα3.2Jα7, Vα1Jα9 Restricted number of β chains including Vβ8.1 and Vβ3.1 segments [9] | Diverse [9] | |
| Restricting elements | Mainly unknown CD1d T10/T22 butyrophilin-like molecules (Skint-1) [10] | Butyrophilin 3A1/2A1 (Vγ9Vδ2) CD1d (Vδ1) CD1c viral glycoproteins [11] | MR1 [12] | CD1d [13] | CD1d [9] | ||||
| TCR ligands | Natural | Unknown Cardiolipin Phycoerythrin [14] | IPP, HMBPP (Vγ9Vδ2) Sulfatide and α-GalCer (Vδ1) EPCR (Vγ4Vδ5) [15] | Microbial-derived vitamin B2 metabolites (5-OP-RU, 5-OE-RU) [16] | α-GalCer Microbial α-derived glycolipids Ganglioside [17,18,19] | Sulfatide, LPC, PG Hydrophobic peptides β-GlcCer, LysoGL1 [20] | Sulfatide, LPC, PG β-GlcCer, LysoGL1 [20] | ||
| Synthetic | - | Zoledronate *, Pamidronate * (Vγ9Vδ2) [21] | 5-OP-RU derivatives [16,22] | KRN7000 ** and derivatives Ganglioside (C24:1 GM3 and GD3) [23,24] | KRN7000 ** and derivatives [25] | - | - | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barsac, E.; de Amat Herbozo, C.; Gonzalez, L.; Baranek, T.; Mallevaey, T.; Paget, C. Regulation and Functions of Protumoral Unconventional T Cells in Solid Tumors. Cancers 2021, 13, 3578. https://doi.org/10.3390/cancers13143578
Barsac E, de Amat Herbozo C, Gonzalez L, Baranek T, Mallevaey T, Paget C. Regulation and Functions of Protumoral Unconventional T Cells in Solid Tumors. Cancers. 2021; 13(14):3578. https://doi.org/10.3390/cancers13143578
Chicago/Turabian StyleBarsac, Emilie, Carolina de Amat Herbozo, Loïc Gonzalez, Thomas Baranek, Thierry Mallevaey, and Christophe Paget. 2021. "Regulation and Functions of Protumoral Unconventional T Cells in Solid Tumors" Cancers 13, no. 14: 3578. https://doi.org/10.3390/cancers13143578
APA StyleBarsac, E., de Amat Herbozo, C., Gonzalez, L., Baranek, T., Mallevaey, T., & Paget, C. (2021). Regulation and Functions of Protumoral Unconventional T Cells in Solid Tumors. Cancers, 13(14), 3578. https://doi.org/10.3390/cancers13143578






