1. Introduction
Head and neck squamous cell carcinoma (HNSCC) was the seventh most common cancer in 2018 accounting for over 890,000 new cases and 450,000 deaths worldwide [
1]. Despite early detection and advances and improvements in treatment, there has been an unsatisfactory 5-year survival rate of approximately 50% [
2]. While most early-stage HNSCCs can be cured with surgical resections, advanced HNSCCs are difficult to eliminate using surgery alone, and multiple combined treatments that include chemotherapy and radiotherapy combined with surgery are required. A subpopulation of cancer cells called cancer stem-like or stem cells (CSCs) has been shown to exhibit therapeutic resistance leading to local recurrences and distant metastases. CSCs are a promising target for cancer treatment, based on their therapeutic resistance; however, identifying CSCs within the heterogenic malignant tumor can be difficult, and there is currently no simple biomarker for their identification [
3]. These drawbacks necessitate novel treatment strategies to target the tumor stroma and the tumor itself. The tumor stroma offers an attractive target for cancer therapy and may provide a new path for therapeutic intervention.
It has been reported that HNSCCs and other types of tumors frequently activate or enhance mediators such as cytokines or chemokines that are generated from stroma components to promote tumor growth and progression. Chemokines are a family of chemoattractant cytokines that play an important role in the control of cell migration in several different human cancers [
3,
4]. They also promote the tumor cell movement required for metastasis [
5]. The receptor CXCR4, and its ligand CXCL12 (also called SDF-1), is the key chemokine essential for HNSCC metastasis. A study has shown that there was significant activation of the CXCR4 in HNSCCs with regard to cell growth, differentiation, and survival and that the CXCR4 was significantly higher in patients with positive lymph node involvements and distant metastases [
6]. The impact of chemokines and their receptors on HNSCCs may be described by their influence on matrix metalloproteinases (MMPs) [
7]. Moreover, tumor cells can stimulate stromal cells to synthesize and secrete growth-promoting chemokines, establishing a reciprocal cross tumor–stromal interaction that favors tumor growth. Accomplice cells in the tumor microenvironment (TME), such as cancer-associated fibroblasts (CAFs) and macrophages have also been shown to produce chemokines and promote tumor growth [
8,
9,
10].
A cytokine, interleukin 33 (IL33), released by CAFs, has been reported to induce treatment failure in HNSCCs [
11] and promote the stemness properties of via crosstalk between tumor cells and CAFs [
12]. In our previous study, we used an organotypic culture to investigate CAFs that promote the aggressive behavior of cancer cells and the paracrine effect of CAF-induced IL-33, which increases the aggressiveness of HNSCCs [
13]. However, the pathogenesis of this complex tumor–stromal signaling remains to be completely elucidated. A better understanding of the mechanisms of the complex communication network of the TME via IL-33 in tumor–stromal signaling, on a cellular and molecular basis, may provide a new potential therapeutic target for the treatment of HNSCCs.
Therefore, we aimed to establish stable clones of the IL-33-overexpressing HNSCC cells and an animal model to confirm whether IL-33 exerts an autocrine effect on cancer cells in addition to the paracrine effect of CAF-induced IL-33, which increases the aggressiveness of HNSCC.
3. Discussion
The stromal cells in the surrounding TME, such as the CAFs, not only act as an active contributor to cancer initiation but also contribute actively to cancer progression [
14,
15,
16]. A recent report indicated that targeting IL-33/ST2 signaling was a therapeutic strategy for inflammatory disorders. Another study claimed that IL-33 was used as a target for patients with treatment-resistant breast cancer via the attenuation of epithelial–mesenchymal transition and cancer stemness [
17,
18]. However, as described earlier, immune cell-associated IL-33 might conversely attenuate tumor progression and provide an option for immunotherapy [
19]. A review of the literature showed a paucity of reports involving IL-33/ST2 or SDF1/CXCR4 signaling to target TME for tumor progression. Instead of functioning initially as an important inflammatory cytokine, the CAF-induced IL-33 expression was responsible for enhancing cancer cell progression via a paracrine effect [
12]. In this study, we found relevant evidence in a step-by-step manner to elucidate the molecular mechanisms underlying the complex communication network between the CAFs and the cancer itself, between the cancer cells, and their transduction signaling axis, which can lead to cancer cell progression with the induction of CSC properties.
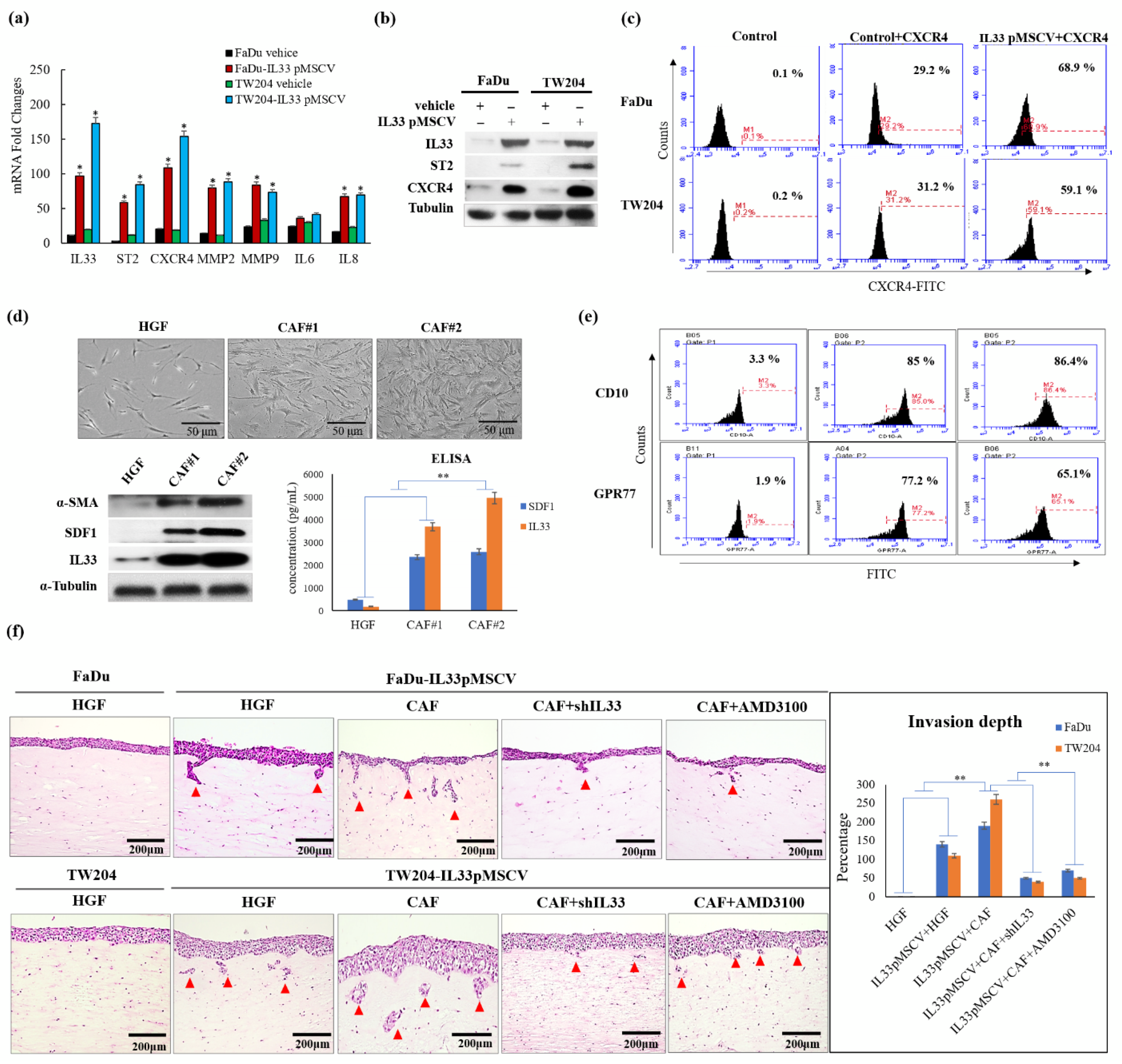
We first proposed to determine the nature and origins of the CAFs [
20]. The CAFs in the TME were generated and transformed from the HGFs. There was a progressive transition that showed functional and morphological alterations between the HGFs and CAFs. Due to the diversity of transformed fibroblasts, the primarily cultured CAFs displayed a higher expression of CD10 and GPR77 than the HGFs, which expressed lower levels of CD10 and GPR77, according to the findings of a previous study [
21]. These findings indicated that the increased CD10 and GPR77 expression implicated a transitional tendency when the HGFs turned into CAFs. The higher expressions of CD10 and GPR77 corresponded to the increased likelihood of HGFs becoming CAFs. Moreover, the CAFs possessed a greater ability to functionally induce HNSCC cells to be more aggressive than the HGFs, as shown by the 3D organotypic raft culture. The induction of invasion was markedly increased by the CAF-induced IL-33 expression via the paracrine effect.
Identifying invasion-related molecules and possible mechanisms that block their pathways can play a crucial role in the diagnosis and treatment of HNSCCs. A recent study [
22] proposed that the IL33-TGF beta niche signaling promoted cancer progression. Furthermore, two recent reports have suggested that the immune cell-associated and proinflammatory cytokine IL-33 might conversely attenuate tumor progression [
23,
24]. However, another study reported the opposite result, and the controversy about the functional role of IL-33 in tumor–stromal signaling, still exists [
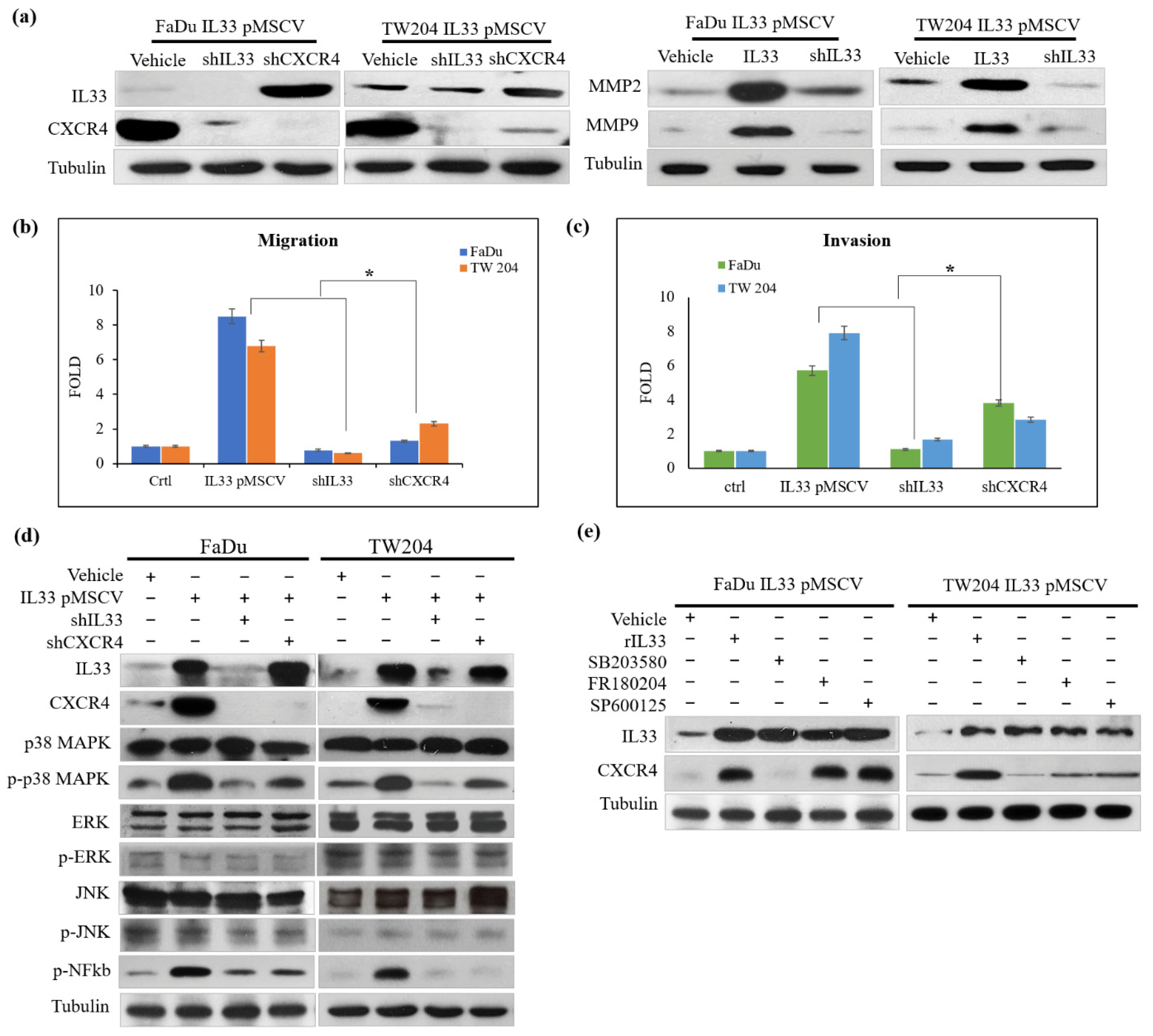
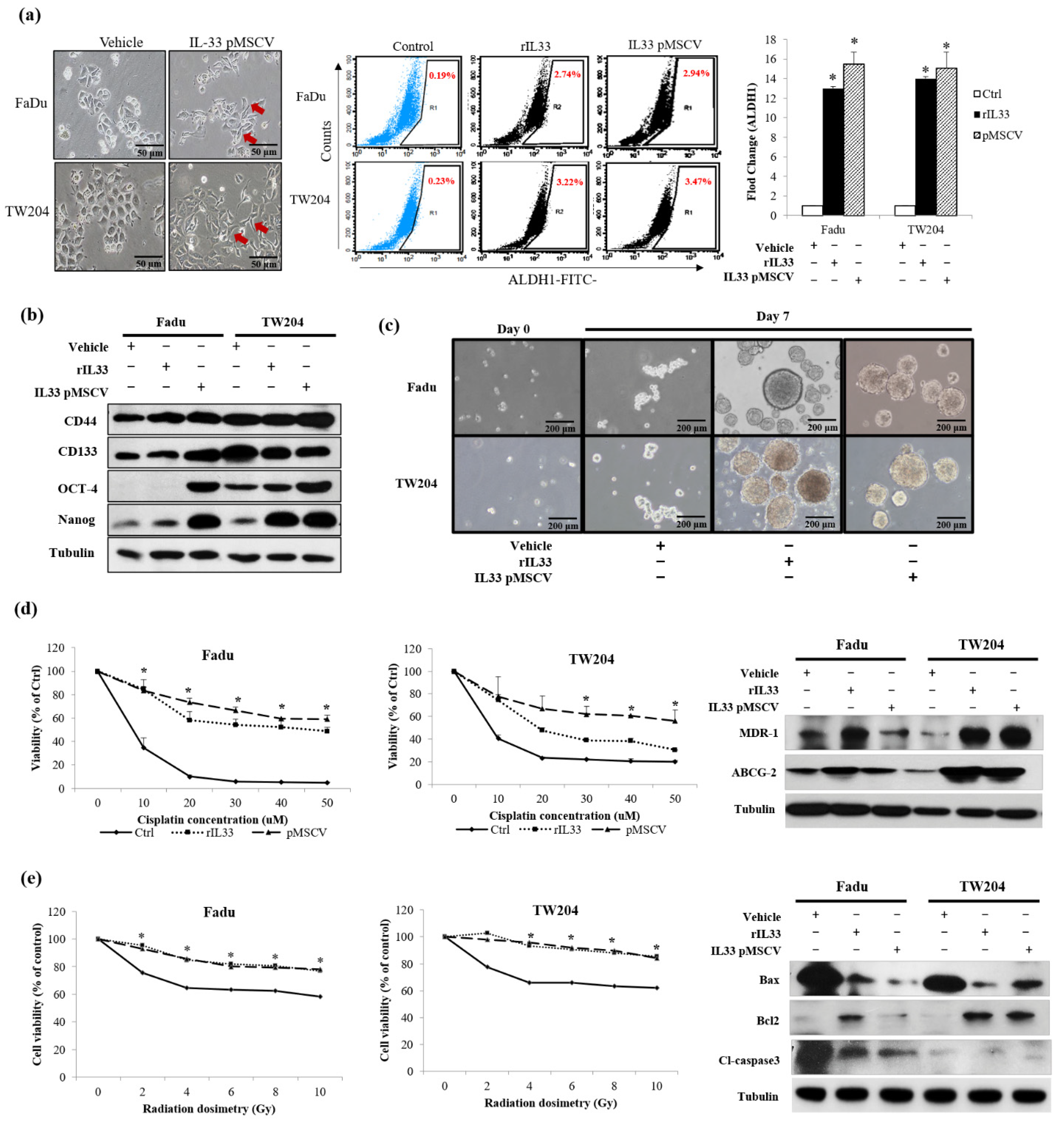
19]. By establishing stable clones of IL-33-overexpressing HNSCC cells, we confirmed the presence of high IL-33 levels in the HNSCC cells. A higher expression of ST2 and CXCR4 was also found in the cloned IL-33-overexpressing HNSCC cells. These data suggested that the established stable clones of IL-33-overexpressing HNSCC cells acted in an autocrine manner and displayed an aggressive phenotype that was closely associated with a higher ST2 and CXCR4. Both the IL-33/ST2 and SDF1/CXCR4 signaling axes are well-established molecular modules. Nevertheless, the results of this study revealed a special interaction of collateral signaling between IL-33 and CXCR4, besides the classical IL-33 linkage to ST2. This novel finding was validated by driving role that the functional CAF-induced IL-33 plays in cancer cells, to regulate CXCR4 expression, wherein the autocrine cancer-secreting IL-33 automatically target the surface receptor ST2 and, subsequently, enhance the IL-33/ST2 signaling cascade transcriptional upregulation of CXCR4, which then generates the newly discovered “IL-33/CXCR4 regulatory circuit,” which undergoes repeated amplification for further activation of the SDF-1/CXCR4 signaling transduction. A higher expression of CXCR4 receptors was found in the cloned IL-33-overexpressing HNSCC cells, along with increased levels of MMP2 and MMP9. A blockade of either IL-33 or CXCR4 decreased the invasion ability. However, the cloned IL-33-overexpressing HNSCC cells together with the autocrine cancer-secreting function increased MMP2 and MMP9 expression spontaneously and, conversely, the blockade of the IL-33 expression enhanced the attenuation of MMP2 and MMP9, suggesting that IL-33 was an upstream mediator of the signaling cascade. The shutdown of IL-33 and CXCR4 elicited the opposite results. Moreover, the inhibition of IL-33 decreased the number of molecules involved in the p38MAPK and pNFkb signaling cascade. The inhibition of CXCR4 did not have a similar effect, again suggesting that IL-33, rather than CXCR4, was an upstream mediator of the signaling cascade. However, using the inhibitors of the phosphorylated p38MAPK, ERK1/2, and JNK did not affect the expression of IL-33 but decreased CXCR4 expression. In this study, we further examined the expression of CSC properties, including the expression of other CSC representative markers, sphere-forming abilities, radioresistance and chemoresistance, drug-resistant genes (
ABCG2 and
MDR-1)
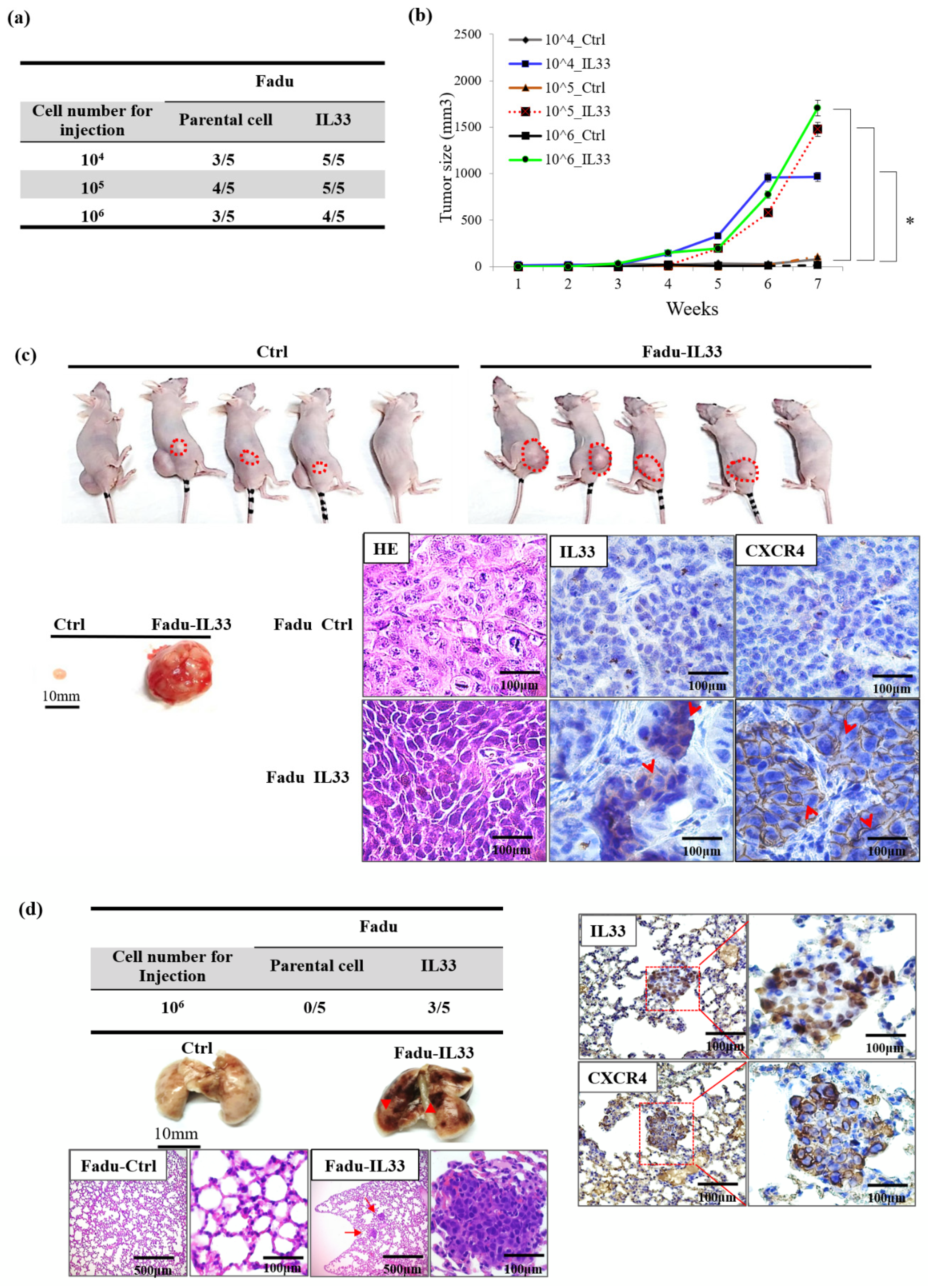
, and antiapoptotic proteins in the IL-33-overexpressing stable clones and HNSCC cells exposed to recombinant IL-33. Thus, all the most important properties of stemness induced by either the paracrine or autocrine IL-33 signaling were enhanced. The IL-33/CXCR4 regulatory circuit was reconfirmed by in vivo experiments. These study data demonstrated a high capability of tumorigenicity and metastases secondary to the induction of the IL-33-overexpressing stably cloned FaDu cells, leading to CXCR4 upregulation in vivo. Immunohistochemistry showed that both IL-33 and CXCR4 were reciprocally positive in both the newly formed subcutaneous tumors and metastatic lung nodules.
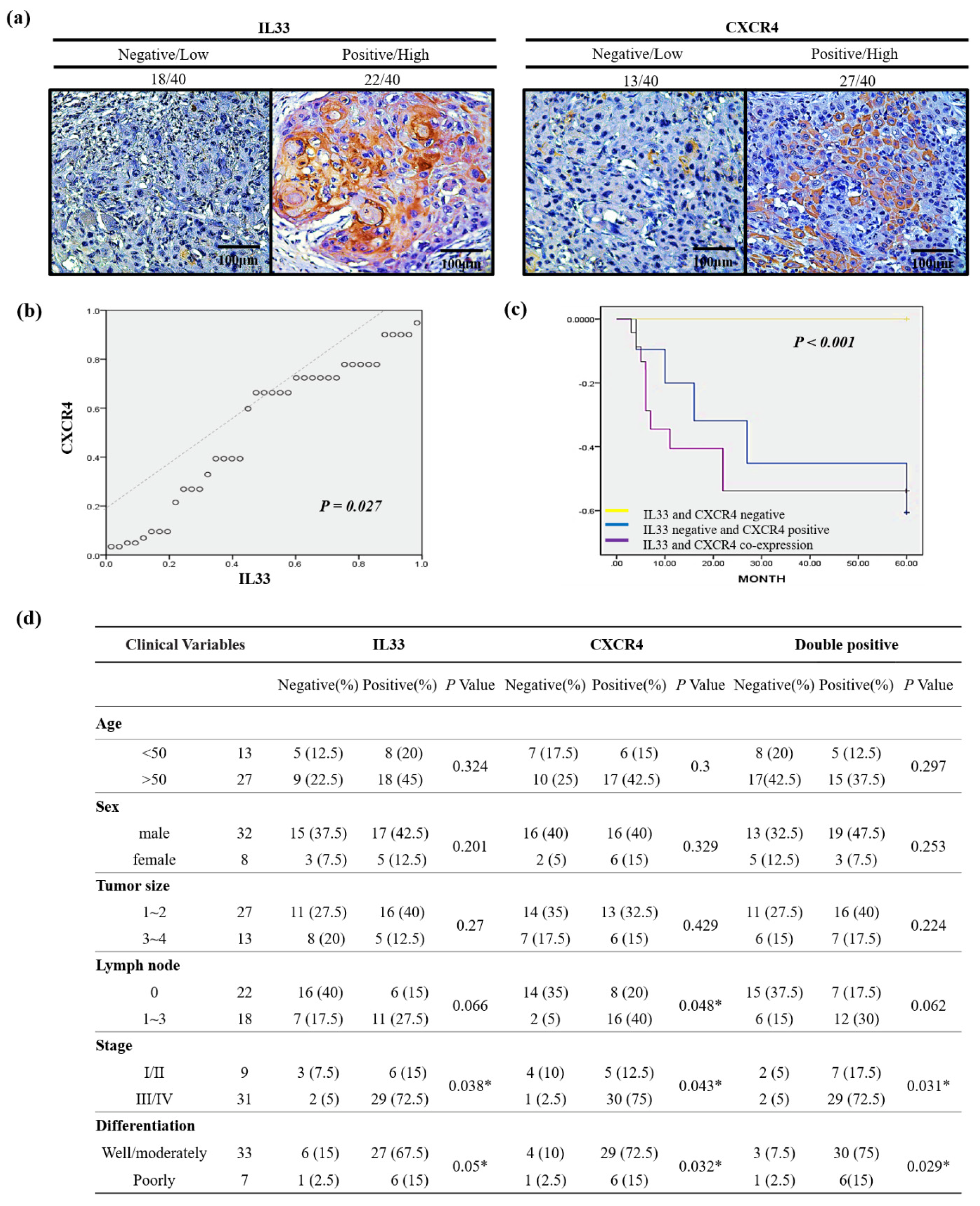
A high CXCR4 expression in cancer cells may be an independent predictor of lymph node metastases and poor survival in colon cancer patients [
25,
26]. A literature review showed that under special conditions, such as marked inflammation, necrosis, or hypoxia, or even in cancer progression. Interestingly, a recent report has shown that HIF-1α enhances IL-33 production by enabling certain signaling pathways, especially the p38MAPK and ERK pathways. IL-33, by turn, induces HIF-1α expression, thus forming a HIF-1α/IL-33 positive feedback loop [
27]. Furthermore, TNF-α promotes HIF-1α expression by both triggering NF-κB signaling and controlling IL-33 expression in a HIF-1α-dependent manner. Therefore, TNF-α facilitates HIF-1α-dependent IL-33 expression on the basis of identifying a functional binding site for HIF-1α in the IL-33 promoter region [
28]. Although the mechanisms are still not clear, these discoveries suggest that IL-33 may be upregulated in hypoxic tumor microenvironments. Both IL-33 and hypoxia-inducible factor 1 (HIF-1)-related CXCR4 levels were markedly increased. This HIF-dependent activation of CXCR4 that is mediated by IL-33 may suggest a novel mechanism for the induction of tumor progression. To confirm the clinical significance of the relationship between the expression of IL-33 and CXCR4, we analyzed samples from 40 representative HNSCC patients whose clinical information was available. Consistent with the results of the clinical tissue samples, the linear regression analysis showed a close relationship between IL-33 and CXCR4 expression. The immunoexpression of IL-33 and CXCR4 showed a significant correlation with disease-free survival, and the co-expression of IL-33 and CXCR4 represented a worse outcome. However, the co-expression of IL-33 and CXCR4 showed a significant correlation with clinical parameters, including TNM stage, nodal involvement, stage, and tumor differentiation.
It is noteworthy that many hypotheses have been proposed to explain the nature and origin of the CAFs [
29,
30,
31]. In this study, we observed that the dual role of the CAFs is mediated by IL-33, that is, normal supporting fibroblasts that are called persevered or putative CAFs for the maintenance of hemostasis, once involved in carcinogenesis, will through their crosstalk with cancer cells, trigger the IL-33/CXCR4 signaling circuit and can contribute to tumor aggressiveness. Finally, the complex crosstalk with plentiful molecular communications proceeds like a symphony that is orchestrated and conducted by both autocrine and paracrine signaling between CAFs and tumor. The diagrammatic illustrations demonstrate our proposal to explain the major mechanism and the relationships between the molecules involved in the IL-33-p38MAPK-CXCR4-SDF-1 loop between the TME and the actual tumor (
Figure S1). Extending these data to clinical practice suggests that modulating the TME by targeting the IL-33/CXCR4 signaling circuit may attenuate the aggressiveness of cancer cells and provide a novel therapeutic strategy in precision medicine to improve the prognosis in HNSCC patients.
4. Materials and Methods
4.1. Cell Cultures and Reagents
Stromal fibroblasts separated from adjacent carcinoma cells and uninvolved intraoral gingival tissues were obtained from five patients undergoing surgical resections of HNSCCs. The CAFs and HGFs were isolated using differential trypsinization with a modified protocol [
12]. This was followed by histological confirmation. The cell line was tested for mycoplasma contamination (Mycoplasma Reagent Set; Euroclone s.p.a, Pavia, Italy). Both fibroblast types were maintained in Dulbecco’s modified Eagle’s medium (DMEM) and Ham’s nutrient mixture F12 culture medium, supplemented with 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin. The FaDu (ATCC HTB-43) and TW204 (from a Chinese nasopharyngeal carcinoma in Taiwan) cell lines [
32], originated from the human hypopharynx geal and nasopharyngeal squamous cell carcinomas, were cultured in a RPMI-1640 medium supplemented with 10% FBS, 2 mM glutamine, 1% penicillin/streptomycin, 1% sodium pyruvate, and 1% of amino-acid solution. Cells were treated for 6 h with recombinant IL33 protein with doses of 50 and 25 μg/mL AMD3100 (Sigma, Zwijndrecht, The Netherlands) [
33] and then, in a series of experiments, were pre-treated for 1 h with various enzymatic inhibitors of different signaling pathways: 50 μM FR180204 (ERK, inhibitor), 10 μM SB203580 (p38MAPK inhibitor) or 4 μM SP600125 (JNK inhibitor).
4.2. Viral Production and Infection of Target Cells
The entire coding sequence was excised from the pMSCV vector (
Table S1) and subcloned into the mammalian expression vector, pMSCV-IL33. The final construct was verified by sequencing. The positive clones were selected using ampicillin and amplified in bacterial culture media. All the plasmids were purified using the Endo-free Plasmid Mini Kit II (OMEGA). For transfection, FaDu and NPC204 cells were plated in six-well plates (Corning, Lowell, MA, USA), cultured in a complete growth medium to 80% confluence, and then cultured in a medium without fetal bovine serum (FBS) for 12–16 h. Transfection was performed using 1 μg (per dish) of either p pMSCV or p pMSCV–IL33 and Turbofect (Thermo Fisher Scientific Inc. Branchburg, NJ, USA) reagent, according to the manufacturer’s protocol.
4.3. Western Blot, RNA Extraction and Quantitative Real-Time PCR
The antibodies and primers used are listed in
Tables S2 and S3. Western blotting was performed according to a standard protocol, as described previously [
12]. For the RNA extraction, the total RNA from the cultured cells was extracted using TRIzol (Invitrogen Life Technologies, Carlsbad, CA, USA) and 1 µg RNA was used for the cDNA synthesis. Quantitative real-time PCR was performed to quantify the gene expression, using the StepOnePlus real-time PCR system (Applied Biosystems, San Francisco, CA, USA). Data from three independent experiments were performed in triplicate and were expressed as mean ± SD.
4.4. Migration and Invasion Assay
The in vitro transwell migration and invasion assays were performed and lower wells were coated with 15 μg/mL collagen type I, incubated for 1 h at 37 °C, and blocked overnight with phosphate-buffered saline (PBS) containing 1% bovine serum albumin at 4 °C. After the blocking buffer was removed, the lower wells were loaded with 300 μL of 10−7 M CXCL12 in serum-free RPMI. The cells were serum-starved overnight and harvested with enzyme-free cell detaching buffer. The inserts were loaded with 2 × 104 cells in 150 μL per condition and were allowed to migrate for 4.5 h at 37 °C. After migration, the nonmigrated cells were removed with a cotton swab wetted in PBS. Cells at the bottom surface were fixed in 75% methanol for 20 min at room temperature, stained with 0.25% Coomassie blue in 45% for 20 min at room temperature, washed, air-dried, and mounted on a microscope slide. The number of migrated cells was calculated by counting the cells from 10 fields of view per slide, using a counting grid and 40× magnification. Data from three independent experiments were performed in triplicate were expressed as mean ± SD.
4.5. Organotypic 3D Culture
For Organotypic 3D culture, eight volumes of collagen I/Matrigel (collagen I:Matrigel = 1:1) were mixed with 1 volume of 10× DMEM and 1 volume of FBS containing fibroblasts (5 × 105). The gel mixture was dispensed into a 12 mm Millicell insert (Millipore, Bedford, MA, USA) inserted into a six-well culture plate. The mixture was allowed to set at 37 °C for 24 h, and the FaDu cells (2 × 105) were seeded atop the gel mixture. After a 24 h incubation period, the cancer cells were exposed to air by removing the medium from the surface. The gel was then fed from underneath with the complete medium, which was changed daily. After 21 days, the cultured tissue was fixed and embedded in paraffin for a histological examination. Data from three independent experiments were performed in triplicate were expressed as mean ± SD.
4.6. Flow Cytometry
The 1 × 106 single-cell suspension from trypsinized cells and spheres were added in 1 mL phosphate-buffered saline (PBS) and stained with aldehyde dehydrogenase 1 (ALDH1; ALDEFLUOR assay kit; Stem Cell Technologies, Durham, NC, USA). After labeling, the cells were washed with PBS three times and stained subsequently with FITC- or PE-labeled secondary antibody for 30 min, in the dark. After three cycles of washing with PBS, FaDu and TW204 cells were incubated with 1:100 polyclonal rabbit anti-hCXCR4 antibody (Abcam) or with PBS (2.7 mM KCl, potassium 1.8 mM KH2PO4, 137 mM NaCl, 10.1 mM Na2HPO4, pH 7.4) for 45 min on ice, followed by 30 min of incubation with mouse–anti rabbit antibody phycoerythrin-labeled (Southern Biotech, Uithoorn, The Netherlands). The cells were analyzed using a flow cytometer, FACSCalibur (Epics Elite; Coulter Electronics, Mijdrecht, The Netherlands). Data analysis was performed using Kaluza software (Beckman Coulter Nederland BV, Woerden, The Netherlands).
4.7. Enzyme-Linked Immunosorbent Assay and Sphere Culture
The medium from the confluent normal fibroblast and the CAF were sampled at 48 h after plating and centrifuged to remove the cell debris. SDF-1 and IL-33 levels in medium were assayed using the Quantikine Human IL-33 and SDF1 Immunoassay kit (R&D Systems, Abingdon, United Kingdom) according to the manufacturer’s instructions. The measured levels of IL-33 were expressed as picograms and SDF-1 as per 1 mg of protein in the cell lysate. The supernatants were collected and analyzed using ELISA, according to the manufacturer’s standard protocol. ELISAs were performed in duplicate on three separate occasions and the data were expressed as means ± SDs.
4.8. Assays for Chemosensitivity and Radiosensitivity
The cells were seeded in a 10 cm dish at a density of 1×106 cells/dish. For the chemosensitivity assay, the cells were treated with 0–30 μM cisplatin (Sigma, St. Louis, MO, USA) for 48 h. For the radioresistance assay, cells were irradiated using a CyberKnife radiosurgery system (Accuray, Sunnyvale, CA, USA) that could deliver different doses (2–10 Gy). The relative survival fraction of the cells was determined using an MTS assay with the Cell Titer 96 Aqueous One Solution Cell Proliferation Assay kit.
4.9. In Vivo Assay
All the animal experiments were approved by the Institutional Animal Care and Use Committee of the National Defense Medical Center (IACUC-13-039). The in vivo tumorigenicity study was performed according to the guidelines of the local ethics committee that had full accreditation awarded to it by the Association for Assessment and Accreditation of Laboratory Animal Care in the National Defense Medical Center. There were five mice in each group. The mice were kept at 18–26 °C, in 30–70% humidity, and independently airconditioned under a 12 h dark/12 h light cycle for seven days before the xenograft injection. The parental FaDu-Ctrl and FaDu-IL33 cells were injected into BALB/c nude mice (five weeks). The cell suspension (100 μL) was injected subcutaneously into each mouse with different tumor cell numbers, from 1 × 106, 1 × 105 and 1 × 104 cells. The tumors formed seven days after injection. The tumor sizes were monitored and measured weekly according to the formula (length × width2)/2. At 30 days after the orthotopic inoculation, the mice were euthanized under anesthesia using chloroform. To evaluate the metastatic capability, stable clones and control cells were injected intravenously into the tail vein. The cancer cells were harvested at a concentration of 1 × 106 cells per 0.1 mL DPBS. A volume of 0.1 mL of the suspended cells was injected through the tail vein into 5-week-old male BALB/c mice using 27-gauge needles. The mice were sacrificed and examined for the growth of metastatic tumors at various time points. The mice injected with FaDu-Ctrl or FaDu-IL33 cells were sacrificed seven weeks after injection. Two independent experiments were performed for each pair of cancer cells. At seven weeks, the mice were euthanized under anesthesia.
4.10. Human Tumor Tissue Collection and Immunohistochemical Staining
Archival tissue specimens from the primary tumors and lymph nodes were obtained from the Tri-Service General Hospital in the Taiwan between 2009 and 2013. Immunohistochemistry was performed on paraffin-embedded sections of the HNSCC specimens. After deparaffinization and dehydration, specimens were brought to the boil in 10 mM sodium citrate buffer (pH 6.0) for 40 min for antigen retrieval and then blocked in peroxidase-blocking solution (Dako Cytomation, Glostrup, Denmark). Rabbit monoclonal anti-CD133 antibody (clone C24B9, Cell Signaling Technology, Danvers, MA, USA) was diluted to 1:200 and incubated at 4 °C overnight. The staining was detected using an Envision detection system (peroxidase/DAB+, rabbit/mouse, Dako Cytomation). The specimens were counterstained with Mayer’s hematoxylin. CXCR4 expression was assessed by staining with IL33 (R & D) and CXCR4 antibody (Abcam), secondary goat anti-rabbit antibody conjugated to peroxidase (DAKO, Heverlee, Belgium), and the subsequent tertiary rabbit anti-goat was conjugated to peroxidase (DAKO). Staining was visualized by 3,3′-diaminobenzidine.
Only clinical cases without any neoadjuvant androgen deprivation were selected. All the tissue specimens were encoded with unique numbers. According to Dutch law, no further institutional review board approval was required (
www.federa.org (accessed on 4 July 2016)). The FFPE tissue specimens were mounted on slides as whole tissue sections and stained with hematoxylin and eosin. We chose one of the most representative and adequate sections from the total positive tissue samples and then counted for intensity and distribution. For immunohistochemistry, the treated but without the primary antibody specimen was used as a negative control, and a human retina with immunoreactivity was used as a positive control. The intensities of IL33 and CXCR4 immunoreactivity of the tumor cells were classified into four categories: 0 (no staining), 1 (weak staining), 2 (moderate staining), and 3 (strongest intensity). The distribution was also measured as the percentages of the positively stained tumor cells (from 0 to 100%) in the total tumor volumes in each section. To compare immunoexpression for each case, the percentage of positive cells of intensity was multiplied by the corresponding intensity to obtain an immunoreactivity score ranging from 0 to 300. The staining results were evaluated independently by two pathologists blinded to the patients’ clinical information. Discrepancies between the pathologists were resolved by consensus.
4.11. Statistical Analysis
The independent Student’s t-test or ANOVA was used to compare the continuous variables between the groups and the Chi-square test was used for the comparison of the dichotomous variables. The level of statistical significance was set at a p-value < 0.05. All the statistical analyses were performed using SPSS version 20 (SPSS, Inc., Chicago, IL, USA).