Pregnancy Inhibits Mammary Carcinogenesis by Persistently Altering the Hypothalamic–Pituitary Axis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
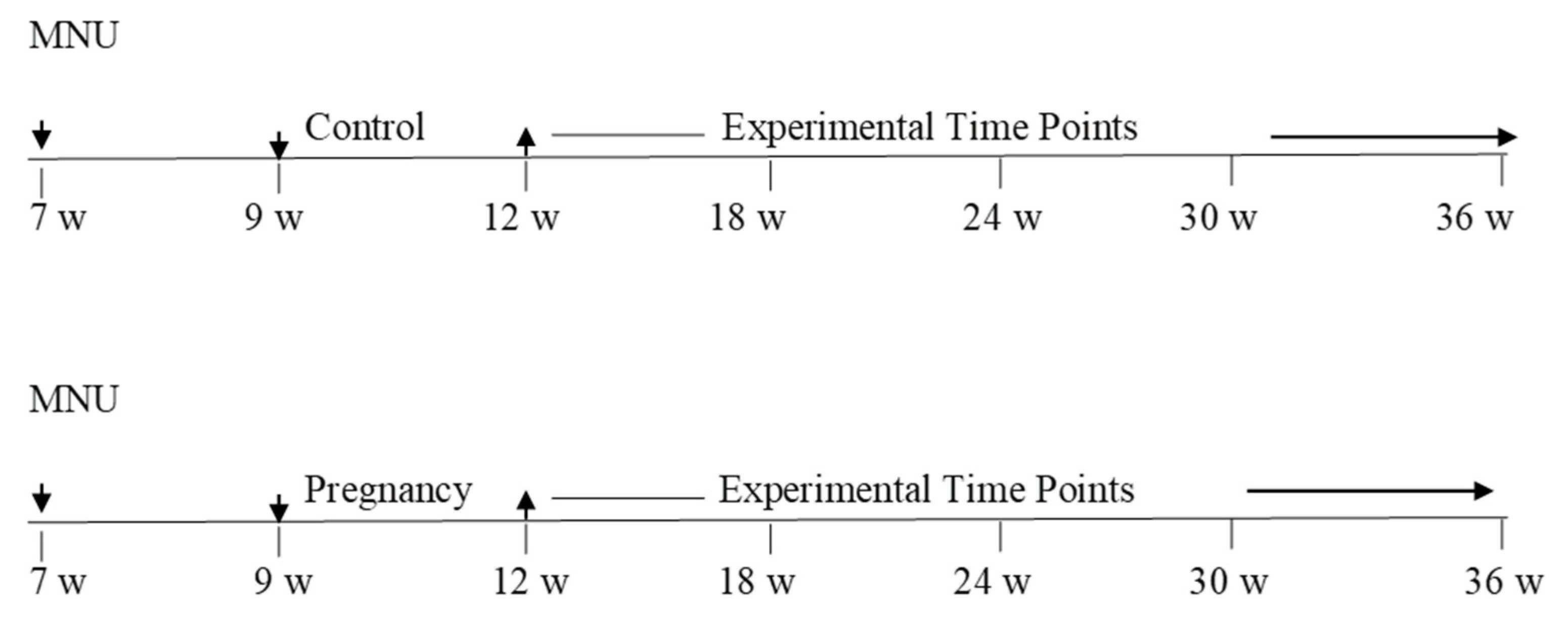
2.2. Experimental Design Line Diagram
2.3. Mammary Carcinogenesis
2.4. Immunohistochemistry
2.5. Measurement of Static Levels of Hypothalamic and Pituitary Hormones in Response to Pregnancy
2.6. The Hypothalamic–Pituitary Axis Response to GH and PRL Secretagogues
2.7. Measuring the Functional Activity of the Pituitary in Response to GH and PRL Secretagogues
3. Results
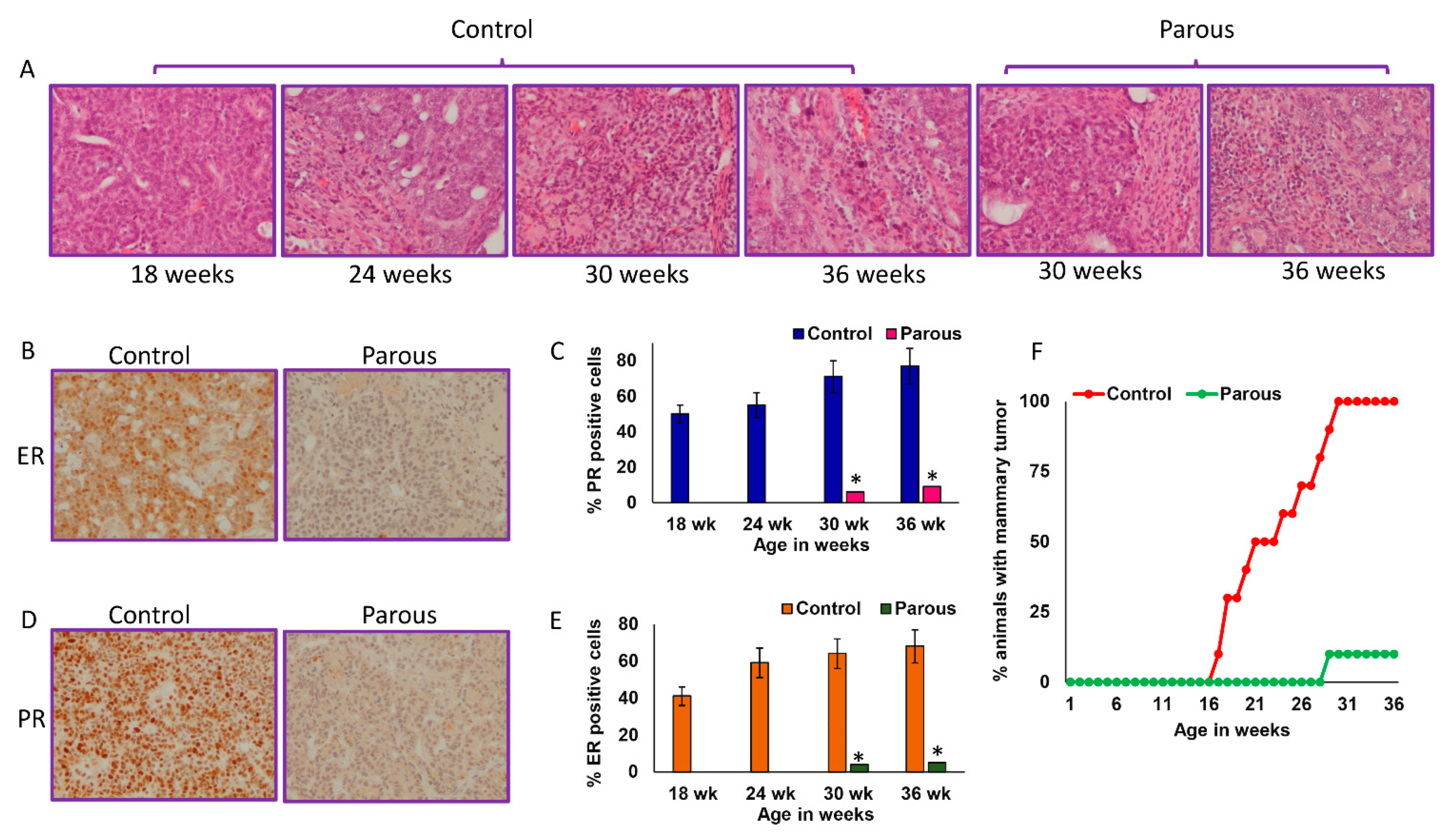
3.1. Parity Inhibits Mammary Carcinogenesis
3.2. The Effect of Parity on Tissue Levels of GHRH, TRH, DA, SS, GH, and PRL
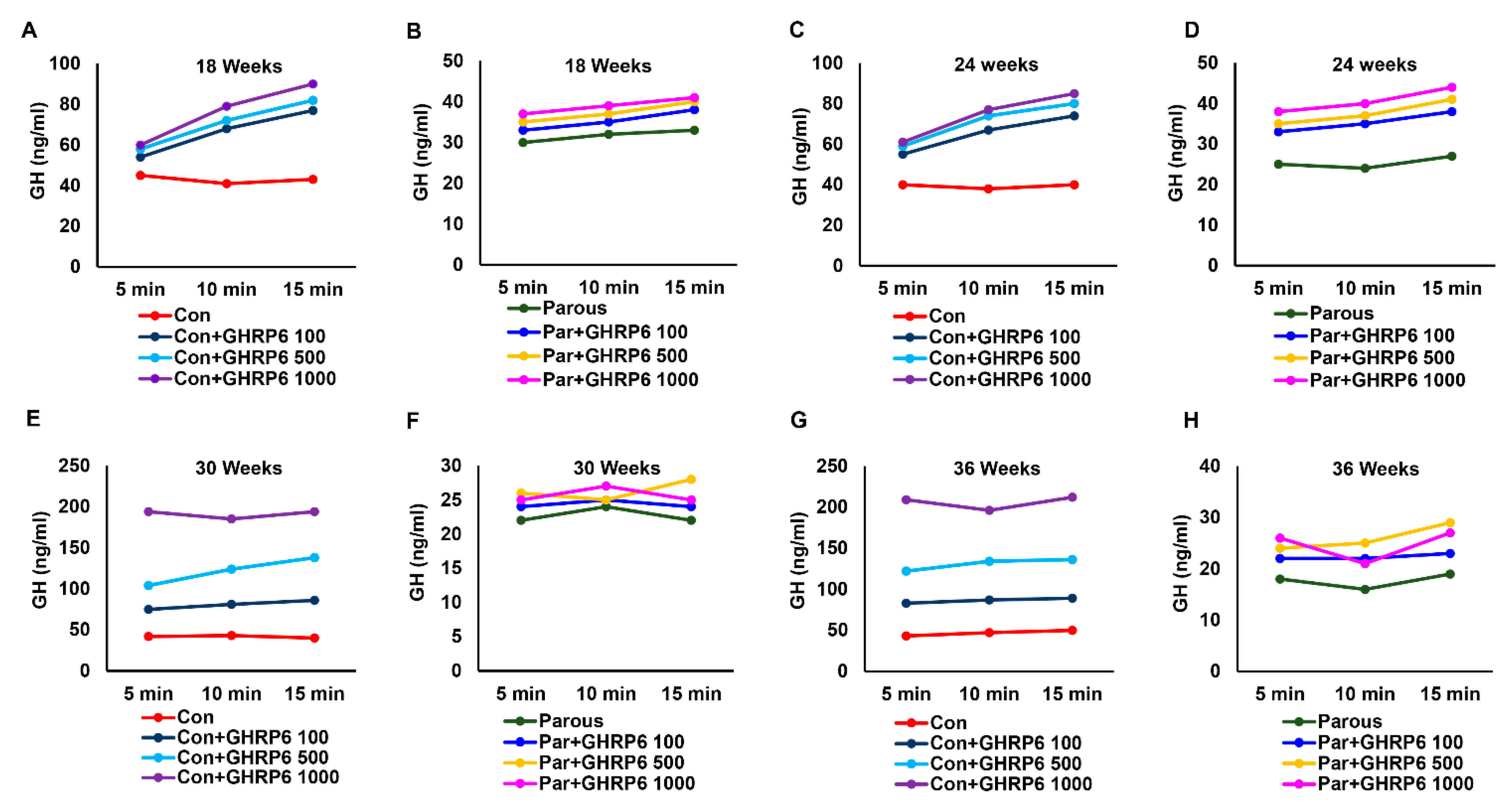
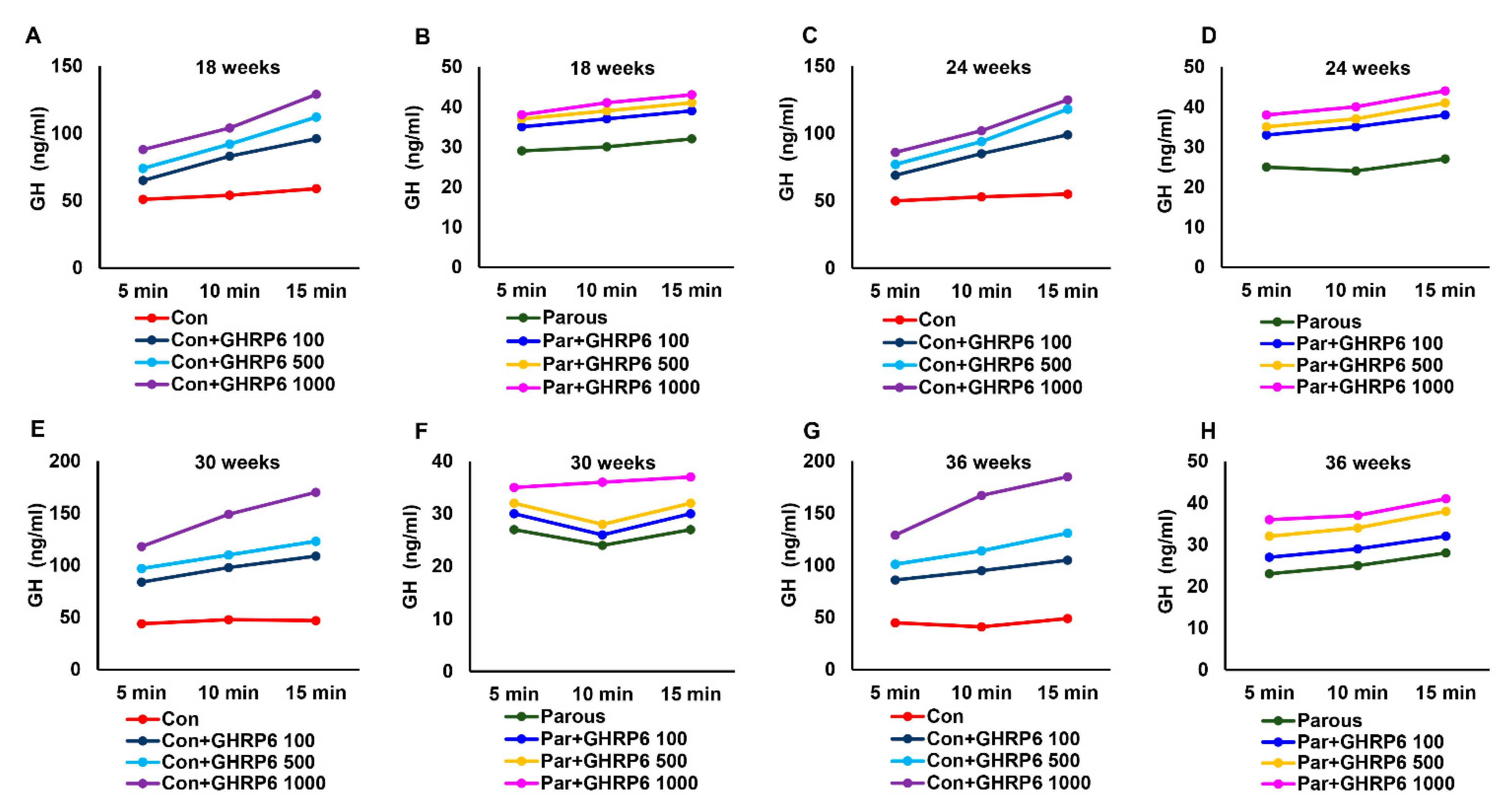
3.3. The Effect of Parity on the Hypothalamic–Pituitary–GH Axis
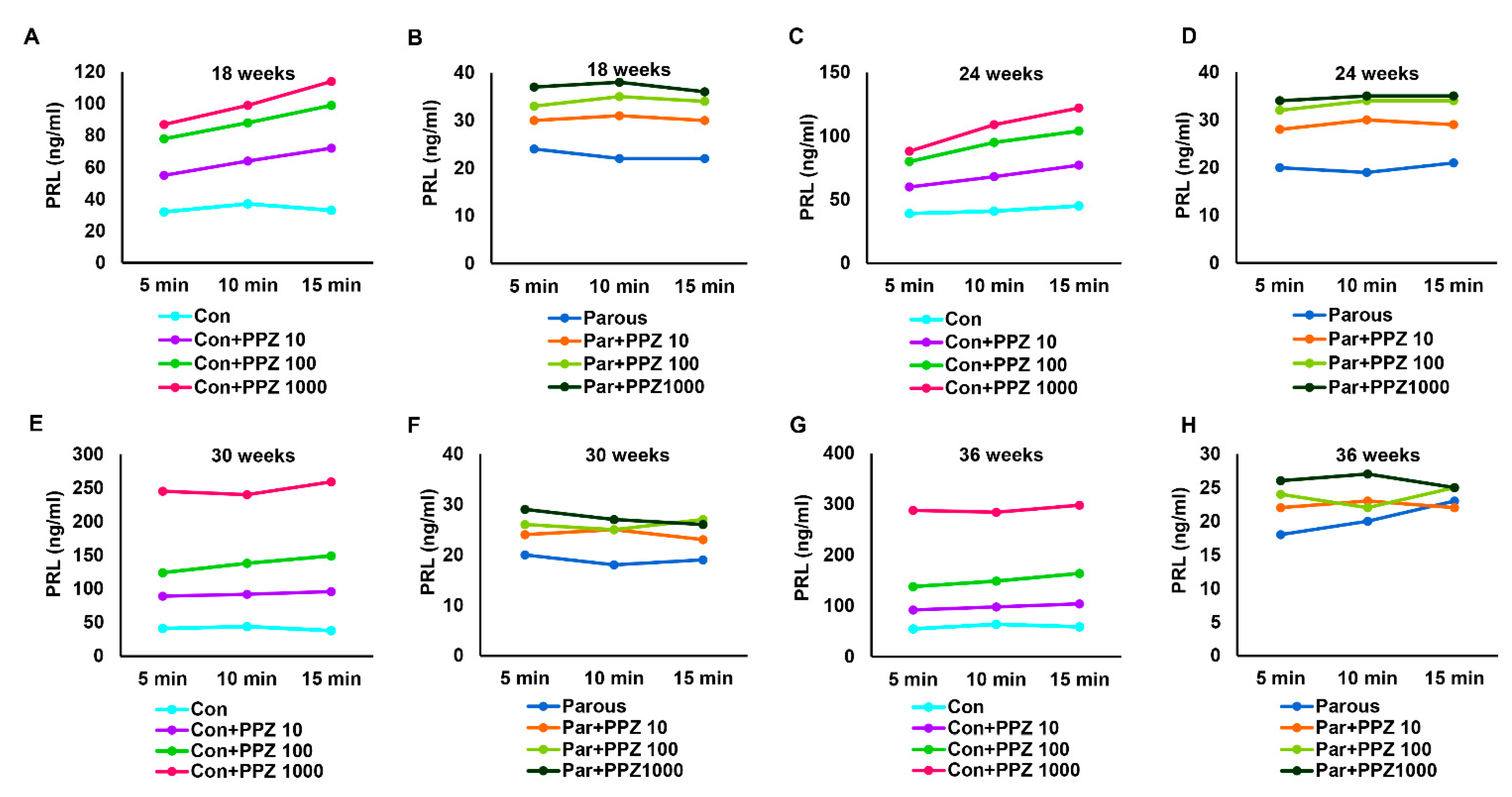
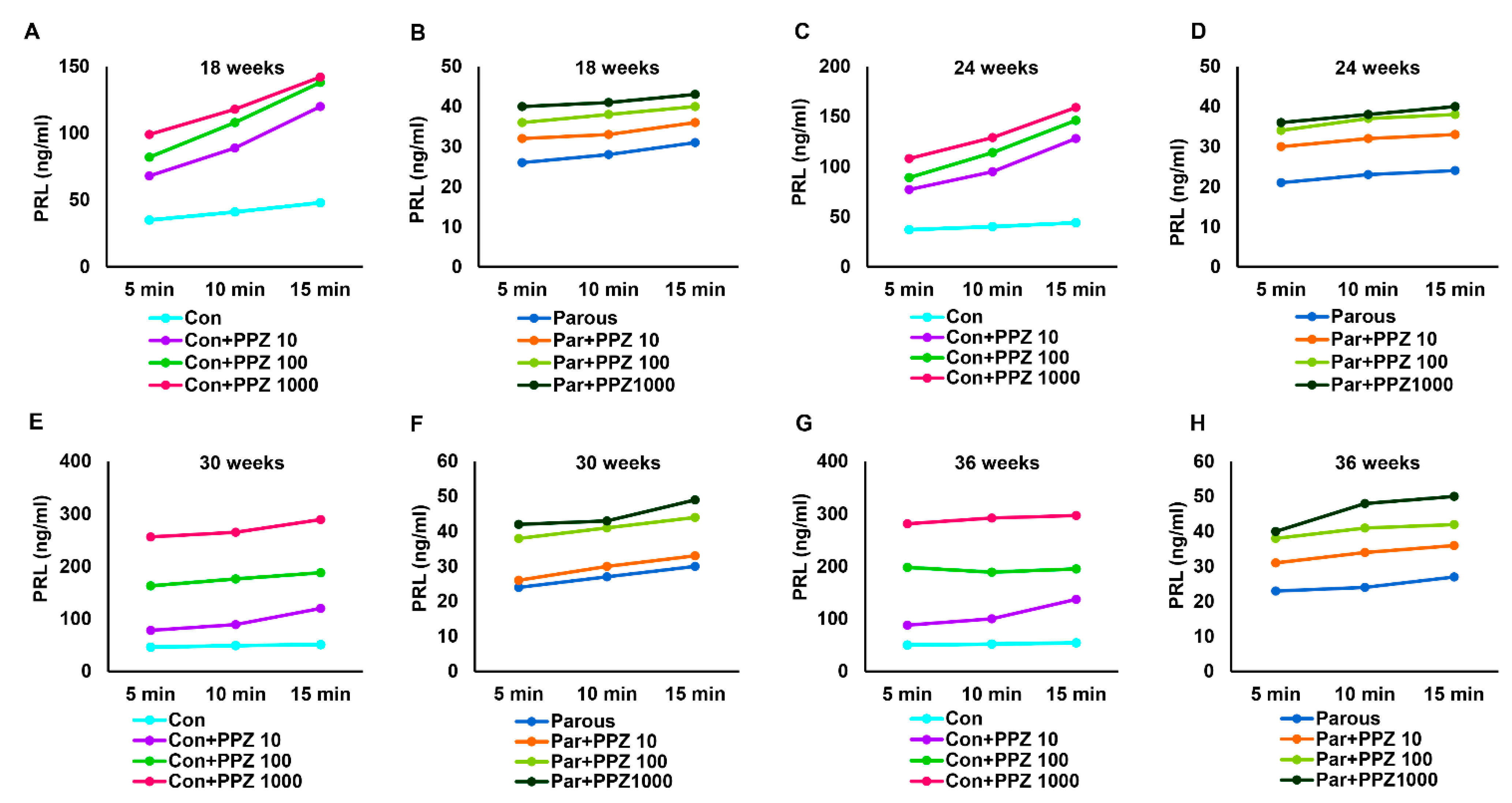
3.4. The Influence of Parity on the Hypothalamic–Pituitary-PRL Axis
3.5. Parity Induces Dynamic Changes in Pituitary Glands
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Subramani, R.; Lakshmanaswamy, R. Pregnancy and Breast Cancer. Prog. Mol. Biol. Transl. Sci. 2017, 151, 81–111. [Google Scholar] [CrossRef]
- Dall, G.V.; Vieusseux, J.; Seyed-Razavi, Y.; Godde, N.; Ludford-Menting, M.; Russell, S.M.; Ashworth, A.; Anderson, R.L.; Risbridger, G.P.; Shackleton, M.; et al. Parity reduces mammary repopulating activity but does not affect mammary stem cells defined as CD24 + CD29/CD49fhi in mice. Breast Cancer Res. Treat. 2020, 183, 565–575. [Google Scholar] [CrossRef]
- MacMahon, B.; Cole, P.; Lin, T.M.; Lowe, C.R.; Mirra, A.P.; Ravnihar, B.; Salber, E.J.; Valaoras, V.G.; Yuasa, S. Age at first birth and breast cancer risk. Bull. World Health Organ. 1970, 43, 209–221. [Google Scholar]
- Henderson, B.E.; Powell, D.; Rosario, I.; Keys, C.; Hanisch, R.; Young, M.; Casagrande, J.; Gerkins, V.; Pike, M.C. An epidemiologic study of breast cancer. J. Natl. Cancer Inst. 1974, 53, 609–614. [Google Scholar] [CrossRef]
- Dupont, W.D.; Page, D.L. Breast cancer risk associated with proliferative disease, age at first birth, and a family history of breast cancer. Am. J. Epidemiol. 1987, 125, 769–779. [Google Scholar] [CrossRef]
- Kelsey, J.L. Breast cancer epidemiology: Summary and future directions. Epidemiol. Rev. 1993, 15, 256–263. [Google Scholar] [CrossRef]
- Thordarson, G.; Jin, E.; Guzman, R.C.; Swanson, S.M.; Nandi, S.; Talamantes, F. Refractoriness to mammary tumorigenesis in parous rats: Is it caused by persistent changes in the hormonal environment or permanent biochemical alterations in the mammary epithelia? Carcinogenesis 1995, 16, 2847–2853. [Google Scholar] [CrossRef]
- Moon, R.C. Relationship between previous reproductive history and chemically induced mammary cancer in rats. Int. J. Cancer J. Int. Cancer 1969, 4, 312–317. [Google Scholar] [CrossRef]
- Arumugam, A.; Subramani, R.; Nandy, S.; Lopez, R.; Boopalan, T.; Lakshmanaswamy, R. Parity and short-term estradiol treatment utilizes similar cellular mechanisms to confer protection against breast cancer. Cell Physiol. Biochem. 2014, 34, 491–505. [Google Scholar] [CrossRef]
- Russo, J.; Lynch, H.; Russo, I.H. Mammary gland architecture as a determining factor in the susceptibility of the human breast to cancer. Breast J. 2001, 7, 278–291. [Google Scholar] [CrossRef]
- Russo, J.; Russo, I.H. The etiopathogenesis of breast cancer prevention. Cancer Lett. 1995, 90, 81–89. [Google Scholar] [CrossRef]
- Russo, J.; Russo, I.H. Toward a physiological approach to breast cancer prevention. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 1994, 3, 353–364. [Google Scholar]
- Britt, K.L.; Kendrick, H.; Regan, J.L.; Molyneux, G.; Magnay, F.A.; Ashworth, A.; Smalley, M.J. Pregnancy in the mature adult mouse does not alter the proportion of mammary epithelial stem/progenitor cells. Breast Cancer Res. BCR 2009, 11, R20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boulanger, C.A.; Wagner, K.U.; Smith, G.H. Parity-induced mouse mammary epithelial cells are pluripotent, self-renewing and sensitive to TGF-beta1 expression. Oncogene 2005, 24, 552–560. [Google Scholar] [CrossRef] [Green Version]
- Russo, J.; Balogh, G.A.; Chen, J.; Fernandez, S.V.; Fernbaugh, R.; Heulings, R.; Mailo, D.A.; Moral, R.; Russo, P.A.; Sheriff, F.; et al. The concept of stem cell in the mammary gland and its implication in morphogenesis, cancer and prevention. Front. Biosci. J. Virtual Libr. 2006, 11, 151–172. [Google Scholar] [CrossRef] [Green Version]
- Nandy, S.B.; Subramani, R.; Rajamanickam, V.; Lopez-Valdez, R.; Arumugam, A.; Boopalan, T.; Lakshmanaswamy, R. microRNA alterations in ALDH positive mammary epithelial cells: A crucial contributing factor towards breast cancer risk reduction in case of early pregnancy. BMC Cancer 2014, 14, 644. [Google Scholar] [CrossRef] [Green Version]
- Schulz, S.; Röcken, C. Immunocytochemical localisation of plasma membrane GHRH receptors in human tumours using a novel anti-peptide antibody. Eur. J. Cancer 2006, 42, 2390–2396. [Google Scholar] [CrossRef]
- Ericsson, M.; Bhuiyan, H.; Yousif, B.; Lehtihet, M.; Ekström, L. The intra-individual stability of GH biomarkers IGF-I and P-III-NP in relation to GHRH administration, menstrual cycle, and hematological parameters. Drug Test. Anal. 2020, 12, 1620–1628. [Google Scholar] [CrossRef]
- Omouessi, S.T.; Leipprandt, J.R.; Akoume, M.Y.; Charbeneau, R.; Wade, S.; Neubig, R.R. Mice with an RGS-insensitive Gα. Mol. Cell Endocrinol. 2021, 521, 111098. [Google Scholar] [CrossRef]
- Oberhaus, E.L.; Thompson, D.L.; Kerrigan, L.E.; Chapman, A.M. Plasma prolactin, thyroid-stimulating hormone, melanocyte-stimulating hormone, and adrenocorticotropin responses to thyrotropin-releasing hormone in mares treated with detomidine and butorphanol. Domest. Anim. Endocrinol. 2021, 74, 106536. [Google Scholar] [CrossRef]
- Yip, S.H.; Araujo-Lopes, R.; Szawka, R.E.; York, J.; Hyland, B.; Grattan, D.R.; Bunn, S.J. Morphological plasticity of the tuberoinfundibular dopaminergic neurones in the rat during the oestrous cycle and lactation. J. Neuroendocr. 2020, 32, e12884. [Google Scholar] [CrossRef]
- Caja, G.; Elhadi, A.; Such, X.; Salama, A.A.K. Suppression of prolactin and reduction of milk secretion by effect of cabergoline in lactating dairy ewes. J. Dairy Sci. 2020, 103, 12033–12044. [Google Scholar] [CrossRef]
- Gutierrez, C.M.; Lopez-Valdez, R.; Subramani, R.; Arumugam, A.; Nandy, S.; Rajamanickam, V.; Ravichandran, V.; Lakshmanaswamy, R. A Breast Tissue Protein Expression Profile Contributing to Early Parity-Induced Protection Against Breast Cancer. Cell Physiol. Biochem. 2015, 37, 1671–1685. [Google Scholar] [CrossRef]
- Bracamontes, C.G.; Lopez-Valdez, R.; Subramani, R.; Arumugam, A.; Nandy, S.; Rajamanickam, V.; Ravichandran, V.; Lakshmanaswamy, R. The serum protein profile of early parity which induces protection against breast cancer. Oncotarget 2016, 7, 82538–82553. [Google Scholar] [CrossRef] [Green Version]
- Baudier, F. Nutrition of French adolescents. Current views. Soins Gynecol. Obs. Pueric. Pediatr. 1990, 111–112, 50–57. [Google Scholar]
- Xu, J.; Zhang, Y.; Berry, P.A.; Jiang, J.; Lobie, P.E.; Langenheim, J.F.; Chen, W.Y.; Frank, S.J. Growth hormone signaling in human T47D breast cancer cells: Potential role for a growth hormone receptor-prolactin receptor complex. Mol. Endocrinol. 2011, 25, 597–610. [Google Scholar] [CrossRef] [Green Version]
- Arumugam, A.; Subramani, R.; Nandy, S.B.; Terreros, D.; Dwivedi, A.K.; Saltzstein, E.; Lakshmanaswamy, R. Silencing growth hormone receptor inhibits estrogen receptor negative breast cancer through ATP-binding cassette sub-family G member 2. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Emerman, J.T.; Leahy, M.; Gout, P.W.; Bruchovsky, N. Elevated growth hormone levels in sera from breast cancer patients. Horm. Metab. Res. 1985, 17, 421–424. [Google Scholar] [CrossRef]
- Jenkins, P.J.; Mukherjee, A.; Shalet, S.M. Does growth hormone cause cancer? Clin. Endocrinol. 2006, 64, 115–121. [Google Scholar] [CrossRef]
- Shevah, O.; Laron, Z. Patients with congenital deficiency of IGF-I seem protected from the development of malignancies: A preliminary report. Growth Horm. IGF Res. 2007, 17, 54–57. [Google Scholar] [CrossRef]
- Swanson, S.M.; Unterman, T.G. The growth hormone-deficient Spontaneous Dwarf rat is resistant to chemically induced mammary carcinogenesis. Carcinogenesis 2002, 23, 977–982. [Google Scholar] [CrossRef] [Green Version]
- Thordarson, G.; Semaan, S.; Low, C.; Ochoa, D.; Leong, H.; Rajkumar, L.; Guzman, R.C.; Nandi, S.; Talamantes, F. Mammary tumorigenesis in growth hormone deficient spontaneous dwarf rats; effects of hormonal treatments. Breast Cancer Res. Treat. 2004, 87, 277–290. [Google Scholar] [CrossRef]
- Lantvit, D.D.; Unterberger, C.J.; Lazar, M.; Arneson, P.D.; Longhurst, C.A.; Swanson, S.M.; Marker, P.C. Mammary Tumors Growing in the Absence of Growth Hormone Are More Sensitive to Doxorubicin Than Wild-Type Tumors. Endocrinology 2021, 162, bqab013. [Google Scholar] [CrossRef]
- Chen, X.; Wu, D.; Zheng, Y.; Liu, X.; Wang, J. Preparation of a Growth Hormone Receptor/Prolactin Receptor Bispecific Antibody Antagonist Which Exhibited Anti-Cancer Activity. Front. Pharm. 2020, 11, 598423. [Google Scholar] [CrossRef]
- Dearth, R.K.; Delgado, D.A.; Hiney, J.K.; Pathiraja, T.; Oesterreich, S.; Medina, D.; Dees, W.L.; Lee, A.V. Parity-induced decrease in systemic growth hormone alters mammary gland signaling: A potential role in pregnancy protection from breast cancer. Cancer Prev. Res. 2010, 3, 312–321. [Google Scholar] [CrossRef] [Green Version]
- Tworoger, S.S.; Hankinson, S.E. Prolactin and breast cancer etiology: An epidemiologic perspective. J. Mammary Gland Biol. Neoplasia 2008, 13, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Swanson, S.M.; Guzman, R.C.; Collins, G.; Tafoya, P.; Thordarson, G.; Talamantes, F.; Nandi, S. Refractoriness to mammary carcinogenesis in the parous mouse is reversible by hormonal stimulation induced by pituitary isografts. Cancer Lett. 1995, 90, 171–181. [Google Scholar] [CrossRef]
- LaPensee, E.W.; Schwemberger, S.J.; LaPensee, C.R.; Bahassi el, M.; Afton, S.E.; Ben-Jonathan, N. Prolactin confers resistance against cisplatin in breast cancer cells by activating glutathione-S-transferase. Carcinogenesis 2009, 30, 1298–1304. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, L.S.; Mayer, I.A. Platinum agents in the treatment of early-stage triple-negative breast cancer: Is it time to change practice? Clin. Adv. Hematol. Oncol. 2014, 12, 654–658. [Google Scholar]
- Plotnikov, A.; Varghese, B.; Tran, T.H.; Liu, C.; Rui, H.; Fuchs, S.Y. Impaired turnover of prolactin receptor contributes to transformation of human breast cells. Cancer Res. 2009, 69, 3165–3172. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| (A): Mammary cancer incidence | ||||
| Group/Timepoint | 18 weeks | 24 weeks | 30 weeks | 36 weeks |
| Control | 30% | 65% | 100% | 100% |
| Parous | 0% | 0% | 10% | 10% |
| (B): Mammary cancer multiplicity | ||||
| Group/Timepoint | 18 weeks | 24 weeks | 30 weeks | 36 weeks |
| Control | 1 tumor/tumor-bearing rat | 2.5 tumors/tumor-bearing rat | 3.2 tumors/tumor-bearing rat | 5.9 tumors/tumor-bearing rat |
| Parous | 0 tumors/tumor-bearing rat | 0 tumors/tumor-bearing rat | 1 tumor/tumor-bearing rat | 1 tumor/tumor-bearing rat |
| (C): Mammary cancer latency | ||||
| Group | Days | |||
| Control | 158 ± 32 | |||
| Parous | 221 ± 25 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subramani, R.; Estrada, A.; Dixon, M.; Parada, M.; Rodriguez, S.; Pedroza, D.A.; Ramirez, M.D.; Clift, A.; Garcia, L.; Lakshmanaswamy, R. Pregnancy Inhibits Mammary Carcinogenesis by Persistently Altering the Hypothalamic–Pituitary Axis. Cancers 2021, 13, 3207. https://doi.org/10.3390/cancers13133207
Subramani R, Estrada A, Dixon M, Parada M, Rodriguez S, Pedroza DA, Ramirez MD, Clift A, Garcia L, Lakshmanaswamy R. Pregnancy Inhibits Mammary Carcinogenesis by Persistently Altering the Hypothalamic–Pituitary Axis. Cancers. 2021; 13(13):3207. https://doi.org/10.3390/cancers13133207
Chicago/Turabian StyleSubramani, Ramadevi, Adriana Estrada, Madeline Dixon, Maria Parada, Sheryl Rodriguez, Diego A. Pedroza, Matthew D. Ramirez, Alexa Clift, Lilia Garcia, and Rajkumar Lakshmanaswamy. 2021. "Pregnancy Inhibits Mammary Carcinogenesis by Persistently Altering the Hypothalamic–Pituitary Axis" Cancers 13, no. 13: 3207. https://doi.org/10.3390/cancers13133207
APA StyleSubramani, R., Estrada, A., Dixon, M., Parada, M., Rodriguez, S., Pedroza, D. A., Ramirez, M. D., Clift, A., Garcia, L., & Lakshmanaswamy, R. (2021). Pregnancy Inhibits Mammary Carcinogenesis by Persistently Altering the Hypothalamic–Pituitary Axis. Cancers, 13(13), 3207. https://doi.org/10.3390/cancers13133207






