A Randomized, Double-Blind, Placebo-Controlled, Cross-Over Study to Evaluate the Efficacy of AqualiefTM Mucoadhesive Tablets in Head and Neck Cancer Patients Who Developed Radiation-Induced Xerostomia
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Product
2.3. Patient Selection
2.4. Patient Treatment and Follow-Up
2.5. Enrollment and Interventions
2.6. Measurement of Saliva Production and Saliva pH
2.7. Patients’ Compliance
2.8. Study Endpoints
- Saliva production without mechanical stimulation: change of saliva produced from baseline to 8 days of treatment.
- 2.
- Determination of pH of the mouth cavity: change from baseline to 8 days of treatment.
- 3.
- Xerostomia evaluation (XQ-I questionnaire) [30]: change from baseline to 8 days of treatment
- 4.
- 5.
- Adherence to the treatment by accountability: total tablets used from baseline to 8 days of treatment.
- 6.
- Patient’s global satisfaction: patients’ report on the ease of use and palatability of the product from baseline to 8 days of treatment.
2.9. Statistical Analysis
3. Results
3.1. Study Population
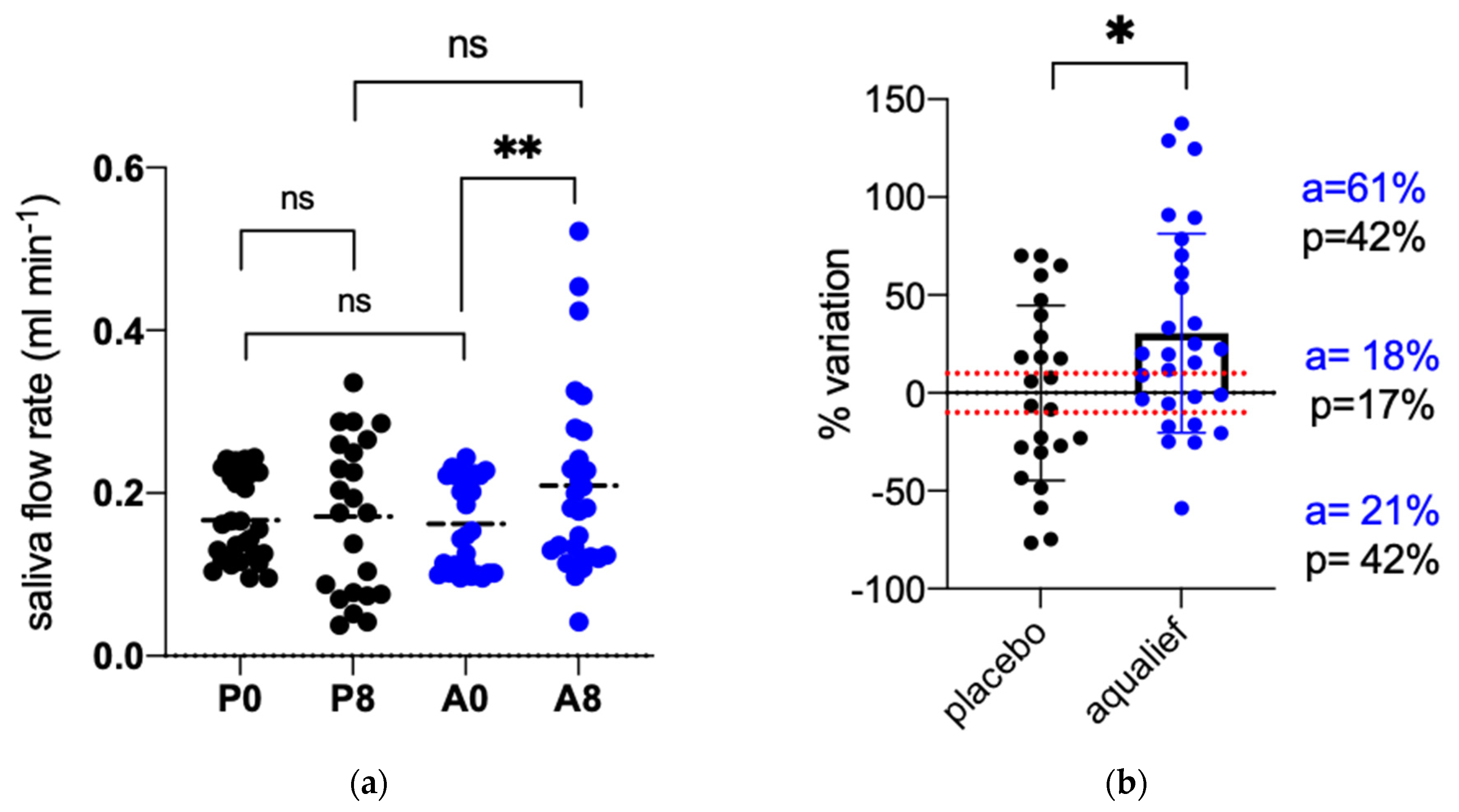
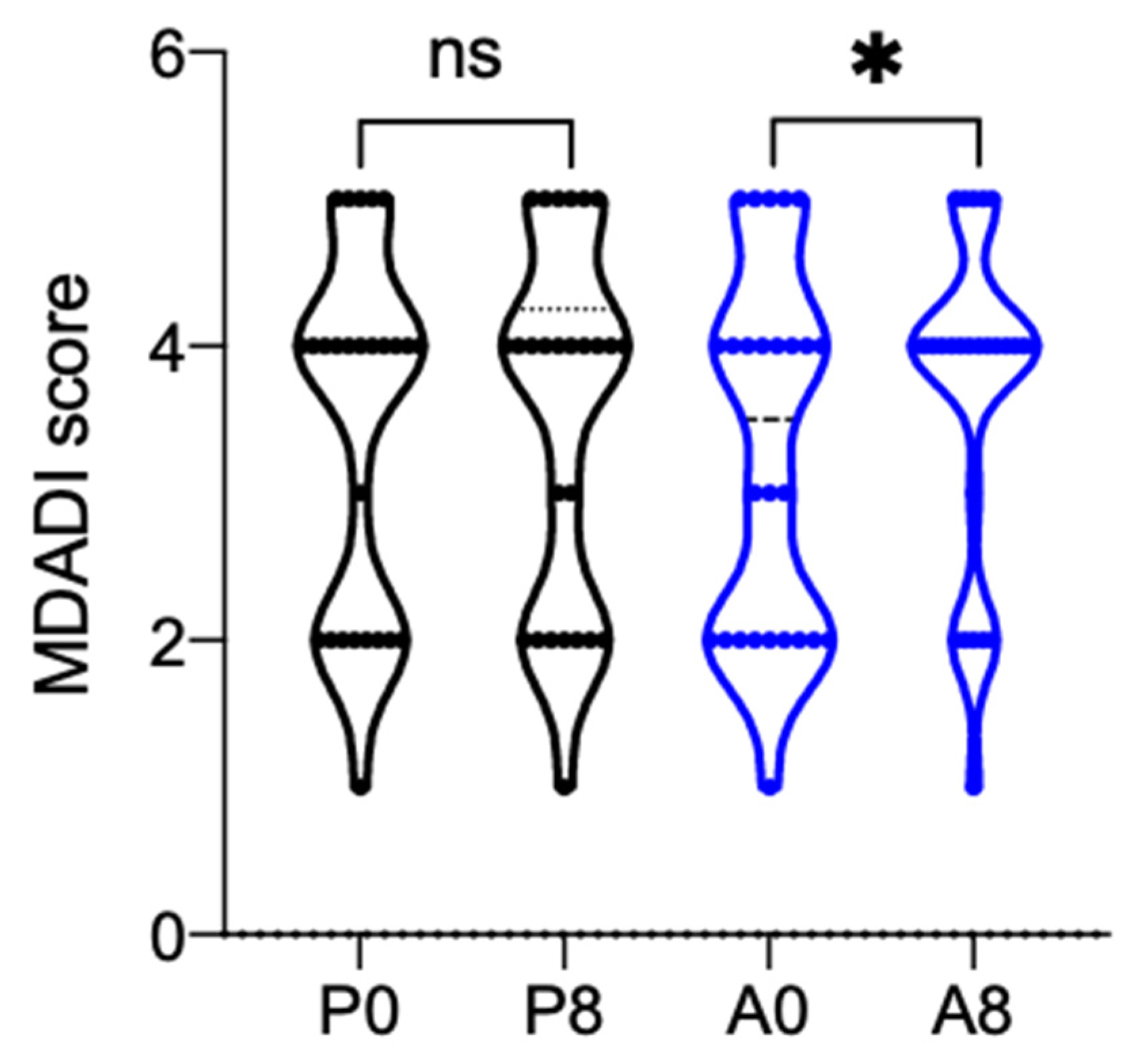
3.2. Primary Endpoints
3.3. Secondary Endpoints
3.4. Adverse Events (AE)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gupta, T.; Agarwal, J.; Jain, S.; Phurailatpam, R.; Kannan, S.; Ghosh-Laskar, S.; Murthy, V.; Budrukkar, A.; Dinshaw, K.; Prabhash, K.; et al. Three-dimensional conformal radiotherapy (3D-CRT) versus intensity modulated radiation therapy (IMRT) in squamous cell carcinoma of the head and neck: A randomized controlled trial. Radiother. Oncol. 2012, 104, 343–348. [Google Scholar] [CrossRef]
- Miah, A.B.; Gulliford, S.L.; Morden, J.; Newbold, K.L.; Bhide, S.A.; Zaidi, S.H.; Hall, E.; Harrington, K.J.; Nutting, C.M. Recovery of salivary function: Contralateral parotid-sparing intensity-modulated radiotherapy versus bilateral superficial lobe parotid-sparing intensity-modulated radiotherapy. Clin. Oncol. (R Coll. Radiol.) 2016, 28, e69–e76. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Jiang, W.; Lakshminarayanan, P.; Han, P.; Cheng, Z.; Bowers, M.; Hui, X.; Shpitser, I.; Siddiqui, S.; Taylor, R.H.; et al. Spatial radiation dose influence on xerostomia recovery and its comparison to acute incidence in patients with head and neck cancer. Adv. Radiat. Oncol. 2019, 5, 221–230. [Google Scholar] [CrossRef]
- Buglione, M.; Cavagnini, R.; Di Rosario, F.; Maddalo, M.; Vassalli, L.; Grisanti, S.; Salgarello, S.; Orlandi, E.; Bossi, P.; Majorana, A.; et al. Oral toxicity management in head and neck cancer patients treated with chemotherapy and radiation: Xerostomia and trismus (Part 2). Literature review and consensus statement. Crit. Rev. Oncol. Hematol. 2016, 102, 47–54. [Google Scholar] [CrossRef]
- Rogers, S.N.; Ahad, S.A.; Murphy, A.P. A structured review and theme analysis of papers published on ’quality of life’ in head and neck cancer: 2000–2005. Oral. Oncol. 2007, 43, 843–868. [Google Scholar] [CrossRef]
- Ettinger, R.L. Xerostomia—A complication of ageing. Aust. Dent. J. 1981, 26, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Delgado, A.J.; Olafsson, V.G. Acidic oral moisturizers with pH below 6.7 may be harmful to teeth depending on formulation: A short report. Clin. Cosmet. Investig. Dent. 2017, 9, 81–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Memtsa, P.T.; Tolia, M.; Tzitzikas, I.; Bizakis, J.; Pistevou-Gombaki, K.; Charalambidou, M.; Iliopoulou, C.; Kyrgias, G. Assessment of xerostomia and its impact on quality of life in head and neck cancer patients undergoing radiation therapy. Mol. Clin. Oncol. 2017, 6, 789–793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vissink, A.; Jansma, J.; Spijkervet, F.K.; Burlage, F.R.; Coppes, R.P. Oral sequelae of head and neck radiotherapy. Crit. Rev. Oral. Biol. Med. 2003, 14, 199–212. [Google Scholar] [CrossRef]
- Beetz, I.; Steenbakkers, R.J.; Chouvalova, O.; Leemans, C.R.; Doornaert, P.; van der Laan, B.F.; Christianen, M.E.; Vissink, A.; Bijl, H.P.; van Luijk, P.; et al. The QUANTEC criteria for parotid gland dose and their efficacy to prevent moderate to severe patient-rated xerostomia. Acta Oncol. 2014, 53, 597–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konings, A.W.; Coppes, R.P.; Vissink, A. On the mechanism of salivary gland radiosensitivity. Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.D. Hyposalivation and xerostomia: Etiology, complications, and medical management. Dent. Clin. N. Am. 2016, 60, 435–443. [Google Scholar] [CrossRef]
- Sim, C.; Soong, Y.L.; Pang, E.; Lim, C.; Walker, G.D.; Manton, D.J.; Reynolds, E.C.; Wee, J. Xerostomia, salivary characteristics and gland volumes following intensity-modulated radiotherapy for nasopharyngeal carcinoma: A two-year follow up. Aust. Dent. J. 2018, 63, 217–223. [Google Scholar] [CrossRef]
- Jensen, S.B.; Vissink, A.; Limesand, K.H.; Reyland, M.E. Salivary gland hypofunction and xerostomia in head and neck radiation patients. J. Natl. Cancer Inst. Monogr. 2019, lgz016. [Google Scholar] [CrossRef]
- Nutting, C.M.; Morden, J.P.; Harrington, K.J.; Urbano, T.G.; Bhide, S.A.; Clark, C.; Miles, E.A.; Miah, A.B.; Newbold, K.; Tanay, M.; et al. PARSPORT trial management group. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): A phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011, 12, 127–136. [Google Scholar] [CrossRef] [Green Version]
- Marta, G.N.; Silva, V.; de Andrade Carvalho, H.; de Arruda, F.F.; Hanna, S.A.; Gadia, R.; da Silva, J.L.; Correa, S.F.; Vita Abreu, C.E.; Riera, R. Intensity-modulated radiation therapy for head and neck cancer: Systematic review and meta-analysis. Radiother. Oncol. 2014, 110, 9–15. [Google Scholar] [CrossRef]
- See, L.; Mohammadi, M.; Han, P.P.; Mulligan, R.; Enciso, R. Efficacy of saliva substitutes and stimulants in the treatment of dry mouth. Spec. Care Dentist. 2019, 39, 287–297. [Google Scholar] [CrossRef]
- Villa, A.; Connell, C.L.; Abati, S. Diagnosis and management of xerostomia and hyposalivation. Ther. Clin. Risk Manag. 2014, 11, 45–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolff, A.; Fox, P.C.; Porter, S.; Konttinen, Y.T. Established and novel approaches for the management of hyposalivation and xerostomia. Curr. Pharm. Des. 2012, 8, 5515–5521. [Google Scholar] [CrossRef] [Green Version]
- Mercadante, V.; Al Hamad, A.; Lodi, G.; Porter, S.; Fedele, S. Interventions for the management of radiotherapy-induced xerostomia and hyposalivation: A systematic review and meta-analysis. Oral. Oncol. 2017, 66, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.B.; Pedersen, A.M.; Vissink, A.; Andersen, E.; Brown, C.G.; Davies, A.N.; Dutilh, J.; Fulton, J.S.; Jankovic, L.; Lopes, N.N.; et al. Salivary Gland Hypofunction/Xerostomia Section; Oral Care Study Group; Multinational Association of Supportive Care in Cancer (MASCC)/International Society of Oral Oncology (ISOO). A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: Management strategies and economic impact. Support Care Cancer 2010, 18, 1061–1079. [Google Scholar] [CrossRef]
- Momm, F.; Volegova-Neher, N.J.; Schulte-Mönting, J.; Guttenberger, R. Different saliva substitutes for treatment of xerostomia following radiotherapy a prospective crossover study. Strahlenther. Onkol. 2005, 181, 231–236. [Google Scholar] [CrossRef]
- Jellema, A.P.; Langendijk, H.; Bergenhenegouwen, L.; van der Reijden, W.; Leemans, R.; Smeele, L.; Slotman, B.J. The efficacy of Xialine in patients with xerostomia resulting from radiotherapy for head and neck cancer. Radiother. Oncol. 2001, 159, 157–160. [Google Scholar] [CrossRef]
- Boldyrev, A.A.; Aldini, G.; Derave, W. Physiology and pathophysiology of carnosine. Physiol. Rev. 2013, 93, 1803–1845. [Google Scholar] [CrossRef] [PubMed]
- Da-Costa-Rocha, I.; Bonnlaender, B.; Sievers, H.; Pischel, I.; Heinrich, M. Hibiscus sabdariffa L.—A phytochemical and pharmacological review. Food Chem. 2014, 165, 424–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levrini, L.; Azzi, L.; Bossi, S. The efficacy of a dietary supplement with carnosine and Hibiscus sabdariffa L. (AqualiefTM) in patients with xerostomia: A randomized, placebo-controlled, double-blind trial. Clin. Ter. 2020, 171, e295–e301. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, E.; Tomatis, S.; Potepan, P.; Bossi, P.; Mongioj, V.; Carrara, M.; Palazzi, M.; Franceschini, M.; Bergamini, C.; Locati, L.; et al. Critical analysis of locoregional failures following intensity-modulated radiotherapy for nasopharyngeal carcinoma. Future Oncol. 2013, 9, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Spreafico, A.; Huang, S.H.; Xu, W.; Granata, R.; Liu, C.S.; Waldron, J.N.; Chen, E.; Ringash, J.; Bayley, A.; Chan, K.K.; et al. Impact of cisplatin dose intensity on human papillomavirus-related and -unrelated locally advanced head and neck squamous cell carcinoma. Eur. J. Cancer 2016, 67, 174–182. [Google Scholar] [CrossRef]
- Granata, R.; Miceli, R.; Orlandi, E.; Perrone, F.; Cortelazzi, B.; Franceschini, M.; Locati, L.D.; Bossi, P.; Bergamini, C.; Mirabile, A.; et al. Tumor stage, human papillomavirus and smoking status affect the survival of patients with oropharyngeal cancer: An Italian validation study. Ann. Oncol. 2012, 7, 1832–1837. [Google Scholar] [CrossRef]
- Marquezin, M.C.S.; Chaves-Júnior, S.C.; Rasera, I.J.r.; Pacheco, E.R.P.; Gavião, M.B.D.; Lamy, E.; Castelo, P.M. Oral health and nutritional characteristics of adults with morbid obesity: A multivariate analysis. Front. Nutr. 2020, 7, 589510. [Google Scholar] [CrossRef]
- Quon, H.; Hui, X.; Cheng, Z.; Robertson, S.; Peng, L.; Bowers, M.; Moore, J.; Choflet, A.; Thompson, A.; Muse, M.; et al. Quantitative evaluation of head and neck cancer treatment-related dysphagia in the development of a personalized treatment deintensification paradigm. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, E.; Miceli, R.; Infante, G.; Mirabile, A.; Alterio, D.; Cossu Rocca, M.; Denaro, N.; Vigna-Taglianti, R.; Merlotti, A.; Schindler, A.; et al. Predictors of patient-reported dysphagia following IMRT plus chemotherapy in oropharyngeal cancer. Dysphagia 2019, 34, 52–62. [Google Scholar] [CrossRef]
- Hegde, M.N.; Attavar, S.H.; Shetty, N.; Hegde, N.D.; Hegde, N.N. Saliva as a biomarker for dental caries: A systematic review. J. Conserv. Dent. 2019, 22, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Kivela, J.; Parkkila, S.; Parkkila, A.K.; Leinonen, J.; Rajaniemi, H. Salivary carbonic anhydrase isoenzyme VI. J. Physiol. 1999, 520, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Vistoli, G.; Aldini, G.; Fumagalli, L.; Dallanoce, C.; Angeli, A.; Supuran, C.T. Activation effects of carnosine- and histidine-containing dipeptides on human carbonic anhydrases: A comprehensive study. Int. J. Mol. Sci. 2020, 21, 1761. [Google Scholar] [CrossRef] [Green Version]
- Sim, Y.Y.; Nyam, K.L. Hibiscus cannabinus L. (kenaf) studies: Nutritional composition, phytochemistry, pharmacology, and potential applications. Food Chem. 2021, 344, 128582. [Google Scholar] [CrossRef] [PubMed]
- Ciulla, M.; Re, D.; Gilardoni, E.; D’Amato, A.; Altomare, A.; Baron, G.; Carugo, S.; Aldini, G. PHoral: Effects of carnosine supplementation on quantity/quality of oral salivae in healthy volunteer and in subjects affected by common oral pathologies. Medicine 2021, 100, e26369. [Google Scholar] [CrossRef]
- Sinjari, B.; Feragalli, B.; Cornelli, U.; Belcaro, G.; Vitacolonna, E.; Santilli, M.; Rexhepi, I.; D’Addazio, G.; Zuccari, F.; Caputi, S. Artificial saliva in diabetic xerostomia (ASDIX): Double blind trial of Aldiamed® versus placebo. Clin. Med. 2020, 9, 2196. [Google Scholar] [CrossRef]
- Łysik, D.; Niemirowicz-Laskowska, K.; Bucki, R.; Tokajuk, G.; Mystkowska, J. Artificial saliva: Challenges and future perspectives for the treatment of xerostomia. Int. J. Mol. Sci. 2019, 20, 3199. [Google Scholar] [CrossRef] [Green Version]
- Niklander, S.; Fuentes, F.; Sanchez, D.; Araya, V.; Chiappini, G.; Martinez, R.; Marshall, M. Impact of 1% malic acid spray on the oral health-related quality of life of patients with xerostomia. J. Oral Sci. 2018, 60, 278–284. [Google Scholar] [CrossRef] [Green Version]
- Ma, S.J.; Rivers, C.I.; Serra, L.M.; Singh, A.K. Long-term outcomes of interventions for radiation-induced xerostomia: A review. World J. Clin. Oncol. 2019, 10, 1–13. [Google Scholar] [CrossRef]
- Jacobs, C.D.; van der Pas, M. A multicenter maintenance study of oral pilocarpine tablets for radiation-induced xerostomia. Oncology 1996, 10 (Suppl. 3), 16–20. [Google Scholar]
- Chitapanarux, I.; Kamnerdsupaphon, P.; Tharavichitkul, E. Effect of oral pilocarpine on post-irradiation xerostomia in head and neck cancer patients: A single-center, single-blind clinical trial. J. Med. Assoc. Thai. 2008, 91, 1410–1415. [Google Scholar]
- Chambers, M.S.; Posner, M.; Jones, C.U.; Biel, M.A.; Hodge, K.M.; Vitti, R.; Armstrong, I.; Yen, C.; Weber, R.S. Cevimeline for the treatment of postirradiation xerostomia in patients with head and neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 1102–1109. [Google Scholar] [CrossRef]
- Witsell, D.L.; Stinnett, S.; Chambers, M.S. Effectiveness of cevimeline to improve oral health in patients with postradiation xerostomia. Head Neck 2012, 34, 1136–1142. [Google Scholar] [CrossRef]
- Brimhall, J.; Jhaveri, M.A.; Yepes, J.F. Efficacy of cevimeline vs. pilocarpine in the secretion of saliva: A pilot study. Spec. Care Dent. 2013, 33, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Hamada, T.; Nakane, T.; Kimura, T.; Arisawa, K.; Yoneda, K.; Yamamoto, T.; Osaki, T. Treatment of xerostomia with the bile secretion-stimulating drug anethole trithione: A clinical trial. Am. J. Med. Sci. 1999, 318, 146–151. [Google Scholar] [CrossRef]
- Bagheri, H.; Schmitt, L.; Berlan, M.; Montastruc, J.L. Effect of 3 weeks treatment with yohimbine on salivary secretion in healthy volunteers and in depressed patients treated with tricyclic antidepressants. Br. J. Clin. Pharmacol. 1992, 34, 555–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, R.S.; Akula, R.; Satyanarayana, T.S.V.; Indugu, V. Recent advances of pacemakers in treatment of xerostomia: A systematic review. Int. Soc. Prev. Community Dent. 2019, 9, 311–315. [Google Scholar] [CrossRef]
- Assy, Z.; Brand, H.S. A systematic review of the effects of acupuncture on xerostomia and hyposalivation. BMC Complement Altern. Med. 2018, 18, 57. [Google Scholar] [CrossRef]

| Characteristics | Placebo-AqualiefTM (N = 14) | AqualiefTM-Placebo (N = 16) | p-Value | |
|---|---|---|---|---|
| Gender | Males | 9 (64.3%) | 10 (62.5%) | 0.919 |
| Females | 5 (38.5%) | 6 (37.5%) | ||
| Age | N | 14 | 16 | |
| Mean (SD) | 58.07 (11.23) | 58.56 (9.37) | 0.897 | |
| Median | 58.50 | 57.50 | ||
| Min–Max | 39.00–74.00 | 40.00–75.00 | ||
| MDADI Global Score | N | 14 | 16 | 0.502 |
| Mean (SD) | 3.29 (1.07) | 3.00 (1.21) | ||
| Median | 4.00 | 2.00 | ||
| Min–Max | 2.00–5.00 | 2.00–5.00 | ||
| MDADI Composite Score | N | 14 | 16 | 0.992 |
| Mean (SD) | 70.15 (14.16) | 70.20 (10.38) | ||
| Median | 71.58 | 72.10 | ||
| Min–Max | 45.26–91.58 | 54.74–89.47 | ||
| XQ1-Questionnaire | N | 14 | 16 | 0.830 |
| Mean (SD) | 45.21 (16.04) | 43.88 (17.51) | ||
| Median | 43.00 | 46.00 | ||
| Min–Max | 19.00–72.00 | 19.00–73.00 |
| Characteristics | Placebo-AqualiefTM (N = 13) | AqualiefTM-Placebo (N = 14) | p-Value | |
|---|---|---|---|---|
| Gender | Females | 8 (61.5%) | 9 (64.3%) | 0.883 |
| Males | 5 (38.5%) | 5 (35.7%) | ||
| Age | N | 13 | 14 | |
| Mean (SD) | 58.62 (11.49) | 58.00 (9.92) | 0.883 | |
| Median | 59.00 | 55.50 | ||
| Min–Max | 39.00–74.00 | 40.00–75.00 | ||
| MDADI Global Score | N | 13 | 14 | 0.614 |
| Mean (SD) | 3.23 (1.09) | 3.00 (1.24) | ||
| Median | 4.00 | 2.00 | ||
| Min–Max | 2.00–5.00 | 2.00–5.00 | ||
| MDADI Composite Score | N | 13 | 14 | 0.609 |
| Mean (SD) | 68.99 (14.02) | 71.35 (9.39) | ||
| Median | 71.58 | 72.10 | ||
| Min–Max | 45.26–91.58 | 54.74–89.47 | ||
| XQ1-Questionnaire | N | 13 | 14 | 0.871 |
| Mean (SD) | 44.38 (16.38) | 43.29 (18.31) | ||
| Median | 39.00 | 46.00 | ||
| Min–Max | 19.00–72.00 | 19.00–73.00 |
| Answer | Placebo | AqualiefTM |
|---|---|---|
| Very poor (not satisfied at all) | 5 (19.2%) | 4 (15.4%) |
| Poor (not very satisfied) | 9 (34.6%) | 9 (34.6%) |
| Medium (on average satisfied) | 10 (38.5%) | 9 (34.6%) |
| Good (quite satisfied) | 2 (7.7%) | 3 (11.5%) |
| Very good (very satisfied) | 0 (0.0%) | 1 (3.8%) |
| p-value | 0.572 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iacovelli, N.A.; Ingargiola, R.; Facchinetti, N.; Franceschini, M.; Romanello, D.A.; Bossi, P.; Bergamini, C.; Alfieri, S.; Cavalieri, S.; Baron, G.; et al. A Randomized, Double-Blind, Placebo-Controlled, Cross-Over Study to Evaluate the Efficacy of AqualiefTM Mucoadhesive Tablets in Head and Neck Cancer Patients Who Developed Radiation-Induced Xerostomia. Cancers 2021, 13, 3456. https://doi.org/10.3390/cancers13143456
Iacovelli NA, Ingargiola R, Facchinetti N, Franceschini M, Romanello DA, Bossi P, Bergamini C, Alfieri S, Cavalieri S, Baron G, et al. A Randomized, Double-Blind, Placebo-Controlled, Cross-Over Study to Evaluate the Efficacy of AqualiefTM Mucoadhesive Tablets in Head and Neck Cancer Patients Who Developed Radiation-Induced Xerostomia. Cancers. 2021; 13(14):3456. https://doi.org/10.3390/cancers13143456
Chicago/Turabian StyleIacovelli, Nicola Alessandro, Rossana Ingargiola, Nadia Facchinetti, Marzia Franceschini, Domenico Attilio Romanello, Paolo Bossi, Cristiana Bergamini, Salvatore Alfieri, Stefano Cavalieri, Giovanna Baron, and et al. 2021. "A Randomized, Double-Blind, Placebo-Controlled, Cross-Over Study to Evaluate the Efficacy of AqualiefTM Mucoadhesive Tablets in Head and Neck Cancer Patients Who Developed Radiation-Induced Xerostomia" Cancers 13, no. 14: 3456. https://doi.org/10.3390/cancers13143456
APA StyleIacovelli, N. A., Ingargiola, R., Facchinetti, N., Franceschini, M., Romanello, D. A., Bossi, P., Bergamini, C., Alfieri, S., Cavalieri, S., Baron, G., Aldini, G., Locati, L., & Orlandi, E. (2021). A Randomized, Double-Blind, Placebo-Controlled, Cross-Over Study to Evaluate the Efficacy of AqualiefTM Mucoadhesive Tablets in Head and Neck Cancer Patients Who Developed Radiation-Induced Xerostomia. Cancers, 13(14), 3456. https://doi.org/10.3390/cancers13143456









