Therapeutic Potential of Antibody-Drug Conjugate-Based Therapy in Head and Neck Cancer: A Systematic Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.2.1. Inclusion Criteria
2.2.2. Exclusion Criteria
2.3. Focused PICO Question
2.4. Selection of Studies
2.5. Data Extraction and Method of Analysis
2.6. Risk of Bias Assessment
2.7. Statistical Analysis
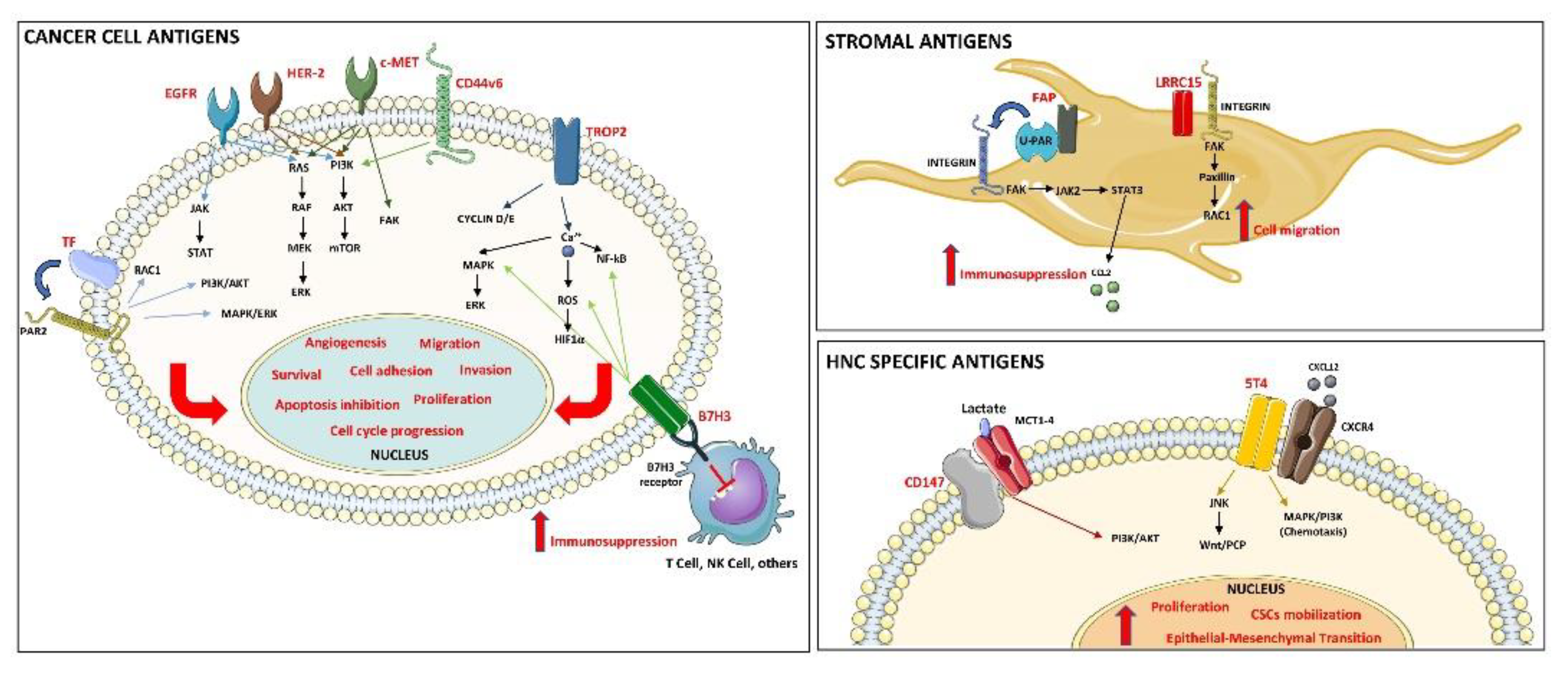
3. Results
3.1. Study Characteristics
3.2. Risk of Bias
3.3. Preclinical Studies
Anderson et al., 2020
Gymnopoulos et al., 2020
Scribner et al., 2020
Ghanemi et al., 2018
Purcell et al., 2018
Theunissen et al., 2018
Wong et al., 2018
Kerk et al., 2017
Strop et al., 2016
Sweeny et al., 2013
Chen et al., 2012
Osterman et al., 2008
Herbert et al., 2003
3.4. Clinical Studies
Cleary et al., 2020
Tsurutani et al., 2020
de Bono et al., 2019
Ocean et al., 2017
Riechelmann et al., 2008
Sauter et al., 2006
3.5. Clinical Trials
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Prim. 2020, 6, 1–22. [Google Scholar] [CrossRef]
- Vigneswaran, N.; Williams, M.D. Epidemiologic Trends in Head and Neck Cancer and Aids in Diagnosis. Oral Maxillofac. Surg. Clin. North Am. 2014, 26, 123–141. [Google Scholar] [CrossRef] [PubMed]
- Svahn, M.F.; Munk, C.; Nielsen, T.S.S.; von Buchwald, C.; Frederiksen, K.; Kjaer, S.K. Trends in all-cause five-year mortality after head and neck cancers diagnosed over a period of 33 years. Focus on estimated degree of association with human papillomavirus. Acta Oncol. 2016, 55, 1084–1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langer, C.J. Targeted therapy in head and neck cancer: State of the art 2007 and review of clinical applications. Cancer 2008, 112, 2635–2645. [Google Scholar] [CrossRef]
- Institute NC Cancer Stat Facts: Oral Cavity and Pharynx Cancer. 2020. Available online: https://seer.cancer.gov/statfacts/html/oralcav.html (accessed on 16 May 2021).
- Bonner, J.A.; Harari, P.M.; Giralt, J.; Azarnia, N.; Shin, D.M.; Cohen, R.B.; Jones, C.U.; Sur, R.; Raben, D.; Jassem, J.; et al. Radiotherapy plus Cetuximab for Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2006, 354, 567–578. [Google Scholar] [CrossRef] [Green Version]
- Vokes, E.E. Induction Chemotherapy for Head and Neck Cancer: Recent Data. Oncologist 2010, 15, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Price, K.A.R.; Cohen, E.E. Current Treatment Options for Metastatic Head and Neck Cancer. Curr. Treat. Options Oncol. 2012, 13, 35–46. [Google Scholar] [CrossRef]
- Choong, N.W.; Cohen, E.E. Epidermal growth factor receptor directed therapy in head and neck cancer. Crit. Rev. Oncol. 2006, 57, 25–43. [Google Scholar] [CrossRef]
- Kim, E.S.; Kies, M.; Herbst, R.S. Novel therapeutics for head and neck cancer. Curr. Opin. Oncol. 2002, 14, 334–342. [Google Scholar] [CrossRef]
- Rajendra, A.; Noronha, V.; Joshi, A.; Patil, V.M.; Menon, N.; Prabhash, K. Palliative chemotherapy in head and neck cancer: Balancing between beneficial and adverse effects. Expert Rev. Anticancer. Ther. 2020, 20, 17–29. [Google Scholar] [CrossRef]
- Xu, G.; McLeod, H.L. Strategies for enzyme/prodrug cancer therapy. Clin. Cancer Res. 2001, 7, 3314–3324. [Google Scholar]
- Vaddepally, R.K.; Kharel, P.; Pandey, R.; Garje, R.; Chandra, A.B. Review of Indications of FDA-Approved Immune Checkpoint Inhibitors per NCCN Guidelines with the Level of Evidence. Cancers 2020, 12, 738. [Google Scholar] [CrossRef] [Green Version]
- Merlano, M.; Occelli, M. Review of cetuximab in the treatment of squamous cell carcinoma of the head and neck. Ther. Clin. Risk Manag. 2007, 3, 871–876. [Google Scholar] [PubMed]
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef]
- Seiwert, T.Y.; Burtness, B.; Mehra, R.; Weiss, J.; Berger, R.; Eder, J.P.; Heath, K.; McClanahan, T.; Lunceford, J.; Gause, C.; et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): An open-label, multicentre, phase 1b trial. Lancet Oncol. 2016, 17, 956–965. [Google Scholar] [CrossRef]
- Kalyankrishna, S.; Grandis, J.R. Epidermal Growth Factor Receptor Biology in Head and Neck Cancer. J. Clin. Oncol. 2006, 24, 2666–2672. [Google Scholar] [CrossRef]
- Okano, S.; Yoshino, T.; Fujii, M.; Onozawa, Y.; Kodaira, T.; Fujii, H.; Akimoto, T.; Ishikura, S.; Oguchi, M.; Zenda, S.; et al. Phase II Study of Cetuximab Plus Concomitant Boost Radiotherapy in Japanese Patients with Locally Advanced Squamous Cell Carcinoma of the Head and Neck. Jpn. J. Clin. Oncol. 2013, 43, 476–482. [Google Scholar] [CrossRef]
- Vermorken, J.B.; Mesia, R.; Rivera, F.; Remenar, E.; Kawecki, A.; Rottey, S.; Erfan, J.; Zabolotnyy, D.; Kienzer, H.-R.; Cupissol, D.; et al. Platinum-Based Chemotherapy plus Cetuximab in Head and Neck Cancer. N. Engl. J. Med. 2008, 359, 1116–1127. [Google Scholar] [CrossRef] [Green Version]
- Yoshino, T.; Hasegawa, Y.; Takahashi, S.; Monden, N.; Homma, A.; Okami, K.; Onozawa, Y.; Fujii, M.; Taguchi, T.; De Blas, B.; et al. Platinum-based Chemotherapy Plus Cetuximab for the First-line Treatment of Japanese Patients with Recurrent and/or Metastatic Squamous Cell Carcinoma of the Head and Neck: Results of a Phase II Trial. Jpn. J. Clin. Oncol. 2013, 43, 524–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braig, F.; Kriegs, M.; Voigtlaender, M.; Habel, B.; Grob, T.; Biskup, K.; Blanchard, V.; Sack, M.; Thalhammer, A.; Ben Batalla, I.; et al. Cetuximab Resistance in Head and Neck Cancer Is Mediated by EGFR-K521 Polymorphism. Cancer Res. 2016, 77, 1188–1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seiwert, T.Y.; Kochanny, S.; Wood, K.; Worden, F.P.; Adkins, D.; Wade, J.L.; Sleckman, B.G.; Anderson, D.; Brisson, R.J.; Karrison, T.; et al. A randomized phase 2 study of temsirolimus and cetuximab versus temsirolimus alone in recurrent/metastatic, cetuximab-resistant head and neck cancer: The MAESTRO study. Cancer 2020, 126, 3237–3243. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, T.L. Head and Neck Carcinoma Immunotherapy: Facts and Hopes. Clin. Cancer Res. 2017, 24, 6–13. [Google Scholar] [CrossRef] [Green Version]
- Petrelli, F.; Coinu, A.; Riboldi, V.; Borgonovo, K.; Ghilardi, M.; Cabiddu, M.; Lonati, V.; Sarti, E.; Barni, S. Concomitant platinum-based chemotherapy or cetuximab with radiotherapy for locally advanced head and neck cancer: A systematic review and meta-analysis of published studies. Oral Oncol. 2014, 50, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.; Huang, J.; Qiao, B.; Lam, A.K.-Y. Immune checkpoint pathways in immunotherapy for head and neck squamous cell carcinoma. Int. J. Oral Sci. 2020, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ang, K.K.; Zhang, Q.; Rosenthal, D.; Nguyen-Tan, P.F.; Sherman, E.J.; Weber, R.S.; Galvin, J.M.; Bonner, J.A.; Harris, J.; El-Naggar, A.K.; et al. Randomized Phase III Trial of Concurrent Accelerated Radiation Plus Cisplatin With or Without Cetuximab for Stage III to IV Head and Neck Carcinoma: RTOG 0522. J. Clin. Oncol. 2014, 32, 2940–2950. [Google Scholar] [CrossRef]
- Dos Santos, L.V.; Abrahão, C.M.; William, W.N.J. Overcoming Resistance to Immune Checkpoint Inhibitors in Head and Neck Squamous Cell Carcinomas. Front. Oncol. 2021, 11, 105. [Google Scholar] [CrossRef]
- Birrer, M.J.; Moore, K.N.; Betella, I.; Bates, R.C. Antibody-Drug Conjugate-Based Therapeutics: State of the Science. J. Natl. Cancer Inst. 2019, 111, 538–549. [Google Scholar] [CrossRef]
- Coats, S.; Williams, M.; Kebble, B.; Dixit, R.; Tseng, L.; Yao, N.-S.; Tice, D.A.; Soria, J.-C. Antibody–Drug Conjugates: Future Directions in Clinical and Translational Strategies to Improve the Therapeutic Index. Clin. Cancer Res. 2019, 25, 5441–5448. [Google Scholar] [CrossRef] [Green Version]
- Wolska-Washer, A.; Robak, T. Safety and Tolerability of Antibody-Drug Conjugates in Cancer. Drug Saf. 2019, 42, 295–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haeuw, J.-F.; Caussanel, V.; Beck, A. Les immunoconjugués, anticorps «armés» pour combattre le cancer. Médecine/Sciences 2009, 25, 1046–1052. [Google Scholar] [CrossRef]
- Joubert, N.; Beck, A.; Dumontet, C.; Denevault-Sabourin, C. Antibody–Drug Conjugates: The Last Decade. Pharmaceuticals 2020, 13, 245. [Google Scholar] [CrossRef]
- Hamadani, M.; Radford, J.; Carlo-Stella, C.; Caimi, P.F.; Reid, E.; O’Connor, O.A.; Feingold, J.M.; Ardeshna, K.M.; Townsend, W.; Solh, M.; et al. Final results of a phase 1 study of loncastuximab tesirine in relapsed/refractory B-cell non-Hodgkin lymphoma. Blood 2021, 137, 2634–2645. [Google Scholar] [CrossRef]
- McGavin, J.K.; Spencer, C.M. Gemtuzumab ozogamicin. Drugs 2001, 61, 1317–1322. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Chen, R.; O’Connor, O.A.; Gopal, A.K.; Ramchandren, R.; Goy, A.; Matous, J.V.; Fasanmade, A.A.; Manley, T.J.; Han, T.H. Brentuximab vedotin, an antibody-drug conjugate, in patients with CD30-positive haematologic malignancies and hepatic or renal impairment. Br. J. Clin. Pharmacol. 2016, 82, 696–705. [Google Scholar] [CrossRef] [Green Version]
- Lamb, Y.N. Inotuzumab Ozogamicin: First Global Approval. Drugs 2017, 77, 1603–1610. [Google Scholar] [CrossRef] [PubMed]
- Sehn, L.H.; Herrera, A.F.; Flowers, C.R.; Kamdar, M.K.; McMillan, A.; Hertzberg, M.; Assouline, S.; Kim, T.M.; Kim, W.S.; Ozcan, M.; et al. Polatuzumab Vedotin in Relapsed or Refractory Diffuse Large B-Cell Lymphoma. J. Clin. Oncol. 2020, 38, 155–165. [Google Scholar] [CrossRef]
- Richardson, P.G.; Lee, H.C.; Abdallah, A.-O.; Cohen, A.D.; Kapoor, P.; Voorhees, P.M.; Hoos, A.; Wang, K.; Baron, J.; Piontek, T.; et al. Single-agent belantamab mafodotin for relapsed/refractory multiple myeloma: Analysis of the lyophilised presentation cohort from the pivotal DREAMM-2 study. Blood Cancer J. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Janus, A.; Robak, T. Moxetumomab pasudotox for the treatment of hairy cell leukemia. Expert Opin. Biol. Ther. 2019, 19, 501–508. [Google Scholar] [CrossRef]
- Dhillon, S. Trastuzumab Emtansine: A Review of Its Use in Patients with HER2-Positive Advanced Breast Cancer Previously Treated with Trastuzumab-Based Therapy. Drugs 2014, 74, 675–686. [Google Scholar] [CrossRef]
- Modi, S.; Saura, C.; Yamashita, T.; Park, Y.H.; Kim, S.-B.; Tamura, K.; Andre, F.; Iwata, H.; Ito, Y.; Tsurutani, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N. Engl. J. Med. 2020, 382, 610–621. [Google Scholar] [CrossRef]
- Bardia, A.; Mayer, I.A.; Vahdat, L.T.; Tolaney, S.M.; Isakoff, S.J.; Diamond, J.R.; O’Shaughnessy, J.; Moroose, R.L.; Santin, A.D.; Abramson, V.G.; et al. Sacituzumab Govitecan-hziy in Refractory Metastatic Triple-Negative Breast Cancer. N. Engl. J. Med. 2019, 380, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, J.E.; O’Donnell, P.H.; Balar, A.V.; McGregor, B.A.; Heath, E.I.; Yu, E.Y.; Galsky, M.D.; Hahn, N.M.; Gartner, E.M.; Pinelli, J.M.; et al. Pivotal Trial of Enfortumab Vedotin in Urothelial Carcinoma After Platinum and Anti-Programmed Death 1/Programmed Death Ligand 1 Therapy. J. Clin. Oncol. 2019, 37, 2592–2600. [Google Scholar] [CrossRef]
- Kaplon, H.; Reichert, J.M. Antibodies to watch in 2021. mAbs 2021, 13, 1860476. [Google Scholar] [CrossRef] [PubMed]
- Forrest, J.L.; Miller, S. Evidence-based decision making in dental hygiene education, practice, and research. J. Dent. Hyg. 2001, 75, 50–63. [Google Scholar] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef] [Green Version]
- Hooijmans, C.R.; Rovers, M.M.; De Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [Green Version]
- Litière, S.; Isaac, G.; de Vries, E.; Bogaerts, J.; Chen, A.; Dancey, J.; Ford, R.; Gwyther, S.; Hoekstra, O.; Huang, E.; et al. RECIST 1.1 for Response Evaluation Apply Not Only to Chemotherapy-Treated Patients But Also to Targeted Cancer Agents: A Pooled Database Analysis. J. Clin. Oncol. 2019, 37, 1102–1110. [Google Scholar] [CrossRef]
- Margulis, A.V.; Pladevall, M.; Riera-Guardia, N.; Varas-Lorenzo, C.; Hazell, L.; Berkman, N.; Viswanathan, M.; Perez-Gutthann, S. Quality assessment of observational studies in a drug-safety systematic review, comparison of two tools: The Newcastle–Ottawa Scale and the RTI item bank. Clin. Epidemiol. 2014, 6, 359–368. [Google Scholar] [CrossRef] [Green Version]
- Gagnier, J.J.; Moher, D.; Boon, H.; Beyene, J.; Bombardier, C. Investigating clinical heterogeneity in systematic reviews: A methodologic review of guidance in the literature. BMC Med Res. Methodol. 2012, 12, 111. [Google Scholar] [CrossRef] [Green Version]
- Anderson, M.G.; Falls, H.D.; Mitten, M.J.; Oleksijew, A.; Vaidya, K.S.; Boghaert, E.R.; Gao, W.; Palma, J.P.; Cao, D.; Chia, P.-L.; et al. Targeting Multiple EGFR-expressing Tumors with a Highly Potent Tumor-selective Antibody–Drug Conjugate. Mol. Cancer Ther. 2020, 19, 2117–2125. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zhang, D.; Mao, Y.; Zhu, J.; Ming, H.; Wen, J.; Ma, J.; Cao, Q.; Lin, H.; Tang, Q.; et al. A Human Fab-Based Immunoconjugate Specific for the LMP1 Extracellular Domain Inhibits Nasopharyngeal Carcinoma Growth In Vitro and In Vivo. Mol. Cancer Ther. 2012, 11, 594–603. [Google Scholar] [CrossRef] [Green Version]
- Cleary, J.M.; Calvo, E.; Moreno, V.; Juric, D.; Shapiro, G.I.; Vanderwal, C.A.; Hu, B.; Gifford, M.; Barch, D.; Roberts-Rapp, L.; et al. A phase 1 study evaluating safety and pharmacokinetics of losatuxizumab vedotin (ABBV-221), an anti-EGFR antibody-drug conjugate carrying monomethyl auristatin E, in patients with solid tumors likely to overexpress EGFR. Investig. New Drugs 2020, 38, 1483–1494. [Google Scholar] [CrossRef] [PubMed]
- De Bono, J.S.; Concin, N.; Hong, D.S.; Thistlethwaite, F.C.; Machiels, J.-P.; Arkenau, H.-T.; Plummer, R.; Jones, R.H.; Nielsen, D.; Windfeld, K.; et al. Tisotumab vedotin in patients with advanced or metastatic solid tumours (InnovaTV 201): A first-in-human, multicentre, phase 1–2 trial. Lancet Oncol. 2019, 20, 383–393. [Google Scholar] [CrossRef]
- Ghanemi, M.; Pourshohod, A.; Ghaffari, M.A.; Kheirollah, A.; Amin, M.; Zeinali, M.; Jamalan, M. Specific Targeting of HER2-Positive Head and Neck Squamous Cell Carcinoma Line HN5 by Idarubicin-ZHER2 Affibody Conjugate. Curr. Cancer Drug Targets 2018, 19, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Gymnopoulos, M.; Betancourt, O.; Blot, V.; Fujita, R.; Galvan, D.; Lieuw, V.; Nguyen, S.; Snedden, J.; Stewart, C.; Villicana, J.; et al. TR1801-ADC: A highly potent cMet antibody–drug conjugate with high activity in patient-derived xenograft models of solid tumors. Mol. Oncol. 2019, 14, 54–68. [Google Scholar] [CrossRef] [PubMed]
- Hebert, C.; Norris, K.; Sauk, J. Targeting of Human Squamous Carcinomas by SPA470-doxorubicin Immunoconjugates. J. Drug Target. 2003, 11, 101–107. [Google Scholar] [CrossRef]
- Kerk, S.A.; Finkel, K.A.; Pearson, A.; Warner, K.A.; Zhang, Z.; Nör, F.; Wagner, V.; Vargas, P.A.; Wicha, M.S.; Hurt, E.M.; et al. 5T4-Targeted Therapy Ablates Cancer Stem Cells and Prevents Recurrence of Head and Neck Squamous Cell Carcinoma. Clin. Cancer Res. 2017, 23, 2516–2527. [Google Scholar] [CrossRef] [Green Version]
- Ocean, A.J.; Starodub, A.N.; Bardia, A.; Vahdat, L.T.; Isakoff, S.J.; Guarino, M.; Messersmith, W.A.; Picozzi, V.J.; Mayer, I.A.; Wegener, W.A.; et al. Sacituzumab govitecan (IMMU-132), an anti-Trop-2-SN-38 antibody-drug conjugate for the treatment of diverse epithelial cancers: Safety and pharmacokinetics. Cancer 2017, 123, 3843–3854. [Google Scholar] [CrossRef] [Green Version]
- Ostermann, E.; Garin-Chesa, P.; Heider, K.H.; Kalat, M.; Lamche, H.; Puri, C.; Kerjaschki, D.; Rettig, W.J.; Adolf, G.R. Effective Immunoconjugate Therapy in Cancer Models Targeting a Serine Protease of Tumor Fibroblasts. Clin. Cancer Res. 2008, 14, 4584–4592. [Google Scholar] [CrossRef] [Green Version]
- Purcell, J.; Tanlimco, S.G.; Hickson, J.A.; Fox, M.; Sho, M.; Durkin, L.; Uziel, T.; Powers, R.; Foster, K.; McGonigal, T.; et al. LRRC15 Is a Novel Mesenchymal Protein and Stromal Target for Antibody–Drug Conjugates. Cancer Res. 2018, 78, 4059–4072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riechelmann, H.; Sauter, A.; Golze, W.; Hanft, G.; Schroen, C.; Hoermann, K.; Erhardt, T.; Gronau, S. Phase I trial with the CD44v6-targeting immunoconjugate bivatuzumab mertansine in head and neck squamous cell carcinoma. Oral Oncol. 2008, 44, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Sauter, A.; Kloft, C.; Gronau, S.; Bogeschdorfer, F.; Erhardt, T.; Golze, W.; Schroen, C.; Staab, A.; Riechelmann, H.; Hoermann, K. Pharmacokinetics, immunogenicity and safety of bivatuzumab mertansine, a novel CD44v6-targeting immunoconjugate, in patients with squamous cell carcinoma of the head and neck. Int. J. Oncol. 2007, 30, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Scribner, J.A.; Brown, J.G.; Son, T.; Chiechi, M.; Li, P.; Sharma, S.; Li, H.; De Costa, A.; Li, Y.; Chen, Y.; et al. Preclinical Development of MGC018, a Duocarmycin-based Antibody–drug Conjugate Targeting B7-H3 for Solid Cancer. Mol. Cancer Ther. 2020, 19, 2235–2244. [Google Scholar] [CrossRef]
- Strop, P.; Tran, T.-T.; Dorywalska, M.; Delaria, K.; Dushin, R.; Wong, O.K.; Thomas-Toan, T.; Zhou, D.; Wu, A.; Kraynov, E.; et al. RN927C, a Site-Specific Trop-2 Antibody–Drug Conjugate (ADC) with Enhanced Stability, Is Highly Efficacious in Preclinical Solid Tumor Models. Mol. Cancer Ther. 2016, 15, 2698–2708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sweeny, L.; Hartman, Y.E.; Zinn, K.R.; Prudent, J.R.; Marshall, D.J.; Shekhani, M.S.; Rosenthal, E.L. A novel extracellular drug conjugate significantly inhibits head and neck squamous cell carcinoma. Oral Oncol. 2013, 49, 991–997. [Google Scholar] [CrossRef] [Green Version]
- Theunissen, J.-W.; Cai, A.G.; Bhatti, M.M.; Cooper, A.B.; Avery, A.D.; Dorfman, R.; Guelman, S.; Levashova, Z.; Migone, T.-S. Treating Tissue Factor–Positive Cancers with Antibody–Drug Conjugates That Do Not Affect Blood Clotting. Mol. Cancer Ther. 2018, 17, 2412–2426. [Google Scholar] [CrossRef] [Green Version]
- Tsurutani, J.; Iwata, H.; Krop, I.; Jänne, P.A.; Doi, T.; Takahashi, S.; Park, H.; Redfern, C.; Tamura, K.; Wise-Draper, T.M.; et al. Targeting HER2 with Trastuzumab Deruxtecan: A Dose-Expansion, Phase I Study in Multiple Advanced Solid Tumors. Cancer Discov. 2020, 10, 688–701. [Google Scholar] [CrossRef] [Green Version]
- Wong, O.K.; Tran, T.-T.; Ho, W.-H.; Casas, M.G.; Au, M.; Bateman, M.; Lindquist, K.C.; Rajpal, A.; Shelton, D.L.; Strop, P.; et al. RN765C, a low affinity EGFR antibody drug conjugate with potent anti-tumor activity in preclinical solid tumor models. Oncotarget 2018, 9, 33446–33458. [Google Scholar] [CrossRef]
- Bachran, C.; Abdelazim, S.; Fattah, R.J.; Liu, S.; Leppla, S.H. Recombinant expression and purification of a tumor-targeted toxin in Bacillus anthracis. Biochem. Biophys. Res. Commun. 2013, 430, 150–155. [Google Scholar] [CrossRef] [Green Version]
- Benedetto, R.; Massicano, A.V.F.; Silva, J.J.; Boas, C.A.W.V.; Mengatti, J.; De Araujo, E.B. Development of radioimmunoconjugate for diagnosis and management of head-and-neck subclinical cancer and colorectal carcinoma. Braz. J. Pharm. Sci. 2018, 53. [Google Scholar] [CrossRef] [Green Version]
- Bera, T.K.; Liu, W.; Leshem, Y.; King, E.; Kozlov, S.; Pastan, I. Generation of a Transgenic BALB/c Mouse Line With Selective Expression of Human Mesothelin in Thyroid Gland: Application in Mesothelin-targeted Immunotherapy. J. Immunother. 2019, 42, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Challita-Eid, P.M.; Satpayev, D.; Yang, P.; An, Z.; Morrison, K.; Shostak, Y.; Raitano, A.; Nadell, R.; Liu, W.; Lortie, D.R.; et al. Enfortumab Vedotin Antibody-Drug Conjugate Targeting Nectin-4 Is a Highly Potent Therapeutic Agent in Multiple Preclinical Cancer Models. Cancer Res. 2016, 76, 3003–3013. [Google Scholar] [CrossRef] [Green Version]
- El-Sayed, I.H.; Huang, X.; El-Sayed, M.A. Selective laser photo-thermal therapy of epithelial carcinoma using anti-EGFR antibody conjugated gold nanoparticles. Cancer Lett. 2006, 239, 129–135. [Google Scholar] [CrossRef]
- Jang, S.; Yu, X.-M.; Montemayor-Garcia, C.; Ahmed, K.; Weinlander, E.; Lloyd, R.V.; Dammalapati, A.; Marshall, D.; Prudent, J.R.; Chen, H. Dysadherin specific drug conjugates for the treatment of thyroid cancers with aggressive phenotypes. Oncotarget 2017, 8, 24457–24468. [Google Scholar] [CrossRef] [Green Version]
- Kawakami, K.; Kawakami, M.; Puri, R.K. Nitric Oxide Accelerates Interleukin-13 Cytotoxin-Mediated Regression in Head and Neck Cancer Animal Model. Clin. Cancer Res. 2004, 10, 5264–5270. [Google Scholar] [CrossRef] [Green Version]
- Lamberts, L.E.; De Groot, D.J.A.; Bense, R.D.; De Vries, E.G.; Fehrmann, R.S. Functional Genomic mRNA Profiling of a large cancer data base demonstrates mesothelin overexpression in a broad range of tumor types. Oncotarget 2015, 6, 28164–28172. [Google Scholar] [CrossRef] [Green Version]
- Maeda, H.; Tominaga, K.; Iwanaga, K.; Nagao, F.; Habu, M.; Tsujisawa, T.; Seta, Y.; Toyoshima, K.; Fukuda, J.-I.; Nishihara, T. Targeted drug delivery system for oral cancer therapy using sonoporation. J. Oral Pathol. Med. 2009, 38, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Zhao, Y.; Li, F.; Li, Z.; Tian, S.; Debinski, W.; Ming, X. P-glycoprotein targeted and near-infrared light-guided depletion of chemoresistant tumors. J. Control Release 2018, 286, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Munasinghe, W.P.; Mittapalli, R.K.; Li, H.; Hoffman, D.M.; Holen, K.D.; Menon, R.M.; Xiong, H. Evaluation of the effect of the EGFR antibody-drug conjugate ABT-414 on QT interval prolongation in patients with advanced solid tumors likely to over-express EGFR. Cancer Chemother. Pharmacol. 2017, 26, 263–922. [Google Scholar] [CrossRef] [PubMed]
- Nagaya, T.; Nakamura, Y.; Okuyama, S.; Ogata, F.; Maruoka, Y.; Choyke, P.L.; Allen, C.; Kobayashi, H. Syngeneic Mouse Models of Oral Cancer Are Effectively Targeted by Anti–CD44-Based NIR-PIT. Mol. Cancer Res. 2017, 15, 1667–1677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandstrom, K.; Haylock, A.-K.; Velikyan, I.; Spiegelberg, D.; Kareem, H.; Tolmachev, V.; Lundqvist, H.; Nestor, M. Improved Tumor-to-Organ Ratios of a Novel 67Ga-Human Epidermal Growth Factor Radionuclide Conjugate with Preadministered Antiepidermal Growth Factor Receptor Affibody Molecules. Cancer Biother. Radiopharm. 2011, 26, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Seok, J.Y.; Astvatsaturyan, K.; De Peralta-Venturina, M.; Lai, J.; Fan, X. TROP-2, 5hmC, and IDH1 Expression in Anaplastic Thyroid Carcinoma. Int. J. Surg. Pathol. 2021, 29, 368–377. [Google Scholar] [CrossRef]
- Strome, S.; Kawakami, K.; Alejandro, D.; Voss, S.; Kasperbauer, J.L.; Salomao, D.; Chen, L.; Maki, R.; Puri, R.K. Interleukin 4 receptor-directed cytotoxin therapy for human head and neck squamous cell carcinoma in animal models. Clin. Cancer Res. 2002, 8, 281–286. [Google Scholar] [PubMed]
- Sunavala-Dossabhoy, G. Smart cell-specific protein therapeutics for head and neck cancer. Oral Dis. 2020, 26, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Takei, J.; Kaneko, M.K.; Ohishi, T.; Kawada, M.; Harada, H.; Kato, Y. A novel anti-EGFR monoclonal antibody (EMab-17) exerts antitumor activity against oral squamous cell carcinomas via antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity. Oncol. Lett. 2020, 19, 2809–2816. [Google Scholar] [CrossRef] [Green Version]
- Van Driel, P.B.; Boonstra, M.C.; Slooter, M.D.; Heukers, R.; Stammes, M.A.; Snoeks, T.J.; De Bruijn, H.S.; Van Diest, P.J.; Vahrmeijer, A.L.; van Bergen en Henegouwen, P.M.; et al. EGFR targeted nanobody–photosensitizer conjugates for photodynamic therapy in a pre-clinical model of head and neck cancer. J. Control Release 2016, 229, 93–105. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, K.; Tominaga, K.; Sukedai, M.; Okinaga, T.; Iwanaga, K.; Nishihara, T.; Fukuda, J.-I. Delivery of cytolethal distending toxin B induces cell cycle arrest and apoptosis in gingival squamous cell carcinoma in vitro. Eur. J. Oral Sci. 2004, 112, 445–451. [Google Scholar] [CrossRef]
- Zhang, D.; Mao, Y.; Xiong, L.; Cao, Q.; Zhu, J.; Chen, R. Characterization of human Fab antibody fragments specific to LMP1 (HLEAFab) in nasopharyngeal carcinoma for potential molecular diagnosis and therapeutic applications. Oncol. Lett. 2013, 5, 1694–1698. [Google Scholar] [CrossRef]
- Zhang, Y.; Cong, L.; He, J.; Wang, Y.; Zou, Y.; Yang, Z.; Hu, Y.; Zhang, S.; He, X. Photothermal treatment with EGFRmAb–AuNPs induces apoptosis in hypopharyngeal carcinoma cells via PI3K/AKT/mTOR and DNA damage response pathways. Acta Biochim. Biophys. Sin. 2018, 50, 567–578. [Google Scholar] [CrossRef] [Green Version]
- Ang, K.K.; Berkey, B.; Tu, X.; Zhang, H.-Z.; Katz, R.; Hammond, E.H.; Fu, K.K.; Milas, L. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 2002, 62, 7350–7356. [Google Scholar]
- Morris, L.G.T.; Taylor, B.S.; Bivona, T.G.; Gong, Y.; Eng, S.; Brennan, C.W.; Kaufman, A.; Kastenhuber, E.R.; Banuchi, V.; Singh, B.; et al. Genomic dissection of the epidermal growth factor receptor (EGFR)/PI3K pathway reveals frequent deletion of the EGFR phosphatase PTPRS in head and neck cancers. Proc. Natl. Acad. Sci. USA 2011, 108, 19024–19029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seiwert, T.Y.; Jagadeeswaran, R.; Faoro, L.; Janamanchi, V.; Nallasura, V.; El Dinali, M.; Yala, S.; Kanteti, R.; Cohen, E.; Lingen, M.W.; et al. The MET Receptor Tyrosine Kinase Is a Potential Novel Therapeutic Target for Head and Neck Squamous Cell Carcinoma. Cancer Res. 2009, 69, 3021–3031. [Google Scholar] [CrossRef] [Green Version]
- Elferink, L.A.; Resto, V.A. Receptor-Tyrosine-Kinase-Targeted Therapies for Head and Neck Cancer. J. Signal Transduct. 2011, 2011, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Tiberghien, A.C.; Levy, J.-N.; Masterson, L.A.; Patel, N.V.; Adams, L.R.; Corbett, S.; Williams, D.G.; Hartley, J.A.; Howard, P.W. Design and Synthesis of Tesirine, a Clinical Antibody–Drug Conjugate Pyrrolobenzodiazepine Dimer Payload. ACS Med. Chem. Lett. 2016, 7, 983–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucas, A.T.; Price, L.S.L.; Schorzman, A.N.; Storrie, M.; Piscitelli, J.A.; Razo, J.; Zamboni, W.C. Factors Affecting the Pharmacology of Antibody–Drug Conjugates. Antibodies 2018, 7, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakada, T.; Sugihara, K.; Jikoh, T.; Abe, Y.; Agatsuma, T. The Latest Research and Development into the Antibody–Drug Conjugate, [fam-] Trastuzumab Deruxtecan (DS-8201a), for HER2 Cancer Therapy. Chem. Pharm. Bull. 2019, 67, 173–185. [Google Scholar] [CrossRef] [Green Version]
- Katayama, A.; Takahara, M.; Kishibe, K.; Nagato, T.; Kunibe, I.; Katada, A.; Hayashi, T.; Harabuchi, Y. Expression of B7-H3 in hypopharyngeal squamous cell carcinoma as a predictive indicator for tumor metastasis and prognosis. Int. J. Oncol. 2011, 38, 1219–1226. [Google Scholar] [CrossRef] [Green Version]
- Sieviläinen, M.; Wirsing, A.M.; Hyytiäinen, A.; Almahmoudi, R.; Rodrigues, P.; Bjerkli, I.-H.; Åström, P.; Toppila-Salmi, S.; Paavonen, T.; Coletta, R.D.; et al. Evaluation Challenges in the Validation of B7-H3 as Oral Tongue Cancer Prognosticator. Head Neck Pathol. 2021, 15, 469–478. [Google Scholar] [CrossRef]
- Christensen, A.; Kiss, K.; Lelkaitis, G.; Juhl, K.; Persson, M.; Charabi, B.W.; Mortensen, J.; Forman, J.L.; Sørensen, A.L.; Jensen, D.H.; et al. Urokinase-type plasminogen activator receptor (uPAR), tissue factor (TF) and epidermal growth factor receptor (EGFR): Tumor expression patterns and prognostic value in oral cancer. BMC Cancer 2017, 17, 572. [Google Scholar] [CrossRef] [Green Version]
- Damelin, M.; Geles, K.G.; Follettie, M.T.; Yuan, P.; Baxter, M.; Golas, J.; DiJoseph, J.F.; Karnoub, M.; Huang, S.; Diesl, V.; et al. Delineation of a Cellular Hierarchy in Lung Cancer Reveals an Oncofetal Antigen Expressed on Tumor-Initiating Cells. Cancer Res. 2011, 71, 4236–4246. [Google Scholar] [CrossRef] [Green Version]
- Heller, M.; Von Der Ohe, M.; Kleene, R.; Mohajeri, M.H.; Schachner, M. The immunoglobulin-superfamily molecule basigin is a binding protein for oligomannosidic carbohydrates: An anti-idiotypic approach. J. Neurochem. 2003, 84, 557–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, A.; Xiong, H.; Wang, W.; Hu, X.; Wang, C.; Mao, T.; Yang, L.; Huang, D.; Xia, K.; Su, T. CD147 promotes proliferation and migration of oral cancer cells by inhibiting junctions between E-cadherin and β-catenin. J. Oral Pathol. Med. 2020, 49, 1019–1029. [Google Scholar] [CrossRef]
- Ma, C.; Wang, J.; Fan, L.; Guo, Y. Inhibition of CD147 expression promotes chemosensitivity in HNSCC cells by deactivating MAPK/ERK signaling pathway. Exp. Mol. Pathol. 2017, 102, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Linz, C.; Brands, R.C.; Kertels, O.; Dierks, A.; Brumberg, J.; Gerhard-Hartmann, E.; Hartmann, S.; Schirbel, A.; Serfling, S.; Zhi, Y.; et al. Targeting fibroblast activation protein in newly diagnosed squamous cell carcinoma of the oral cavity—Initial experience and comparison to [18F]FDG PET/CT and MRI. Eur. J. Nucl. Med. Mol. Imaging 2021. [Google Scholar] [CrossRef]
- Ollins, G.J.; Nikitakis, N.; Norris, K.; Herbert, C.; Siavash, H.; Sauk, J.J. The production of the endostatin precursor collagen XVIII in head and neck carcinomas is modulated by CBP2/Hsp47. Anticancer Res. 2002, 22, 1977–1982. [Google Scholar]
- Chen, J.; Zhou, J.; Lu, J.; Xiong, H.; Shi, X.; Gong, L. Significance of CD44 expression in head and neck cancer: A systemic review and meta-analysis. BMC Cancer 2014, 14, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Argiris, A.; Karamouzis, M.V.; Raben, D.; Ferris, R.L. Head and neck cancer. Lancet 2008, 371, 1695–1709. [Google Scholar] [CrossRef]
- Al Qaraghuli, M.M. Biotherapeutic Antibodies for the Treatment of Head and Neck Cancer: Current Approaches and Future Considerations of Photothermal Therapies. Front. Oncol. 2020, 10, 2710. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015, 517, 576–582. [Google Scholar] [CrossRef] [Green Version]
- Barak, V.; Meirovitz, A.; Leibovici, V.; Rachmut, J.; Peretz, T.; Eliashar, R.; Gross, M. The Diagnostic and Prognostic Value of Tumor Markers (CEA, SCC, CYFRA 21-1, TPS) in Head and Neck Cancer Patients. Anticancer. Res. 2015, 35, 5519–5524. [Google Scholar]
- Rabassa, M.E.; Croce, M.V.; Pereyra, A.; Segal-Eiras, A. MUC1 expression and anti-MUC1 serum immune response in head and neck squamous cell carcinoma (HNSCC): A multivariate analysis. BMC Cancer 2006, 6, 253. [Google Scholar] [CrossRef] [Green Version]
- Stransky, N.; Egloff, A.M.; Tward, A.D.; Kostic, A.; Cibulskis, K.; Sivachenko, A.; Kryukov, G.; Lawrence, M.S.; Sougnez, C.; McKenna, A.; et al. The Mutational Landscape of Head and Neck Squamous Cell Carcinoma. Science 2011, 333, 1157–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Witzleben, A.; Wang, C.; Laban, S.; Savelyeva, N.; Ottensmeier, C.H. HNSCC: Tumour Antigens and Their Targeting by Immunotherapy. Cells 2020, 9, 2103. [Google Scholar] [CrossRef]
- Micaily, I.; Johnson, J.; Argiris, A. An update on angiogenesis targeting in head and neck squamous cell carcinoma. Cancers Head Neck 2020, 5, 5–7. [Google Scholar] [CrossRef]
- Day, C.-P.; Merlino, G.; Van Dyke, T. Preclinical Mouse Cancer Models: A Maze of Opportunities and Challenges. Cell 2015, 163, 39–53. [Google Scholar] [CrossRef] [Green Version]
- Gillenwater, A.M.; Cognetti, D.; Johnson, J.M.; Curry, J.; Kochuparambil, S.T.; McDonald, D.; Fidler, M.J.; Stenson, K.; Vasan, N.; Razaq, M.; et al. RM-1929 photo-immunotherapy in patients with recurrent head and neck cancer: Results of a multicenter phase 2a open-label clinical trial. J. Clin. Oncol. 2018, 36, 6039. [Google Scholar] [CrossRef]
- Cognetti, D.M.; Johnson, J.M.; Curry, J.M.; Mott, F.; Kochuparambil, S.T.; McDonald, D.; Fidler, M.J.; Stenson, K.; Vasan, N.R.; Razaq, M.; et al. Results of a phase 2a, multicenter, open-label, study of RM-1929 photoimmunotherapy (PIT) in patients with locoregional, recurrent head and neck squamous cell carcinoma (rHNSCC). J. Clin. Oncol. 2019, 37, 6014. [Google Scholar] [CrossRef]
- Kobayashi, H.; Furusawa, A.; Rosenberg, A.; Choyke, P.L. Near-infrared photoimmunotherapy of cancer: A new approach that kills cancer cells and enhances anti-cancer host immunity. Int. Immunol. 2021, 33, 7–15. [Google Scholar] [CrossRef] [PubMed]

| Study | Reasons for Exclusion |
|---|---|
| Seok et al., 2020 [83] | Anaplastic thyroid carcinoma |
| Sunavala-Dossabhoy et al., 2020 [85] | Invited commentary |
| Takei et al., 2020 [86] | mAb |
| Bera et al., 2019 [72] | Thyroid peroxidase (TPO)-mesothelin (MSLN) mouse model development and antitumor efficacy of LMB-100 (hMSLN-targeted immunotoxin) and anti-CTLA-4 (cytotoxic T-lymphocyte antigen 4) |
| Mao et al., 2018 [79] | Photodynamic therapy |
| Zhang et al., 2018 [90] | Near-infrared photoimmunotherapy using gold nanoparticles (AuNPs) conjugated with a mAb targeting the EGFR |
| Benedetto et al., 2017 [71] | 111In-DTPA-cetuximab radioimmunoconjugate preparation |
| Jang et al., 2017 [75] | Study performed on patients with thyroid cancers |
| Munasinghe et al., 2017 [80] | Investigation on specific adverse effect on QT interval prolongation |
| Nagaya et al., 2017 [81] | Near-infrared photoimmunotherapy using anti-CD44 monoclonal antibodies conjugated to the photoabsorber IR700DX |
| Challita-Eid et al., 2016 [73] | Immunohistochemical expression of Nectin-4 |
| vanDriel et al., 2016 [87] | Photodynamic therapy |
| Lamberts et al., 2015 [77] | Expression of membrane-bound glycoprotein mesothelin (MSLN) by functional genomic mRNA profiling in 41 tumor types |
| Bachran et al., 2013 [70] | Cytotoxicity of Bacillus anthracis lethal factor (LFn), N-terminal 389 aminoacids of diphtheria toxin (DT389) and human transforming growth factor alpha (TGFalpha) against EGFR-expressing cell line |
| Zhang et al., 2013 [89] | Immunohistochemical expression of anti-latent membrane protein 1 (LMP1) in the treatment of advanced nasopharyngeal carcinoma (NPC) |
| Sandstrom et al., 2011 [82] | (67Ga)Ga-NOTA-Bn-NCS-hEGF radioimmunoconjugate for the diagnostic imaging of EGFR-expressing tumors |
| Maeda et al., 2009 [78] | Effect of sonoporation and anti-EGFR antibody as a drug-delivery system for treating squamous cell carcinoma |
| El-Sayed et al., 2006 [74] | Photodynamic therapy |
| Kawakami et al., 2004 [76] | Effect of nitric oxide (NO) inhibiter on IL-13-PE38QQR (Pseudomonas exotoxin) cytotoxin-mediated cytotoxicity |
| Yamamoto et al., 2004 [88] | Actinobacillus actinomycetemcomitans cytolethal distending toxin (Cdt-B) |
| Strome et al., 2002 [84] | IL--4R-PE38KDEL (Pseudomonas exotoxin) |
| ADC | Target Antigen | Payload | Linker Type | Tumor Type (s) | Models | Company | References | Notes |
|---|---|---|---|---|---|---|---|---|
| Serclutamab talirine/ABBV-321 | EGFR | PBD dimer SGD-1882 with a fixed DAR of 2.0 | Cathepsin-cleavable maleimidocaproyl-valine-alanine (MC-Val-Ala) type linker | Colorectal cancer, glioblastoma, HNC, lung cancer, malignant mesothelioma | HNC cell lines: FaDu, A253 HNC PDX models: CTG-505, CTG-152, CTG-149, CTG-786, CTG-434 | AbbVie | Anderson 2020 [51] | NCT03234712—https://adc.expert/2MKZSp2 (accessed on May 16th, 2021) |
| TR1801-ADC/MT-8633 | c-Met | PBD toxin-linker tesirine (SG3249) | Cleavable (Val-Ala) | Biliary tract cancer, colon cancer, gastric cancer, HNC, lung cancer | HNC cell lines: Detroit 562, FaDu. Ten HuPrime HNC PDX models, among three specified: HN3533; HN0635; HN0696 | Tanabe Research Laboratories USA in collaboration with Open Innovation Partners and MedImmune/AstraZeneca | Gymnopoulos 2020 [56] | TR1801-ADC in patients with tumors that express c-Met | https://clinicaltrials.gov/ct2/show/NCT03859752 (accessed on May 16th, 2021) |
| MGC018; ANTI-B7-H3 ADC | B7-H3 (CD276) | Synthetic duocarmycin analogs | Cleavable valine-citrulline-seco duocarmycin hydroxy-benzamide azaindole (vc-seco-DUBA) | Breast cancer, HNC, lung cancer, melanoma, ovarian cancer | HNC PDX model: Not specified | MacroGenics, Inc. | Scribner 2020 [64] | |
| Idarubicin-Z HER2:342 | HER 2 | Idarubicin | Cleavable | HNC | HNC cell lines: HN5 | // | Ghanemi 2018 [55] | |
| Samrotamab vedotin/ABBV-085 | LRRC15 | MMAE | Protease cleavable Val-cit | Breast cancer, colorectal cancer, gastric cancer, glioblastoma, HNC, lung cancer, melanoma, osteosarcoma, ovarian cancer, pancreatic cancer, pleomorphic undifferentiated sarcoma, testicular cancer | HNC xenograft models: SCC15 | AbbVie. S.E., AbbVie. E.D. | Purcell 2018 [61] | NCT02565758—https://clinicaltrials.gov/ct2/show/NCT02565758 (accessed on May 16th, 2021) |
| Anti-TF ADCs | TF (CD142) | MMAE | Cleavable | gastric cancer, HNC, ovarian cancer | HNC PDX models: not specified | Iconic Therapeutics, Inc. | Theunissen 2018 [67] | |
| RN765C | EGFR | PF-06380101 (AUR0101) an auristatin microtubule inhibitor (a cytotoxic dolastatin 10 analogue) | AcLys-VC (valine-citruline)-PABC (cleavable linker) | Breast cancer, colorectal cancer, glioblastoma, HNC, lung cancer | HNC cell lines: FADu | Pfizer/Rinat | Wong 2018 [69] | |
| MEDI0641 | 5T4 | PBD | Cleavable (dipeptide) | HNC | HNC cell lines: UM-SCC-11B, UM-SCC-22B, HNC PDX models: PDX-SCC-M0, PDX-SCC-M1, PDX-SCC-M11 | MedImmune LLC | Kerk 2017 [58] | |
| RN927C | Trop-2 | PF-06380101 (AUR0101) an auristatin microtubule inhibitor (a cytotoxic Dolastatin 10 analogue) | Cleavable AcLys-VC-PABC | Breast cancer, colon cancer, HNC, lung cancer, ovarian cancer, pancreatic cancer, skin cancer | HNC cell lines: Fadu | Pfizer/Rinat | Strop 2016 [65] | |
| EDC22 | CD147 | Na/K-ATPase inhibitor | Non cleavable heterobifunctional linker | HNC | HNC cell lines: FaDu, OSC-19, Cal27, SCC-1 HNC xenograft models: SCC-1HNC orthotopic models: OSC-19 | Centrose, LLC | Sweeny 2013 [66] | |
| HLEAFab-MMC | LMP1 | Mytomicin C | Cleavable N-succinimidyl 3-(2-pyridyldithio) propionate (SPDP) | Nasopharyngeal cancer | HNC cell lines: HNE2 and HNE2/LMP1 transfected, HNC xenograft models: HNE2/LMP1 transfected | // | Chen 2012 [52] | |
| FAP5-SPP-DM1, FAP5-SPDB-DM4, FAP5-SMCC-DM1 | FAPα | Maytansinoids DM1/DM4 | Cleavable SPP, Cleavable SPDB, Non cleavable SMCC | Colon cancer, fibrosarcoma, HNC, lung cancer, pancreatic cancer | HNC cell lines: FaDu HNC xenograft model: FaDu | ImmunoGen and Oncotest | Ostermann 2008 [60] | |
| SPA470-doxorubicin | Hsp47/CBP2 | doxorubicin | Cleavable acylhydrazone linker | HNC | HNC cell lines: SCC-4, -9, -15 and -25; UMB2/Hsp47 transfected | // | Herbert 2003 [57] |
| ADC | Target Antigen | Payload | Linker Type | Tumor Type(s) | Phase | Sample Size (Total/HNC) | Stage | Primary Outcomes | Secondary Outcomes | Sponsor/ Collaborator | References | Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Losatuxizumab vedotin/ABBV-221 | EGFR | MMAE | Cleavable (Mc-Val-Cit-PABC) | Breast cancer, colorectal cancer, glioblastoma, HNC, lung cancer, malignant mesothelioma | I | 45/5 | ECOG: 0–2 | Safety (TEAE, MTD, DLT), PK profile | In vivo efficacy (CR, DOR, ORR, OS, PD, PR, SD, TTP), change in ECOG | AbbVie | Cleary 2020 [53] | stopped for high frequency of infusion-related reactions |
| Trastuzumab deruxtecan (DS-8201A, T-Dxd) | HER2 | Camptothecin analog exatecan (DXd; DX-8951 derivative) | Cleavable tetrapeptide linker, Gly-Phe-Leu-Gly (GFLG) | Biliary tract cancer, breast cancer, colorectal cancer, endometrial cancer, lung cancer, salivary glands cancer | I | 60/8 | ECOG: 0–1 | Safety (TEAE), tolerability | In vivo efficacy (CR, DCR, ORR PD, PFS, PR, SD, TTR) | Daiichi Sankyo Inc. | Tsurutani 2020 [68] | NCT03248492—http://adc.expert/2eYaukS (accessed on May, 16th 2021) NCT03734029—https://clinicaltrials.gov/ct2/show/NCT03734029 (accessed on May 16th, 2021) NCT03523585—https://clinicaltrials.gov/ct2/show/NCT03523585 (accessed on May 16th, 2021) NCT03529110—https://clinicaltrials.gov/ct2/show/NCT03529110 (accessed onMay 16th, 2021) |
| Tisotumab vedotin/TF-011-MMAE/HUMAX-TF-ADC | TF (CD142) | MMAE | Cleavable (Val-Cit) | Bladder cancer, cervix cancer, endometrial cancer, HNC, lung cancer, oesophagus cancer, ovaric cancer | I-II | 27/1 | ECOG: 0–1 | Safety (CTCAE) | MTD, PK profile, in vivo efficacy (CR, DCR, DOR, ORR, PFS, PR, SD) | Genmab/Seattle Genetics | de Bono 2019 [54] | |
| Sacituzumab govitecan/IMMU-132/HRS7-SN38 | Trop-2 | Camptothecin analog (SN38) Irinotecan metabolite 7-ethyl-10 hydroxycamptothecin | Cleavable carbonate | Bladder cancer, colorectal cancer, gastrointestinal cancer, HNC, kidney cancer, lung cancer, ovaric cancer, pancreas cancer, prostate cancer | I-II | 178/2 | ECOG: 0–1 | Safety (CTCAE), PK profile | In vivo efficacy | Immunomedics | Ocean 2017 [59] | NCT01631552—https://clinicaltrials.gov/ct2/show/NCT01631552 (accessed on May 16th, 2021) NCT02161679—https://clinicaltrials.gov/ct2/show/NCT02161679 (accessed on May 16th, 2021) |
| Bivatuzumab mertansine/BIWI-1 | CD44v6 | DM1 | Cleavable disulfide | HNC | I | 31/31 | ECOG: 0–2 | Safety (CTC, DLT, MTD,) PK profile | In vivo efficacy (PR, TTP) | Boehringer lngelheim Pharma GmbH | Riechelmann 2008 [62] | |
| Bivatuzumab mertansine/BIWI-1 | CD44v6 | DM1 | Cleavable disulfide | HNC | I | 31/31 | NS | Safety (CTC, DLT, MTD), PK profile, immunogenicity | Boehringer Ingelheim/ImmunoGen | Sauter 2007 [63] |
| ADC | Target | Payload | Linker | Weblink | Trial Identifier/Study Phase | Sponsor | Status |
|---|---|---|---|---|---|---|---|
| ABBV-085 | LRRC15 | MMAE | Non-cleavable | https://clinicaltrials.gov/ct2/show/study/NCT02565758 (accessed on May 16th, 2021) | NCT02565758/I | AbbVie | Completed |
| A166 | HER2 | MMAF derivative | NS | https://clinicaltrials.gov/ct2/show/NCT03602079 (accessed on May 16th, 2021) | NCT03602079/I-II | Klus Pharma Inc. | Recruiting |
| CX-2029 | CD71 | MMAE | Valine-citrulline (VC) peptide | https://clinicaltrials.gov/ct2/show/NCT03543813 (accessed on May 16th, 2021) | NCT03543813/I-II | CytomX Therapeutics | Recruiting |
| CX-2009 | CD71 | MMAE | Valine-citrulline (VC) peptide | https://clinicaltrials.gov/ct2/show/NCT03149549 (accessed on May 16th, 2021) | NCT03149549/I-II | CytomX Therapeutics | Completed |
| SBT6050 | HER2/TLR8 | TLR8 agonist | NS | https://clinicaltrials.gov/ct2/show/NCT04460456 (accessed on May 16th, 2021) | NCT04460456/I | Silverback Therapeutics | Recruiting |
| ABBV-321 | EGFR | PBD | Cathepsin-cleavable maleimidocaproyl-valine-alanine | https://clinicaltrials.gov/ct2/show/study/NCT03234712 (accessed on May 16th, 2021) | NCT03234712/I | AbbVie | Ongoing |
| MGC018 | B7-H3 (CD276) | Synthetic duocarmycin analogs | Cleavable valine-citrulline-seco duocarmycin hydroxy-benzamide azaindole (vc-seco-DUBA) | https://clinicaltrials.gov/ct2/show/NCT03729596 (accessed on May 16th, 2021) | NCT03729596 | MacroGenics | Recruiting |
| MRG003 | EGFR | MMAE | NS | https://clinicaltrials.gov/ct2/show/NCT03729596 (accessed on May 16th, 2021) | NCT04868162 | Shanghai Miracogen Inc. | Recruiting |
| ADC | Target | Payload | Linker | Government Approval | Disease | Developer |
|---|---|---|---|---|---|---|
| Gemtuzumab ozogamicin | CD33 | N-acetyl-γ calicheamicin 1,2-dimethyl hydrazine dichloride | 4-(4-acetylphenoxy)butanoic acid (AcBut linker) | FDA | Relapsed acute myelogenous leukemia | Pfizer/Wyeth |
| Brentuximab vedotin | CD30 | Monomethyl Auristatin E | Thiolreactive maleimidocaproyl spacer, the dipeptide valine–citrulline linker, and a self-immolative, p-amino-benzyloxycarbony (PABC) spacer | FDA | Hodgkin lymphoma and systemic anaplastic large-cell lymphoma | Seattle Genetics, Millennium/Takeda |
| Trastuzumab emtansine | HER2 | Maytansinoid DM1 | Non-reducible tioether linker: N-succinimidyl-4-(N-maleimidomethyl) cyclohexane-1-carboxylate linker (SMCC) | FDA | HER2-positive metastatic breast cancer following treatment with trastuzumab and a maytansinoid | Genentech, Roche |
| Inotuzumab ozogamicin | CD22 | N-acetyl- γ Calicheamicin | Acid-labile(4-(4’-acetylphenoxy) butanoic acid) linker | FDA | Relapsed or refractory CD22-positive B-cell precursor acute lymphoblastic leukemia | Pfizer/Wyeth |
| Polatuzumab vedotin | CD79b | Monomethyl Auristatin E | Protease-cleavable peptide linker : maleimidocaproylvaline-citrulline-p-aminobenzoyloxycarbonyl linker (MC-VC-PABC) | FDA | Relapsed or refractory diffuse large B-cell lymphoma | Genentech, Roche |
| Enfortumab vedotin | Cell Surface Protein Nectin 4 | Monomethyl Auristatin E | Protease-cleavable peptide linker : maleimidocaproylvaline-citrulline-p-aminobenzoyloxycarbonyl linker (MC-VC-PABC) | FDA | Adult patients with locally advanced or metastatic urothelial cancer who have received a PD-1 or PD-L1 inhibitor and a Pt-containing therapy | Astellas/Seattle Genetics |
| Trastuzumab deruxtecan | HER2 | A topoisomerase I inhibitor payload, a derivative of the camptothecin analog exatecan (DXd) | A tetrapeptide linker, Gly-Phe-Leu-Gly (GFLG) | FDA | Adult patients with unresectable or metastatic HER2-positive breast cancer who have received two or more prior anti-HER2 based regimens | AstraZeneca/Daiichi Sankyo |
| Sacituzumab govitecan | TROP-2 | SN-38 (active metabolite of irinotecan) | Hydrolyzable CL2A linker | FDA | Adult patients with metastatic triple-negative breast cancer who have received at least two prior therapies for patients with relapsed or refractory metastatic disease | Immunomedics |
| Belantamab mafodotin | TNFRSF17 | Monomethyl Auristatin F | A non-cleavable maleimidocaproyl (MC) linker | FDA | Multiple myeloma patients whose disease has progressed despite prior treatment with an immunomodulatory agent, proteasome inhibitor and anti-CD38 antibody | GlaxoSmithKline |
| Loncastuximab tesirine | CD19 | SG3199/ Pyrrolobenzodiazepine (PBD) dimer SCX | A cleavable (valine-alanine dipeptide as cathepsin B cleavage site) maleimide type linker containing a PEG spacer | Japan | Relapsed or refractory large B-cell lymphoma (including diffuse large B-cell lymphoma not otherwise specified, arising from low-grade lymphoma, and high-grade B-cell lymphoma) after two or more lines of systemic therapy | ADC Therapeutics |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perrotti, V.; Caponio, V.C.A.; Mascitti, M.; Lo Muzio, L.; Piattelli, A.; Rubini, C.; Capone, E.; Sala, G. Therapeutic Potential of Antibody-Drug Conjugate-Based Therapy in Head and Neck Cancer: A Systematic Review. Cancers 2021, 13, 3126. https://doi.org/10.3390/cancers13133126
Perrotti V, Caponio VCA, Mascitti M, Lo Muzio L, Piattelli A, Rubini C, Capone E, Sala G. Therapeutic Potential of Antibody-Drug Conjugate-Based Therapy in Head and Neck Cancer: A Systematic Review. Cancers. 2021; 13(13):3126. https://doi.org/10.3390/cancers13133126
Chicago/Turabian StylePerrotti, Vittoria, Vito Carlo Alberto Caponio, Marco Mascitti, Lorenzo Lo Muzio, Adriano Piattelli, Corrado Rubini, Emily Capone, and Gianluca Sala. 2021. "Therapeutic Potential of Antibody-Drug Conjugate-Based Therapy in Head and Neck Cancer: A Systematic Review" Cancers 13, no. 13: 3126. https://doi.org/10.3390/cancers13133126
APA StylePerrotti, V., Caponio, V. C. A., Mascitti, M., Lo Muzio, L., Piattelli, A., Rubini, C., Capone, E., & Sala, G. (2021). Therapeutic Potential of Antibody-Drug Conjugate-Based Therapy in Head and Neck Cancer: A Systematic Review. Cancers, 13(13), 3126. https://doi.org/10.3390/cancers13133126









