Explainable Artificial Intelligence Reveals Novel Insight into Tumor Microenvironment Conditions Linked with Better Prognosis in Patients with Breast Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
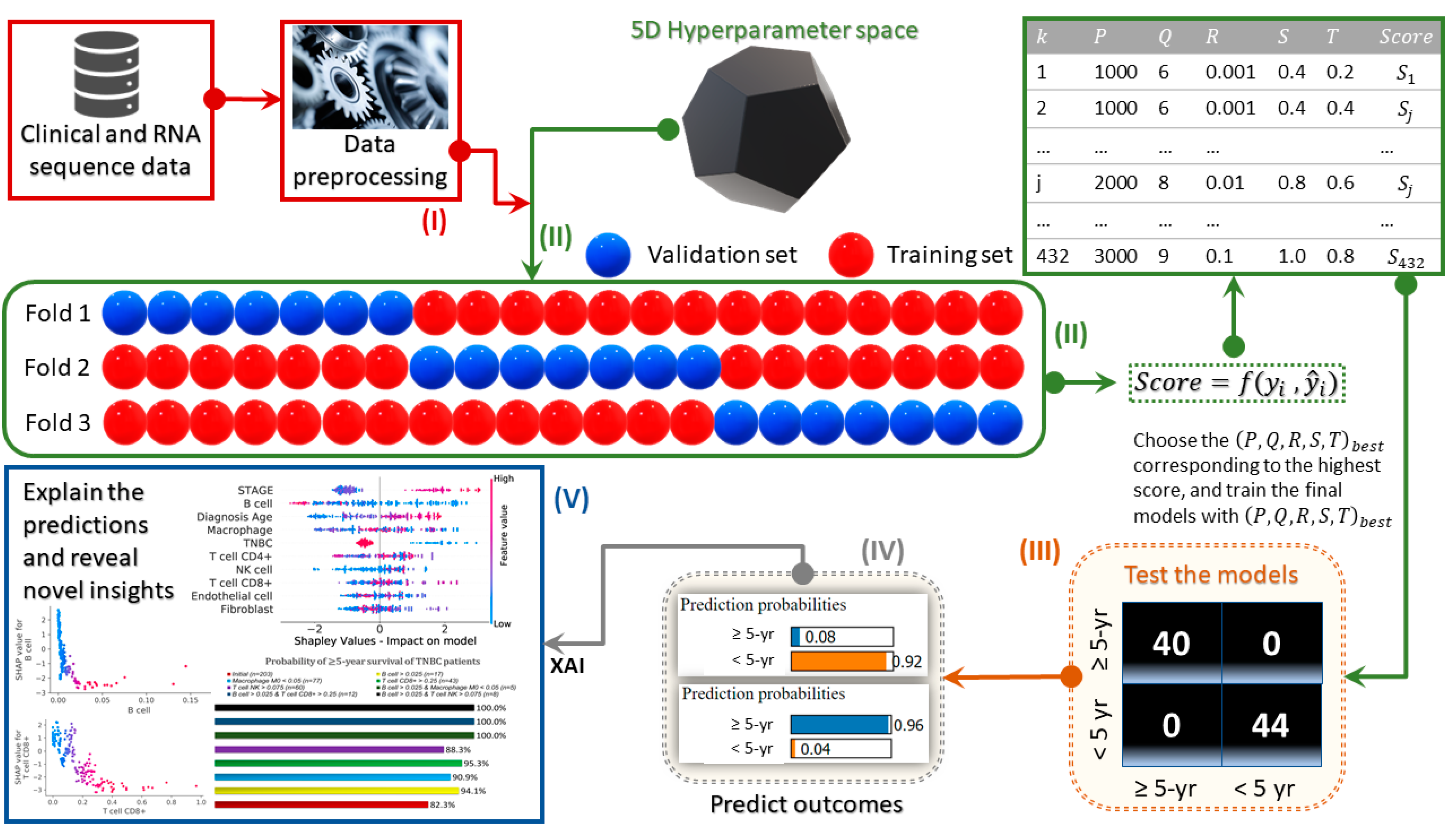
2. Materials and Methods
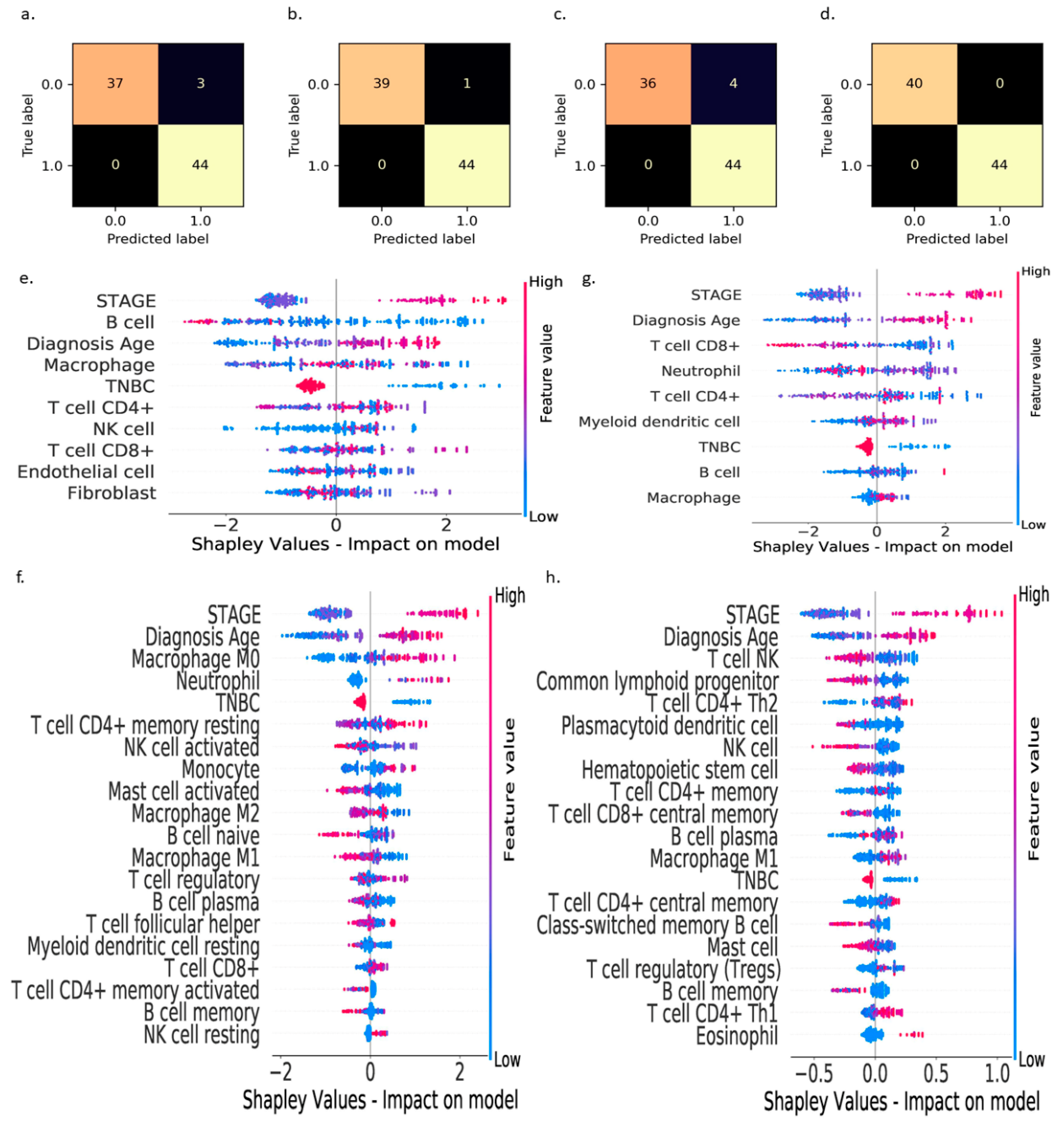
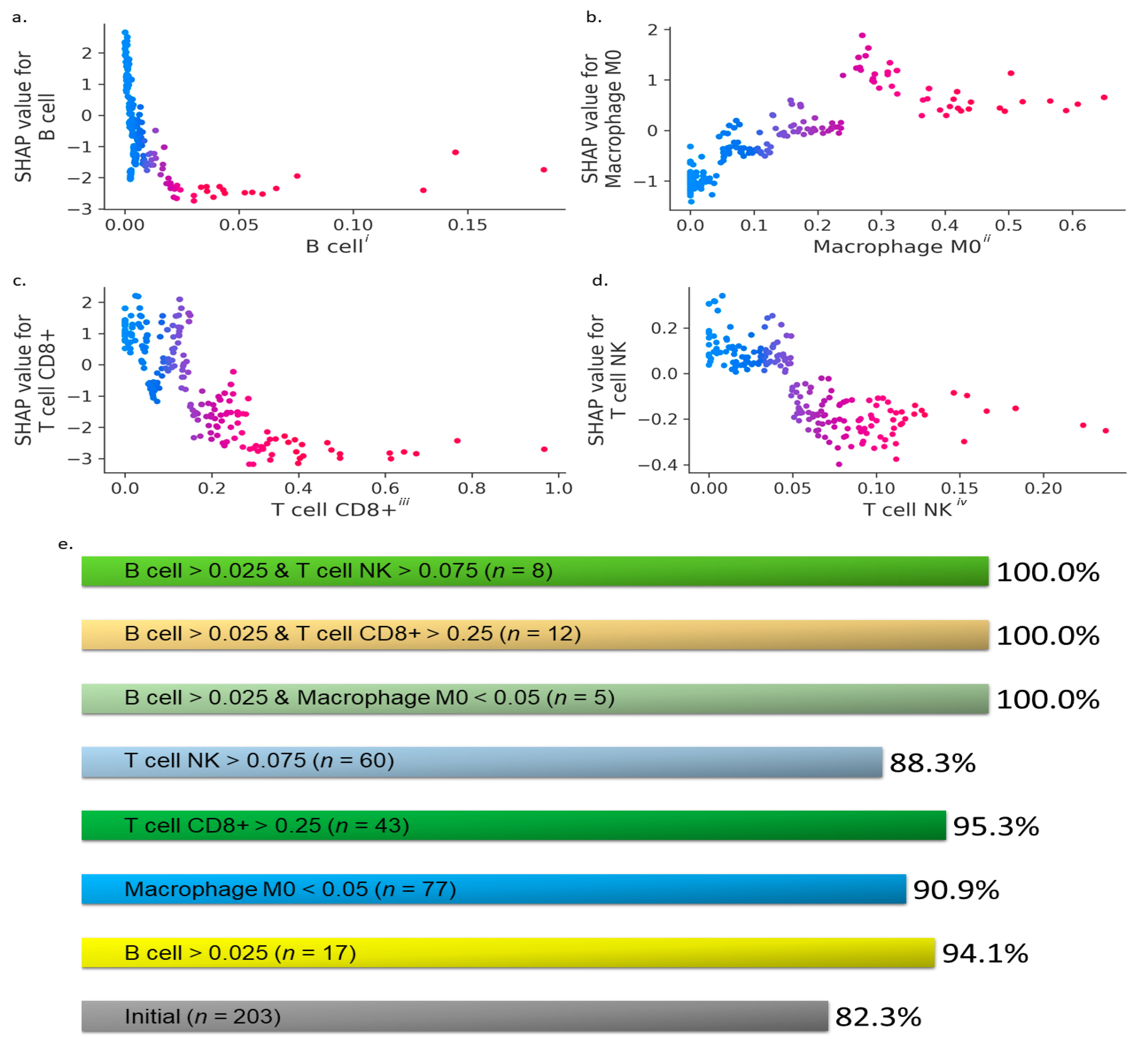
3. Results
- (i)
- : B cells > 0.025,
- (ii)
- : CD8+ T cells > 0.25,
- (iii)
- : M0 macrophages < 0.05,
- (iv)
- : NK T cells > 0.075,
- (v)
- : B cells > 0.025 and M0 macrophages < 0.05,
- (vi)
- : B cells > 0.025 and CD8+ T cells > 0.25, and
- (vii)
- : B cells > 0.025 and NK T cells > 0.075,
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Allaoui, R.; Bergenfelz, C.; Mohlin, S.; Hagerling, C.; Salari, K.; Werb, Z.; Anderson, R.L.; Ethier, S.P.; Jirstrom, K.; Pahlman, S.; et al. Cancer-associated fibroblast-secreted CXCL16 attracts monocytes to promote stroma activation in triple-negative breast cancers. Nat. Commun. 2016, 7, 13050. [Google Scholar] [CrossRef]
- Liedtke, C.; Mazouni, C.; Hess, K.R.; Andre, F.; Tordai, A.; Mejia, J.A.; Symmans, W.F.; Gonzalez-Angulo, A.M.; Hennessy, B.; Green, M.; et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J. Clin. Oncol. 2008, 26, 1275–1281. [Google Scholar] [CrossRef]
- Bareche, Y.; Buisseret, L.; Gruosso, T.; Girard, E.; Venet, D.; Dupont, F.; Desmedt, C.; Larsimont, D.; Park, M.; Rothe, F.; et al. Unraveling Triple-Negative Breast Cancer Tumor Microenvironment Heterogeneity: Towards an Optimized Treatment Approach. J. Natl. Cancer Inst. 2020, 112, 708–719. [Google Scholar] [CrossRef] [Green Version]
- Nagl, L.; Horvath, L.; Pircher, A.; Wolf, D. Tumor Endothelial Cells (TECs) as Potential Immune Directors of the Tumor Microenvironment—New Findings and Future Perspectives. Front Cell Dev. Biol. 2020, 8, 766. [Google Scholar] [CrossRef] [PubMed]
- Allinen, M.; Beroukhim, R.; Cai, L.; Brennan, C.; Lahti-Domenici, J.; Huang, H.; Porter, D.; Hu, M.; Chin, L.; Richardson, A.; et al. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell 2004, 6, 17–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gebremeskel, S.; Clattenburg, D.R.; Slauenwhite, D.; Lobert, L.; Johnston, B. Natural killer T cell activation overcomes immunosuppression to enhance clearance of postsurgical breast cancer metastasis in mice. Oncoimmunology 2015, 4, e995562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obeid, E.; Nanda, R.; Fu, Y.X.; Olopade, O.I. The role of tumor-associated macrophages in breast cancer progression (review). Int. J. Oncol. 2013, 43, 5–12. [Google Scholar] [CrossRef] [Green Version]
- Bianchini, G.; Balko, J.M.; Mayer, I.A.; Sanders, M.E.; Gianni, L. Triple-negative breast cancer: Challenges and opportunities of a heterogeneous disease. Nat. Rev. Clin. Oncol. 2016, 13, 674–690. [Google Scholar] [CrossRef]
- Chokr, N.; Chokr, S. Immune Checkpoint Inhibitors in Triple Negative Breast Cancer: What is the Evidence? J. Neoplasm. 2018, 3. [Google Scholar] [CrossRef]
- Liu, T.; Han, C.; Wang, S.; Fang, P.; Ma, Z.; Xu, L.; Yin, R. Cancer-associated fibroblasts: An emerging target of anti-cancer immunotherapy. J. Hematol. Oncol. 2019, 12, 86. [Google Scholar] [CrossRef]
- Li, X.; Gruosso, T.; Zuo, D.; Omeroglu, A.; Meterissian, S.; Guiot, M.C.; Salazar, A.; Park, M.; Levine, H. Infiltration of CD8+ T cells into tumor cell clusters in triple-negative breast cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 3678–3687. [Google Scholar] [CrossRef] [Green Version]
- Shen, M.; Wang, J.; Ren, X. New Insights into Tumor-Infiltrating B Lymphocytes in Breast Cancer: Clinical Impacts and Regulatory Mechanisms. Front Immunol. 2018, 9, 470. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, M.; Zhang, Y.; Rosenblatt, J.D. B cell regulation of the anti-tumor response and role in carcinogenesis. J. Immunother. Cancer 2016, 4, 40. [Google Scholar] [CrossRef] [Green Version]
- Cassetta, L.; Kitamura, T. Targeting Tumor-Associated Macrophages as a Potential Strategy to Enhance the Response to Immune Checkpoint Inhibitors. Front Cell. Dev. Biol. 2018, 6, 38. [Google Scholar] [CrossRef]
- Tay, R.E.; Richardson, E.K.; Toh, H.C. Revisiting the role of CD4+ T cells in cancer immunotherapy-new insights into old paradigms. Cancer Gene Ther. 2021, 28, 5–17. [Google Scholar] [CrossRef]
- DeNardo, D.G.; Ruffell, B. Macrophages as regulators of tumour immunity and immunotherapy. Nat. Rev. Immunol. 2019, 19, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Camacho, D.M.; Collins, K.M.; Powers, R.K.; Costello, J.C.; Collins, J.J. Next-Generation Machine Learning for Biological Networks. Cell 2018, 173, 1581–1592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Chen, H.; Wang, Y.; Chen, M.M.; Liang, H. Next-Generation Analytics for Omics Data. Cancer Cell 2021, 39, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Hutter, C.; Zenklusen, J.C. The Cancer Genome Atlas: Creating Lasting Value beyond Its Data. Cell 2018, 173, 283–285. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Ghosh, S.; Kagan, J.; Mazurchuk, R.; National Cancer Institute’s, H.I. The Making of a PreCancer Atlas: Promises, Challenges, and Opportunities. Trends Cancer 2018, 4, 523–536. [Google Scholar] [CrossRef]
- Dragoni, M.; Donadello, I.; Eccher, C. Explainable AI meets persuasiveness: Translating reasoning results into behavioral change advice. Artif. Intell. Med. 2020, 105, 101840. [Google Scholar] [CrossRef]
- Lou, S.J.; Hou, M.F.; Chang, H.T.; Chiu, C.C.; Lee, H.H.; Yeh, S.J.; Shi, H.Y. Machine Learning Algorithms to Predict Recurrence within 10 Years after Breast Cancer Surgery: A Prospective Cohort Study. Cancers 2020, 12, 3817. [Google Scholar] [CrossRef] [PubMed]
- Richter, A.N.; Khoshgoftaar, T.M. A review of statistical and machine learning methods for modeling cancer risk using structured clinical data. Artif. Intell. Med. 2018, 90, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferroni, P.; Zanzotto, F.M.; Riondino, S.; Scarpato, N.; Guadagni, F.; Roselli, M. Breast Cancer Prognosis Using a Machine Learning Approach. Cancers 2019, 11, 328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greshock, J.; Lewi, M.; Hartog, B.; Tendler, C. Harnessing Real-World Evidence for the Development of Novel Cancer Therapies. Trends Cancer 2020, 6, 907–909. [Google Scholar] [CrossRef]
- Gilvary, C.; Madhukar, N.; Elkhader, J.; Elemento, O. The Missing Pieces of Artificial Intelligence in Medicine. Trends Pharmacol. Sci. 2019, 40, 555–564. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.H.; Wright, S.N.; Hamblin, M.; McCloskey, D.; Alcantar, M.A.; Schrubbers, L.; Lopatkin, A.J.; Satish, S.; Nili, A.; Palsson, B.O.; et al. A White-Box Machine Learning Approach for Revealing Antibiotic Mechanisms of Action. Cell 2019, 177, 1649–1661. [Google Scholar] [CrossRef] [PubMed]
- Lamy, J.B.; Sekar, B.; Guezennec, G.; Bouaud, J.; Seroussi, B. Explainable artificial intelligence for breast cancer: A visual case-based reasoning approach. Artif. Intell. Med. 2019, 94, 42–53. [Google Scholar] [CrossRef]
- Tjoa, E.; Guan, C. A Survey on Explainable Artificial Intelligence (XAI): Toward Medical XAI. IEEE Trans Neural. Netw. Learn. Syst. 2020. [Google Scholar] [CrossRef]
- Andreson, C. Ready for Prime Time?: AI Influencing Precision Medicine but May Not Match the Hype. Clin. OMICs 2018, 5, 44–46. [Google Scholar] [CrossRef]
- Shaywitz, D. AI Doesn’t Ask Why—But Physicians and Drug Developers Want to Know. Forbes, 9 November 2018. [Google Scholar]
- Gu, D.; Su, K.; Zhao, H. A case-based ensemble learning system for explainable breast cancer recurrence prediction. Artif. Intell. Med. 2020, 107, 101858. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Guestrin, C. XGBoost: A Scalable Tree Boosting System. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; ACM: New York, NY, USA, 2016; pp. 785–794. [Google Scholar]
- Lundberg, S.M.; Lee, S.-I. A unified approach to interpreting model predictions. In Proceedings of the 31st International Conference on Neural Information Processing Systems, Long Beach, CA, USA, 4–9 December 2017; pp. 4768–4777. [Google Scholar]
- Lundberg, S.M.; Erion, G.; Chen, H.; DeGrave, A.; Prutkin, J.M.; Nair, B.; Katz, R.; Himmelfarb, J.; Bansal, N.; Lee, S.-I. From local explanations to global understanding with explainable AI for trees. Nat. Mach. Intell. 2020, 2, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Racle, J.; de Jonge, K.; Baumgaertner, P.; Speiser, D.E.; Gfeller, D. Simultaneous enumeration of cancer and immune cell types from bulk tumor gene expression data. eLife 2017, 6. [Google Scholar] [CrossRef]
- Newman, A.M.; Liu, C.L.; Green, M.R.; Gentles, A.J.; Feng, W.; Xu, Y.; Hoang, C.D.; Diehn, M.; Alizadeh, A.A. Robust enumeration of cell subsets from tissue expression profiles. Nat. Methods 2015, 12, 453–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Severson, E.; Pignon, J.C.; Zhao, H.; Li, T.; Novak, J.; Jiang, P.; Shen, H.; Aster, J.C.; Rodig, S.; et al. Comprehensive analyses of tumor immunity: Implications for cancer immunotherapy. Genome Biol. 2016, 17, 174. [Google Scholar] [CrossRef] [Green Version]
- Aran, D.; Hu, Z.; Butte, A.J. xCell: Digitally portraying the tissue cellular heterogeneity landscape. Genome Biol. 2017, 18, 220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sturm, G.; Finotello, F.; Petitprez, F.; Zhang, J.D.; Baumbach, J.; Fridman, W.H.; List, M.; Aneichyk, T. Comprehensive evaluation of transcriptome-based cell-type quantification methods for immuno-oncology. Bioinformatics 2019, 35, i436–i445. [Google Scholar] [CrossRef]
- Rakha, E.A.; Reis-Filho, J.S.; Ellis, I.O. Combinatorial biomarker expression in breast cancer. Breast Cancer Res. Treat. 2010, 120, 293–308. [Google Scholar] [CrossRef] [Green Version]
- Kourou, K.; Exarchos, T.P.; Exarchos, K.P.; Karamouzis, M.V.; Fotiadis, D.I. Machine learning applications in cancer prognosis and prediction. Comput. Struct. Biotechnol. J. 2015, 13, 8–17. [Google Scholar] [CrossRef] [Green Version]
- Kawakami, E.; Tabata, J.; Yanaihara, N.; Ishikawa, T.; Koseki, K.; Iida, Y.; Saito, M.; Komazaki, H.; Shapiro, J.S.; Goto, C.; et al. Application of Artificial Intelligence for Preoperative Diagnostic and Prognostic Prediction in Epithelial Ovarian Cancer Based on Blood Biomarkers. Clin. Cancer Res. 2019, 25, 3006–3015. [Google Scholar] [CrossRef] [Green Version]
- Rudin, C. Stop explaining black box machine learning models for high stakes decisions and use interpretable models instead. Nat. Mach. Intell. 2019, 1, 206–215. [Google Scholar] [CrossRef] [Green Version]
- Kernbach, J.M.; Staartjes, V.E. Predicted Prognosis of Pancreatic Cancer Patients by Machine Learning-Letter. Clin. Cancer Res. 2020, 26, 3891. [Google Scholar] [CrossRef]
- Ichimasa, K.; Kudo, S.E.; Mori, Y.; Misawa, M.; Matsudaira, S.; Kouyama, Y.; Baba, T.; Hidaka, E.; Wakamura, K.; Hayashi, T.; et al. Artificial intelligence may help in predicting the need for additional surgery after endoscopic resection of T1 colorectal cancer. Endoscopy 2018, 50, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Ito, N.; Kawahira, H.; Nakashima, H.; Uesato, M.; Miyauchi, H.; Matsubara, H. Endoscopic Diagnostic Support System for cT1b Colorectal Cancer Using Deep Learning. Oncology 2019, 96, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Janizek, J.D.; Celik, S.; Lee, S.-I. Explainable machine learning prediction of synergistic drug combinations for precision cancer medicine. bioRxiv 2018. [Google Scholar] [CrossRef]
- Bindea, G.; Mlecnik, B.; Tosolini, M.; Kirilovsky, A.; Waldner, M.; Obenauf, A.C.; Angell, H.; Fredriksen, T.; Lafontaine, L.; Berger, A.; et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 2013, 39, 782–795. [Google Scholar] [CrossRef] [Green Version]
- Garnelo, M.; Tan, A.; Her, Z.; Yeong, J.; Lim, C.J.; Chen, J.; Lim, K.H.; Weber, A.; Chow, P.; Chung, A.; et al. Interaction between tumour-infiltrating B cells and T cells controls the progression of hepatocellular carcinoma. Gut 2017, 66, 342–351. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Jiang, Z.; Chen, C.; Wang, X. Classification of triple-negative breast cancers based on Immunogenomic profiling. J. Exp. Clin. Cancer Res. 2018, 37, 327. [Google Scholar] [CrossRef]
- He, T.F.; Yost, S.E.; Frankel, P.H.; Dagis, A.; Cao, Y.; Wang, R.; Rosario, A.; Tu, T.Y.; Solomon, S.; Schmolze, D.; et al. Multi-panel immunofluorescence analysis of tumor infiltrating lymphocytes in triple negative breast cancer: Evolution of tumor immune profiles and patient prognosis. PLoS ONE 2020, 15, e0229955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janeway, C.A., Jr.; Travers, P.; Walport, M.; Shlomchik, M.J. Part V. The Immune System in Health and Disease. In Immunobiology: The Immune System in Health and Disease, 5th ed.; Garland Science: New York, NY, USA, 2001. [Google Scholar]
- Doherty, D.G.; Melo, A.M.; Moreno-Olivera, A.; Solomos, A.C. Activation and Regulation of B Cell Responses by Invariant Natural Killer T Cells. Front Immunol. 2018, 9, 1360. [Google Scholar] [CrossRef]
- Huang, Y.; Ma, C.; Zhang, Q.; Ye, J.; Wang, F.; Zhang, Y.; Hunborg, P.; Varvares, M.A.; Hoft, D.F.; Hsueh, E.C.; et al. CD4+ and CD8+ T cells have opposing roles in breast cancer progression and outcome. Oncotarget 2015, 6, 17462–17478. [Google Scholar] [CrossRef] [Green Version]
- Hollern, D.P.; Xu, N.; Thennavan, A.; Glodowski, C.; Garcia-Recio, S.; Mott, K.R.; He, X.; Garay, J.P.; Carey-Ewend, K.; Marron, D.; et al. B Cells and T Follicular Helper Cells Mediate Response to Checkpoint Inhibitors in High Mutation Burden Mouse Models of Breast Cancer. Cell 2019, 179, 1191–1206.e21. [Google Scholar] [CrossRef] [PubMed]
- Gunderson, A.J.; Coussens, L.M. B cells and their mediators as targets for therapy in solid tumors. Exp. Cell Res. 2013, 319, 1644–1649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Zhao, Q.; Liao, J.Y.; Song, E.; Xia, Q.; Pan, J.; Li, Y.; Li, J.; Zhou, B.; Ye, Y.; et al. Complement Signals Determine Opposite Effects of B Cells in Chemotherapy-Induced Immunity. Cell 2020, 180, 1081–1097. [Google Scholar] [CrossRef]
- Godfrey, D.I.; Kronenberg, M. Going both ways: Immune regulation via CD1d-dependent NKT cells. J. Clin. Invest. 2004, 114, 1379–1388. [Google Scholar] [CrossRef] [Green Version]
- Kmieciak, M.; Basu, D.; Payne, K.K.; Toor, A.; Yacoub, A.; Wang, X.Y.; Smith, L.; Bear, H.D.; Manjili, M.H. Activated NKT cells and NK cells render T cells resistant to myeloid-derived suppressor cells and result in an effective adoptive cellular therapy against breast cancer in the FVBN202 transgenic mouse. J. Immunol. 2011, 187, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Favreau, M.; Vanderkerken, K.; Elewaut, D.; Venken, K.; Menu, E. Does an NKT-cell-based immunotherapeutic approach have a future in multiple myeloma? Oncotarget 2016, 7, 23128–23140. [Google Scholar] [CrossRef] [Green Version]
- Lao, L.; Fan, S.; Song, E. Tumor Associated Macrophages as Therapeutic Targets for Breast Cancer. Adv. Exp. Med. Biol. 2017, 1026, 331–370. [Google Scholar] [CrossRef]
- Zhou, J.; Tang, Z.; Gao, S.; Li, C.; Feng, Y.; Zhou, X. Tumor-Associated Macrophages: Recent Insights and Therapies. Front Oncol. 2020, 10, 188. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, L.; Su, H.; Liu, Q.; Shen, J.; Dai, H.; Zheng, W.; Lu, Y.; Zhang, W.; Bei, Y.; et al. Chimeric antigen receptor macrophage therapy for breast tumours mediated by targeting the tumour extracellular matrix. Br. J. Cancer 2019, 121, 837–845. [Google Scholar] [CrossRef] [PubMed]
- La Forgia, D.; Fanizzi, A.; Campobasso, F.; Bellotti, R.; Didonna, V.; Lorusso, V.; Moschetta, M.; Massafra, R.; Tamborra, P.; Tangaro, S.; et al. Radiomic Analysis in Contrast-Enhanced Spectral Mammography for Predicting Breast Cancer Histological Outcome. Diagnostics 2020, 10, 708. [Google Scholar] [CrossRef] [PubMed]
- Dilorenzo, G.; Telegrafo, M.; La Forgia, D.; Stabile Ianora, A.A.; Moschetta, M. Breast MRI background parenchymal enhancement as an imaging bridge to molecular cancer sub-type. Eur. J. Radiol. 2019, 113, 148–152. [Google Scholar] [CrossRef] [PubMed]
| TME Immune Cell Estimation Method | Accuracy (%) | Precision (%) | Recall (%) | F1 Score (%) |
|---|---|---|---|---|
| EPIC | 96.4 | 93.6 | 100.0 | 96.7 |
| CIBERSORT | 98.8 | 97.8 | 100.0 | 98.9 |
| TIMER | 95.2 | 91.7 | 100.0 | 95.7 |
| xCell | 100.0 | 100.0 | 100.0 | 100.0 |
| TCGA Patient ID | Stage | Age | Months | B Cell | M0 Macrophage | CD8+ T Cell | NK T Cell |
|---|---|---|---|---|---|---|---|
| A1-A0SK | II | 54 | 31.8 | 0.003 | 0.325 | 0.000 | 0.000 |
| A2-A0CM | II | 40 | 24.8 | 0.008 | 0.129 | 0.025 | 0.013 |
| A2-A0T2 | IV | 66 | 8.4 | 0.001 | 0.077 | 0.098 | 0.183 |
| A2-A3XY | II | 49 | 35.9 | 0.013 | 0.261 | 0.049 | 0.072 |
| AC-A2QJ | III | 48 | 14.7 | 0.000 | 0.196 | 0.000 | 0.146 |
| AR-A5QQ | III | 68 | 10.6 | 0.017 | 0.109 | 0.259 | 0.061 |
| B6-A3ZX | IV | 50 | 37.9 | 0.145 | 0.058 | 0.110 | 0.058 |
| B6-A409 | III | 44 | 18.8 | 0.002 | 0.052 | 0.000 | 0.096 |
| BH-A1EW | II | 38 | 55.7 | 0.004 | 0.000 | 0.244 | 0.047 |
| C8-A3M7 | III | 60 | 34.0 | 0.008 | 0.000 | 0.216 | 0.014 |
| E2-A1LK | III | 84 | 8.7 | 0.005 | 0.650 | 0.000 | 0.034 |
| EW-A1P8 | III | 58 | 7.9 | 0.003 | 0.000 | 0.034 | 0.101 |
| TCGA Patient ID | Stage | Age | Months | B cell | M0 Macrophage | CD8+ T Cell | NK T Cell |
|---|---|---|---|---|---|---|---|
| A2-A0SV | IV | 63 | 27.1 | 0.000 | 0.173 | 0.016 | 0.038 |
| A7-A13E | II | 62 | 20.2 | 0.001 | 0.504 | 0.118 | 0.005 |
| A8-A08J | IV | 52 | 37.1 | 0.003 | 0.240 | 0.014 | 0.050 |
| AC-A23H | II | 90 | 5.7 | 0.002 | 0.419 | 0.150 | 0.003 |
| AR-A0TY | II | 54 | 55.9 | 0.007 | 0.270 | 0.030 | 0.043 |
| BH-A0C1 | III | 61 | 46.4 | 0.002 | 0.000 | 0.284 | 0.034 |
| BH-A18J | IV | 56 | 20.1 | 0.001 | 0.375 | 0.084 | 0.081 |
| BH-A18P | I | 60 | 30.3 | 0.003 | 0.159 | 0.230 | 0.049 |
| BH-A18T | II | 70 | 7.4 | 0.001 | 0.157 | 0.000 | 0.008 |
| BH-A1EV | III | 45 | 12.0 | 0.001 | 0.310 | 0.087 | 0.053 |
| BH-A1EX | II | 67 | 49.6 | 0.003 | 0.061 | 0.147 | 0.035 |
| BH-A1EY | II | 79 | 17.7 | 0.001 | 0.072 | 0.250 | 0.025 |
| BH-A1F8 | III | 90 | 25.1 | 0.005 | 0.069 | 0.188 | 0.000 |
| BH-A1FD | I | 68 | 33.2 | 0.000 | 0.130 | 0.051 | 0.004 |
| C8-A12Q | III | 78 | 12.7 | 0.007 | 0.264 | 0.177 | 0.038 |
| D8-A1XC | III | 85 | 12.4 | 0.004 | 0.266 | 0.126 | 0.041 |
| D8-A1Y1 | III | 80 | 9.9 | 0.000 | 0.000 | 0.050 | 0.010 |
| D8-A73W | III | 79 | 12.7 | 0.002 | 0.290 | 0.000 | 0.072 |
| E2-A14Z | I | 64 | 18.5 | 0.005 | 0.018 | 0.147 | 0.067 |
| E2-A1LE | III | 71 | 28.9 | 0.002 | 0.173 | 0.219 | 0.085 |
| E9-A1N6 | II | 52 | 22.3 | 0.000 | 0.313 | 0.081 | 0.038 |
| E9-A1NF | II | 60 | 35.2 | 0.000 | 0.280 | 0.124 | 0.033 |
| LL-A73Z | IV | 55 | 7.5 | 0.006 | 0.045 | 0.130 | 0.104 |
| UU-A93S | IV | 63 | 3.8 | 0.001 | 0.275 | 0.038 | 0.069 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chakraborty, D.; Ivan, C.; Amero, P.; Khan, M.; Rodriguez-Aguayo, C.; Başağaoğlu, H.; Lopez-Berestein, G. Explainable Artificial Intelligence Reveals Novel Insight into Tumor Microenvironment Conditions Linked with Better Prognosis in Patients with Breast Cancer. Cancers 2021, 13, 3450. https://doi.org/10.3390/cancers13143450
Chakraborty D, Ivan C, Amero P, Khan M, Rodriguez-Aguayo C, Başağaoğlu H, Lopez-Berestein G. Explainable Artificial Intelligence Reveals Novel Insight into Tumor Microenvironment Conditions Linked with Better Prognosis in Patients with Breast Cancer. Cancers. 2021; 13(14):3450. https://doi.org/10.3390/cancers13143450
Chicago/Turabian StyleChakraborty, Debaditya, Cristina Ivan, Paola Amero, Maliha Khan, Cristian Rodriguez-Aguayo, Hakan Başağaoğlu, and Gabriel Lopez-Berestein. 2021. "Explainable Artificial Intelligence Reveals Novel Insight into Tumor Microenvironment Conditions Linked with Better Prognosis in Patients with Breast Cancer" Cancers 13, no. 14: 3450. https://doi.org/10.3390/cancers13143450
APA StyleChakraborty, D., Ivan, C., Amero, P., Khan, M., Rodriguez-Aguayo, C., Başağaoğlu, H., & Lopez-Berestein, G. (2021). Explainable Artificial Intelligence Reveals Novel Insight into Tumor Microenvironment Conditions Linked with Better Prognosis in Patients with Breast Cancer. Cancers, 13(14), 3450. https://doi.org/10.3390/cancers13143450











