Albumin Nanostructures for Nucleic Acid Delivery in Cancer: Current Trend, Emerging Issues, and Possible Solutions
Abstract
Simple Summary
Abstract
1. Introduction
2. Nucleic Acids in Cancer Therapy
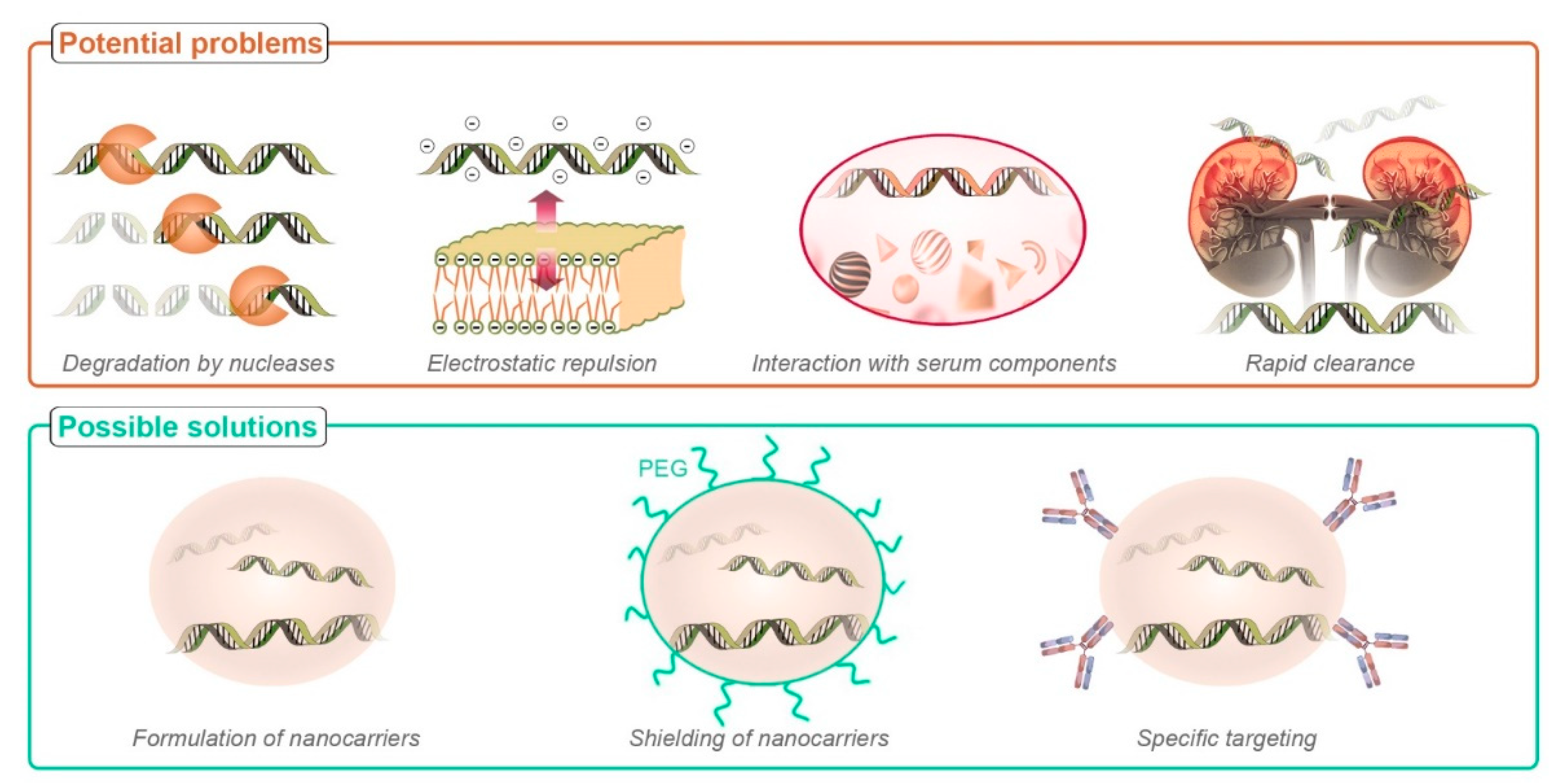
2.1. Limitations Associated with Nucleic Acid Delivery
2.2. Nanocarriers for Nucleic Acid Delivery
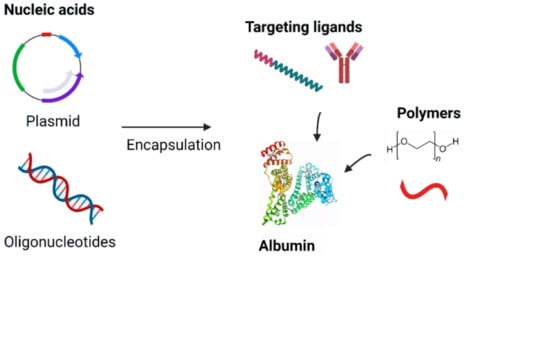
3. Albumin-Based Nanocarriers
3.1. Albumin
3.2. Albumin in Cancer Therapy
3.3. Albumin Nanocarrier for Gene Therapy in Cancer
3.4. Albumin Nanoparticles
3.4.1. Plasmid
3.4.2. Oligonucleotides
3.4.3. siRNA
3.5. Polyplexes
3.6. Nanoconjugates
4. Albumin as a Coating Agent
5. Nucleic Acid-Loaded Albumin Nanocarriers for Immunotherapy
6. Emerging Issues and Possible Solutions
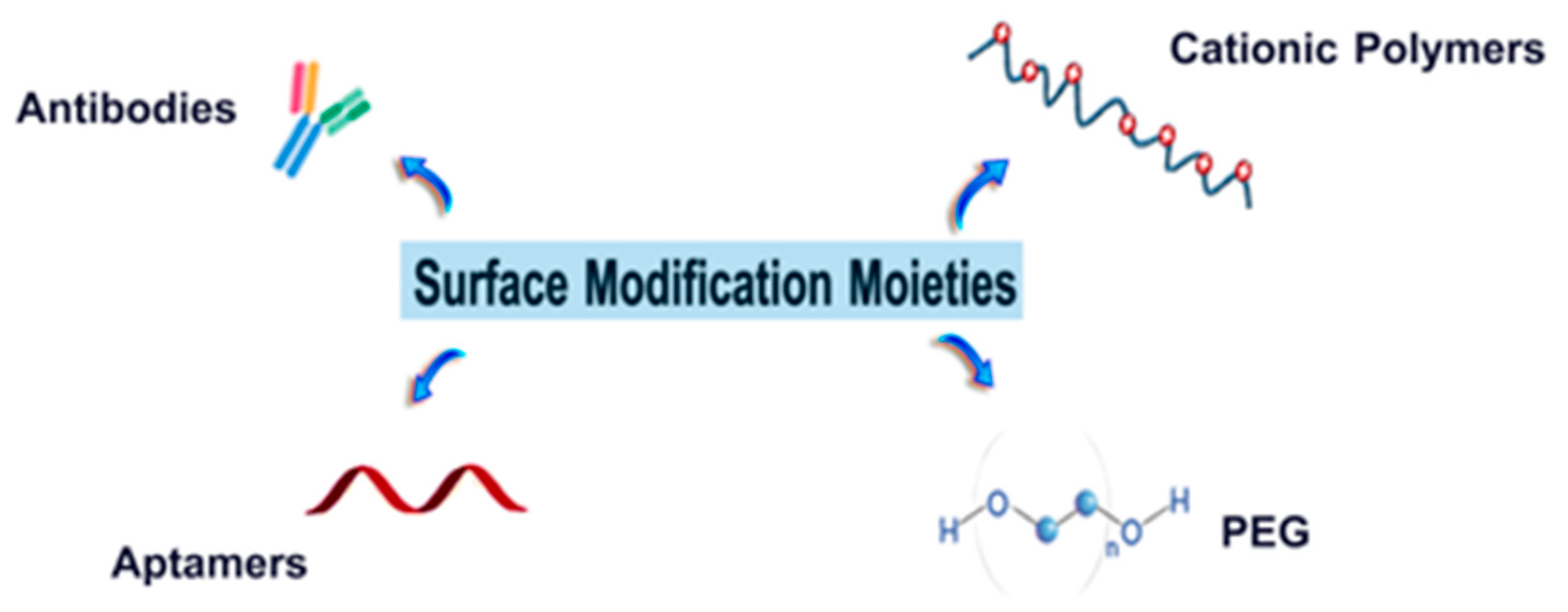
6.1. Polymers
6.1.1. Cationic Polymers
6.1.2. PEG
6.2. Targeting Ligands
6.2.1. Antibodies
6.2.2. Aptamers
7. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Fitzmaurice, C.; Allen, C.; Barber, R.M.; Barregard, L.; Bhutta, Z.A.; Brenner, H.; Dicker, D.J.; Chimed-Orchir, O.; Dandona, R.; Dandona, L.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A systematic analysis for the global burden of disease study. JAMA Oncol. 2017, 3, 524–548. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, D.A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Sawyers, C.L. Targeted cancer therapy. Nature 2004, 432, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.M.; Chaudhari, P.D.; Sahu, M.; Duragkar, N.J. Review article on gene therapy. Int. J. Genet. 2012, 4, 74–79. [Google Scholar]
- Elsabahy, M.; Nazarali, A.; Foldvari, M. Non-viral nucleic acid delivery: Key challenges and future directions. Curr. Drug Deliv. 2011, 8, 235–244. [Google Scholar] [CrossRef]
- Silva, A.C.; Lopes, C.M.; Lobo, J.M.S.; Amaral, M.H. Nucleic acids delivery systems: A challenge for pharmaceutical technologists. Curr. Drug Metab. 2015, 16, 3–16. [Google Scholar] [CrossRef]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef]
- Jeong, J.H.; Park, T.G.; Kim, S.H. Self-Assembled and Nanostructured siRNA Delivery Systems. Pharm. Res. 2011, 28, 2072–2085. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, P.; Kumar, S.U.; Matai, I.; Bhushan, B.; Malwal, D.; Sachdev, A.; Dubey, P. Cancer Nanotheranostics; Springer: Singapore, 2015; ISBN 9789812874351. [Google Scholar]
- Prabhakar, U.; Maeda, H.; Jain, R.K.; Sevick-Muraca, E.M.; Zamboni, W.; Farokhzad, O.C.; Barry, S.T.; Gabizon, A.; Grodzinski, P.; Blakey, D.C. Challenges and Key Considerations of the Enhanced Permeability and Retention Effect for Nanomedicine Drug Delivery in Oncology. Cancer Res. 2013, 73, 2412–2417. [Google Scholar] [CrossRef]
- Dharap, S.S.; Wang, Y.; Chandna, P.; Khandare, J.J.; Qiu, B.; Gunaseelan, S.; Sinko, P.J.; Stein, S.; Farmanfarmaian, A.; Minko, T.; et al. Tumor-specific targeting of an anticancer drug delivery system by LHRH peptide. Proc. Natl. Acad. Sci. USA 2005, 102, 12962–12967. [Google Scholar] [CrossRef]
- Rothdiener, M.; Beuttler, J.; Messerschmidt, S.K.E.; Kontermann, R.E. Antibody targeting of nanoparticles to tumor-specific receptors: Immunoliposomes. Cancer Nanotechnol. 2010, 1, 295–308. [Google Scholar] [CrossRef]
- Wang, C.-H.; Kang, S.-T.; Lee, Y.-H.; Luo, Y.-L.; Huang, Y.-F.; Yeh, C.-K. Aptamer-conjugated and drug-loaded acoustic droplets for ultrasound theranosis. Biomaterials 2012, 33, 1939–1947. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Aebersold, P.; Cornetta, K.; Kasid, A.; Morgan, R.A.; Moen, R.; Karson, E.M.; Lotze, M.T.; Yang, J.C.; Topalian, S.L.; et al. Gene Transfer into Humans—Immunotherapy of Patients with Advanced Melanoma, Using Tumor-Infiltrating Lymphocytes Modified by Retroviral Gene Transduction. N. Engl. J. Med. 1990, 323, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Salas, L.M. Nucleic acids as therapeutic agents. Curr. Top. Med. Chem. 2008, 8, 1379–1404. [Google Scholar] [CrossRef] [PubMed]
- Steinhauser, I.; Langer, K.; Strebhardt, K.; Spänkuch, B. Uptake of plasmid-loaded nanoparticles in breast cancer cells and effect on Plk1 expression. J. Drug Target. 2009, 17, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Y.; Zhu, Y.; Oupický, D. Recent advances in delivery of drug–nucleic acid combinations for cancer treatment. J. Control. Release 2013, 172, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, M. Short interfering RNAs as a tool for cancer gene therapy. Cancer Gene Ther. 2005, 12, 217–227. [Google Scholar] [CrossRef]
- Shir, A.; Ogris, M.; Wagner, E.; Levitzki, A. EGF Receptor-Targeted Synthetic Double-Stranded RNA Eliminates Glioblastoma, Breast Cancer, and Adenocarcinoma Tumors in Mice. PLoS Med. 2005, 3, e266. [Google Scholar] [CrossRef]
- Zhou, G.; Wilson, G.; Hebbard, L.; Duan, W.; Liddle, C.; George, J.; Qiao, L. Aptamers: A promising chemical antibody for cancer therapy. Oncotarget 2016, 7, 13446–13463. [Google Scholar] [CrossRef]
- Parsel, S.M.; Grandis, J.R.; Thomas, S.M. Nucleic acid targeting: Towards personalized therapy for head and neck cancer. Oncogene 2016, 35, 3217–3226. [Google Scholar] [CrossRef]
- Ramasamy, T.; Munusamy, S.; Ruttala, H.B.; Kim, J.O. Smart Nanocarriers for the Delivery of Nucleic Acid-Based Therapeutics: A Comprehensive Review. Biotechnol. J. 2021, 16, 1900408. [Google Scholar] [CrossRef]
- Lu, W.; Sun, Q.; Wan, J.; She, Z.; Jiang, X.-G. Cationic Albumin–Conjugated Pegylated Nanoparticles Allow Gene Delivery into Brain Tumors via Intravenous Administration. Cancer Res. 2006, 66, 11878–11887. [Google Scholar] [CrossRef]
- Zhu, Q.; Pan, X.; Sun, Y.; Wang, Z.; Liu, F.; Li, A.; Zhao, Z.; Wang, Y.; Li, K.; Mi, L. Biological nanoparticles carrying the Hmda-7 gene are effective in inhibiting pancreatic cancer in vitro and in vivo. PLoS ONE 2017, 12, e0185507. [Google Scholar] [CrossRef] [PubMed]
- Dachs, G.U.; Dougherty, G.J.; Stratford, I.J.; Chaplin, D.J. Targeting gene therapy to cancer: A review. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 1997, 9, 313–325. [Google Scholar]
- Harrison, E.B.; Azam, S.H.; Pecot, C.V. Targeting Accessories to the Crime: Nanoparticle Nucleic Acid Delivery to the Tumor Microenvironment. Front. Pharmacol. 2018, 9, 307. [Google Scholar] [CrossRef] [PubMed]
- Harada-Shiba, M.; Yamauchi, K.; Harada, A.; Takamisawa, I.; Shimokado, K.; Kataoka, K. Polyion complex micelles as vectors in gene therapy–pharmacokinetics and in vivo gene transfer. Gene Ther. 2002, 9, 407–414. [Google Scholar] [CrossRef]
- Karikó, K.; Bhuyan, P.; Capodici, J.; Weissman, D. Small Interfering RNAs Mediate Sequence-Independent Gene Suppression and Induce Immune Activation by Signaling through Toll-Like Receptor 3. J. Immunol. 2004, 172, 6545–6549. [Google Scholar] [CrossRef]
- Oh, Y.-K.; Park, T.G. siRNA delivery systems for cancer treatment. Adv. Drug Deliv. Rev. 2009, 61, 850–862. [Google Scholar] [CrossRef] [PubMed]
- Lundstrom, K.; Boulikas, T. Viral and Non-viral Vectors in Gene Therapy: Technology Development and Clinical Trials. Technol. Cancer Res. Treat. 2003, 2, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Elzoghby, A.O.; Samy, W.M.; Elgindy, N.A. Protein-based nanocarriers as promising drug and gene delivery systems. J. Control. Release 2012, 161, 38–49. [Google Scholar] [CrossRef]
- Larsen, M.T.; Kuhlmann, M.; Hvam, M.L.; Howard, K.A. Albumin-based drug delivery: Harnessing nature to cure disease. Mol. Cell. Ther. 2016, 4, 3. [Google Scholar] [CrossRef]
- Hoogenboezem, E.N.; Duvall, C.L. Harnessing albumin as a carrier for cancer therapies. Adv. Drug Deliv. Rev. 2018, 130, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Kragh-Hansen, U.; Chuang, V.T.G.; Otagiri, M. Practical Aspects of the Ligand-Binding and Enzymatic Properties of Human Serum Albumin. Biol. Pharm. Bull. 2002, 25, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, G.J.; Martin, G.S.; Evans, T.W. Albumin: Biochemical properties and therapeutic potential. Hepatology 2005, 41, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Merlot, A.M.; Kalinowski, D.S.; Richardson, D.R. Unraveling the mysteries of serum albumin—More than just a serum protein. Front. Physiol. 2014, 5, 299. [Google Scholar] [CrossRef]
- Elzoghby, A.O.; Samy, W.M.; Elgindy, N.A. Albumin-based nanoparticles as potential controlled release drug delivery systems. J. Control. Release 2012, 157, 168–182. [Google Scholar] [CrossRef] [PubMed]
- Evans, T.W. Review article: Albumin as a drug—Biological effects of albumin unrelated to oncotic pressure. Aliment. Pharmacol. Ther. 2002, 16, 6–11. [Google Scholar] [CrossRef]
- Kratz, F. Albumin, a versatile carrier in oncology. Int. J. Clin. Pharmacol. Ther. 2010, 48, 453–455. [Google Scholar] [CrossRef]
- An, F.-F.; Zhang, X.-H. Strategies for Preparing Albumin-based Nanoparticles for Multifunctional Bioimaging and Drug Delivery. Theranostics 2017, 7, 3667–3689. [Google Scholar] [CrossRef]
- Chatterjee, M.; Ben-Josef, E.; Robb, R.; Vedaie, M.; Seum, S.; Thirumoorthy, K.; Palanichamy, K.; Harbrecht, M.; Chakravarti, A.; Williams, T.M. Caveolae-Mediated Endocytosis Is Critical for Albumin Cellular Uptake and Response to Albumin-Bound Chemotherapy. Cancer Res. 2017, 77, 5925–5937. [Google Scholar] [CrossRef]
- Langer, K.; Anhorn, M.G.; Steinhauser, I.; Dreis, S.; Celebi, D.; Schrickel, N.; Faust, S.; Vogel, V. Human serum albumin (HSA) nanoparticles: Reproducibility of preparation process and kinetics of enzymatic degradation. Int. J. Pharm. 2008, 347, 109–117. [Google Scholar] [CrossRef]
- Karimi, M.; Bahrami, S.; Ravari, S.B.; Zangabad, P.S.; Mirshekari, H.; Bozorgomid, M.; Shahreza, S.; Sori, M.; Hamblin, M.R. Albumin nanostructures as advanced drug delivery systems. Expert Opin. Drug Deliv. 2016, 13, 1609–1623. [Google Scholar] [CrossRef] [PubMed]
- Syga, M.-I.; Nicolì, E.; Kohler, E.; Shastri, V.P. Albumin Incorporation in Polyethylenimine–DNA Polyplexes Influences Transfection Efficiency. Biomacromolecules 2016, 17, 200–207. [Google Scholar] [CrossRef]
- Rhaese, S.; Von Briesen, H.; Rubsamen-waigmann, H.; Langer, K. Human serum albumin–polyethylenimine nanoparticles for gene delivery. J. Control. Release 2003, 92, 199–208. [Google Scholar] [CrossRef]
- Ming, X.; Carver, K.; Wu, L. Albumin-based nanoconjugates for targeted delivery of therapeutic oligonucleotides. Biomaterials 2013, 34, 7939–7949. [Google Scholar] [CrossRef]
- Wartlick, H.; Spänkuch-Schmitt, B.; Strebhardt, K.; Kreuter, J.; Langer, K. Tumour cell delivery of antisense oligonuclceotides by human serum albumin nanoparticles. J. Control. Release 2004, 96, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, Y.; Chen, L.; Liu, Q.; Qi, S.; Cheng, X.; Lee, Y.B.; Ahn, C.-H.; Kim, D.J.; Lee, R.J. Folate receptor-targeted lipid-albumin nanoparticles (F-LAN) for therapeutic delivery of an Akt1 antisense oligonucleotide. J. Drug Target. 2018, 26, 466–473. [Google Scholar] [CrossRef]
- Son, S.; Song, S.; Lee, S.J.; Min, S.; Kim, S.A.; Yhee, J.Y.; Huh, M.S.; Kwon, I.C.; Jeong, S.Y.; Byun, Y.; et al. Self-crosslinked human serum albumin nanocarriers for systemic delivery of polymerized siRNA to tumors. Biomaterials 2013, 34, 9475–9485. [Google Scholar] [CrossRef]
- Choi, J.-H.; Hwang, H.-J.; Shin, S.W.; Choi, J.-W.; Um, S.H.; Oh, B.-K. A novel albumin nanocomplex containing both small interfering RNA and gold nanorods for synergetic anticancer therapy. Nanoscale 2015, 7, 9229–9237. [Google Scholar] [CrossRef] [PubMed]
- Piao, L.; Li, H.; Teng, L.; Yung, B.C.; Sugimoto, Y.; Brueggemeier, R.W.; Lee, R.J. Human serum albumin-coated lipid nanoparticles for delivery of siRNA to breast cancer. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Lynn, G.M.; Jacobson, O.; Chen, K.; Liu, Y.; Zhang, H.; Ma, Y.; Zhang, F.; Tian, R.; Ni, Q.; et al. Albumin/vaccine nanocomplexes that assemble in vivo for combination cancer immunotherapy. Nat. Commun. 2017, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Sheu, M.; Lin, H. Stearyl polyethylenimine complexed with plasmids as the core of human serum albumin nanoparticles noncovalently bound to CRISPR/Cas9 plasmids or siRNA for disrupting or silencing PD-L1 expression for immunotherapy. Int. J. Nanomed. 2018, 13, 7079–7094. [Google Scholar] [CrossRef] [PubMed]
- Yedomon, B.H.; Fessi, H.; Charcosset, C. Preparation of Bovine Serum Albumin (BSA) nanoparticles by desolvation using a membrane contactor: A new tool for large scale production. Eur. J. Pharm. Biopharm. 2013, 85, 398–405. [Google Scholar] [CrossRef]
- Mo, Y.; Barnett, M.E.; Takemoto, D.; Davidson, H.; Kompella, U.B. Human serum albumin nanoparticles for efficient delivery of Cu, Zn superoxide dismutase gene. Mol. Vis. 2007, 13, 746–757. [Google Scholar]
- Wagh, J.; Patel, K.J.; Soni, P.; Desai, K.; Upadhyay, P.; Soni, H.P. Transfecting pDNA to E. coli DH5α using bovine serum albumin nanoparticles as a delivery vehicle. Luminescence 2015, 30, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Xiang, P.; Li, Q. Investigations of the effect of DNA size in transient transfection assay using dual luciferase system. Anal. Biochem. 2005, 346, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Liu, Y.; Li, J.; Hou, L.; Lei, F.; Huang, S.; Feng, L.; Zhao, X.; Feng, L.; Zhao, X. Human serum albumin-mediated apoptin delivery suppresses breast cancer cell growth in vitro and in vivo. Oncol. Lett. 2017, 13, 579–586. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, H.; Li, H.; Zhang, D. Magnetic albumin immuno-nanospheres as an efficient gene delivery system for a potential use in lung cancer: Preparation, in vitro targeting and biological effect analysis. J. Drug Target. 2016, 24, 247–256. [Google Scholar] [CrossRef]
- Zhang, H.; Liang, C.; Hou, X.; Wang, L.; Zhang, D. Study of the combined treatment of lung cancer using gene-loaded immunomagnetic albumin nanospheres in vitro and in vivo. Int. J. Nanomed. 2016, 11, 1039–1050. [Google Scholar] [CrossRef][Green Version]
- Bennett, C.F.; Swayze, E.E. RNA Targeting Therapeutics: Molecular Mechanisms of Antisense Oligonucleotides as a Therapeutic Platform. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 259–293. [Google Scholar] [CrossRef]
- Srinivasan, S.K.; Tewary, H.K.; Iversen, P.L. Characterization of Binding Sites, Extent of Binding, and Drug Interactions of Oligonucleotides with Albumin. Antisense Res. Dev. 1995, 5, 131–139. [Google Scholar] [CrossRef]
- Chernikov, I.V.; Vlassov, V.V.; Chernolovskaya, E.L. Current Development of siRNA Bioconjugates: From Research to the Clinic. Front. Pharmacol. 2019, 10, 444. [Google Scholar] [CrossRef] [PubMed]
- Pecot, C.V.; Calin, G.A.; Coleman, R.L.; Lopez-Berestein, G.; Sood, A.K. RNA interference in the clinic: Challenges and future directions. Nat. Rev. Cancer 2011, 11, 59–67. [Google Scholar] [CrossRef]
- Malhotra, A.; Mittal, B.R. SiRNA gene therapy using albumin as a carrier. Pharm. Genom. 2014, 24, 582–587. [Google Scholar] [CrossRef]
- Hall, A.; Lächelt, U.; Bartek, J.; Wagner, E.; Moghimi, S.M. Polyplex Evolution: Understanding Biology, Optimizing Performance. Mol. Ther. 2017, 25, 1476–1490. [Google Scholar] [CrossRef]
- Boussif, O.; Lezoualc’H, F.; Zanta, M.A.; Mergny, M.D.; Scherman, D.; Demeneix, B.; Behr, J.P. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: Polyethylenimine. Proc. Natl. Acad. Sci. USA 1995, 92, 7297–7301. [Google Scholar] [CrossRef]
- Kumari, M.; Liu, C.-H.; Wu, W.-C. Efficient gene delivery by oligochitosan conjugated serum albumin: Facile synthesis, polyplex stability, and transfection. Carbohydr. Polym. 2018, 183, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Nicolì, E.; Syga, M.I.; Bosetti, M.; Shastri, V.P. Enhanced Gene Silencing through Human Serum Albumin-Mediated Delivery of Polyethylenimine-siRNA Polyplexes. PLoS ONE 2015, 10, e0122581. [Google Scholar] [CrossRef]
- Kudarha, R.R.; Sawant, K.K. Albumin based versatile multifunctional nanocarriers for cancer therapy: Fabrication, surface modification, multimodal therapeutics and imaging approaches. Mater. Sci. Eng. C 2017, 81, 607–626. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Alam, R.; Dixit, V.; Fisher, M.; Juliano, R.L. Cellular Delivery and Biological Activity of Antisense Oligonucleotides Conjugated to a Targeted Protein Carrier. Bioconjug. Chem. 2008, 19, 2182–2188. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar]
- Carver, K.; Ming, X.; Juliano, R.L. Multicellular Tumor Spheroids as a Model for Assessing Delivery of Oligonucleotides in Three Dimensions. Mol. Ther. Nucleic Acids 2014, 3, e153. [Google Scholar] [CrossRef]
- Sarett, S.M.; Werfel, T.A.; Lee, L.; Jackson, M.A.; Kilchrist, K.V.; Brantley-Sieders, D.; Duvall, C.L. Lipophilic siRNA targets albumin in situ and promotes bioavailability, tumor penetration, and carrier-free gene silencing. Proc. Natl. Acad. Sci. USA 2017, 114, E6490–E6497. [Google Scholar] [CrossRef] [PubMed]
- Boisguérin, P.; Konate, K.; Josse, E.; Vivès, E.; Deshayes, S. Peptide-Based Nanoparticles for Therapeutic Nucleic Acid Delivery. Biomedicines 2021, 9, 583. [Google Scholar] [CrossRef]
- Ghahremani, F.; Shahbazi-Gahrouei, D.; Kefayat, A.; Motaghi, H.; Mehrgardi, M.A.; Javanmard, S.H. AS1411 aptamer conjugated gold nanoclusters as a targeted radiosensitizer for megavoltage radiation therapy of 4T1 breast cancer cells. RSC Adv. 2018, 8, 4249–4258. [Google Scholar] [CrossRef]
- Xu, B.; Xu, Y.; Su, G.; Zhu, H.; Zong, L. A multifunctional nanoparticle constructed with a detachable albumin outer shell and a redox-sensitive inner core for efficient siRNA delivery to hepatocellular carcinoma cells. J. Drug Target. 2018, 26, 941–954. [Google Scholar] [CrossRef] [PubMed]
- Weecharangsan, W.; Yu, B.; Zheng, Y.; Liu, S.; Pang, J.X.; Lee, L.J.; Marcucci, G.; Lee, R.J. Efficient Delivery of Antisense Oligodeoxyribonucleotide G3139 by Human Serum Albumin-Coated Liposomes. Mol. Pharm. 2009, 6, 1848–1855. [Google Scholar] [CrossRef]
- Kirkwood, J.M.; Butterfield, L.H.; Tarhini, A.A.; Zarour, H.; Kalinski, P.; Ferrone, S. Immunotherapy of cancer in 2012. CA Cancer J. Clin. 2012, 62, 309–335. [Google Scholar] [CrossRef]
- Hargadon, K.M.; Johnson, C.E.; Williams, C.J. Immune checkpoint blockade therapy for cancer: An overview of FDA-approved immune checkpoint inhibitors. Int. Immunopharmacol. 2018, 62, 29–39. [Google Scholar] [CrossRef]
- Hager, S.; Fittler, F.J.; Wagner, E.; Bros, M. Nucleic Acid-Based Approaches for Tumor Therapy. Cells 2020, 9, 2061. [Google Scholar] [CrossRef]
- Shen, T.; Zhang, Y.; Zhou, S.; Lin, S.; Zhang, X.-B.; Zhu, G. Nucleic Acid Immunotherapeutics for Cancer. ACS Appl. Bio Mater. 2020, 3, 2838–2849. [Google Scholar] [CrossRef]
- Fala, L. Kymriah (Tisagenlecleucel) for Young Patients with Acute Lymphoblastic Leukemia: First FDA-Approved Gene Therapy. Oncol. Guid. FDA Approv. 2018, 11, 37–38. [Google Scholar]
- Rupp, L.J.; Schumann, K.; Roybal, K.T.; Gate, R.E.; Chun, J.Y.; Lim, W.A.; Marson, A. CRISPR/Cas9-mediated PD-1 disruption enhances anti-tumor efficacy of human chimeric antigen receptor T cells. Sci. Rep. 2017, 7, 737. [Google Scholar] [CrossRef]
- Look, J.; Wilhelm, N.; Von Briesen, H.; Noske, N.; Günther, C.; Langer, K.; Gorjup, E. Ligand-Modified Human Serum Albumin Nanoparticles for Enhanced Gene Delivery. Mol. Pharm. 2015, 12, 3202–3213. [Google Scholar] [CrossRef] [PubMed]
- Fischer, D.; Bieber, T.; Bru, S.; Elsa, H.; Kissel, T. Cationized human serum albumin as a non-viral vector system for gene delivery? Characterization of complex formation with plasmid DNA and transfection efficiency. Int. J. Pharm. 2001, 225, 97–111. [Google Scholar] [CrossRef]
- Steinhauser, I.; Spänkuch, B.; Strebhardt, K.; Langer, K. Trastuzumab-modified nanoparticles: Optimisation of preparation and uptake in cancer cells. Biomaterials 2006, 27, 4975–4983. [Google Scholar] [CrossRef]
- Choi, J.-S.; Meghani, N. Impact of surface modification in BSA nanoparticles for uptake in cancer cells. Colloids Surf. B Biointerfaces 2016, 145, 653–661. [Google Scholar] [CrossRef]
- Yogasundaram, H.; Bahniuk, M.S.; Singh, H.-D.; Aliabadi, H.M.; Uludag, H.; Unsworth, L.D. BSA Nanoparticles for siRNA Delivery: Coating Effects on Nanoparticle Properties, Plasma Protein Adsorption, and In Vitro siRNA Delivery. Int. J. Biomater. 2012, 2012, 584060. [Google Scholar] [CrossRef]
- Abbasi, S.; Paul, A.; Prakash, S. Investigation of siRNA-Loaded Polyethylenimine-Coated Human Serum Albumin Nanoparticle Complexes for the Treatment of Breast Cancer. Cell Biochem. Biophys. 2011, 61, 277–287. [Google Scholar] [CrossRef]
- Lv, J.; Chang, H.; Wang, Y.; Wang, M.; Xiao, J.; Zhang, Q.; Cheng, Y. Fluorination on polyethylenimine allows efficient 2D and 3D cell culture gene delivery. J. Mater. Chem. B 2015, 3, 642–650. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, M.; Meng, F.; Zhang, J.; Peng, R.; Zhong, Z. Branched Polyethylenimine Derivatives with Reductively Cleavable Periphery for Safe and Efficient In Vitro Gene Transfer. Biomacromolecules 2011, 12, 1032–1040. [Google Scholar] [CrossRef]
- Liu, D.-E.; An, J.; Pang, C.; Yan, X.; Li, W.; Ma, J.; Gao, H. Construction of Bovine Serum Albumin/AIE-Based Quaternary Complexes for Efficient Gene Transfection. Macromol. Biosci. 2019, 19, e1800359. [Google Scholar] [CrossRef] [PubMed]
- Gosselin, M.A.; Guo, W.; Lee, R.J. Efficient Gene Transfer Using Reversibly Cross-Linked Low Molecular Weight Polyethylenimine. Bioconjug. Chem. 2001, 12, 989–994. [Google Scholar] [CrossRef]
- Lu, W.; Tan, Y.-Z.; Hu, K.-L.; Jiang, X.-G. Cationic albumin conjugated pegylated nanoparticle with its transcytosis ability and little toxicity against blood–brain barrier. Int. J. Pharm. 2005, 295, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Ogris, M.; Brunner, S.; Schu, S.; Kircheis, R.; Wagner, E. PEGylated DNA/transferrin–PEI complexes: Reduced interaction with blood components, extended circulation in blood and potential for systemic gene delivery. Gene Ther. 1999, 6, 595–605. [Google Scholar] [CrossRef]
- Abuchowski, A.; Van Es, T.; Palczuk, N.C.; Davis, F.F. Alteration of immunological properties of bovine serum albumin by covalent attachment of polyethylene glycol. J. Biol. Chem. 1977, 252, 3578–3581. [Google Scholar] [CrossRef]
- Kouchakzadeh, H.; Shojaosadati, S.A.; Tahmasebi, F.; Shokri, F. Optimization of an anti-HER2 monoclonal antibody targeted delivery system using PEGylated human serum albumin nanoparticles. Int. J. Pharm. 2013, 447, 62–69. [Google Scholar] [CrossRef]
- Allen, T.M. Ligand-targeted therapeutics in anticancer therapy. Nat. Rev. Cancer 2002, 2, 750–763. [Google Scholar] [CrossRef]
- Juliano, R.L.; Ming, X.; Nakagawa, O.; Xu, R.; Yoo, H. Integrin Targeted Delivery of Gene Therapeutics. Theranostics 2011, 1, 211–219. [Google Scholar] [CrossRef]
- Steinhauser, I.M.; Langer, K.; Strebhardt, K.M.; Spänkuch, B. Effect of trastuzumab-modified antisense oligonucleotide-loaded human serum albumin nanoparticles prepared by heat denaturation. Biomaterials 2008, 29, 4022–4028. [Google Scholar] [CrossRef]
- Altintas, I.; Heukers, R.; van der Meel, R.; Lacombe, M.; Amidi, M.; van Bergen en Henegouwen, P.M.P.; Hennink, W.E.; Schiffelers, R.M.; Kok, R.J. Nanobody-albumin nanoparticles (NANAPs) for the delivery of a multikinase inhibitor 17864 to EGFR overexpressing tumor cells. J. Control. Release 2013, 165, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Brennan, F.R.; Shaw, L.; Wing, M.G.; Robinson, C. Preclinical safety testing of biotechnology-derived pharmaceuticals understanding the issues and addressing the challenges. Mol. Biotechnol. 2004, 27, 59–74. [Google Scholar] [CrossRef]
- Gu, F.X.; Karnik, R.; Wang, A.Z.; Alexis, F.; Levy-Nissenbaum, E.; Hong, S.; Langer, R.S.; Farokhzad, O.C. Targeted nanoparticles for cancer therapy. Nano Today 2007, 2, 14–21. [Google Scholar] [CrossRef]
- Mongelard, F.; Bouvet, P. AS-1411, a guanosine-rich oligonucleotide aptamer targeting nucleolin for the potential treatment of cancer, including acute myeloid leukemia. Curr. Opin. Mol. Ther. 2010, 12, 107–114. [Google Scholar]
- Gong, G.; Xu, Y.; Zhou, Y.; Meng, Z.; Ren, G.; Zhao, Y.; Zhang, X.; Wu, J.; Hu, Y. Molecular Switch for the Assembly of Lipophilic Drug Incorporated Plasma Protein Nanoparticles and In Vivo image. Biomacromolecules 2012, 13, 23–28. [Google Scholar] [CrossRef]
- Hassanin, I.; Elzoghby, A. Albumin-based nanoparticles: A promising strategy to overcome cancer drug resistance. Cancer Drug Resist. 2020, 3, 930–946. [Google Scholar] [CrossRef]

| Therapeutic Nucleic Acid | Type of Nanocarrier | Size (nm) | Z-Potential (mV) | Model System |
|---|---|---|---|---|
| Plasmid | ||||
| Plasmid pORF-hTRAIL (pDNA) | BSA NPs | 115.7 | −15.4 (pH 7) +11.3 (pH 2) | BALB/c mice bearing i.c. C6 gliomas (Brain Tumor [23]) |
| Plasmid pCMV-EGFP-C | PEI Polyplex | 140–450 | NA | HeLa cells [44] |
| hMDA-7 plasmid | BSA NPs | 115.6 | +33.8 | PANC-1 and BXPC-3 human pancreatic cell lines and tumor-induced BALB/c nude mice [24] |
| pGL3 vector coding for the firefly luciferase gene | HSA-PEI NPs | 300 to 700 | −7 in H2O +16 in 1 mM KCl | Human epithelial kidney 293-cells [45] |
| Oligonucleotides | ||||
| Oligonucleotide | Nanoconjugate | 13 | NA | Tumor spheroids of A375/GFP cells [46] |
| Antisense Oligonucleotides (ASOs) | HSA NPs | 290–330 | NA | MCF-7 cells [47] |
| Akt1 ASOs | Lipid-HSA NPs | 108.6 | 10.5 | KB cells and A549 cells [48] |
| siRNAs | ||||
| VEGF siRNA | Self-crosslinked HSA NPs | 169.3 | NA | B16F10 murine melanoma cells, squamous cell carcinoma cells (SCC7), and human prostatic carcinoma cells (PC-3) [49] |
| Bcl-2-specific siRNA | Anti-ErbB-2 antibody conjugated BSA nanocomplex | 278 | −39.6 | SK-BR-3 and MCF-7 breast cancer cells [50] |
| phrGFP-targeted siRNA | HSA-coated lipid NPs | 79.5 | +15.3 | MCF-7, MDA-MB-231, SK-BR-3 cells, and phrGFP-transfected MCF-7 xenograft tumor mice model [51] |
| Immunotherapeutic biologics | ||||
| Vaccine conjugated with Evans blue (EB) and CpG | Albumin/vaccine nanocomplexes | ~13 | NA | Female C57BL/6 mice s.c. inoculated with EL4 cells, or EG7.OVA cells, B16F10 cells, MC38 cells on the shoulder [52] |
| PD-L1 plasmid (CRISPR/Cas9) | Stearyl PEI complexed HSA NPs | 203 | 13 | Mouse colon carcinoma CT26 cells [53] |
| Targeting Moiety | Description of Nanocarrier | Advantage | Reference |
|---|---|---|---|
| Anti-ErbB-2 antibodies | BSA nanocomplexes with Bcl-2-specific siRNA and gold nanorods | 5-fold greater internalization of BSA nanocomplexes | [50] |
| Folate | Lipid-albumin nanoparticles encapsulating Akt1 ASOs | Provided enhanced selectivity towards folate positive KB cells | [48] |
| RGD peptide | HSA nanoconjugates with splice-switching oligonucleotides (SSOs) | 61-fold enhanced uptake in the A375/Luc705 tumor cells compared to the non-targeted control nanoconjugates | [46] |
| Trastuzumab | ASOs against Plk1 (polo-like kinase 1) loaded HSA nanoparticles | PlK-1 protein levels were decreased to 46% in BT474 breast cancer cells compared to the controls, while no significant effect was shown with PEGylated albumin nanoparticles | [101] |
| Anti-EGFR-1 nanobody | Multikinase inhibitor 17864, a platinum-bound sunitinib analog loaded HSA nanoparticles | 40-fold higher binding to EGFR-positive 14C squamous head and neck cancer cells in comparison with PEGylated nanoparticles | [102] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prajapati, R.; Somoza, Á. Albumin Nanostructures for Nucleic Acid Delivery in Cancer: Current Trend, Emerging Issues, and Possible Solutions. Cancers 2021, 13, 3454. https://doi.org/10.3390/cancers13143454
Prajapati R, Somoza Á. Albumin Nanostructures for Nucleic Acid Delivery in Cancer: Current Trend, Emerging Issues, and Possible Solutions. Cancers. 2021; 13(14):3454. https://doi.org/10.3390/cancers13143454
Chicago/Turabian StylePrajapati, Rama, and Álvaro Somoza. 2021. "Albumin Nanostructures for Nucleic Acid Delivery in Cancer: Current Trend, Emerging Issues, and Possible Solutions" Cancers 13, no. 14: 3454. https://doi.org/10.3390/cancers13143454
APA StylePrajapati, R., & Somoza, Á. (2021). Albumin Nanostructures for Nucleic Acid Delivery in Cancer: Current Trend, Emerging Issues, and Possible Solutions. Cancers, 13(14), 3454. https://doi.org/10.3390/cancers13143454