Neurocognitive Outcomes in Pediatric Patients Following Brain Irradiation
Abstract
:Simple Summary
Abstract
1. Introduction
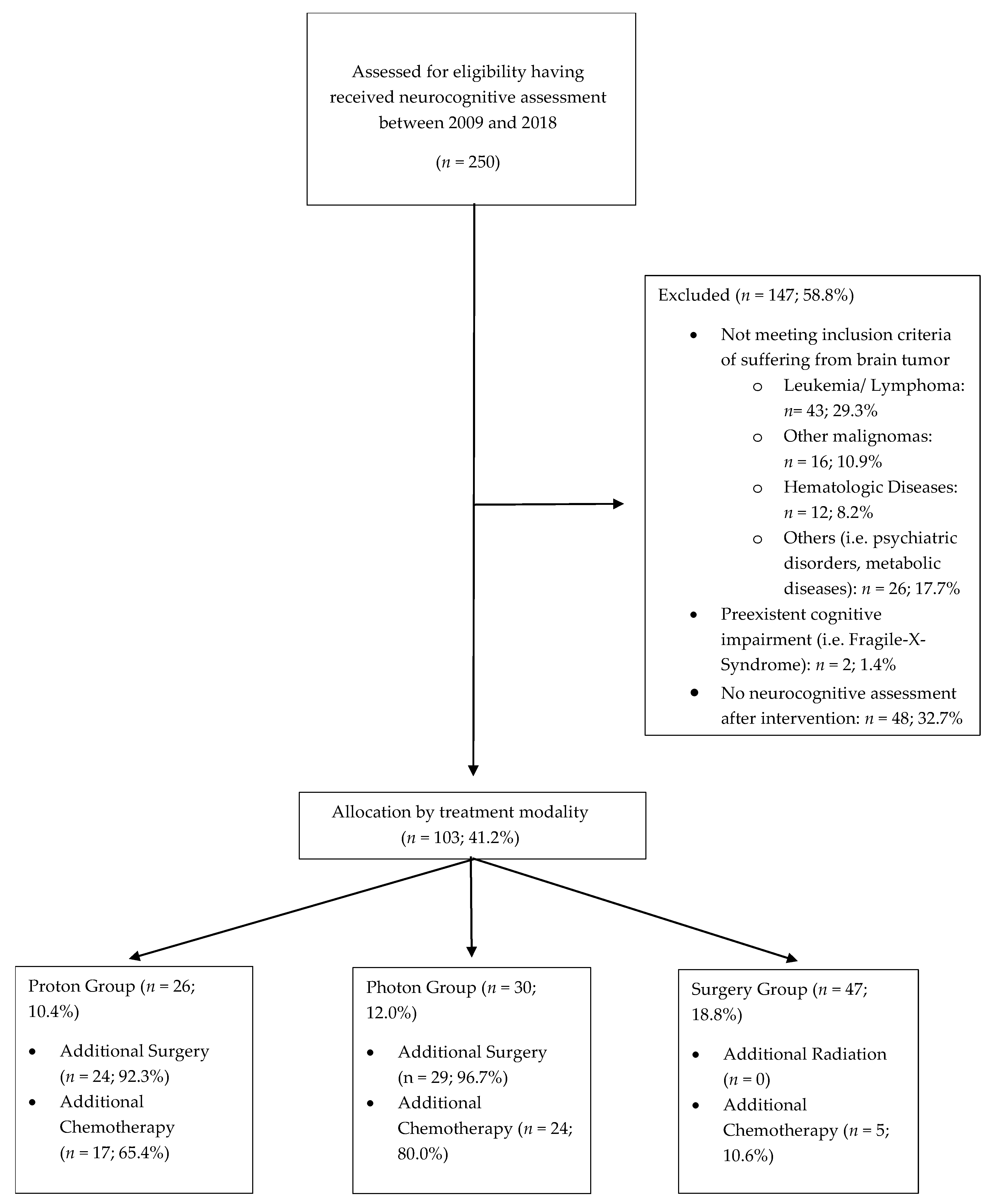
2. Materials and Methods
2.1. Patient and Treatment Characteristics
2.2. Neuropsychological Assessment
2.3. Statistical Analysis
2.4. Ethics Approval
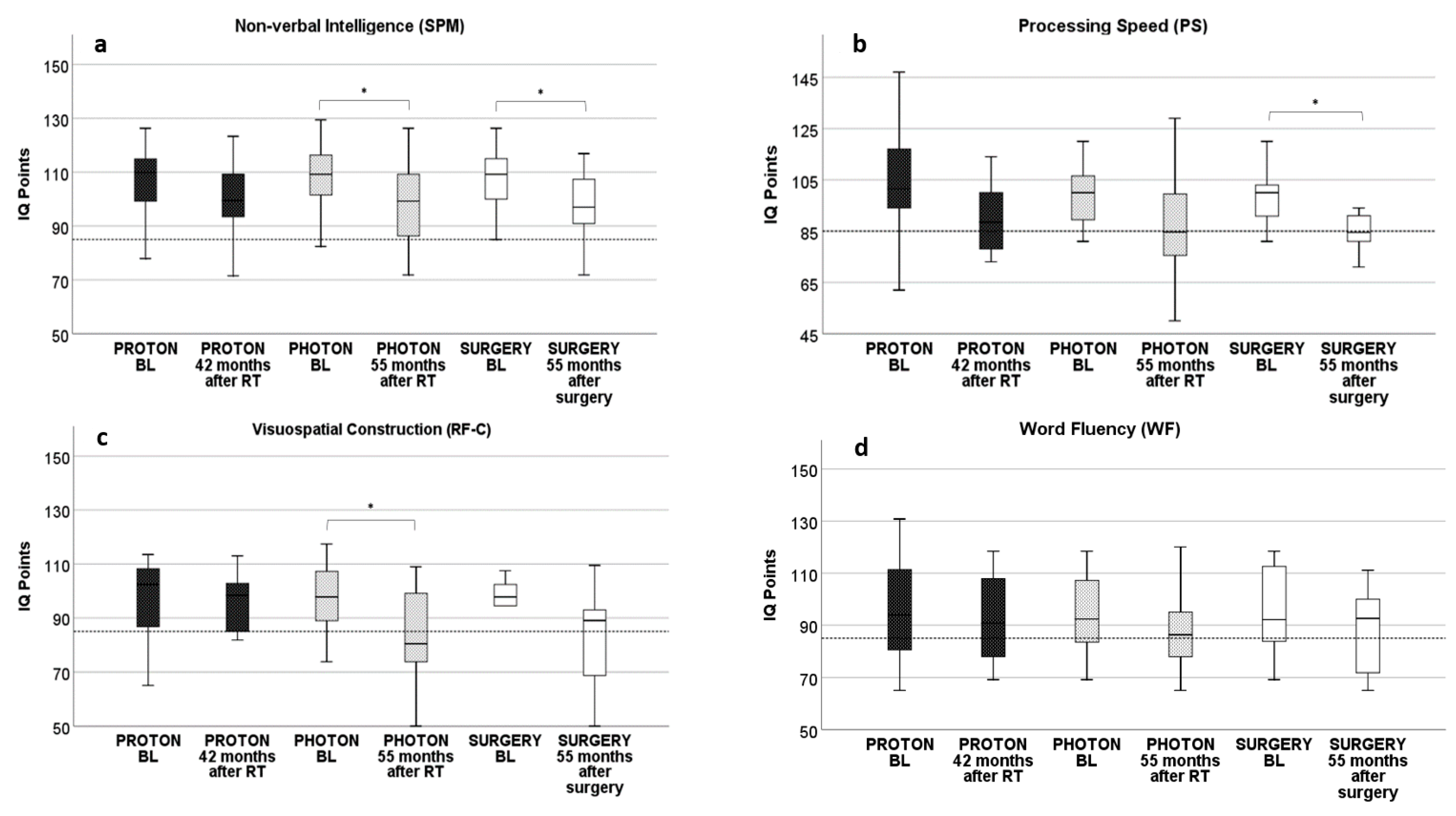
3. Results
3.1. Proton RT: Longitudinal Development from Baseline to 42 Months after Proton RT
3.2. Photon RT: Longitudinal Development from Baseline to 55 Months after Photon RT
3.3. Surgery: Longitudinal Development from Baseline to 55 Months after Surgery
3.4. Intermodal Comparison of Neurocognitive Results after RT and Surgery
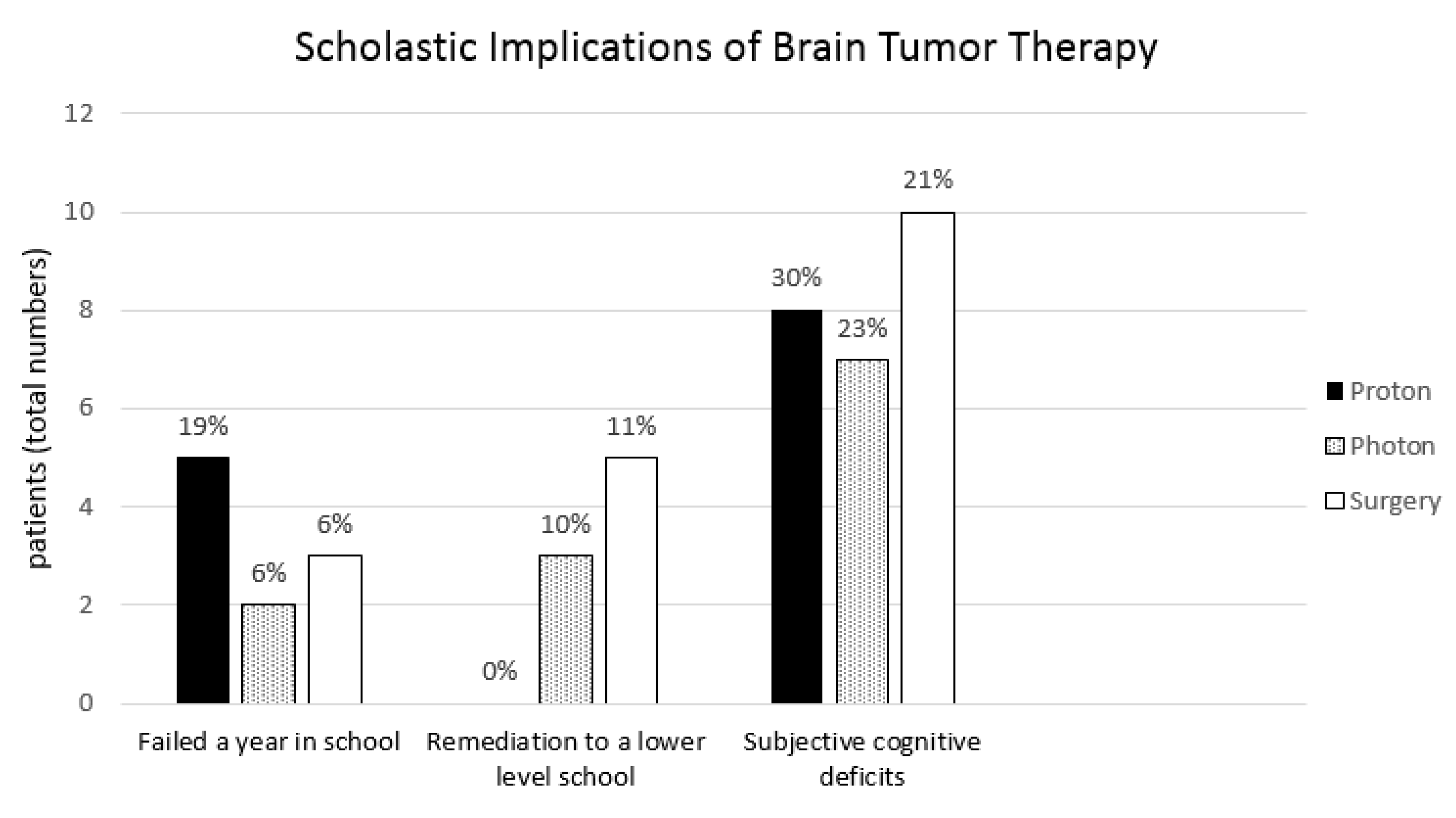
3.5. Scholastic Implications of Brain Tumor Treatment
3.6. Subgroup Analysis: Tumor Localization
3.7. Subgroup Analysis: Extent of Tumor Resection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steliarova-Foucher, E.; Colombet, M.; Ries, L.A.G.; Moreno, F.; Dolya, A.; Bray, F.; Hesseling, P.; Shin, H.Y.; Stiller, C.A.; Bouzbid, S.; et al. International incidence of childhood cancer, 2001–2010: A population-based registry study. Lancet Oncol. 2017, 18, 719–731. [Google Scholar] [CrossRef]
- Frühwald, M.C.; Rutkowski, S. ZNS-Tumoren bei Kindern und Jugendlichen. Dtsch. Arztebl. Int. 2011, 108, 390–397. [Google Scholar] [PubMed]
- Glauser, T.A.; Packer, R.J. Cognitive deficits in long-term survivors of childhood brain tumors. Childs Nerv. Syst. 1991, 7, 2–12. [Google Scholar] [CrossRef]
- Chadderton, R.D.; West, C.G.H.; Schulz, S.; Quirke, D.C.; Gattamaneni, R.; Taylor, R. Radiotherapy in the treatment of low-grade astrocytomas. Childs Nerv. Syst. 1995, 11, 443–448. [Google Scholar] [CrossRef]
- Hoppe-Hirsch, E.; Renier, D.; Lellouch-Tubiana, A.; Sainte-Rose, C.; Pierre-Kahn, A.; Hirsch, J.F. Medulloblastoma in childhood: Progressive intellectual deterioration. Childs Nerv. Syst. 1990, 6, 60–65. [Google Scholar] [CrossRef]
- Douw, L.; Klein, M.; Fagel, S.S.A.A.; van den Heuvel, J.; Taphoorn, M.J.B.; Aaronson, N.K.; Postma, T.J.; Vandertop, W.P.; Mooij, J.J.; Boerman, R.H.; et al. Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: Long-term follow-up. Lancet Neurol. 2009, 8, 810–818. [Google Scholar] [CrossRef]
- Mulhern, R.K.; Merchant, T.E.; Gajjar, A.; Reddick, W.E.; Kun, L.E. Late neurocognitive sequelae in survivors of brain tumours in childhood. Lancet Oncol. 2004, 5, 399–408. [Google Scholar] [CrossRef]
- Mulhern, R.K.; Palmer, S.L.; Merchant, T.E.; Wallace, D.; Kocak, M.; Brouwers, P.; Krull, K.; Chintagumpala, M.; Stargatt, R.; Ashley, D.M.; et al. Neurocognitive Consequences of Risk-Adapted Therapy for Childhood Medulloblastoma. J. Clin. Oncol. 2005, 23, 5511–5519. [Google Scholar] [CrossRef]
- Harrabi, S.B.; Bougatf, N.; Mohr, A.; Haberer, T.; Herfarth, K.; Combs, S.E.; Debus, J.; Adeberg, S. Dosimetric advantages of proton therapy over conventional radiotherapy with photons in young patients and adults with low-grade glioma. Strahlenther. Onkol. 2016, 192, 759–769. [Google Scholar] [CrossRef] [Green Version]
- Adeberg, S.; Harrabi, S.B.; Bougatf, N.; Bernhardt, D.; Rieber, J.; Koerber, S.A.; Syed, M.; Sprave, T.; Mohr, A.; Abdollahi, A.; et al. Intensity-modulated proton therapy, volumetric-modulated arc therapy, and 3D conformal radiotherapy in anaplastic astrocytoma and glioblastoma. Strahlenther. Onkol. 2016, 192, 770–779. [Google Scholar] [CrossRef]
- Merchant, T.E.; Kiehna, E.N.; Li, C.; Xiong, X.; Mulhern, R.K. Radiation dosimetry predicts IQ after conformal radiation therapy in pediatric patients with localized ependymoma. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 1546–1554. [Google Scholar] [CrossRef] [PubMed]
- Merchant, T.E.; Schreiber, J.E.; Wu, S.; Lukose, R.; Xiong, X.; Gajjar, A. Critical Combinations of Radiation Dose and Volume Predict Intelligence Quotient and Academic Achievement Scores After Craniospinal Irradiation in Children With Medulloblastoma. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 554–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merchant, T.E.; Hua, C.-H.; Shukla, H.; Ying, X.; Nill, S.; Oelfke, U. Proton versus photon radiotherapy for common pediatric brain tumors: Comparison of models of dose characteristics and their relationship to cognitive function. Pediatric Blood Cancer 2008, 51, 110–117. [Google Scholar] [CrossRef]
- Kahalley, L.S.; Douglas Ris, M.; Mahajan, A.; Fatih Okcu, M.; Chintagumpala, M.; Paulino, A.C.; Whitehead, W.E.; Minard, C.G.; Stancel, H.H.; Orobio, J.; et al. Prospective, longitudinal comparison of neurocognitive change in pediatric brain tumor patients treated with proton radiotherapy versus surgery only. Neuro-Oncology 2019, 21, 809–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leavitt, V.M. Standard Progressive Matrices. In Encyclopedia of Clinical Neuropsychology; Kreutzer, J.S., DeLuca, J., Caplan, B., Eds.; Springer: New York, NY, USA, 2011; p. 2368. [Google Scholar]
- Crotty, K.; Baron, I.S. Beery Developmental Test of Visual-Motor Integration (VMI). In Encyclopedia of Clinical Neuropsychology; Kreutzer, J.S., DeLuca, J., Caplan, B., Eds.; Springer: New York, NY, USA, 2011; pp. 364–365. [Google Scholar]
- Jacobson, L.A.; Mahone, E.M. Wechsler Intelligence Scale for Children. In Encyclopedia of Clinical Neuropsychology; Kreutzer, J.S., DeLuca, J., Caplan, B., Eds.; Springer: New York, NY, USA, 2011; pp. 2682–2688. [Google Scholar]
- Aschenbrenner, S.; Tucha, O.; Lange, K.W. Regensburger Wortflüssigkeits-Test: RWT; Hogrefe, Verl. für Psychologie: Göttingen, Germany; Bern, Switzerland; Toronto, ON, Canada; Seattle, WA, USA, 2001. [Google Scholar]
- Willard, V.W.; Conklin, H.M.; Wu, S.; Merchant, T.E. Prospective longitudinal evaluation of emotional and behavioral functioning in pediatric patients with low-grade glioma treated with conformal radiation therapy. J. Neuro-Oncol. 2015, 122, 161–168. [Google Scholar] [CrossRef]
- Di Pinto, M.; Conklin, H.M.; Li, C.; Xiong, X.; Merchant, T.E. Investigating Verbal and Visual Auditory Learning After Conformal Radiation Therapy for Childhood Ependymoma. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 1002–1008. [Google Scholar] [CrossRef] [Green Version]
- Di Pinto, M.; Conklin, H.M.; Li, C.; Merchant, T.E. Learning and Memory Following Conformal Radiation Therapy for Pediatric Craniopharyngioma and Low-Grade Glioma. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, e363–e369. [Google Scholar] [CrossRef] [Green Version]
- Pulsifer, M.B.; Duncanson, H.; Grieco, J.; Evans, C.; Tseretopoulos, I.D.; MacDonald, S.; Tarbell, N.J.; Yock, T.I. Cognitive and Adaptive Outcomes After Proton Radiation for Pediatric Patients With Brain Tumors. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 391–398. [Google Scholar] [CrossRef]
- Pulsifer, M.B.; Sethi, R.V.; Kuhlthau, K.A.; MacDonald, S.M.; Tarbell, N.J.; Yock, T.I. Early Cognitive Outcomes Following Proton Radiation in Pediatric Patients With Brain and Central Nervous System Tumors. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 400–407. [Google Scholar] [CrossRef] [Green Version]
- Kahalley, L.S.; Ris, M.D.; Grosshans, D.R.; Okcu, M.F.; Paulino, A.C.; Chintagumpala, M.; Moore, B.D.; Guffey, D.; Minard, C.G.; Stancel, H.H.; et al. Comparing Intelligence Quotient Change After Treatment With Proton Versus Photon Radiation Therapy for Pediatric Brain Tumors. J. Clin. Oncol. 2016, 34, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Kahalley, L.S.; Conklin, H.M.; Tyc, V.L.; Hudson, M.M.; Wilson, S.J.; Wu, S.; Xiong, X.; Hinds, P.S. Slower processing speed after treatment for pediatric brain tumor and acute lymphoblastic leukemia. Psycho-Oncology 2013, 22, 1979–1986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mabbott, D.J.; Penkman, L.; Witol, A.; Strother, D.; Bouffet, E. Core neurocognitive functions in children treated for posterior fossa tumors. Neuropsychology 2008, 22, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Law, N.; Bouffet, E.; Laughlin, S.; Laperriere, N.; Brière, M.-E.; Strother, D.; McConnell, D.; Hukin, J.; Fryer, C.; Rockel, C.; et al. Cerebello–thalamo–cerebral connections in pediatric brain tumor patients: Impact on working memory. NeuroImage 2011, 56, 2238–2248. [Google Scholar] [CrossRef] [PubMed]
- Law, N.; Greenberg, M.; Bouffet, E.; Laughlin, S.; Taylor, M.D.; Malkin, D.; Liu, F.; Moxon-Emre, I.; Scantlebury, N.; Skocic, J.; et al. Visualization and segmentation of reciprocal cerebrocerebellar pathways in the healthy and injured brain. Hum. Brain Mapp. 2015, 36, 2615–2628. [Google Scholar] [CrossRef]
- Morris, E.B.; Phillips, N.S.; Laningham, F.H.; Patay, Z.; Gajjar, A.; Wallace, D.; Boop, F.; Sanford, R.; Ness, K.K.; Ogg, R.J. Proximal dentatothalamocortical tract involvement in posterior fossa syndrome. Brain 2009, 132, 3087–3095. [Google Scholar] [CrossRef] [PubMed]
- Sortor, J.M.; Kulp, M.T. Are the Results of the Beery-Buktenica Developmental Test of Visual-Motor Integration and Its Subtests Related to Achievement Test Scores? Optom. Vis. Sci. 2003, 80, 758–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, M.-S.; Park, S.-Y.; Park, S.-R.; Seol, S.-H.; Kwon, J.S. Clinical and empirical applications of the Rey–Osterrieth Complex Figure Test. Nat. Protoc. 2006, 1, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Ogino, T.; Nakano, K.; Hattori, J.; Kado, Y.; Sanada, S.; Ohtsuka, Y. The Rey–Osterrieth Complex Figure as a measure of executive function in childhood. Brain Dev. 2005, 27, 564–569. [Google Scholar] [CrossRef] [Green Version]
- Dockstader, C.; Gaetz, W.; Bouffet, E.; Tabori, U.; Wang, F.; Bostan, S.R.; Laughlin, S.; Mabbott, D.J. Neural correlates of delayed visual–motor performance in children treated for brain tumours. Cortex 2013, 49, 2140–2150. [Google Scholar] [CrossRef]
- Gourovitch, M.L.; Kirkby, B.S.; Goldberg, T.E.; Weinberger, D.R.; Gold, J.M.; Esposito, G.; Van Horn, J.D.; Berman, K.F. A comparison of rCBF patterns during letter and semantic fluency. Neuropsychology 2000, 14, 353–360. [Google Scholar] [CrossRef]
- Aita, S.L. Executive, language, or both? An examination of the construct validity of verbal fluency measures. Appl. Neuropsychol. Adult 2019, 26, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Squire, L.R.; Zola-Morgan, S. The Medial Temporal Lobe Memory System. Science 1991, 253, 1380–1386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McConley, R.; Martin, R.; Palmer, C.A.; Kuzniecky, R.; Knowlton, R.; Faught, E. Rey Osterrieth complex figure test spatial and figural scoring: Relations to seizure focus and hippocampal pathology in patients with temporal lobe epilepsy. Epilepsy Behav. 2008, 13, 174–177. [Google Scholar] [CrossRef]
- Markham, J.A.; Greenough, W.T. Experience-driven brain plasticity: Beyond the synapse. Neuron Glia Biol. 2004, 1, 351–363. [Google Scholar] [CrossRef] [Green Version]
- Sur, M.; Leamey, C.A. Development and plasticity of cortical areas and networks. Nat. Rev. Neurosci. 2001, 2, 251–262. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Specification | Proton RT n = 26 | Photon RT n = 30 | Surgery n = 47 |
|---|---|---|---|---|
| Gender | male (%) | 16 (61.5) | 15 (50.0) | 25 (53.2) |
| female (%) | 10 (38.5) | 15 (50.0) | 22 (46.8) | |
| Follow-up (in years) | after initial diagnosis (range) | 6.6 (2.0–17.2) | 9.2 (3.4–16.8) | 6.3 (0.7–14.2) |
| Alive at last follow-up | yes (%) | 23 (88.5) | 28 (93.3) | 44 (93.6) |
| no (%) | 3 (11.5) | 2 (6.7) | 3 (6.4) | |
| Age at Intervention (in years) | mean (range) | 9.4 (3.2–19.0) | 9.6 (2.3–17.3) | 10.5 (1.6–17.9) |
| Diagnosis | glioma (%) | 11 (42.3) *,† | 8 (26.7) † | 42 (89.4) *,† |
| medulloblastoma (%) | 2 (7.7) | 13 (43.3) | 0 (0) | |
| ependymoma (%) | 5 (19.2) | 3 (10.0) | 1 (2.1) | |
| craniopharyngeoma (%) | 1 (3.8) | 0 (0) | 3 (6.4) | |
| germinoma (%) | 3 (11.5) | 4 (13.3) | 0 (0) | |
| others (%) | 4 (15.4) | 2 (6.7) | 1 (2.1) | |
| Grading | low-grade (WHO °I & °II) | 12 (46.1) * | 6 (20.0) † | 39 (83.0) *,† |
| high grade (WHO °III & °IV) | 10 (38.5) * | 18 (60.0) † | 4 (8.5) *,† | |
| unknown | 4 (15.4) | 6 (20) | 4 (8.5) | |
| Localization | supratentorial (%) | 15 (57.7) | 14 (46.7) | 23 (48.9) |
| infratentorial (%) | 9 (34.6) | 16 (53.3) | 21 (44.7) | |
| extraaxial (%) | 2 (7.7) | 0 (0) | 3 (6.4) | |
| Radiation Therapy | irradiation (%) | 26 (100), proton | 30 (100), photon | 0 (0) |
| total dose, mean (range) in Gy (RBE) | 51.3 (16.0–74.0) | 53.3 (30–68) | 0 | |
| PTV, mean in ccm (range) | 262.9 (27.7–1691.4) | 463.13 (13.05–4078.9) | 0 | |
| CTV, mean in ccm (range) | 205.9 (16.1–1465.6) | 46.5 (10.99–166.93) | 0 | |
| Total craniospinal irradiation | 1 (3.8) ** | 14 (46.7) ** | 0 (0) | |
| Chemotherapy | yes (%) | 17 (65.4) * | 24 (80.0) † | 5 (10.6) *,† |
| no (%) | 9 (34.6) | 6 (20.0) | 42 (89.4) | |
| Surgery | no (%) | 2 (7.7) | 1 (3.3) | 0 (0) |
| STR (%) | 15 (57.7) | 15 (50.0) | 21 (44.7) | |
| GTR (%) | 9 (34.6) | 14 (46.7) | 26 (55.3) |
| Domains | Specification | Proton (Follow-Up 42 Months after RT) | Photon (Follow-Up 55 Months after RT) | Surgery (Follow-Up 55 Months after Surgery) |
|---|---|---|---|---|
| non-verbal intelligence (CPM/SPM) | baseline (IQ-points) | 107.2 | 108.5 | 108.3 |
| follow-up (IQ-points) | 99.9 | 98.1 | 96.6 | |
| change to baseline | −6.8% (p = 0.15) | −9.6% (p = 0.01) | −10.7% (p = 0.01) | |
| visuomotor integration (VMI) | baseline (IQ-points) | 98.4 | 95.2 | 94.3 |
| follow-up (IQ-points) | 88.6 | 86.3 | 89.9 | |
| change to baseline | −9.9% (p = 0.05) | −9.4% (p = 0.06) | −6.9% (p = 0.12) | |
| word fluency (WF-C) categorial | baseline (IQ-points) | 95.1 | 94.0 | 95.2 |
| follow-up (IQ-points) | 91.2 | 89.8 | 88.7 | |
| change to baseline | −4.1% (p = 0.60) | −4.4% (p = 0.67) | −6.8% (p = 0.31) | |
| word fluency (WF-L) lexical | baseline (IQ-points) | 92.0 | 94.2 | 91.5 |
| follow-up (IQ-points) | 91.0 | 92.6 | 86.9 | |
| change to baseline | −1.0% (p = 0.89) | −1.6% (p = 0.76) | −5.0% (p = 0.42) | |
| processing speed (PS) | baseline (IQ-points) | 102.7 | 99.2 | 99.2 |
| follow-up (IQ-points) | 90.5 | 88.9 | 84.4 | |
| change to baseline | −11.9% (p = 0.06) | −10.4% (p = 0.12) | −14.9% (p = 0.002) | |
| working memory (WM) (digit span) | baseline (IQ-points) | 97.7 | 99.1 | 97.6 |
| follow-up (IQ-points) | 93.9 | 93.6 | 93.5 | |
| change to baseline | −3.8% (p = 0.48) | −5.6% (p = 0.19) | −4.3% (p = 0.53) | |
| visuospatial construction (ROCF-C) | baseline (IQ-points) | 95.93 | 96.5 | 95.6 |
| follow-up (IQ-points) | 95.9 | 82.1 | 81.2 | |
| change to baseline | −0.03% (p = 0.99) | −14.9% (p = 0.02) | −15.1% (p = 0.09) | |
| visuospatial memory (ROCF-R) | baseline (IQ-points) | 95.5 | 89.84 | 88.9 |
| follow-up (IQ-points) | 91.4 | 89.78 | 89.1 | |
| change to baseline | −4.3% (p = 0.59) | −0.1% (p = 0.99) | +0.20 (p = 0.98) |
| Equivalence Analysis (Significance Equates to Equivalence) | Specification | Proton (Follow-Up 42 Months after RT) | Photon (Follow-Up 55 Months after RT) | Surgery (Follow-Up 55 Months after Surgery) |
|---|---|---|---|---|
| non-verbal intelligence (CPM/SPM) | follow up (IQ-points) | 99.9 | 98.1 | 96.6 |
| difference (equivalence) to proton | - | −1.8 (p = 0.01) | −3.3 (p = 0.01) | |
| difference (equivalence) to photon | +1.8 (p = 0.01) | - | −1.5 (p = 0.002) | |
| difference (equivalence) to surgery | +3.3 (p = 0.01) | +1.5 (p = 0.01) | - | |
| visuomotor integration (VMI) | follow-up (IQ-points) | 88.6 | 86.3 | 89.9 |
| difference (equivalence) to proton | - | −2.3 (p = 0.09) | −0.7 (p = 0.02) | |
| difference (equivalence) to photon | +2.3 (p = 0.09) | - | +1.6 (p = 0.005) | |
| difference (equivalence) to surgery | +0.7 (p = 0.02) | −1.6 (p = 0.005) | - | |
| word fluency (WF-C) categorial | follow-up (IQ-points) | 91.2 | 89.8 | 88.7 |
| difference (equivalence) to proton | - | −1.4 (p = 0.05) | −2.5 (p = 0.149) | |
| difference (equivalence) to photon | +1.4 (p = 0.05) | - | −1.2 (p = 0.01) | |
| difference (equivalence) to surgery | +2.5 (p = 0.149) | +1.2 (p = 0.01) | - | |
| word fluency (WF-L) lexical | follow-up (IQ-points) | 91.0 | 92.6 | 86.9 |
| difference (equivalence) to proton | - | +1.6 (p = 0.04) | −4.1 (p = 0.07) | |
| difference (equivalence) to photon | −1.6 (p = 0.04) | - | −5.7 (p = 0.10) | |
| difference (equivalence) to surgery | +4.1 (p = 0.07) | +5.7 (p = 0.10) | - | |
| processing speed (PS) | follow-up (IQ-points) | 90.5 | 88.9 | 84.4 |
| difference (equivalence) to proton | - | −1.6 (p = 0.04) | −6.1 (p = 0.03) | |
| difference (equivalence) to photon | +1.6 (p = 0.04) | - | −4.5 (p = 0.11) | |
| difference (equivalence) to surgery | +6.1 (p = 0.03) | +4.5 (p = 0.11) | - | |
| working memory (WM) (digit span) | follow-up (IQ-points) | 93.9 | 93.6 | 93.5 |
| difference (equivalence) to proton | - | −0.3 (p = 0.005) | −0.4 (p = 0.03) | |
| difference (equivalence) to photon | +0.3 (p = 0.005) | - | −0.1 (p = 0.002) | |
| difference (equivalence) to surgery | +0.4 (p = 0.03) | +0.1 (p = 0.002) | - | |
| visuospatial construction (ROCF-C) | follow-up (IQ-points) | 95.9 | 82.1 | 81.2 |
| difference (equivalence) to proton | - | −13.8 (p = 0.49) | −14.7 (p = 0.54) | |
| difference (equivalence) to photon | +13.8 (p = 0.49) | - | −0.9 (p = 0.04) | |
| difference (equivalence) to surgery | +14.7 (p = 0.54) | +0.9 (p = 0.04) | - | |
| visuospatial memory (ROCF-R) | follow-up (IQ-points) | 91.4 | 89.8 | 89.1 |
| difference (equivalence) to proton | - | −1.6 (p = 0.12) | −2.3 (p = 0.11) | |
| difference (equivalence) to photon | +1.6 (p = 0.12) | - | −0.7 (p = 0.01) | |
| difference (equivalence) to surgery | +2.3 (p = 0.11) | +0.7 (p = 0.01) | - |
| Surgery Cohort | Specification | 2 Months after Surgery | 2 Months after Surgery | 19 Months after Surgery | 19 Months after Surgery | 32 Months after Surgery | 32 Months after Surgery | 55 Months after Surgery | 55 Months after Surgery |
|---|---|---|---|---|---|---|---|---|---|
| PTR | GTR | PTR | GTR | PTR | GTR | PTR | GTR | ||
| non-verbal | IQ points | 105.7 | 105.6 | 103.6 | 102.5 | 104.5 | 100.5 | 104.1 | 92.1 |
| intelligence | difference PTR & GTR | +0.1% (p = 0.99) | −0.1% (p = 0.99) | +1.1% (p = 0.84) | −1.1% (p = 0.84) | +3.8% (p = 0.42) | −3.8% (p = 0.42) | +11.5% (p = 0.07) | −11.5% (p = 0.07) |
| visuomotor | IQ points | 88.7 | 97.3 | 90.1 | 95.5 | 90.3 | 92.2 | 91.0 | 86.0 |
| integration (VMI) | difference PTR & GTR | −9.7% (p = 0.34) | +9.7% (p = 0.34) | −6.0% (p = 0.19) | +6.0% (p = 0.19) | −2.1% (p = 0.63) | +2.1% (p = 0.63) | +5.5% (p = 0.39) | −5.5% (p = 0.39) |
| word fluency | IQ points | 88.9 | 92.8 | 93.5 | 89.1 | 96.5 | 87.4 | 90.1 | 87.8 |
| categorial | difference PTR & GTR | -4.4% (p = 0.72) | +4.4% (p = 0.72) | +4.7% (p = 0.51) | −4.7% (p = 0.51) | +9.4% (p = 0.11) | −9.4% (p = 0.11) | +2.6% (p = 0.81) | −2.6% (p = 0.81) |
| word fluency | IQ points | 96.0 | 91.3 | 99.4 | 85.9 | 96.1 | 88.5 | 88.4 | 85.6 |
| lexical | difference PTR & GTR | +4.9% (p = 0.62) | −4.9% (p = 0.62) | +13.6% (p = 0.04) | −13.6% (p = 0.04) | +7.9% (p = 0.23) | −7.9% (p = 0.23) | +3.1% (p = 0.73) | −3.1% (p = 0.73) |
| processing speed | IQ points | 109.3 | 89.0 | 100.4 | 90.3 | 96.8 | 92.9 | 87.8 | 82.2 |
| difference PTR & GTR | +18.6% (p = 0.009) | −18.6% (p = 0.009) | +10.1% (p = 0.09) | −10.1% (p = 0.09) | +4.0% (p = 0.49) | −4.0% (p = 0.49) | +6.4% (p = 0.24) | -6.4% (p = 0.24) | |
| working memory | IQ points | 115.5 | 101.4 | 100.8 | 98.9 | 103.0 | 99.3 | 91.3 | 94.4 |
| (digit span) | difference PTR & GTR | +12.2% (p = 0.15) | −12.2% (p = 0.15) | +2.0% (p = 0.78) | −2.0% (p = 0.78) | +3.6% (p = 0.60) | −3.6% (p = 0.60) | −3.4% (p = 0.74) | +3.4% (p = 0.74) |
| visuospatial | IQ points | 90.7 | 104.1 | 84.7 | 92.7 | 84.0 | 90.9 | 76.5 | 85.2 |
| construction | difference PTR & GTR | −14.8% (p = 0.02) | +14.8% (p = 0.02) | −9.4% (p = 0.42) | +9.4% (p = 0.42) | −8.2% (p = 0.44) | +8.2% (p = 0.44) | −11.4% (p = 0.54) | +11.4% (p = 0.54) |
| visuospatial | IQ points | 81.0 | 100.6 | 90.3 | 98.3 | 87.4 | 92.9 | 92.1 | 86.5 |
| memory | difference PTR & GTR | −24.2% (p = 0.05) | +24.2% (p = 0.05) | −8.9% (p = 0.25) | +8.9% (p = 0.25) | −6.3% (p = 0.37) | +6.3% (p = 0.37) | +6.1% (p = 0.50) | −6.1% (p = 0.50) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weusthof, K.; Lüttich, P.; Regnery, S.; König, L.; Bernhardt, D.; Witt, O.; Herfarth, K.; Unterberg, A.; Jungk, C.; Farnia, B.; et al. Neurocognitive Outcomes in Pediatric Patients Following Brain Irradiation. Cancers 2021, 13, 3538. https://doi.org/10.3390/cancers13143538
Weusthof K, Lüttich P, Regnery S, König L, Bernhardt D, Witt O, Herfarth K, Unterberg A, Jungk C, Farnia B, et al. Neurocognitive Outcomes in Pediatric Patients Following Brain Irradiation. Cancers. 2021; 13(14):3538. https://doi.org/10.3390/cancers13143538
Chicago/Turabian StyleWeusthof, Katharina, Peggy Lüttich, Sebastian Regnery, Laila König, Denise Bernhardt, Olaf Witt, Klaus Herfarth, Andreas Unterberg, Christine Jungk, Benjamin Farnia, and et al. 2021. "Neurocognitive Outcomes in Pediatric Patients Following Brain Irradiation" Cancers 13, no. 14: 3538. https://doi.org/10.3390/cancers13143538
APA StyleWeusthof, K., Lüttich, P., Regnery, S., König, L., Bernhardt, D., Witt, O., Herfarth, K., Unterberg, A., Jungk, C., Farnia, B., Combs, S. E., Debus, J., Rieken, S., Harrabi, S., & Adeberg, S. (2021). Neurocognitive Outcomes in Pediatric Patients Following Brain Irradiation. Cancers, 13(14), 3538. https://doi.org/10.3390/cancers13143538







