Contemporary Analysis of Electronic Frailty Measurement in Older Adults with Multiple Myeloma Treated in the National US Veterans Affairs Healthcare System
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source and Population
2.2. Measurement of Frailty and Covariates
2.3. Outcomes
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Study Population
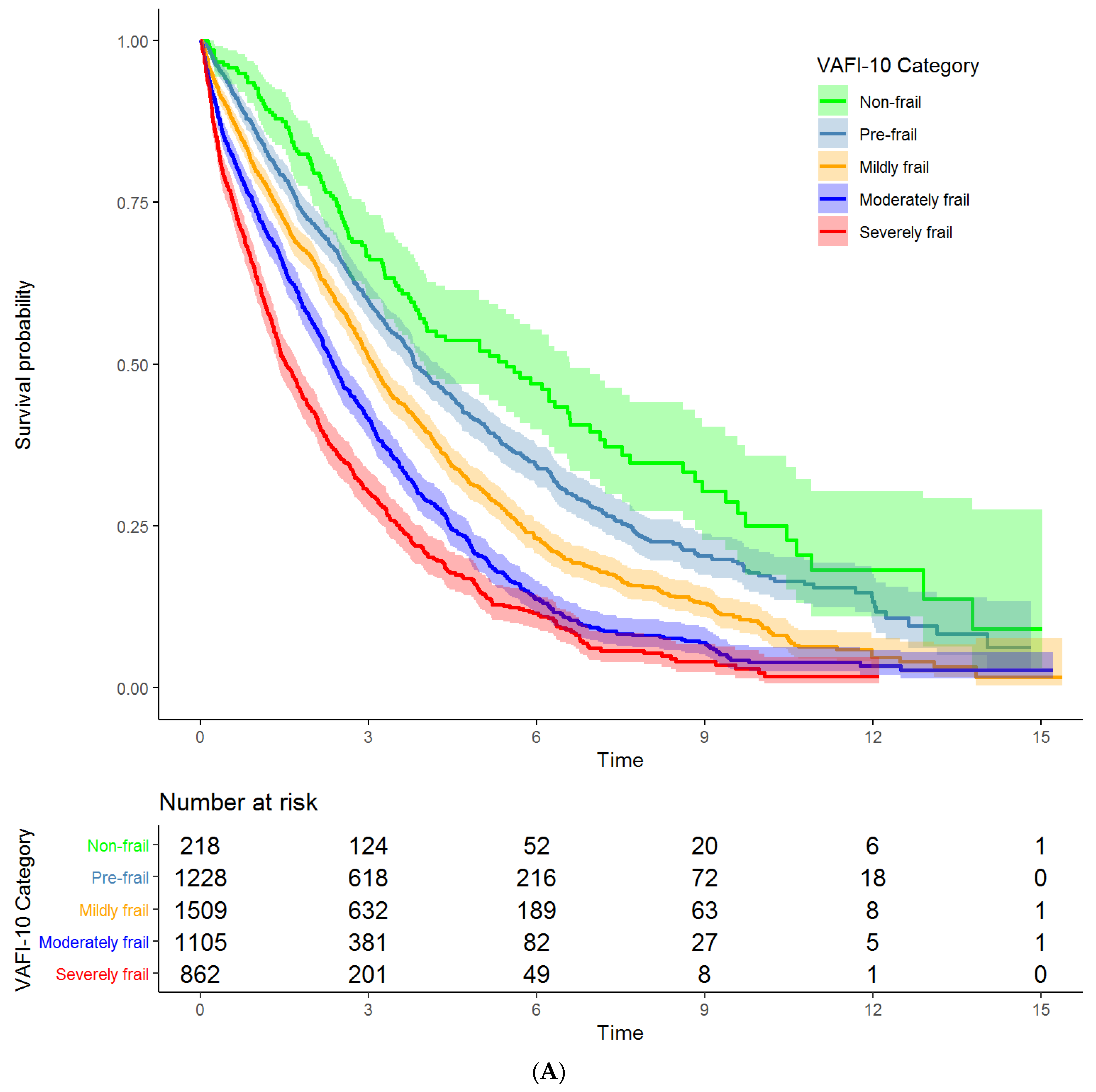
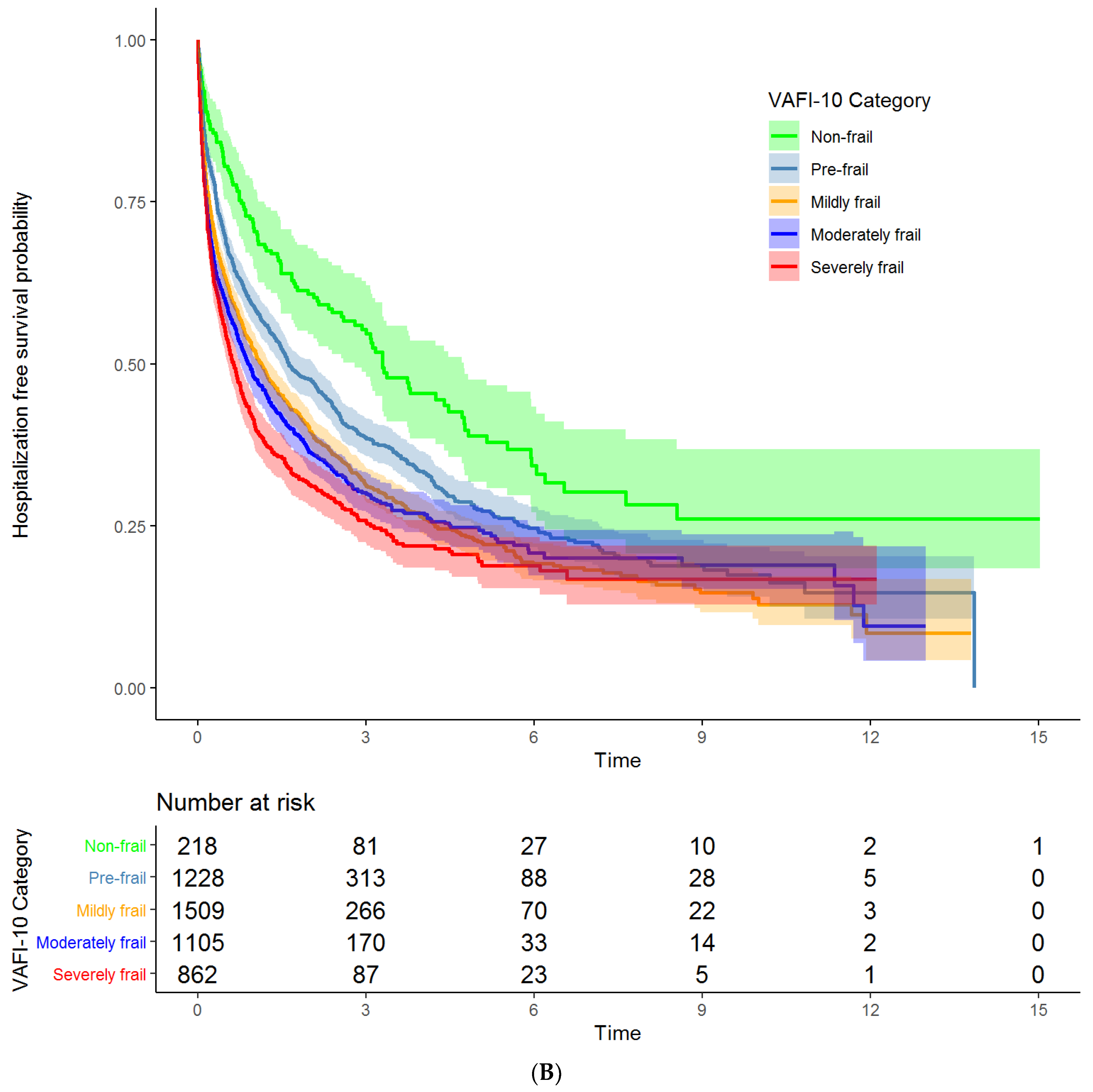
3.2. Associations of VA-FI-10 with Mortality and Unplanned Hospitalizations
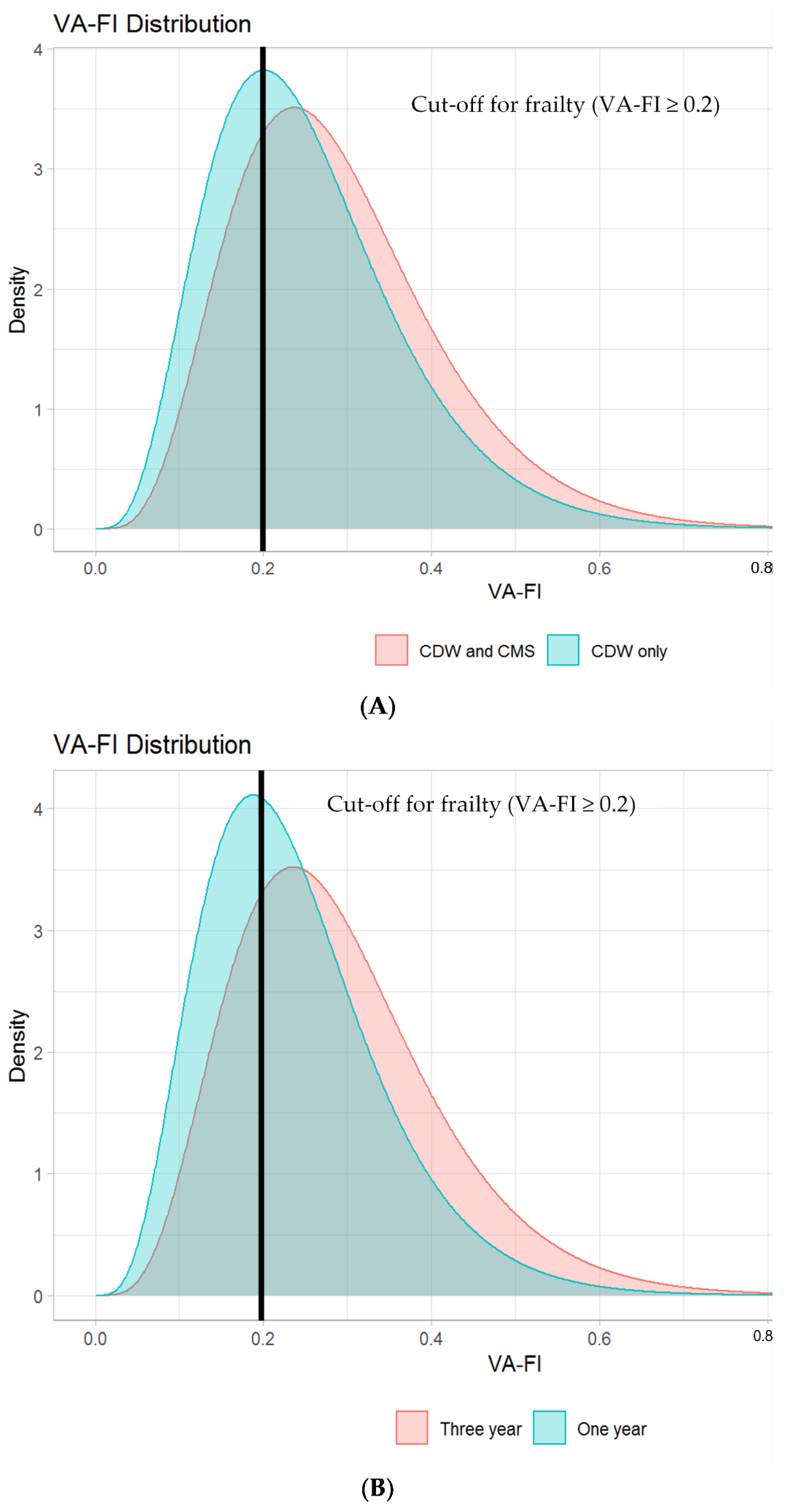
3.3. Impact on VA-FI-10 of Adding CMS Data to Capture External Deficits and of Varying Assessment Periods
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Disclaimer
Appendix A
References
- Mohile, S.G.; Dale, W.; Somerfield, M.R.; Schonberg, M.A.; Boyd, C.M.; Burhenn, P.S.; Canin, B.; Cohen, H.J.; Holmes, H.M.; Hopkins, J.O.; et al. Practical Assessment and Management of Vulnerabilities in Older Patients Receiving Chemotherapy: ASCO Guideline for Geriatric Oncology. J. Clin. Oncol. 2018, 36, 2326–2347. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. Older Adult Oncology (Version 1.2020). Available online: https://www.nccn.org/professionals/physician_gls/pdf/senior.pdf (accessed on 18 January 2021).
- Wildiers, H.; Heeren, P.; Puts, M.; Topinkova, E.; Janssen-Heijnen, M.L.G.; Extermann, M.; Falandry, C.; Artz, A.; Brain, E.; Colloca, G.; et al. International Society of Geriatric Oncology Consensus on Geriatric Assessment in Older Patients with Cancer. J. Clin. Oncol. 2014, 32, 2595–2603. [Google Scholar] [CrossRef] [PubMed]
- Mikhael, J.; Ismaila, N.; Cheung, M.C.; Costello, C.; Dhodapkar, M.V.; Kumar, S.; Lacy, M.; Lipe, B.; Little, R.F.; Nikonova, A.; et al. Treatment of Multiple Myeloma: ASCO and CCO Joint Clinical Practice Guideline. J. Clin. Oncol. 2019, 37, 1228–1263. [Google Scholar] [CrossRef] [PubMed]
- Cook, G.; LaRocca, A.; Facon, T.; Zweegman, S.; Engelhardt, M. Defining the vulnerable patient with myeloma—a frailty position paper of the European Myeloma Network. Leukemia 2020, 34, 2285–2294. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A.; Bringhen, S.; Mateos, M.-V.; LaRocca, A.; Facon, T.; Kumar, S.; Offidani, M.; McCarthy, P.; Evangelista, A.; Lonial, S.; et al. Geriatric assessment predicts survival and toxicities in elderly myeloma patients: An International Myeloma Working Group report. Blood 2015, 125, 2068–2074. [Google Scholar] [CrossRef]
- Engelhardt, M.; Domm, A.S.; Dold, S.M.; Ihorst, G.; Reinhardt, H.; Zober, A.; Hieke, S.; Baayen, C.; Müller, S.J.; Einsele, H.; et al. A concise revised Myeloma Comorbidity Index as a valid prognostic instrument in a large cohort of 801 multiple myeloma patients. Haematologica 2017, 102, 910–921. [Google Scholar] [CrossRef]
- Mian, H.; Brouwers, M.; Kouroukis, C.T.; Wildes, T.M. Comparison of Frailty Scores in Newly Diagnosed Patients with Multiple Myeloma: A Review. J. Frailty Aging 2019, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Salazar, A.S.; Recinos, L.M.; Mian, H.S.; Stoll, C.; Simon, L.E.; Sekhon, S.; Colditz, G.; Wildes, T.M. Geriatric Assessment and Frailty Scores Predict Mortality in Myeloma: Systematic Review and Meta-analysis. Clin. Lymphoma Myeloma Leuk. 2019, 19, 488–496.e6. [Google Scholar] [CrossRef]
- Zweegman, S.; Engelhardt, M.; LaRocca, A. Elderly patients with multiple myeloma: Towards a frailty approach? Curr. Opin. Oncol. 2017, 29, 315–321. [Google Scholar] [CrossRef]
- Dale, W.; Williams, G.R.; MacKenzie, A.R.; Soto-Perez-De-Celis, E.; Maggiore, R.J.; Merrill, J.K.; Katta, S.; Smith, K.T.; Klepin, H.D. How Is Geriatric Assessment Used in Clinical Practice for Older Adults with Cancer? A Survey of Cancer Providers by the American Society of Clinical Oncology. JCO Oncol. Pr. 2021, 17, 336–344. [Google Scholar] [CrossRef]
- Abel, G.A.; Klepin, H.D. Frailty and the management of hematologic malignancies. Blood 2018, 131, 515–524. [Google Scholar] [CrossRef]
- Kim, D.H.; Patorno, E.; Pawar, A.; Lee, H.; Schneeweiss, S.; Glynn, R.J. Measuring Frailty in Administrative Claims Data: Comparative Performance of Four Claims-Based Frailty Measures in the U.S. Medicare Data. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2019, 75, 1120–1125. [Google Scholar] [CrossRef]
- Clegg, A.; Bates, C.; Young, J.; Ryan, R.; Nichols, L.; Teale, E.A.; Mohammed, M.A.; Parry, J.; Marshall, T. Development and validation of an electronic frailty index using routine primary care electronic health record data. Age Ageing 2017, 47, 319. [Google Scholar] [CrossRef]
- Pajewski, N.M.; Lenoir, K.; Wells, B.J.; Williamson, J.D.; Callahan, E.K. Frailty Screening Using the Electronic Health Record Within a Medicare Accountable Care Organization. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2019, 74, 1771–1777. [Google Scholar] [CrossRef] [PubMed]
- Levit, L.A.; Kaltenbaugh, M.W.; Magnuson, A.; Hershman, D.L.; Goncalves, P.H.; Garrett-Mayer, E.; Bruinooge, S.S.; Miller, R.S.; Klepin, H.D. Challenges and opportunities to developing a frailty index using electronic health record data. J. Geriatr. Oncol. 2021, 12, 851–854. [Google Scholar] [CrossRef]
- Kim, D.H.; Glynn, R.J.; Avorn, J.; Lipsitz, A.L.; Rockwood, K.; Pawar, A.; Schneeweiss, S. Validation of a Claims-Based Frailty Index Against Physical Performance and Adverse Health Outcomes in the Health and Retirement Study. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2019, 74, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- National Health Service. Electronic Frailty Index. Available online: https://www.england.nhs.uk/ourwork/clinical-policy/older-people/frailty/efi/ (accessed on 9 April 2021).
- US Department of Veterans Affairs. Veterans Health Administration. Available online: https://www.va.gov/health/ (accessed on 4 May 2021).
- Orkaby, A.R.; Nussbaum, L.; Ho, Y.-L.; Gagnon, D.; Quach, L.; Ward, R.; Quaden, R.; Yaksic, E.; Harrington, K.; Paik, J.M.; et al. The Burden of Frailty Among U.S. Veterans and Its Association with Mortality, 2002–2012. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2019, 74, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; DuMontier, C.; Yildirim, C.; Charest, B.; Hawley, E.C.; Zhuo, M.; Paik, J.M.; Yaksic, E.; Gaziano, J.M.; Do, N.; et al. Updating and Validating the U.S. Veterans Affairs Frailty Index: Transitioning from ICD-9 to ICD-10. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2021, 76, 1318–1325. [Google Scholar] [CrossRef]
- Aggarwal, N.K. Ramifications of the VA MISSION Act of 2018 on Mental Health. JAMA Psychiatry 2020, 77, 337. [Google Scholar] [CrossRef] [PubMed]
- Gilbertson, D.T.; Bradbury, B.D.; Wetmore, J.B.; Weinhandl, E.D.; Monda, K.L.; Liu, J.; Brookhart, M.A.; Gustafson, S.K.; Roberts, T.; Collins, A.J.; et al. Controlling confounding of treatment effects in administrative data in the presence of time-varying baseline confounders. Pharmacoepidemiol. Drug Saf. 2015, 25, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Nakasian, S.S.; Rassen, J.; Franklin, J.M. Effects of expanding the look-back period to all available data in the assessment of covariates. Pharmacoepidemiol. Drug Saf. 2017, 26, 890–899. [Google Scholar] [CrossRef]
- Price, L.E.; Shea, K.; Gephart, S. The Veterans Affairs’s Corporate Data Warehouse. Nurs. Adm. Q. 2015, 39, 311–318. [Google Scholar] [CrossRef]
- Shortliffe, E.H.; Millet, L.I.; Committee on Future Information Architectures Processes and Strategies for the Centers for Medicare and Medicaid Services; National Research Council (U.S.); Division on Engineering and Physical Sciences; National Research Council (U.S.); Computer Science and Telecommunications Board; ebrary Inc. Strategies and Priorities for Information Tech-nology at the Centers for Medicare and Medicaid Services; National Academies Press: Washington, DC, USA, 2012; Available online: https://yale.idm.oclc.org/login?URL=http://site.ebrary.com/lib/yale/Doc?id=10531101 (accessed on 10 January 2020).
- Fihn, S.D.; Francis, J.; Clancy, C.; Nielson, C.; Nelson, K.; Rumsfeld, J.; Cullen, T.; Bates, J.; Graham, G.L. Insights from Advanced Analytics at The Veterans Health Administration. Heal. Aff. 2014, 33, 1203–1211. [Google Scholar] [CrossRef]
- Research Data and Assistance Center. Strengths and Limitations of CMS Administrative Data 2018. Available online: https://resdac.org/articles/strengths-and-limitations-cms-administrative-data-research (accessed on 6 June 2021).
- Fillmore, N.R.; DuMontier, C.; Yildirim, C.; La, J.; Epstein, M.M.; Cheng, D.; Cirstea, D.; Yellapragada, S.; Abel, A.G.; Gaziano, J.M.; et al. Defining Multimorbidity and Its Impact in Older United States Veterans Newly Treated for Multiple Myeloma. J. Natl. Cancer Inst. 2021, djab007. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, K.; Mitnitski, A. Frailty Defined by Deficit Accumulation and Geriatric Medicine Defined by Frailty. Clin. Geriatr. Med. 2011, 27, 17–26. [Google Scholar] [CrossRef]
- Searle, S.D.; Mitnitski, A.; Gahbauer, E.A.; Gill, T.M.; Rockwood, K. A standard procedure for creating a frailty index. BMC Geriatr. 2008, 8, 24. [Google Scholar] [CrossRef]
- Armstrong, J.J.; Andrew, M.K.; Mitnitski, A.; Launer, L.J.; White, L.R.; Rockwood, K. Social vulnerability and survival across levels of frailty in the Honolulu-Asia Aging Study. Age Ageing 2015, 44, 709–712. [Google Scholar] [CrossRef] [PubMed]
- Blodgett, J.; Theou, O.; Kirkland, S.; Andreou, P.; Rockwood, K. Frailty in NHANES: Comparing the frailty index and phenotype. Arch. Gerontol. Geriatr. 2015, 60, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Hoover, M.; Rotermann, M.; Sanmartin, C.; Bernier, J. Validation of an index to estimate the prevalence of frailty among commu-nity-dwelling seniors. Health Rep. 2013, 24, 10–17. [Google Scholar]
- Pajewski, N.M.; Williamson, J.D.; Applegate, W.B.; Berlowitz, D.R.; Bolin, L.; Chertow, G.M.; Krousel-Wood, M.A.; Lopez-Barrera, N.; Powell, J.R.; Roumie, C.L.; et al. Characterizing Frailty Status in the Systolic Blood Pressure Intervention Trial. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2016, 71, 649–655. [Google Scholar] [CrossRef]
- Greipp, P.R.; Miguel, J.S.; Durie, B.G.; Crowley, J.J.; Barlogie, B.; Bladé, J.; Boccadoro, M.; Child, J.A.; Avet-Loiseau, H.; Kyle, R.A.; et al. International Staging System for Multiple Myeloma. J. Clin. Oncol. 2005, 23, 3412–3420. [Google Scholar] [CrossRef]
- Kyle, R.A.; Gertz, M.A.; Witzig, T.E.; Lust, J.A.; Lacy, M.Q.; Dispenzieri, A.; Fonseca, R.; Rajkumar, S.V.; Offord, J.R.; Larson, D.R.; et al. Review of 1027 Patients with Newly Diagnosed Multiple Myeloma. Mayo Clin. Proc. 2003, 78, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A.; Rajkumar, S.V.; Miguel, J.F.S.; LaRocca, A.; Niesvizky, R.; Morgan, G.; Landgren, O.; Hajek, R.; Einsele, H.; Anderson, K.C.; et al. International Myeloma Working Group Consensus Statement for the Management, Treatment, and Supportive Care of Patients with Myeloma Not Eligible for Standard Autologous Stem-Cell Transplantation. J. Clin. Oncol. 2014, 32, 587–600. [Google Scholar] [CrossRef] [PubMed]
- Sohn, M.-W.; Arnold, N.; Maynard, C.; Hynes, D.M. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul. Heal. Metr. 2006, 4, 2. [Google Scholar] [CrossRef]
- van Buuren, S.; Groothuis-Oudshoorn, K. Mice: Multivariate Imputation by Chained Equations inR. J. Stat. Softw. 2011, 45, 1–67. [Google Scholar] [CrossRef]
- van Buuren, S. Flexible Imputation of Missing Data; CRC Press: Boca Raton, FL, USA, 2012; Available online: https://yale.idm.oclc.org/login?URL=https://www.taylorfrancis.com/books/9781439868256 (accessed on 10 January 2020).
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. STROBE Initiative. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Patel, B.G.; Luo, S.; Wildes, T.M.; Sanfilippo, K.M. Frailty in Older Adults with Multiple Myeloma: A Study of US Veterans. JCO Clin. Cancer Inform. 2020, 4, 117–127. [Google Scholar] [CrossRef]
- Sheikh, A.R.; Cheng, D.; Fillmore, N.; Do, N.; Brophy, M.T.; Tuck, D.P. Predictive value of an electronic frailty index (FI) in U.S. Veterans with newly diagnosed non-small cell lung cancer (NSCLC). J. Clin. Oncol. 2019, 37, 11545. [Google Scholar] [CrossRef]
- Shahrokni, A.; Tin, A.; Alexander, K.; Sarraf, S.; Afonso, A.; Filippova, O.; Harris, J.; Downey, R.J.; Vickers, A.J.; Korc-Grodzicki, B. Development and Evaluation of a New Frailty Index for Older Surgical Patients with Cancer. JAMA Netw. Open 2019, 2, e193545. [Google Scholar] [CrossRef]
- Guerard, E.J.; Deal, A.M.; Chang, Y.; Williams, G.; Nyrop, K.A.; Pergolotti, M.; Muss, H.B.; Sanoff, H.K.; Lund, J.L. Frailty Index Developed from a Cancer-Specific Geriatric Assessment and the Association with Mortality Among Older Adults with Cancer. J. Natl. Compr. Cancer Netw. 2017, 15, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Cohen, H.J.; Smith, D.; Sun, C.-L.; Tew, W.; Mohile, S.G.; Owusu, C.; Klepin, H.D.; Gross, C.P.; Lichtman, S.M.; Gajra, A.; et al. Frailty as determined by a comprehensive geriatric assessment-derived deficit-accumulation index in older patients with cancer who receive chemotherapy. Cancer 2016, 122, 3865–3872. [Google Scholar] [CrossRef]
- Engelhardt, M.; Ihorst, G.; Duque-Afonso, J.; Wedding, U.; Spät-Schwalbe, E.; Goede, V.; Kolb, G.; Stauder, R.; Wäsch, R. Structured assessment of frailty in multiple myeloma as a paradigm of individualized treatment algorithms in cancer patients at advanced age. Haematologica 2020, 105, 1183–1188. [Google Scholar] [CrossRef]
- Iezzoni, L.I. 4. Using Administrative Data to Study Persons with Disabilities. Milbank Q. 2002, 80, 347–379. [Google Scholar] [CrossRef]
- Festa, N.; Shi, S.M.; Kim, D.H. Accuracy of diagnosis and health service codes in identifying frailty in Medicare data. BMC Geriatr. 2020, 20, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, A.; Fiala, M.; Tuchman, S.; Wildes, T.M. A comparison of three different approaches to defining frailty in older patients with multiple myeloma. J. Geriatr. Oncol. 2020, 11, 311–315. [Google Scholar] [CrossRef]
- Giri, S.; Williams, G.; Rosko, A.; Grant, S.J.; Mian, H.S.; Tuchman, S.; Zweegman, S.; Wildes, T.M. Simplified frailty assessment tools: Are we really capturing frailty or something else? Leukemia 2020, 34, 1967–1969. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.R.; Weaver, K.E.; Lesser, G.J.; Dressler, E.; Winkfield, K.M.; Neuman, H.B.; Kazak, A.E.; Carlos, R.; Gansauer, L.J.; Kamen, C.S.; et al. Capacity to Provide Geriatric Specialty Care for Older Adults in Community Oncology Practices. Oncologist 2020, 25, 1032–1038. [Google Scholar] [CrossRef]
- Callahan, K.E.; Clark, C.J.; Edwards, A.F.; Harwood, T.N.; Williamson, J.D.; Moses, A.W.; Willard, J.J.; Cristiano, J.A.; Meadows, K.; Hurie, J.; et al. Automated Frailty Screening At-Scale for Pre-Operative Risk Stratification Using the Electronic Frailty Index. J. Am. Geriatr. Soc. 2021, 69, 1357–1362. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.K.; Vij, R.; Noga, S.J.; Berg, D.; Brent, L.; Dollar, L.; Chari, A. Treating Multiple Myeloma Patients with Oral Therapies. Clin. Lymphoma Myeloma Leuk. 2017, 17, 243–251. [Google Scholar] [CrossRef]
- Jang, I.-Y.; Jung, H.-W.; Lee, H.Y.; Park, H.; Lee, E.; Kim, D.H. Evaluation of Clinically Meaningful Changes in Measures of Frailty. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2020, 75, 1143–1147. [Google Scholar] [CrossRef]
- Zweegman, S.; Larocca, A. Frailty in multiple myeloma: The need for harmony to prevent doing harm. Lancet Haematol. 2019, 6, e117–e118. [Google Scholar] [CrossRef]
- Dumontier, C.; Loh, K.P.; Bain, P.A.; Silliman, R.A.; Hshieh, T.; Abel, G.A.; Djulbegovic, B.; Driver, J.A.; Dale, W. Defining Undertreatment and Overtreatment in Older Adults with Cancer: A Scoping Literature Review. J. Clin. Oncol. 2020, 38, 2558–2569. [Google Scholar] [CrossRef]
- Kim, D.H.; Schneeweiss, S.; Glynn, R.J.; Lipsitz, A.L.; Rockwood, K.; Avorn, J. Measuring Frailty in Medicare Data: Development and Validation of a Claims-Based Frailty Index. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2018, 73, 980–987. [Google Scholar] [CrossRef]
- Brundle, C.; Heaven, A.; Brown, L.; Teale, E.; Young, J.; West, R.; Clegg, A. Convergent validity of the electronic frailty index. Age Ageing 2019, 48, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, N.A.; Phillips, S.; Wells, K.E.; Woodcroft, K.J.; Amend, K.L.; Enger, C.; Oliveria, S.A. Validating an algorithm for multiple myeloma based on administrative data using a SEER tumor registry and medical record review. Pharmacoepidemiol. Drug Saf. 2019, 28, 256–263. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Overall | Non-Frail (VA-FI ≤ 0.1) | Pre-Frail (VA-FI > 0.1–0.2) | Mildly Frail (VA-FI > 0.2–0.3) | Moderately Frail (VA-FI > 0.3–0.4) | Severely Frail (VA-FI > 0.4) |
|---|---|---|---|---|---|---|
| n | 4924 | 219 | 1228 | 1510 | 1105 | 862 |
| Age at Diagnosis (median (IQR)) | 75.1 (69.9, 80.8) | 72.6 (68.7, 77.3) | 73.1 (68.7, 78.5) | 75.0(69.9, 80.6) | 76.5 (70.5, 81.7) | 77.4 (72.0, 82.8) |
| Gender = M (%) | 4857 (98.6) | 217 (99.1) | 1220 (99.3) | 1486 (98.4) | 1088 (98.5) | 846 (98.1) |
| Race (%) | ||||||
| White | 3273 (66.5) | 144 (65.8) | 818 (66.6) | 983 (65.1) | 726 (65.7) | 602 (69.8) |
| Black | 1117 (22.7) | 42 (19.2) | 283 (23.0) | 369 (24.4) | 252 (22.8) | 171 (19.8) |
| Other | 64 (1.3) | 5 (2.3) | 19 (1.5) | 15 (1.0) | 14 (1.3) | 11 (1.3) |
| Missing | 470 (9.5) | 28 (12.8) | 108 (8.8) | 143 (9.5) | 113 (10.2) | 78 (9.0) |
| Income in US dollars (median (IQR)) | 27,519 (16,368, 38,647) | 32,491 (19,892, 46,254) | 29,078 (17,828, 42,646) | 26,461 (16,000, 37,716) | 26,507 (16,235, 37,678) | 26,720 (15,506, 35,916) |
| ISS Stage (%) | ||||||
| 1 | 495 (10.1) | 37 (16.9) | 171 (13.9) | 154 (10.2) | 87 (7.9) | 46 (5.3) |
| 2 | 924 (18.8) | 40 (18.3) | 262 (21.3) | 286 (18.9) | 204 (18.5) | 132 (15.3) |
| 3 | 626 (12.7) | 22 (10.0) | 127 (10.3) | 210 (13.9) | 156 (14.1) | 111 (12.9) |
| Missing | 2879 (58.5) | 120 (54.8) | 668 (54.4) | 860 (57.0) | 658 (59.5) | 573 (66.5) |
| Calcium ≥ 11 mg/dL (%) | 151 (3.1) | 4 (1.8) | 40 (3.3) | 46 (3.0) | 39 (3.5) | 22 (2.6) |
| Missing | 422 (8.6) | 27 (12.3) | 108 (8.8) | 131 (8.7) | 83 (7.5) | 73 (8.5) |
| Creatinine > 2 mg/dL (%) | 997 (20.2) | 11 (5.0) | 160 (13.0) | 330 (21.9) | 259 (23.4) | 237 (27.5) |
| Missing | 347 (7.0) | 23 (10.5) | 92 (7.5) | 105 (7.0) | 62 (5.6) | 65 (7.5) |
| Hemoglobin < 10 g/dL (%) | 1727 (35.1) | 41 (18.7) | 362 (29.5) | 532 (35.2) | 425 (38.5) | 367 (42.6) |
| Missing | 347 (7.0) | 27 (12.3) | 92 (7.5) | 100 (6.6) | 71 (6.4) | 57 (6.6) |
| Platelet < 150,000/microL (%) | 1116 (22.7) | 38 (17.4) | 262 (21.3) | 328 (21.7) | 274 (24.8) | 214 (24.8) |
| Missing | 626 (12.7) | 37 (16.9) | 154 (12.5) | 191 (12.6) | 142 (12.9) | 102 (11.8) |
| Novel Therapy at Induction (%) | 4231 (85.9) | 198 (90.4) | 1056 (86.0) | 1308 (86.6) | 937 (84.8) | 732 (84.9) |
| Thalidomide (%) | 1089 (22.1) | 48 (21.9) | 252 (20.5) | 355 (23.5) | 250 (22.6) | 184 (21.3) |
| Lenalidomide (%) | 1870 (38.0) | 108 (49.3) | 526 (42.8) | 574 (38.0) | 383 (34.7) | 279 (32.4) |
| Bortezomib (%) | 1990 (40.4) | 77 (35.2) | 497 (40.5) | 618 (40.9) | 434 (39.3) | 364 (42.2) |
| Thalidomide and Bortezomib (%) | 70 (1.4) | 1 (0.2) | 28 (1.7) | 25 (1.7) | 10 (1.2) | 6 (1.1) |
| Lenalidomide and Bortezomib (%) | 633 (12.9) | 44 (10.7) | 211 (13.1) | 211 (14.0) | - | - |
| Health Deficit | Overall | Non-Frail (VA-FI ≤ 0.1) | Pre-Frail (VA-FI > 0.1–0.2) | Mildly Frail (VA-FI > 0.2–0.3) | Moderately Frail (VA-FI > 0.3–0.4) | Severely Frail (VA-FI > 0.4) |
|---|---|---|---|---|---|---|
| n (%) | 4924 (100) | 219 (4.4) | 1228 (24.9) | 1510 (30.7) | 1105 (22.4) | 862 (17.5) |
| Morbidity | ||||||
| Atrial Fibrillation | 928 (18.8) | 2 (0.9) | 70 (5.7) | 211 (14.0) | 289 (26.2) | 356 (41.3) |
| Anemia | 3629 (73.7) | 55 (25.1) | 690 (56.2) | 1147 (76.0) | 946 (85.6) | 791 (91.8) |
| Coronary Artery Disease | 2071 (42.1) | 15 (6.8) | 227 (18.5) | 560 (37.1) | 632 (57.2) | 637 (73.9) |
| Cancer | 4813 (97.7) | 199 (90.9) | 1185 (96.5) | 1486 (98.4) | 1088 (98.5) | 855 (99.2) |
| Cerebral Vascular Disease | 996 (20.2) | 1 (0.5) | 61 (5.0) | 216 (14.3) | 304 (27.5) | 414 (48.0) |
| Chronic Kidney Disease | 2043 (41.5) | 7 (3.2) | 272 (22.1) | 587 (38.9) | 583 (52.8) | 594 (68.9) |
| Diabetes | 2019 (41.0) | 15 (6.8) | 293 (23.9) | 588 (38.9) | 559 (50.6) | 564 (65.4) |
| Heart Failure | 1144 (23.2) | 2 (0.9) | 47 (3.8) | 236 (15.6) | 377 (34.1) | 482 (55.9) |
| Hypertension | 4375 (88.9) | 116 (53.0) | 1002 (81.6) | 1357 (89.9) | 1051 (95.1) | 849 (98.5) |
| Liver Disease | 540 (11.0) | 4 (1.8) | 59 (4.8) | 143 (9.5) | 141 (12.8) | 193 (22.4) |
| Lung Disease | 1780 (36.1) | 9 (4.1) | 221 (18.0) | 496 (32.8) | 522 (47.2) | 532 (61.7) |
| Thyroid Disease | 739 (15.0) | 4 (1.8) | 87 (7.1) | 226 (15.0) | 182 (16.5) | 240 (27.8) |
| Osteoporosis or Osteoporosis-Related Fracture | 842 (17.1) | 10 (4.6) | 94 (7.7) | 215 (14.2) | 243 (22.0) | 280 (32.5) |
| Incontinence | 388 (7.9) | 0 (0.0) | 35 (2.9) | 78 (5.2) | 101 (9.1) | 174 (20.2) |
| Function | ||||||
| Arthritis | 2754 (55.9) | 36 (16.4) | 462 (37.6) | 851 (56.4) | 743 (67.2) | 662 (76.8) |
| Durable Medical Equipment | 1102 (22.4) | 6 (2.7) | 104 (8.5) | 277 (18.3) | 315 (28.5) | 400 (46.4) |
| Falls | 550 (11.2) | 3 (1.4) | 25 (2.0) | 115 (7.6) | 150 (13.6) | 257 (29.8) |
| Fatigue | 1274 (25.9) | 7 (3.2) | 91 (7.4) | 301 (19.9) | 375 (33.9) | 500 (58.0) |
| Gait Abnormality | 1019 (20.7) | 0 (0.0) | 61 (5.0) | 209 (13.8) | 321 (29.0) | 428 (49.7) |
| Muscular impairment/Debility | 941 (19.1) | 3 (1.4) | 54 (4.4) | 165 (10.9) | 278 (25.2) | 441 (51.2) |
| Parkinson’s Disease | 151 (3.1) | 0 (0.0) | 14 (1.1) | 31 (2.1) | 37 (3.3) | 69 (8.0) |
| Peripheral Vascular Disease/Claudication | 1512 (30.7) | 7 (3.2) | 143 (11.6) | 371 (24.6) | 457 (41.4) | 534 (61.9) |
| Cognition and Mood | ||||||
| Dementia | 685 (13.9) | 1 (0.5) | 47 (3.8) | 130 (8.6) | 208 (18.8) | 299 (34.7) |
| Anxiety | 622 (12.6) | 3 (1.4) | 68 (5.5) | 150 (9.9) | 163 (14.8) | 238 (27.6) |
| Depression | 1155 (23.5) | 8 (3.7) | 137 (11.2) | 275 (18.2) | 325 (29.4) | 410 (47.6) |
| Sensory Loss | ||||||
| Peripheral Neuropathy | 582 (11.8) | 2 (0.9) | 25 (2.0) | 115 (7.6) | 181 (16.4) | 259 (30.0) |
| Hearing Impairment | 1693 (34.4) | 23 (10.5) | 282 (23.0) | 491 (32.5) | 459 (41.5) | 438 (50.8) |
| Vision Impairment | 1510 (30.7) | 15 (6.8) | 225 (18.3) | 433 (28.7) | 422 (38.2) | 415 (48.1) |
| Other | ||||||
| Chronic Pain | 1416 (28.8) | 10 (4.6) | 166 (13.5) | 389 (25.8) | 386 (34.9) | 465 (53.9) |
| Failure to Thrive | 86 (1.7) | 0 (0.0) | 1 (0.1) | 12 (0.8) | 26 (2.4) | 47 (5.5) |
| Weight Loss | 598 (12.1) | 2 (0.9) | 64 (5.2) | 148 (9.8) | 186 (16.8) | 198 (23.0) |
| VA-FI-10 Severity | Mortality Unadjusted HR (95% CI) | Mortality Adjusted HR (95% CI) | Hospitalization Unadjusted HR (95% CI) | Hospitalization Adjusted HR (95% CI) |
|---|---|---|---|---|
| Non-frail | Reference | Reference | Reference | Reference |
| Pre-frail | 1.33 (1.10 to 1.61) | 1.25 (1.04 to 1.52) | 1.42 (1.18 to 1.73) | 1.32 (1.08 to 1.60) |
| Mildly frail | 1.76 (1.46 to 2.13) | 1.54 (1.27 to 1.86) | 1.72 (1.42 to 2.08) | 1.58 (1.31 to 1.92) |
| Moderately frail | 2.35 (1.94 to 2.84) | 1.95 (1.61 to 2.37) | 1.82 (1.50 to 2.21) | 1.69 (1.39 to 2.06) |
| Severely frail | 3.11 (2.56 to 3.77) | 2.50 (2.05 to 3.04) | 2.11 (1.73 to 2.58) | 1.93 (1.58 to 2.36) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
DuMontier, C.; Fillmore, N.R.; Yildirim, C.; Cheng, D.; La, J.; Orkaby, A.R.; Charest, B.; Cirstea, D.; Yellapragada, S.; Gaziano, J.M.; et al. Contemporary Analysis of Electronic Frailty Measurement in Older Adults with Multiple Myeloma Treated in the National US Veterans Affairs Healthcare System. Cancers 2021, 13, 3053. https://doi.org/10.3390/cancers13123053
DuMontier C, Fillmore NR, Yildirim C, Cheng D, La J, Orkaby AR, Charest B, Cirstea D, Yellapragada S, Gaziano JM, et al. Contemporary Analysis of Electronic Frailty Measurement in Older Adults with Multiple Myeloma Treated in the National US Veterans Affairs Healthcare System. Cancers. 2021; 13(12):3053. https://doi.org/10.3390/cancers13123053
Chicago/Turabian StyleDuMontier, Clark, Nathanael R. Fillmore, Cenk Yildirim, David Cheng, Jennifer La, Ariela R. Orkaby, Brian Charest, Diana Cirstea, Sarvari Yellapragada, John Michael Gaziano, and et al. 2021. "Contemporary Analysis of Electronic Frailty Measurement in Older Adults with Multiple Myeloma Treated in the National US Veterans Affairs Healthcare System" Cancers 13, no. 12: 3053. https://doi.org/10.3390/cancers13123053
APA StyleDuMontier, C., Fillmore, N. R., Yildirim, C., Cheng, D., La, J., Orkaby, A. R., Charest, B., Cirstea, D., Yellapragada, S., Gaziano, J. M., Do, N., Brophy, M. T., Kim, D. H., Munshi, N. C., & Driver, J. A. (2021). Contemporary Analysis of Electronic Frailty Measurement in Older Adults with Multiple Myeloma Treated in the National US Veterans Affairs Healthcare System. Cancers, 13(12), 3053. https://doi.org/10.3390/cancers13123053






