Is It Definitely Clear That Long-Term Survival after Breast Cancer Surgery Is Not Affected by Anaesthetics?
Abstract
Simple Summary
Abstract
1. Introduction
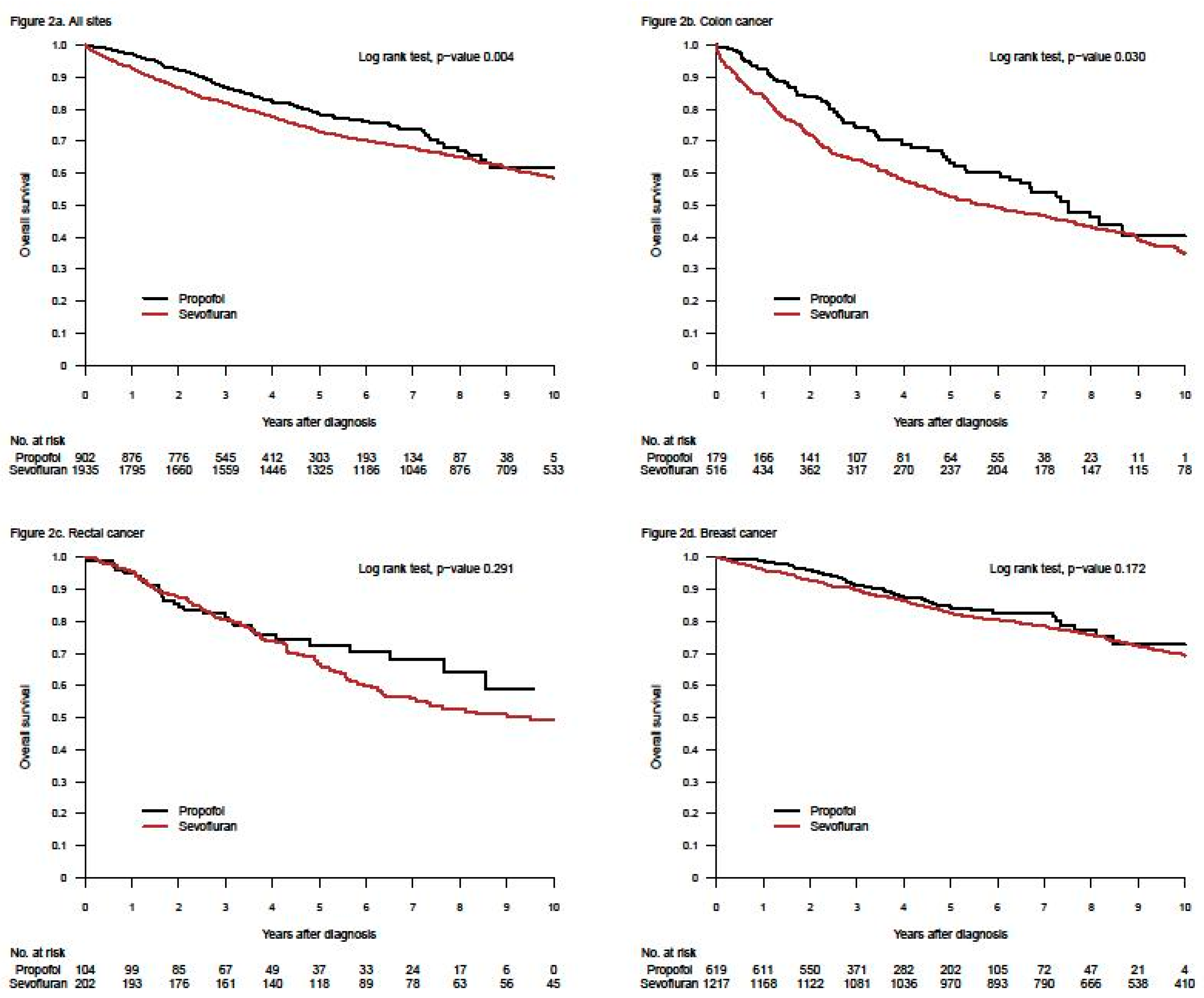
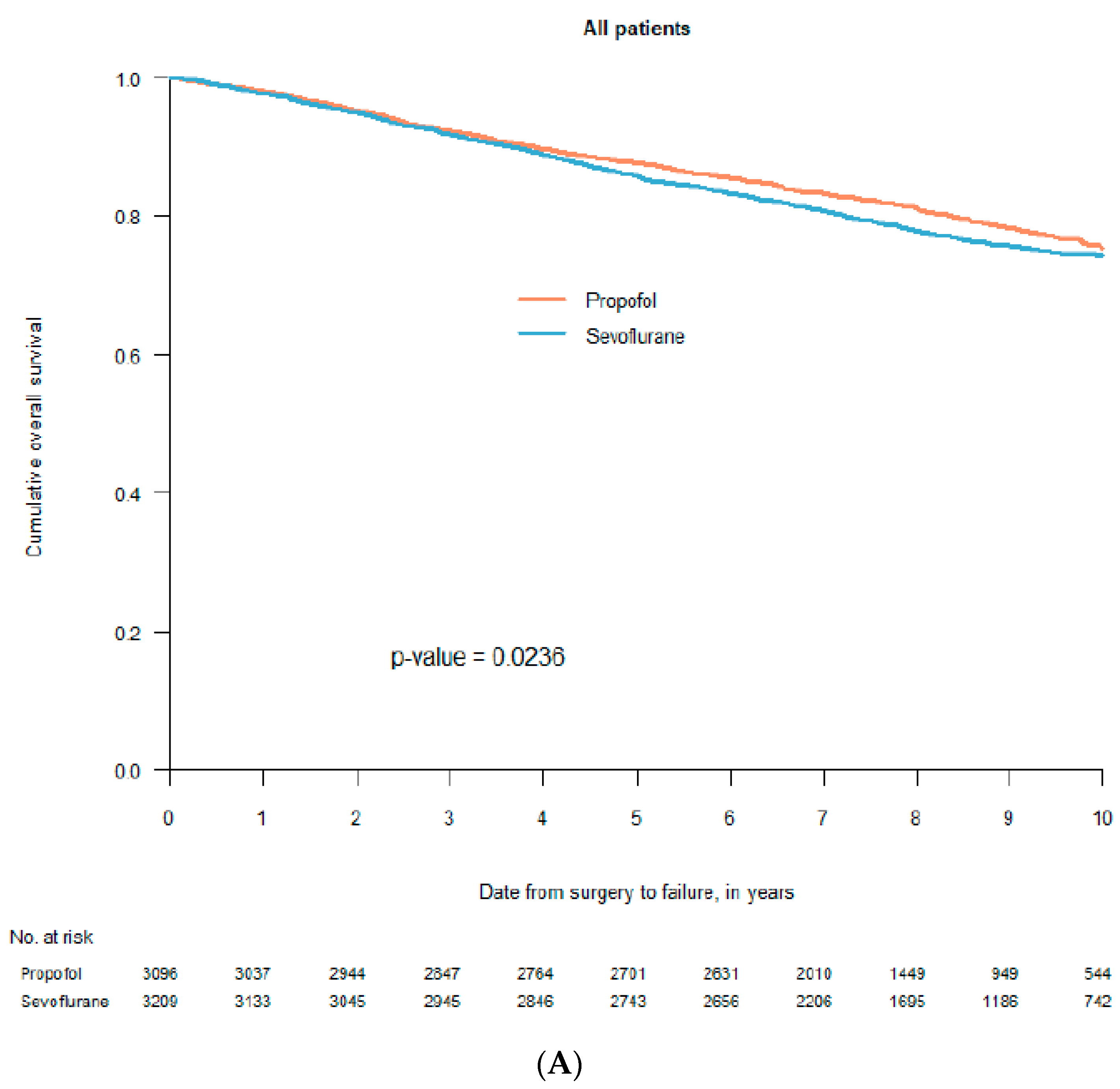
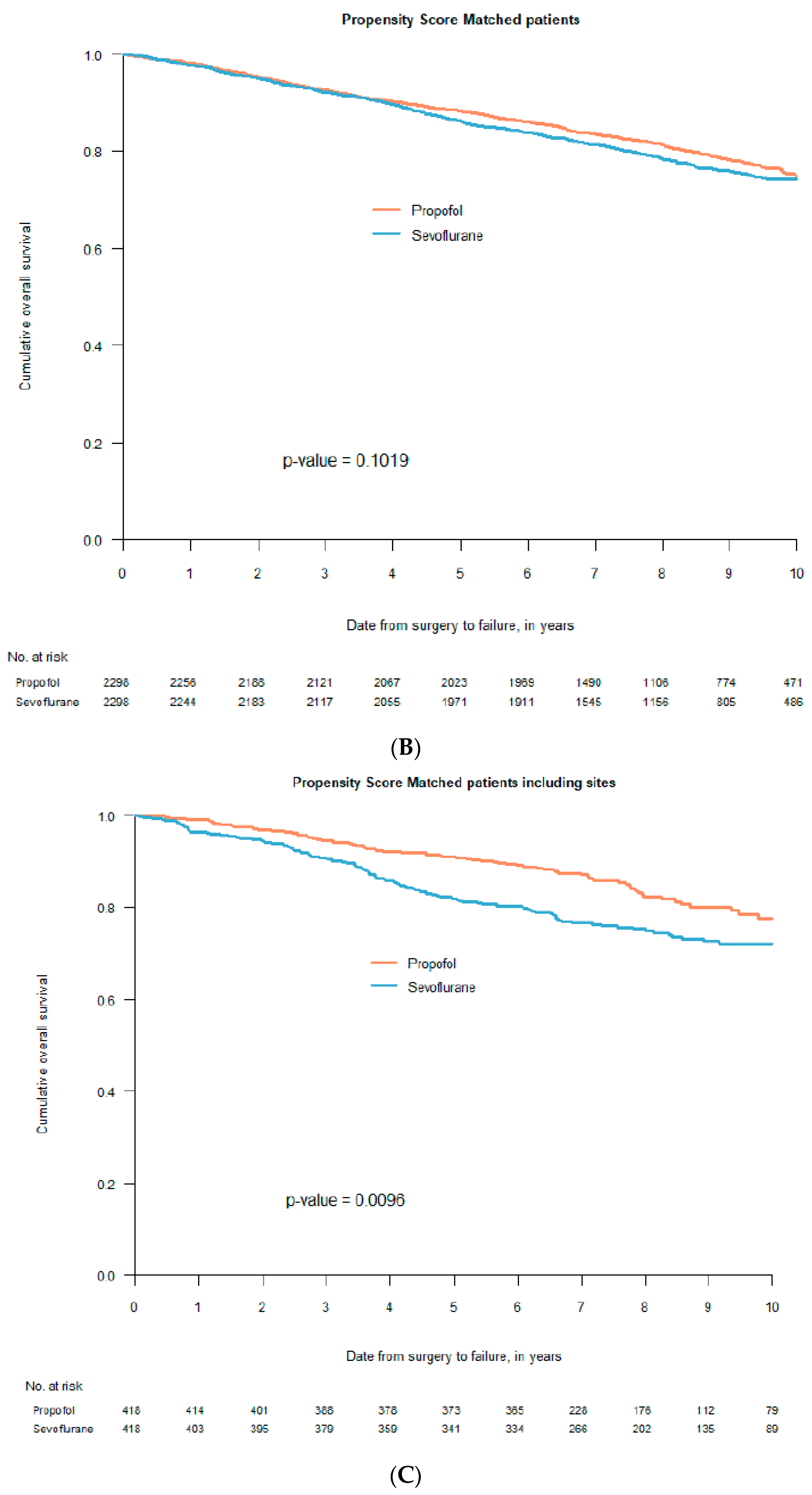
2. Retrospective Patient Studies
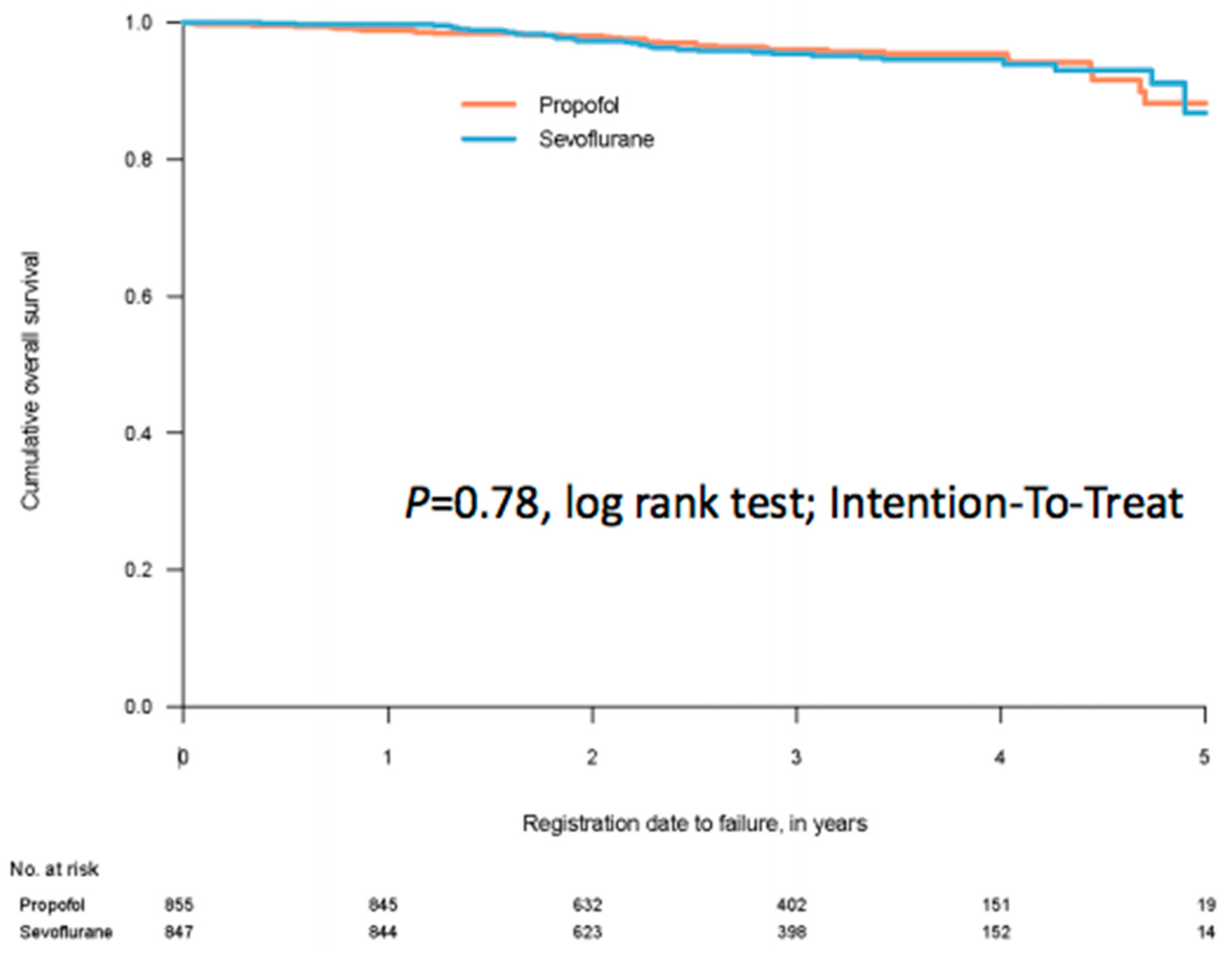
3. Randomised Clinical Trials (RCTs)
4. Discussion
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Shapiro, J.; Jersky, J.; Katzav, S.; Feldman, M.; Segal, S. Anesthetic drugs accelerate the progression of postoperative metastases of mouse tumors. J. Clin. Investig. 1981, 68, 678–685. [Google Scholar] [CrossRef]
- Matsuoka, H.; Kurosawa, S.; Horinouchi, T.; Kato, M.; Hashimoto, Y. Inhalation anesthetics induce apoptosis in normal peripheral lymphocytes in vitro. Anesthesiology 2001, 95, 1467–1472. [Google Scholar] [CrossRef] [PubMed]
- Melamed, R.; Bar-Yosef, S.; Shakhar, G.; Shakhar, K.; Ben-Eliyahu, S. Suppression of natural killer cell activity and promotion of tumor metastasis by ketamine, thiopental, and halothane, but not by propofol: Mediating mechanisms and prophylactic measures. Anesth. Analg. 2003, 97, 1331–1339. [Google Scholar] [CrossRef]
- Loop, T.; Dovi-Akue, D.; Frick, M.; Roesslein, M.; Egger, L.; Humar, M.; Hoetzel, A.; Schmidt, R.; Borner, C.; Pahl, H.L.; et al. Volatile anesthetics induce caspase-dependent, mitochondria-mediated apoptosis in human T lymphocytes in vitro. Anesthesiology 2005, 102, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Kushida, A.; Inada, T.; Shingu, K. Enhancement of antitumor immunity after propofol treatment in mice. Immunopharmacol. Immunotoxicol. 2007, 29, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Inada, T.; Kubo, K.; Kambara, T.; Shingu, K. Propofol inhibits cyclo-oxygenase activity in human monocytic THP-1 cells. Can. J. Anaesth. 2009, 56, 222–229. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Inada, T.; Yamanouchi, Y.; Jomura, S.; Sakamoto, S.; Takahashi, M.; Kambara, T.; Shingu, K. Effect of propofol and isoflurane anaesthesia on the immune response to surgery. Anaesthesia 2004, 59, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Stollings, L.M.; Jia, L.J.; Tang, P.; Dou, H.; Lu, B.; Xu, Y. Immune Modulation by Volatile Anesthetics. Anesthesiology 2016, 125, 399–411. [Google Scholar] [CrossRef]
- Yuki, K.; Eckenhoff, R.G. Mechanisms of the Immunological Effects of Volatile Anesthetics: A Review. Anesth. Analg. 2016, 123, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, Y.; Sawada, S.; Yoshioka, I.; Ohashi, Y.; Matsuo, M.; Harimaya, Y.; Tsukada, K.; Saiki, I. Increased surgical stress promotes tumor metastasis. Surgery 2003, 133, 547–555. [Google Scholar] [CrossRef]
- Schneemilch, C.E.; Ittenson, A.; Ansorge, S.; Hachenberg, T.; Bank, U. Effect of 2 anesthetic techniques on the postoperative proinflammatory and anti-inflammatory cytokine response and cellular immune function to minor surgery. J. Clin. Anesth. 2005, 17, 517–527. [Google Scholar] [CrossRef]
- Ke, J.J.; Zhan, J.; Feng, X.B.; Wu, Y.; Rao, Y.; Wang, Y.L. A comparison of the effect of total intravenous anaesthesia with propofol and remifentanil and inhalational anaesthesia with isoflurane on the release of pro- and anti-inflammatory cytokines in patients undergoing open cholecystectomy. Anaesth. Intensive Care 2008, 36, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Heaney, A.; Buggy, D.J. Can anaesthetic and analgesic techniques affect cancer recurrence or metastasis? Br. J. Anaesth. 2012, 109 (Suppl. 1), i17–i28. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.G.; Chung, C.K.E.; Ip, K.Y.; Wiles, M.D. Influence of propofol-based total intravenous anaesthesia on peri-operative outcome measures: A narrative review. Anaesthesia 2020, 75 (Suppl. 1), e90–e100. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhou, S. Effect of Compound Propofol Nanoemulsion on Immune Function in Patients with Pancreatic Cancer. J. Nanosci. Nanotechnol. 2021, 21, 1390–1396. [Google Scholar] [CrossRef] [PubMed]
- Hoerauf, K.H.; Wiesner, G.; Schroegendorfer, K.F.; Jobst, B.P.; Spacek, A.; Harth, M.; Sator-Katzenschlager, S.; Rudiger, H.W. Waste anaesthetic gases induce sister chromatid exchanges in lymphocytes of operating room personnel. Br. J. Anaesth. 1999, 82, 764–766. [Google Scholar] [CrossRef]
- Wiesner, G.; Harth, M.; Hoerauf, K.; Szulc, R.; Jurczyk, W.; Sobczynski, P.; Hobbhahn, J.; Taeger, K. Occupational exposure to inhaled anaesthetics: A follow-up study on anaesthetists of an eastern European university hospital. Acta Anaesthesiol. Scand. 2000, 44, 804–806. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, G.; Hoerauf, K.; Schroegendorfer, K.; Sobczynski, P.; Harth, M.; Ruediger, H.W. High-level, but not low-level, occupational exposure to inhaled anesthetics is associated with genotoxicity in the micronucleus assay. Anesth. Analg. 2001, 92, 118–122. [Google Scholar] [CrossRef]
- Wiesner, G.; Schiewe-Langgartner, F.; Lindner, R.; Gruber, M. Increased formation of sister chromatid exchanges, but not of micronuclei, in anaesthetists exposed to low levels of sevoflurane. Anaesthesia 2008, 63, 861–864. [Google Scholar] [CrossRef]
- Krause, T.K.; Jansen, L.; Scholz, J.; Bottcher, H.; Wappler, F.; Burmeister, M.A.; am Esch, J.S. Propofol anesthesia in children does not induce sister chromatid exchanges in lymphocytes. Mutat. Res. 2003, 542, 59–64. [Google Scholar] [CrossRef]
- Takabuchi, S.; Hirota, K.; Nishi, K.; Oda, S.; Oda, T.; Shingu, K.; Takabayashi, A.; Adachi, T.; Semenza, G.L.; Fukuda, K. The intravenous anesthetic propofol inhibits hypoxia-inducible factor 1 activity in an oxygen tension-dependent manner. FEBS Lett. 2004, 577, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Tavare, A.N.; Perry, N.J.; Benzonana, L.L.; Takata, M.; Ma, D. Cancer recurrence after surgery: Direct and indirect effects of anesthetic agents. Int. J. Cancer 2012, 130, 1237–1250. [Google Scholar] [CrossRef]
- Benzonana, L.L.; Perry, N.J.; Watts, H.R.; Yang, B.; Perry, I.A.; Coombes, C.; Takata, M.; Ma, D. Isoflurane, a commonly used volatile anesthetic, enhances renal cancer growth and malignant potential via the hypoxia-inducible factor cellular signaling pathway in vitro. Anesthesiology 2013, 119, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Takabuchi, S.; Nishi, K.; Oda, S.; Wakamatsu, T.; Daijo, H.; Fukuda, K.; Hirota, K. The intravenous anesthetic propofol inhibits lipopolysaccharide-induced hypoxia-inducible factor 1 activation and suppresses the glucose metabolism in macrophages. J. Anesth. 2010, 24, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Buckley, A.; McQuaid, S.; Johnson, P.; Buggy, D.J. Effect of anaesthetic technique on the natural killer cell anti-tumour activity of serum from women undergoing breast cancer surgery: A pilot study. Br. J. Anaesth. 2014, 113 (Suppl. 1), i56–i62. [Google Scholar] [CrossRef] [PubMed]
- Jaura, A.I.; Flood, G.; Gallagher, H.C.; Buggy, D.J. Differential effects of serum from patients administered distinct anaesthetic techniques on apoptosis in breast cancer cells in vitro: A pilot study. Br. J. Anaesth. 2014, 113 (Suppl. 1), i63–i67. [Google Scholar] [CrossRef]
- Looney, M.; Doran, P.; Buggy, D.J. Effect of anesthetic technique on serum vascular endothelial growth factor C and transforming growth factor beta in women undergoing anesthesia and surgery for breast cancer. Anesthesiology 2010, 113, 1118–1125. [Google Scholar] [CrossRef]
- Freier, D.O.; Fuchs, B.A. A mechanism of action for morphine-induced immunosuppression: Corticosterone mediates morphine-induced suppression of natural killer cell activity. J. Pharmacol. Exp. Ther. 1994, 270, 1127–1133. [Google Scholar]
- Yeager, M.P.; Colacchio, T.A.; Yu, C.T.; Hildebrandt, L.; Howell, A.L.; Weiss, J.; Guyre, P.M. Morphine inhibits spontaneous and cytokine-enhanced natural killer cell cytotoxicity in volunteers. Anesthesiology 1995, 83, 500–508. [Google Scholar] [CrossRef]
- Flores, L.R.; Dretchen, K.L.; Bayer, B.M. Potential role of the autonomic nervous system in the immunosuppressive effects of acute morphine administration. Eur. J. Pharmacol. 1996, 318, 437–446. [Google Scholar] [CrossRef]
- Cronin-Fenton, D.P.; Heide-Jorgensen, U.; Ahern, T.P.; Lash, T.L.; Christiansen, P.M.; Ejlertsen, B.; Sjogren, P.; Kehlet, H.; Sorensen, H.T. Opioids and breast cancer recurrence: A Danish population-based cohort study. Cancer 2015, 121, 3507–3514. [Google Scholar] [CrossRef]
- Wigmore, T.; Farquhar-Smith, P. Opioids and cancer: Friend or foe? Curr. Opin. Support. Palliat. Care 2016, 10, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Enlund, M.; Berglund, A.; Andreasson, K.; Cicek, C.; Enlund, A.; Bergkvist, L. The choice of anaesthetic-sevoflurane or propofol-and outcome from cancer surgery: A retrospective analysis. Ups. J. Med. Sci. 2014, 119, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Wigmore, T.J.; Mohammed, K.; Jhanji, S. Long-term Survival for Patients Undergoing Volatile versus IV Anesthesia for Cancer Surgery: A Retrospective Analysis. Anesthesiology 2015, 124, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kang, S.H.; Kim, Y.; Kim, H.A.; Kim, B.S. Effects of propofol-based total intravenous anesthesia on recurrence and overall survival in patients after modified radical mastectomy: A retrospective study. Korean J. Anesthesiol. 2016, 69, 126–132. [Google Scholar] [CrossRef]
- Jun, I.J.; Jo, J.Y.; Kim, J.I.; Chin, J.H.; Kim, W.J.; Kim, H.R.; Lee, E.H.; Choi, I.C. Impact of anesthetic agents on overall and recurrence-free survival in patients undergoing esophageal cancer surgery: A retrospective observational study. Sci. Rep. 2017, 7, 14020. [Google Scholar] [CrossRef]
- Kim, M.H.; Kim, D.W.; Kim, J.H.; Lee, K.Y.; Park, S.; Yoo, Y.C. Does the type of anesthesia really affect the recurrence-free survival after breast cancer surgery? Oncotarget 2017, 8, 90477–90487. [Google Scholar] [CrossRef]
- Oh, T.K.; Kim, K.; Jheon, S.; Lee, J.; Do, S.H.; Hwang, J.W.; Song, I.A. Long-Term Oncologic Outcomes for Patients Undergoing Volatile Versus Intravenous Anesthesia for Non-Small Cell Lung Cancer Surgery: A Retrospective Propensity Matching Analysis. Cancer Control 2018, 25, 1073274818775360. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wang, Y.; Dong, L.; Zhao, S.; Wang, L.; Chen, H.; Xu, Y.; Wang, G. Effects of propofol-based total intravenous anesthesia on gastric cancer: A retrospective study. Oncol. Targets Ther. 2018, 11, 1141–1148. [Google Scholar] [CrossRef]
- Wu, Z.F.; Lee, M.S.; Wong, C.S.; Lu, C.H.; Huang, Y.S.; Lin, K.T.; Lou, Y.S.; Lin, C.; Chang, Y.C.; Lai, H.C. Propofol-based Total Intravenous Anesthesia Is Associated with Better Survival Than Desflurane Anesthesia in Colon Cancer Surgery. Anesthesiology 2018, 129, 932–941. [Google Scholar] [CrossRef]
- Lai, H.C.; Lee, M.S.; Lin, C.; Lin, K.T.; Huang, Y.H.; Wong, C.S.; Chan, S.M.; Wu, Z.F. Propofol-based total intravenous anaesthesia is associated with better survival than desflurane anaesthesia in hepatectomy for hepatocellular carcinoma: A retrospective cohort study. Br. J. Anaesth. 2019, 123, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.; Lee, H.-B.; Han, W.; Noh, D.-Y.; Park, S.-U.; Kim, W.; Kim, J.-T. Total intravenous anesthesia versus inhalation anesthesia for breast cancer surgery. Anesthesiology 2019, 130, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-H.; Lee, M.-S.; Lou, Y.-S.; Lai, H.-C.; Yu, J.-C.; Lu, C.-H.; Wong, C.-S.; Wu, Z.-F. Propofol-based total intravenous anesthesia did not improve survival compared to desflurane anesthesia in breast cancer surgery. PLoS ONE 2019, 14, e0224728. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.K.; Kim, H.H.; Jeon, Y.T. Retrospective analysis of 1-year mortality after gastric cancer surgery: Total intravenous anesthesia versus volatile anesthesia. Acta Anaesthesiol. Scand. 2019, 63, 1169–1177. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.; Lee, S.; Kim, Y.; Lee, M.; Youn, A.M.; Rhim, H.; Hong, S.H.; Kim, Y.H.; Yoon, S.H.; Lim, C. Anesthetics and long-term survival after cancer surgery-total intravenous versus volatile anesthesia: A retrospective study. BMC Anesthesiol. 2019, 19, 233. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Zeng, M.; Ji, N.; Hao, S.; Zhou, Y.; Gao, Z.; Gu, H.; Zhang, L.; Ma, D.; Peng, Y.; et al. Impact of Anesthesia on Long-term Outcomes in Patients With Supratentorial High-grade Glioma Undergoing Tumor Resection: A Retrospective Cohort Study. J. Neurosurg. Anesthesiol. 2020, 32, 227–233. [Google Scholar] [CrossRef]
- Grau, S.J.; Löhr, M.; Taurisano, V.; Trautner, H.; Timmer, M.; Schwab, S.G.; Hampl, J.; Annecke, T. The choice of anaesthesia for glioblastoma surgery does not impact the time to recurrence. Sci. Rep. 2020, 10, 5556. [Google Scholar] [CrossRef] [PubMed]
- Shiono, S.; Shibata, S.C.; Kabata, D.; Shintani, A.; Ikeda, T.; Fujino, Y. Comparison of 1-year recurrence-free survival between sevoflurane and propofol use for general anesthesia management in primary breast cancer surgery. J. Anesth. 2020, 34, 694–701. [Google Scholar] [CrossRef]
- Schmoch, T.; Jungk, C.; Bruckner, T.; Haag, S.; Zweckberger, K.; von Deimling, A.; Brenner, T.; Unterberg, A.; Weigand, M.A.; Uhle, F.; et al. The anesthetist’s choice of inhalational vs. intravenous anesthetics has no impact on survival of glioblastoma patients. Neurosurg. Rev. 2020. [Google Scholar] [CrossRef]
- Crone, V.; Hasselager, R.P.; Fransgaard, T.; Gögenur, I. Anaesthetic technique and outcomes after colorectal cancer surgery. Dan. Med. J. 2020, 67, 1–6. [Google Scholar]
- Lai, H.C.; Lee, M.S.; Liu, Y.T.; Lin, K.T.; Hung, K.C.; Chen, J.Y.; Wu, Z.F. Propofol-based intravenous anesthesia is associated with better survival than desflurane anesthesia in pancreatic cancer surgery. PLoS ONE 2020, 15, e0233598. [Google Scholar] [CrossRef]
- Lai, H.C.; Lee, M.S.; Lin, K.T.; Huang, Y.H.; Chen, J.Y.; Lin, Y.T.; Hung, K.C.; Wu, Z.F. Propofol-based total intravenous anesthesia is associated with better survival than desflurane anesthesia in robot-assisted radical prostatectomy. PLoS ONE 2020, 15, e0230290. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.C.; Lee, M.S.; Lai, H.C.; Lin, H.T.; Huang, Y.H.; Lu, C.H.; Hsu, C.H.; Wu, Z.F. Propofol-based total intravenous anesthesia improves survival compared to desflurane anesthesia in gastric cancer surgery: A retrospective analysis. Medicine 2020, 99, e20714. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.W.; Lim, D.J.; Oh, A.Y.; Na, H.S. Retrospective Comparison between the Effects of Propofol and Inhalation Anesthetics on Postoperative Recurrence of Early- and Intermediate-Stage Hepatocellular Carcinoma. Med. Princ. Pract. 2020, 29, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.Y.; Zhang, X.P.; Sun, Z.; Wang, H.Q.; Yu, W.F. Distant survival for patients undergoing surgery using volatile versus IV anesthesia for hepatocellular carcinoma with portal vein tumor thrombus: A retrospective study. BMC Anesthesiol. 2020, 20, 233. [Google Scholar] [CrossRef] [PubMed]
- Hayasaka, K.; Shiono, S.; Miyata, S.; Takaoka, S.; Endoh, M.; Okada, Y. Prognostic significance of propofol-based intravenous anesthesia in early-stage lung cancer surgery. Surg. Today 2021. [Google Scholar] [CrossRef]
- Soltanizadeh, S.; Degett, T.H.; Gogenur, I. Outcomes of cancer surgery after inhalational and intravenous anesthesia: A systematic review. J. Clin. Anesth. 2017, 42, 19–25. [Google Scholar] [CrossRef]
- Jin, Z.; Li, R.; Liu, J.; Lin, J. Long-term prognosis after cancer surgery with inhalational anesthesia and total intravenous anesthesia: A systematic review and meta-analysis. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 83–94. [Google Scholar]
- Yap, A.; Lopez-Olivo, M.A.; Dubowitz, J.; Hiller, J.; Riedel, B. Correction to: Anesthetic technique and cancer outcomes: A meta-analysis of total intravenous versus volatile anesthesia. Can. J. Anaesth. 2019, 66, 1007–1008. [Google Scholar] [CrossRef]
- Chang, C.Y.; Wu, M.Y.; Chien, Y.J.; Su, I.M.; Wang, S.C.; Kao, M.C. Anesthesia and Long-Term Oncological Outcomes: A Systematic Review and Meta-analysis. Anesth. Analg. 2021, 132, 623–634. [Google Scholar] [CrossRef]
- Enlund, M.; Berglund, A.; Ahlstrand, R.; Walldén, J.; Lundberg, J.; Wärnberg, F.; Ekman, A.; Sjöblom Widfeldt, N.; Enlund, A.; Bergkvist, L. Survival after primary breast cancer surgery following propofol or sevoflurane general anesthesia-A retrospective, multicenter, database analysis of 6305 Swedish patients. Acta Anaesthesiol. Scand. 2020, 64, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Makito, K.; Matsui, H.; Fushimi, K.; Yasunaga, H. Volatile versus Total Intravenous Anesthesia for Cancer Prognosis in Patients Having Digestive Cancer Surgery. Anesthesiology 2020, 133, 764–773. [Google Scholar] [CrossRef] [PubMed]
- Hasselager, R.P.; Hallas, J.; Gögenur, I. Inhalation or total intravenous anaesthesia and recurrence after colorectal cancer surgery: A propensity score matched Danish registry-based study. Br. J. Anaesth. 2021, 126, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Enlund, M.; Enlund, A.; Berglund, A.; Bergkvist, L. Rationale and Design of the CAN Study: An RCT of Survival after Propofol- or Sevoflurane-based Anesthesia for Cancer Surgery. Curr. Pharm. Des. 2019, 25, 3028–3033. [Google Scholar] [CrossRef]
- Enlund, M.; Enlund, A.; Berglund, A.; Bergkvist, L. The Cancer and Anaesthesia study (CAN), an RCT of survival after propofol- or sevoflurane-based anesthesia for cancer surgery. First results for breast cancer. Eur. J. Anaesthesiol. 2020, 37, 68. [Google Scholar]
- Hovaguimian, F.; Braun, J.; Roth Z’graggen, B.; Schläpfer, M.; Dumrese, C.; Ewald, C.; Dedes, K.J.; Fink, D.; Rölli, U.; Seeberger, M.; et al. Anesthesia and Circulating Tumor Cells in Primary Breast Cancer Patients: A Randomized Controlled Trial. Anesthesiology 2020, 133, 548–558. [Google Scholar] [CrossRef]
- Sessler, D.I.; Pei, L.; Huang, Y.; Fleischmann, E.; Marhofer, P.; Kurz, A.; Mayers, D.B.; Meyer-Treschan, T.A.; Grady, M.; Tan, E.Y.; et al. Recurrence of breast cancer after regional or general anaesthesia: A randomised controlled trial. Lancet 2019, 394, 1807–1815. [Google Scholar] [CrossRef]
- Yan, T.; Zhang, G.H.; Wang, B.N.; Sun, L.; Zheng, H. Effects of propofol/remifentanil-based total intravenous anesthesia versus sevoflurane-based inhalational anesthesia on the release of VEGF-C and TGF-beta and prognosis after breast cancer surgery: A prospective, randomized and controlled study. BMC Anesthesiol. 2018, 18, 131. [Google Scholar] [CrossRef] [PubMed]
- Kurozumi, S.; Kaira, K.; Matsumoto, H.; Hirakata, T.; Yokobori, T.; Inoue, K.; Horiguchi, J.; Katayama, A.; Koshi, H.; Shimizu, A.; et al. β(2)-Adrenergic receptor expression is associated with biomarkers of tumor immunity and predicts poor prognosis in estrogen receptor-negative breast cancer. Breast Cancer Res. Treat. 2019, 177, 603–610. [Google Scholar] [CrossRef]
| Journal | Year | First Author | Cancer Localisation | Total Number of Patients |
|---|---|---|---|---|
| Upsala J. Med. Sci. [33] | 2014 | Enlund, M. | Breast, colo-rectal | 2838 |
| Oncotarget [37] | 2017 | Kim, M.H. | Breast | 2645 |
| Cancer Control. [38] | 2018 | Oh, T.K. | Lung | 943 (392 in PSM) |
| Anesthesiology [42] | 2019 | Yoo, S. | Breast | 5331 (1766 in PSM) |
| PLoS ONE [43] | 2019 | Huang, Y.-H. | Breast | 976 (888) |
| Acta Anaesthesiol. Scand. [44] | 2019 | Oh, T.K. | Gastric | 4607 (1538 in PSM) |
| BMC Anesthesiol. [45] | 2019 | Hong, B. | Mixed locations | 1458 |
| J. Neurosurg. Anesthesiol. [46] | 2019 | Dong, J. | Brain (glioma) | 294 |
| Sci. Rep. [47] | 2020 | Grau, S.J. | Brain (glioma) | 158 (158 in PSM) |
| J. Anesth. [48] | 2020 | Shiono, S. | Breast | 1026 |
| Neurosurg. Rev. [49] | 2020 | Schmoch, T. | Brain (glioblastom) | 144 |
| Dan. Med. J. [50] | 2020 | Hasselager | Colorectal | 534 |
| Total number of patients | 18,324 |
| Journal | Year | First Author | Cancer Localisation | Total Number of Patients |
|---|---|---|---|---|
| Anesthesiology [34] | 2016 | Wigmore, T. | Mixed cancers | 7030 |
| Korean J. Anestesiol. [35] | 2016 | Lee, J.H. | Breast | 325 |
| Sci. Rep. [36] | 2017 | Jun, I.J. | Esophagus | 922 |
| Onco. Targets Ther. [39] | 2018 | Zheng, X. | Gastric | 2856 (897 in PSM) |
| Anesthesiology [40] | 2018 | Wu, Z.F. | Colon | 1363 (1158 in PSM) |
| Br. J. Anaesth. [41] | 2019 | Lai, H.-C. | Liver | 944 (670 in PSM) |
| PLoS ONE [51] | 2020 | Lai, H.-C. | Pancreas | 140 (116 in PSM) |
| PLoS ONE [52] | 2020 | Lai, H.-C. | Prostate | 631 (528 in PSM) |
| Medicine (Baltim.) [53] | 2020 | Huang, N.C. | Gastric | 408 (334 in PSM) |
| Med. Princ. Prac. [54] | 2020 | Koo, B.-W. | Liver | 535 |
| BMC Anesthesiol. [55] | 2020 | Meng, X.Y. | Liver | 1513 |
| Surg. Today [56] | 2021 | Hayasaka, K. | Lung | 230 |
| Total number of patients | 16,897 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enlund, M. Is It Definitely Clear That Long-Term Survival after Breast Cancer Surgery Is Not Affected by Anaesthetics? Cancers 2021, 13, 3390. https://doi.org/10.3390/cancers13143390
Enlund M. Is It Definitely Clear That Long-Term Survival after Breast Cancer Surgery Is Not Affected by Anaesthetics? Cancers. 2021; 13(14):3390. https://doi.org/10.3390/cancers13143390
Chicago/Turabian StyleEnlund, Mats. 2021. "Is It Definitely Clear That Long-Term Survival after Breast Cancer Surgery Is Not Affected by Anaesthetics?" Cancers 13, no. 14: 3390. https://doi.org/10.3390/cancers13143390
APA StyleEnlund, M. (2021). Is It Definitely Clear That Long-Term Survival after Breast Cancer Surgery Is Not Affected by Anaesthetics? Cancers, 13(14), 3390. https://doi.org/10.3390/cancers13143390