Intensity Modulated Proton Beam Therapy versus Volumetric Modulated Arc Therapy for Patients with Nasopharyngeal Cancer: A Propensity Score-Matched Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Pre-Treatment Evaluation, Data Collection, and Definition
2.3. Radiotherapy and Chemotherapy
2.4. Statistical Analysis
3. Results
3.1. Patient and Tumor Characteristics
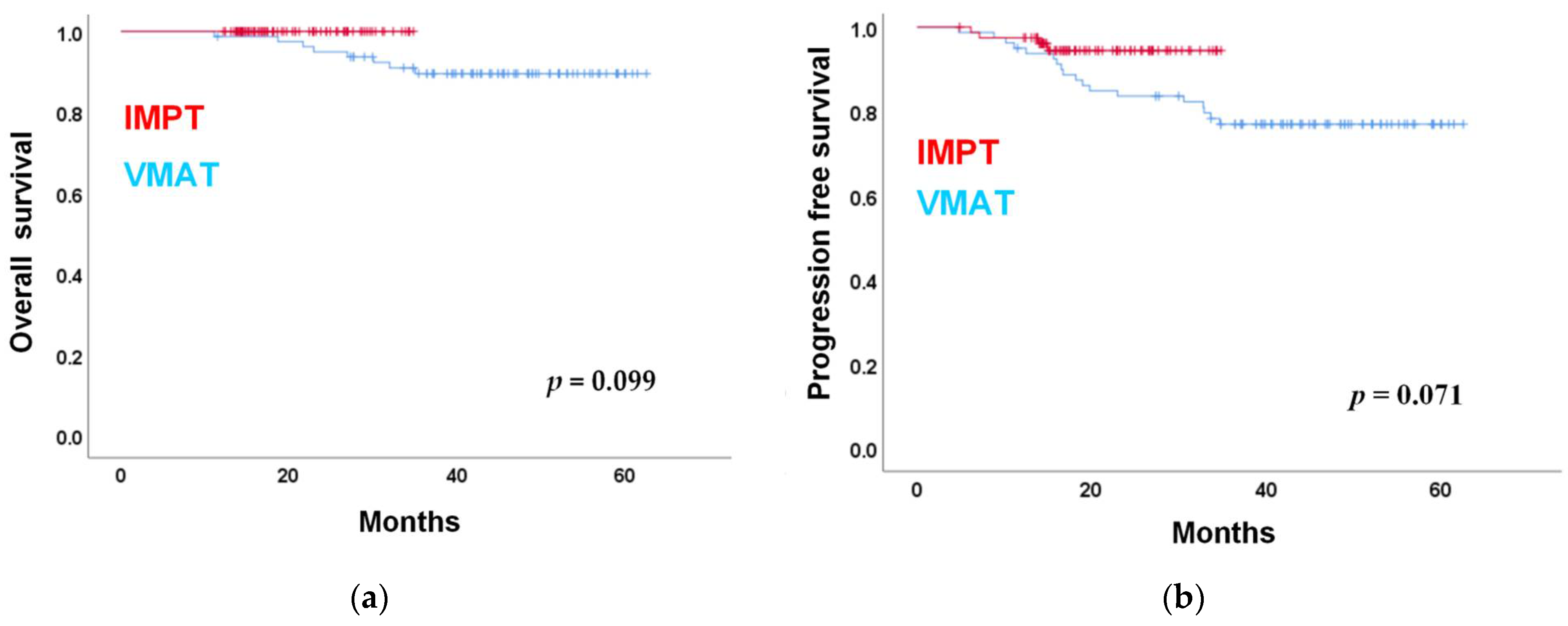
3.2. Oncological Outcome
3.3. NG Tube Placement, BWL, and Radiation Dose Difference
3.4. Common Acute AEs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Al-Sarraf, M.; Leblanc, M.; Giri, P.G.; Fu, K.K.; Cooper, J.; Vuong, T.; Forastiere, A.A.; Adams, G.; Sakr, W.A.; Schuller, D.E.; et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: Phase III randomized Intergroup study 0099. J. Clin. Oncol. 1998, 16, 1310–1317. [Google Scholar] [CrossRef] [PubMed]
- Langendijk, J.A.; Leemans, C.; Buter, J.; Berkhof, J.; Slotman, B. The Additional Value of Chemotherapy to Radiotherapy in Locally Advanced Nasopharyngeal Carcinoma: A Meta-Analysis of the Published Literature. J. Clin. Oncol. 2004, 22, 4604–4612. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Harris, J.; Garden, A.; Straube, W.; Glisson, B.; Xia, P.; Bosch, W.; Morrison, W.H.; Quivey, J.; Thorstad, W.; et al. Intensity-Modulated Radiation Therapy With or Without Chemotherapy for Nasopharyngeal Carcinoma: Radiation Therapy Oncology Group Phase II Trial 0225. J. Clin. Oncol. 2009, 27, 3684–3690. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.-Z.; Li, W.-F.; Chen, L.; Luo, W.; Chen, Y.-Y.; Liu, L.-Z.; Sun, Y.; Lin, A.-H.; Liu, M.-Z.; Ma, J. How Does Intensity-Modulated Radiotherapy Versus Conventional Two-Dimensional Radiotherapy Influence the Treatment Results in Nasopharyngeal Carcinoma Patients? Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 661–668. [Google Scholar] [CrossRef]
- Rosenthal, D.I.; Chambers, M.S.; Fuller, C.D.; Rebueno, N.C.; Garcia, J.; Kies, M.S.; Morrison, W.H.; Ang, K.K.; Garden, A. Beam Path Toxicities to Non-Target Structures During Intensity-Modulated Radiation Therapy for Head and Neck Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 747–755. [Google Scholar] [CrossRef]
- Trotti, A.; Bellm, L.; Epstein, J.B.; Frame, D.; Fuchs, H.J.; Gwede, C.K.; Komaroff, E.; Nalysnyk, L.; Zilberberg, M.D. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: A systematic literature review. Radiother. Oncol. 2003, 66, 253–262. [Google Scholar] [CrossRef]
- Feng, F.Y.; Kim, H.M.; Lyden, T.H.; Haxer, M.J.; Worden, F.P.; Feng, M.; Moyer, J.S.; Prince, M.E.; Carey, T.E.; Wolf, G.T.; et al. Intensity-Modulated Chemoradiotherapy Aiming to Reduce Dysphagia in Patients With Oropharyngeal Cancer: Clinical and Functional Results. J. Clin. Oncol. 2010, 28, 2732–2738. [Google Scholar] [CrossRef]
- Barrachina, J.G.-M.; Sainz, I.J.; Rozos, A.P.; Ros, J.C.R.; Serrano, M.D.T.; Perez, Y.L.; Carmona, J.A.M. Potential advantages of volumetric arc therapy in head and neck cancer. Head Neck 2014, 37, 909–914. [Google Scholar] [CrossRef]
- Chan, A.; Adams, J.A.; Weyman, E.; Parambi, R.J.; Goldsmith, T.; Holman, A.; Truong, M.; Busse, P.; Delaney, T.F. A Phase II Trial of Proton Radiation Therapy with Chemotherapy for Nasopharyngeal Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, S151–S152. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, X.; Li, Y.; Mohan, R. Robust optimization of intensity modulated proton therapy. Med. Phys. 2012, 39, 1079–1091. [Google Scholar] [CrossRef]
- Leeman, J.E.; Romesser, P.B.; Zhou, Y.; McBride, S.; Riaz, N.; Sherman, E.; Cohen, M.A.; Cahlon, O.; Lee, N. Proton therapy for head and neck cancer: Expanding the therapeutic window. Lancet Oncol. 2017, 18, e254–e265. [Google Scholar] [CrossRef]
- Widesott, L.; Pierelli, A.; Fiorino, C.; Dell’Oca, I.; Broggi, S.; Cattaneo, G.M.; Di Muzio, N.; Fazio, F.; Calandrino, R.; Schwarz, M. Intensity-Modulated Proton Therapy Versus Helical Tomotherapy in Nasopharynx Cancer: Planning Comparison and NTCP Evaluation. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Taheri-Kadkhoda, Z.; Björk-Eriksson, T.; Nill, S.; Wilkens, J.J.; Oelfke, U.; Johansson, K.-A.; Huber, P.E.; Münter, M.W. Intensity-modulated radiotherapy of nasopharyngeal carcinoma: A comparative treatment planning study of photons and protons. Radiat. Oncol. 2008, 3, 4. [Google Scholar] [CrossRef]
- Lewis, G.D.; Holliday, E.B.; Kocak-Uzel, E.; Ms, M.H.; Garden, A.; Rosenthal, D.; Frank, S.J. Intensity-modulated proton therapy for nasopharyngeal carcinoma: Decreased radiation dose to normal structures and encouraging clinical outcomes. Head Neck 2016, 38 (Suppl. S1), E1886–E1895. [Google Scholar] [CrossRef]
- Holliday, E.B.; Garden, A.S.; Rosenthal, D.I.; Fuller, C.D.; Morrison, W.H.; Gunn, G.B.; Phan, J.; Beadle, B.M.; Zhu, X.R.; Zhang, X.; et al. Proton Therapy Reduces Treatment-Related Toxicities for Patients with Nasopharyngeal Cancer: A Case-Match Control Study of Intensity-Modulated Proton Therapy and Intensity-Modulated Photon Therapy. Int. J. Part. Ther. 2015, 2, 19–28. [Google Scholar] [CrossRef]
- Zhao, L.-N.; Zhou, B.; Shi, M.; Wang, J.-H.; Xiao, F.; Xu, M.; Luo, S.-Q.; Xue, Y.; Li, J.-P.; Tan, L.-N. Clinical outcome for nasopharyngeal carcinoma with predominantly WHO II histology treated with intensity-modulated radiation therapy in non-endemic region of China. Oral Oncol. 2012, 48, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Fareed, M.M.; AlAmro, A.S.; Bayoumi, Y.; Tunio, M.A.; Ismail, A.S.; Akasha, R.; Mubasher, M.; Al Asiri, M. Intensity-modulated radiotherapy with simultaneous modulated accelerated boost technique and chemotherapy in patients with nasopharyngeal carcinoma. BMC Cancer 2013, 13, 318. [Google Scholar] [CrossRef][Green Version]
- Peng, G.; Wang, T.; Yang, K.-Y.; Zhang, S.; Zhang, T.; Li, Q.; Han, J.; Wu, G. A prospective, randomized study comparing outcomes and toxicities of intensity-modulated radiotherapy vs conventional two-dimensional radiotherapy for the treatment of nasopharyngeal carcinoma. Radiother. Oncol. 2012, 104, 286–293. [Google Scholar] [CrossRef]
- Brown, T.E.; Banks, M.D.; Hughes, B.; Lin, C.Y.; Kenny, L.M.; Bauer, J.D. Randomised controlled trial of early prophylactic feeding vs standard care in patients with head and neck cancer. Br. J. Cancer 2017, 117, 15–24. [Google Scholar] [CrossRef]
- Shen, L.-J.; Chen, C.; Li, B.-F.; Gao, J.; Xia, Y.-F. High Weight Loss during Radiation Treatment Changes the Prognosis in Under-/Normal Weight Nasopharyngeal Carcinoma Patients for the Worse: A Retrospective Analysis of 2433 Cases. PLoS ONE 2013, 8, e68660. [Google Scholar] [CrossRef]
- Zeng, Q.; Shen, L.-J.; Guo, X.; Guo, X.-M.; Qian, C.-N.; Wu, P.-H. Critical weight loss predicts poor prognosis in nasopharyngeal carcinoma. BMC Cancer 2016, 16, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Datema, F.R.; Ferrier, M.B.; de Jong, R.J.B. Impact of severe malnutrition on short-term mortality and overall survival in head and neck cancer. Oral Oncol. 2011, 47, 910–914. [Google Scholar] [CrossRef] [PubMed]
- Capuano, G.; Grosso, A.; Gentile, P.C.; Battista, M.; Bianciardi, F.; Di Palma, A.; Pavese, I.; Satta, F.; Tosti, M.; Palladino, A.; et al. Influence of weight loss on outcomes in patients with head and neck cancer undergoing concomitant chemoradiotherapy. Head Neck 2008, 30, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Zhang, F.; Gao, T.-S.; Zhang, W.; Lawrence, W.R.; Zhu, B.-T.; Zhou, G.-Q.; Ma, J.; Wang, S.-Y.; Sun, Y. Survival impact of radiotherapy interruption in nasopharyngeal carcinoma in the intensity-modulated radiotherapy era: A big-data intelligence platform-based analysis. Radiother. Oncol. 2019, 132, 178–187. [Google Scholar] [CrossRef]
- Kwong, D.L.; Sham, J.S.; Chua, D.T.; Choy, D.T.; Au, G.K.; Wu, P. The effect of interruptions and prolonged treatment time in radiotherapy for nasopharyngeal carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 1997, 39, 703–710. [Google Scholar] [CrossRef]
- Peng, H.; Chen, L.; Zhang, Y.; Li, W.-F.; Mao, Y.-P.; Zhang, F.; Guo, R.; Liu, L.-Z.; Lin, A.-H.; Sun, Y.; et al. Prognostic Value of the Cumulative Cisplatin Dose During Concurrent Chemoradiotherapy in Locoregionally Advanced Nasopharyngeal Carcinoma: A Secondary Analysis of a Prospective Phase III Clinical Trial. Oncologist 2016, 21, 1369–1376. [Google Scholar] [CrossRef]
- DeCesaris, C.M.; Rice, S.; Bentzen, S.M.; Jatczak, J.; Mishra, M.V.; Nichols, E.M. Quantification of Acute Skin Toxicities in Patients With Breast Cancer Undergoing Adjuvant Proton versus Photon Radiation Therapy: A Single Institutional Experience. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 1084–1090. [Google Scholar] [CrossRef]
- Rzepecki, A.; Birnbaum, M.; Ohri, N.; Daily, J.; Fox, J.; Bodner, W.; Kabarriti, R.; Garg, M.; Mehta, K.; Kalnicki, S.; et al. Characterizing the Effects of Radiation Dermatitis on Quality of Life: A Prospective Survey-Based Study. J. Am. Acad. Dermatol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, S.; McCauley, S.; Vairamani, K.; Speth, J.; Girdhani, S.; Abel, E.; Sharma, R.; Perentesis, J.; Wells, S.; Mascia, A.; et al. FLASH Proton Pencil Beam Scanning Irradiation Minimizes Radiation-Induced Leg Contracture and Skin Toxicity in Mice. Cancers 2021, 13, 1012. [Google Scholar] [CrossRef]
| Characteristics | IMPT (n = 80) | VMAT (n = 80) | p-Value |
|---|---|---|---|
| Age at diagnosis, mean (IQR), y | 47.6 (22.6–79.2) | 50.1 (27.3–79.2) | |
| Age, n (%) | 0.844 | ||
| >60 y/o | 65 (81.3) | 63 (78.8) | |
| <60 y/o | 15(18.7) | 17 (21.2) | |
| Sex, n (%) | 0.284 | ||
| Male | 64 (80) | 70 (87.5) | |
| Female | 16 (20) | 10 (12.5) | |
| Charlson Comorbidity Index | 0.298 | ||
| 0–1 | 69 (86.3) | 63 (78.8) | |
| ≥2 | 11 (13.8) | 17 (21.3) | |
| WHO type, n (%) | 0.845 | ||
| I | 1 (1.3) | 1 (1.3) | |
| II | 24 (30.0) | 21 (26.3) | |
| III | 55 (68.8) | 58 (72.5) | |
| T-stage, n (%) | 0.760 | ||
| T1 | 32 (40.0) | 25 (43.8) | |
| T2 | 11 (13.8) | 8 (12.5) | |
| T3 | 19 (23.8) | 7 (17.5) | |
| T4 | 18 (22.5) | 14 (26.3) | |
| N-stage, n (%) | 0.909 | ||
| N0 | 13 (16.3) | 10 (20.0) | |
| N1 | 39 (48.8) | 34 (46.3) | |
| N2 | 15 (18.8) | 12 (16.3) | |
| N3 | 13 (16.3) | 18 (17.5) | |
| AJCC 8th stage, n (%) | 0.945 | ||
| I | 8 (10.0) | 9 (11.3) | |
| II | 21 (26.3) | 21 (26.3) | |
| III | 19 (23.8) | 16 (20.0) | |
| IV | 32 (40.0) | 34 (42.6) | |
| EBV PCR titer, n (%) | 0.257 | ||
| >200 | 45 (56.3) | 52 (65.0) | |
| <200 | 35 (43.8) | 28 (35.0) | |
| Treatment modality, n (%) | 1.000 | ||
| Chemo with CCRT | 10 (12.5) | 10 (12.5) | |
| CCRT | 59 (73.8) | 59 (73.8) | |
| RT alone | 11(13.7) | 11(13.7) | |
| Induction chemotherapy | 0.531 | ||
| GP | 9 (90.0) | 8 (80.0) | |
| TP | 1 (10.0) | 2 (20.0) | |
| Concurrent chemotherapy regimen | 0.459 | ||
| PUL | 58 (84.1) | 61 (88.4) | |
| Weekly cisplatin | 11 (15.9) | 8 (11.6) | |
| Cisplatin total dose (mg/m2) | 0.111 | ||
| <200 | 5 (7.2) | 11 (15.9) | |
| ≥200 | 64 (92.8) | 58 (84.1) | |
| Smoking at diagnosis, n (%) | 0.817 | ||
| No | 43 (54.4) | 45 (56.3) | |
| Yes | 36 (45.6) | 35 (43.8) |
| Characteristics | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | |
| Age | ||||||
| <60 | 1 | |||||
| ≥60 | 1.618 | 0.532–4.920 | 0.396 | |||
| Sex | ||||||
| female | 1 | 1 | ||||
| male | 0.852 | 0.195–3.717 | 0.831 | 1.618 | 0.321–8.155 | 0.560 |
| Pathology | ||||||
| WHO I, II | 1 | 1 | ||||
| WHO III | 1.023 | 0.363–2.879 | 0.966 | 1.257 | 0.381–4.143 | 0.707 |
| T stage | ||||||
| I–II | 1 | 1 | ||||
| III–IV | 1.358 | 0.539–3.425 | 0.516 | 1.267 | 0.437–3.672 | 0.663 |
| N stage | ||||||
| 0–I | 1 | 1 | ||||
| II–III | 3.995 | 1.539–10.374 | 0.004 * | 6.912 | 1.877–25.456 | 0.004 * |
| Radiation modality | ||||||
| VMAT | 1 | 1 | ||||
| IMPT | 0.372 | 0.122–1.133 | 0.082 | 0.298 | 0.088–1.011 | 0.052 |
| EBV titer | ||||||
| ≥200 | 1 | 1 | ||||
| <200 | 0.447 | 0.059–3.362 | 0.434 | 0.326 | 0.075–1.409 | 0.133 |
| Induction chemotherapy | ||||||
| No | 1 | 1 | ||||
| Yes | 2.679 | 0.952–7.541 | 0.062 | 2.235 | 0.669–7.466 | 0.191 |
| Smoking at diagnosis | ||||||
| No | 1 | 1 | ||||
| Yes | 1.933 | 0.749–4.988 | 0.173 | 2.484 | 0.886–6.912 | 0.084 |
| Weight loss ≥7% | ||||||
| No | 1 | 1 | ||||
| Yes | 2.952 | 1.052–8.283 | 0.040 * | 3.216 | 1.062–9.742 | 0.039 * |
| Nasogastric tube insertion | ||||||
| No | 1 | 1 | ||||
| Yes | 0.631 | 0.144–2.759 | 0.541 | 0.566 | 0.121–2.644 | 0.470 |
| Charlson Comorbidity Index | ||||||
| 0–1 | 1 | 1 | ||||
| ≥2 | 1.383 | 0.455–4.205 | 0.567 | 1.927 | 0.601–6.180 | 0.270 |
| Cisplatin total dose (mg/m2) | ||||||
| <200 | 1 | 1 | ||||
| ≥200 | 0.367 | 0.049–2743 | 0.328 | 0.318 | 0.038–2.660 | 0.290 |
| Variables | IMPT (n = 80) | VMAT (n = 80) | p-Value |
|---|---|---|---|
| NG tube placement, No. (%) | 4 (5.0) | 12(15.0) | 0.026 |
| Percentage Body weight loss (SD) | 4.87 (3.94) | 6.21 (4.15) | 0.038 |
| Weight loss over 7%, No. (%) | 24 (32.4) | 41 (54.7) | 0.006 |
| Weight loss over 7% or NG tube during treatment, No. (%) | 25 (32.9) | 46 (57.5) | 0.002 |
| Grade 3 dermatitis with wound care, No. (%) | 28 (35) | 6 (7.5) | <0.000 |
| Grade 3 mucositis, No. (%) | 8 (10.0) | 14 (17.5) | 0.178 |
| Grade 2–4 Xerostomia, No. (%) | 9 (11.3) | 13 (16.3) | 0.358 |
| Emergency Room Visit | 7 (8.8) | 13 (16.3) | 0.151 |
| Unscheduled Hospitalization | 8 (10.0) | 7 (8.8) | 0.786 |
| Characteristics | Univariate Analysis | MVA without Radiation Dose | MVA with Radiation Dose | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Age | |||||||||
| <60 | 1 | 1 | 1 | ||||||
| ≥60 | 0.562 | 0.234–1.350 | 0.197 | 0.419 | 0.156–1.126 | 0.085 | 0.445 | 0.167–1.183 | 0.105 |
| Sex | |||||||||
| female | 1 | 1 | 1 | ||||||
| Male | 0.995 | 0.429–2.311 | 0.991 | 1.110 | 0.455–2.712 | 0.818 | 1.168 | 0.468–2.913 | 0.739 |
| Pathology | |||||||||
| WHO I, II | 1 | 1 | 1 | ||||||
| WHO III | 0.858 | 0.434–1.697 | 0.660 | 0.864 | 0.406–1.839 | 0.704 | 0.956 | 0.439–2.081 | 0.909 |
| T stage | |||||||||
| I–II | 1 | 1 | 1 | ||||||
| III–IV | 1.985 | 1.055–3.737 | 0.034 * | 2.195 | 1.072–4.493 | 0.031 * | 1.827 | 0.865–3.858 | 0.114 |
| N stage | |||||||||
| 0–I | 1 | 1 | 1 | ||||||
| II–III | 1.487 | 0.772–2.864 | 0.235 | 1.742 | 0.751–4.042 | 0.196 | 1.429 | 0.590–3.460 | 0.429 |
| Radiationmodality | |||||||||
| VMAT | 1 | 1 | 1 | ||||||
| IMPT | 0.358 | 0.188–0.680 | 0.002 * | 0.302 | 0.150–0.607 | 0.001 * | 0.828 | 0.238–2.888 | 0.768 |
| Mean oralcavity dose | 1.063 | 1.031–1.095 | 0.000 * | 1.069 | 1.003–1.140 | 0.038 * | |||
| Mean superior Constrictor muscle dose | 0.997 | 0.989–1.005 | 0.441 | 0.994 | 0.977–1.011 | 0.472 | |||
| Mean middle Constrictor muscle dose | 1.035 | 1.002–1.069 | 0.036 * | 0.984 | 0.946–1.023 | 0.407 | |||
| Mean inferior Constrictor muscle dose | 1.043 | 1.001–1.085 | 0.042 * | 1.010 | 0.980–1040 | 0.531 | |||
| EBV titer | |||||||||
| ≥200 | 1 | 1 | 1 | ||||||
| <200 | 1.729 | 0.906–3.298 | 0.097 | 1.024 | 0.438–2.394 | 0.957 | 1.186 | 0.481–2.922 | 0.711 |
| Chemotherapy modality | |||||||||
| No | 1 | 1 | 1 | ||||||
| Concurrent | 2.373 | 0.854–6.591 | 0.097 | 1.691 | 0.521–5.487 | 0.382 | 1.368 | 0.412–4.542 | 0.608 |
| Induction | 1.436 | 0.391–5.269 | 0.585 | 0.759 | 0.165–3.497 | 0.724 | 0.612 | 0.129–2.908 | 0.537 |
| Smoking | |||||||||
| No | 1 | 1 | 1 | ||||||
| Yes | 1.017 | 0.544–1.900 | 0.959 | 0.981 | 0.489–1.970 | 0.958 | 1.094 | 0.526–2.275 | 0.811 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chou, Y.-C.; Fan, K.-H.; Lin, C.-Y.; Hung, T.-M.; Huang, B.-S.; Chang, K.-P.; Kang, C.-J.; Huang, S.-F.; Chang, P.-H.; Hsu, C.-L.; et al. Intensity Modulated Proton Beam Therapy versus Volumetric Modulated Arc Therapy for Patients with Nasopharyngeal Cancer: A Propensity Score-Matched Study. Cancers 2021, 13, 3555. https://doi.org/10.3390/cancers13143555
Chou Y-C, Fan K-H, Lin C-Y, Hung T-M, Huang B-S, Chang K-P, Kang C-J, Huang S-F, Chang P-H, Hsu C-L, et al. Intensity Modulated Proton Beam Therapy versus Volumetric Modulated Arc Therapy for Patients with Nasopharyngeal Cancer: A Propensity Score-Matched Study. Cancers. 2021; 13(14):3555. https://doi.org/10.3390/cancers13143555
Chicago/Turabian StyleChou, Yung-Chih, Kang-Hsing Fan, Chien-Yu Lin, Tsung-Min Hung, Bing-Shen Huang, Kai-Ping Chang, Chung-Jan Kang, Shiang-Fu Huang, Po-Hung Chang, Cheng-Lung Hsu, and et al. 2021. "Intensity Modulated Proton Beam Therapy versus Volumetric Modulated Arc Therapy for Patients with Nasopharyngeal Cancer: A Propensity Score-Matched Study" Cancers 13, no. 14: 3555. https://doi.org/10.3390/cancers13143555
APA StyleChou, Y.-C., Fan, K.-H., Lin, C.-Y., Hung, T.-M., Huang, B.-S., Chang, K.-P., Kang, C.-J., Huang, S.-F., Chang, P.-H., Hsu, C.-L., Wang, H.-M., Hsieh, J. C.-H., Cheng, A.-J., & Chang, J. T.-C. (2021). Intensity Modulated Proton Beam Therapy versus Volumetric Modulated Arc Therapy for Patients with Nasopharyngeal Cancer: A Propensity Score-Matched Study. Cancers, 13(14), 3555. https://doi.org/10.3390/cancers13143555






