The Importance of Clinical Examination under General Anesthesia: Improving Parametrial Assessment in Cervical Cancer Patients
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
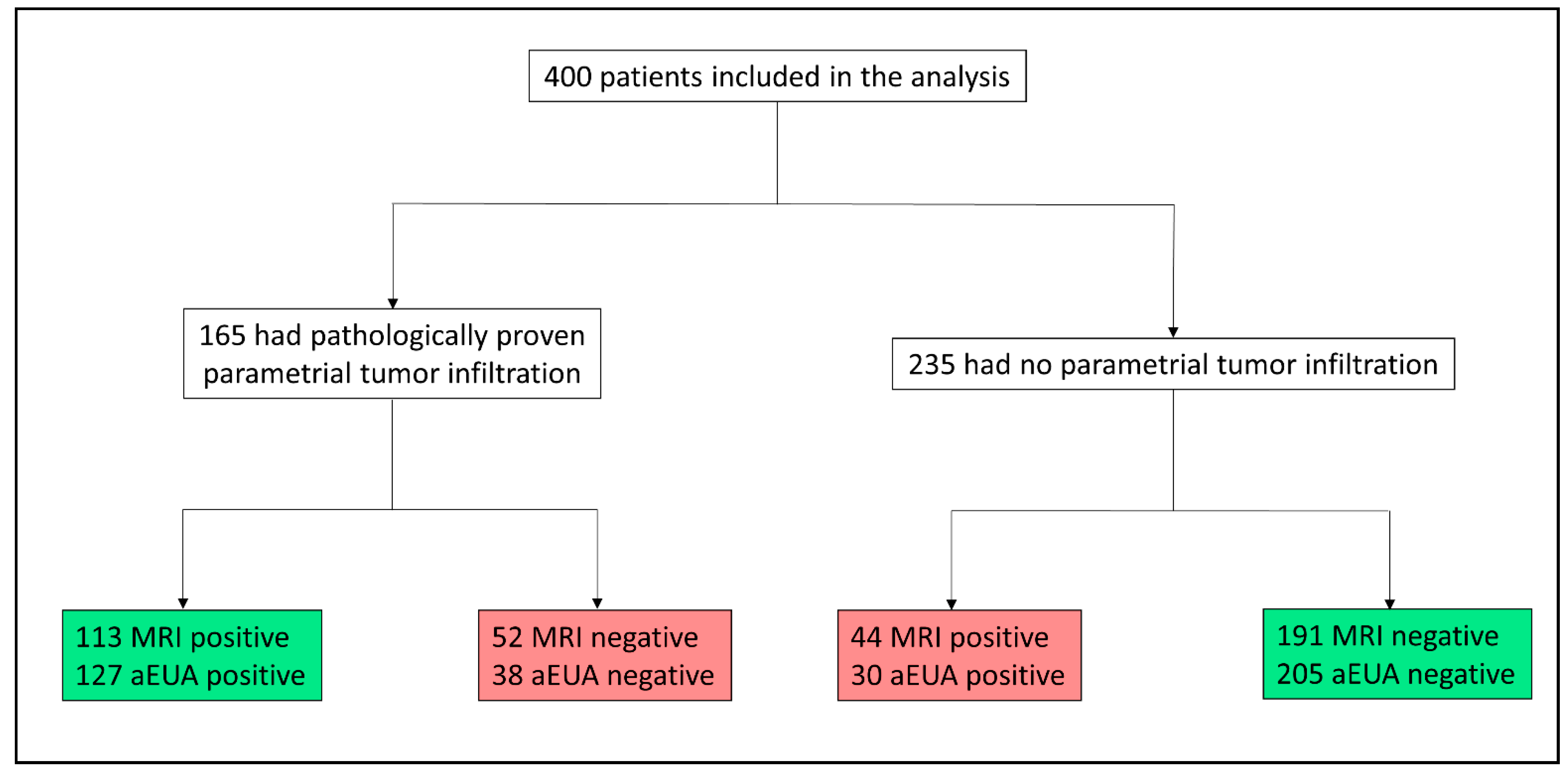
2.1. Patient Selection
2.2. Statistical Analysis
3. Results
3.1. Patient characteristics
3.2. Assessment for Parametrial Involvement by aEUA Versus MRI
3.3. Tumor Size as an Influencing Factor on Test Performance
3.4. The Influence of BMI on Testing for Parametrial Invasion
3.5. Incremental Value of Performing Both, Clinical and Radiological Assessment
3.6. Accuracy and Net-Sensitivity and Specificity with Combined Examination
3.7. Change of Diagnostic Test Performance over Time
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Statistical Analysis in Detail
References
- Bhatla, N.; Berek, J.S.; Cuello Fredes, M.; Denny, L.A.; Grenman, S.; Karunaratne, K.; Kehoe, S.T.; Konishi, I.; Olawaiye, A.B.; Prat, J.; et al. Revised FIGO staging for carcinoma of the cervix uteri. Int. J. Gynaecol. Obstet. 2019, 145, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Kraljević, Z.; Visković, K.; Ledinsky, M.; Zadravec, D.; Grbavac, I.; Bilandzija, M.; Soljacić-Vranes, H.; Kuna, K.; Klasnić, K.; Krolo, I. Primary uterine cervical cancer: Correlation of preoperative magnetic resonance imaging and clinical staging (FIGO) with histopathology findings. Coll. Antropol. 2013, 37, 561–568. [Google Scholar]
- Narayan, K.; Lin, M.Y. Staging for cervix cancer: Role of radiology, surgery and clinical assessment. Best Pract. Res. Clin. Obstet. Gynaecol. 2015, 29, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Thomeer, M.G.; Gerestein, C.; Spronk, S.; van Doorn, H.C.; van der Ham, E.; Hunink, M.G. Clinical examination versus magnetic resonance imaging in the pretreatment staging of cervical carcinoma: Systematic review and meta-analysis. Eur. Radiol. 2013, 23, 2005–2018. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.; Suh, C.H.; Kim, S.Y.; Cho, J.Y.; Kim, S.H. Magnetic resonance imaging for detection of parametrial invasion in cervical cancer: An updated systematic review and meta-analysis of the literature between 2012 and 2016. Eur. Radiol. 2018, 28, 530–541. [Google Scholar] [CrossRef]
- Ozsarlak, O.; Tjalma, W.; Schepens, E.; Corthouts, B.; Beeck, B.O.d.; van Marck, E.; Parizel, P.M.; de Schepper, A.M. The correlation of preoperative CT, MR imaging, and clinical staging (FIGO) with histopathology findings in primary cervical carcinoma. Eur. Radiol. 2003, 13, 2338–2345. [Google Scholar] [CrossRef]
- Bipat, S.; Glas, A.S.; van der Velden, J.; Zwinderman, A.H.; Bossuyt, P.M.M.; Stoker, J. Computed tomography and magnetic resonance imaging in staging of uterine cervical carcinoma: A systematic review. Gynecol. Oncol. 2003, 91, 59–66. [Google Scholar] [CrossRef]
- Hricak, H.; Gatsonis, C.; Chi, D.S.; Amendola, M.A.; Brandt, K.; Schwartz, L.H.; Koelliker, S.; Siegelman, E.S.; Brown, J.J.; McGhee, R.B.; et al. Role of imaging in pretreatment evaluation of early invasive cervical cancer: Results of the intergroup study American College of Radiology Imaging Network 6651-Gynecologic Oncology Group 183. J. Clin. Oncol. 2005, 23, 9329–9337. [Google Scholar] [CrossRef]
- Hancke, K.; Heilmann, V.; Straka, P.; Kreienberg, R.; Kurzeder, C. Pretreatment staging of cervical cancer: Is imaging better than palpation?: Role of CT and MRI in preoperative staging of cervical cancer: Single institution results for 255 patients. Ann. Surg. Oncol. 2008, 15, 2856–2861. [Google Scholar] [CrossRef]
- Chung, H.H.; Kang, S.-B.; Cho, J.Y.; Kim, J.W.; Park, N.-H.; Song, Y.-S.; Kim, S.H.; Lee, H.-P. Can preoperative MRI accurately evaluate nodal and parametrial invasion in early stage cervical cancer? Jpn. J. Clin. Oncol. 2007, 37, 370–375. [Google Scholar] [CrossRef]
- Qin, Y.; Peng, Z.; Lou, J.; Liu, H.; Deng, F.; Zheng, Y. Discrepancies between clinical staging and pathological findings of operable cervical carcinoma with stage IB-IIB: A retrospective analysis of 818 patients. Aust. N. Z. J. Obstet. Gynaecol. 2009, 49, 542–544. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, C.; Liu, P.; Li, W.; Hao, M.; Zhao, W.; Lu, A.; Ni, Y. Impact of pelvic MRI in routine clinical practice on staging of IB1-IIA2 cervical cancer. Cancer Manag. Res. 2019, 11, 3603–3609. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.-R.; Qin, L.; Li, X.; Luo, J.-P.; Li, J.; Zhang, H.-K.; Wang, L.; Shao, N.-N.; Zhang, S.-N.; Li, Y.-L.; et al. Predicting Parametrial Invasion in Cervical Carcinoma (Stages IB1, IB2, and IIA): Diagnostic Accuracy of T2-Weighted Imaging Combined with DWI at 3 T. AJR Am. J. Roentgenol. 2018, 210, 677–684. [Google Scholar] [CrossRef]
- Shweel, M.A.; Abdel-Gawad, E.A.; Abdel-Gawad, E.A.; Abdelghany, H.S.; Abdel-Rahman, A.M.; Ibrahim, E.M. Uterine Cervical Malignancy: Diagnostic Accuracy of MRI with Histopathologic Correlation. J. Clin. Imaging Sci. 2012, 2. [Google Scholar] [CrossRef] [PubMed]
- Marth, C.; Landoni, F.; Mahner, S.; McCormack, M.; Gonzalez-Martin, A.; Colombo, N. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv72–iv83. [Google Scholar] [CrossRef]
- Koh, W.-J.; Abu-Rustum, N.R.; Bean, S.; Bradley, K.; Campos, S.M.; Cho, K.R.; Chon, H.S.; Chu, C.; Clark, R.; Cohn, D.; et al. Cervical Cancer, Version 3.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2019, 17, 64–84. [Google Scholar] [CrossRef]
- Yang, K.; Park, W.; Huh, S.J.; Park, B.K.; Kim, C.K.; Kim, B.-G.; Bae, D.-S.; Lee, J.-W. Parametrial Involvement on Magnetic Resonance Imaging Has No Effect on the Survival of Early-Stage Cervical Cancer Patients. Int. J. Gynecol. Cancer 2017, 27, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Zreik, T.G.; Chambers, J.T.; Chambers, S.K. Parametrial involvement, regardless of nodal status: A poor prognostic factor for cervical cancer. Obstet. Gynecol. 1996, 87, 741–746. [Google Scholar] [CrossRef]
- Höckel, M.; Wolf, B.; Schmidt, K.; Mende, M.; Aktas, B.; Kimmig, R.; Dornhöfer, N.; Horn, L.-C. Surgical resection based on ontogenetic cancer field theory for cervical cancer: Mature results from a single-centre, prospective, observational, cohort study. Lancet Oncol. 2019, 20, 1316–1326. [Google Scholar] [CrossRef]
- Pecorelli, S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int. J. Gynaecol. Obstet. 2009, 105, 103–104. [Google Scholar] [CrossRef]
- Woo, S.; Moon, M.H.; Cho, J.Y.; Kim, S.H.; Kim, S.Y. Diagnostic Performance of MRI for Assessing Parametrial Invasion in Cervical Cancer: A Head-to-Head Comparison between Oblique and True Axial T2-Weighted Images. Korean J. Radiol. 2019, 20, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Dappa, E.; Elger, T.; Hasenburg, A.; Düber, C.; Battista, M.J.; Hötker, A.M. The value of advanced MRI techniques in the assessment of cervical cancer: A review. Insights Imaging 2017, 8, 471–481. [Google Scholar] [CrossRef]
- Balcacer, P.; Shergill, A.; Litkouhi, B. MRI of cervical cancer with a surgical perspective: Staging, prognostic implications and pitfalls. Abdom. Radiol. 2019, 44, 2557–2571. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Silverman, P.M.; Iyer, R.B.; Verschraegen, C.F.; Eifel, P.J.; Charnsangavej, C. Diagnosis, staging, and surveillance of cervical carcinoma. AJR Am. J. Roentgenol. 2003, 180, 1621–1631. [Google Scholar] [CrossRef]
- Leeflang, M.M.G.; Rutjes, A.W.S.; Reitsma, J.B.; Hooft, L.; Bossuyt, P.M.M. Variation of a test’s sensitivity and specificity with disease prevalence. CMAJ 2013, 185, E537–E544. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Suh, D.H.; Kim, K.; Lee, H.J.; Kim, Y.B.; No, J.H. Magnetic Resonance Imaging as a Valuable Tool for Predicting Parametrial Invasion in Stage IB1 to IIA2 Cervical Cancer. Int. J. Gynecol. Cancer 2017, 27, 332–338. [Google Scholar] [CrossRef]
- Canaz, E.; Ozyurek, E.S.; Erdem, B.; Aldikactioglu Talmac, M.; Yildiz Ozaydin, I.; Akbayir, O.; Numanoglu, C.; Ulker, V. Preoperatively Assessable Clinical and Pathological Risk Factors for Parametrial Involvement in Surgically Treated FIGO Stage IB-IIA Cervical Cancer. Int. J. Gynecol. Cancer 2017, 27, 1722–1728. [Google Scholar] [CrossRef]
- Frumovitz, M.; Sun, C.C.; Schmeler, K.M.; Deavers, M.T.; Dos Reis, R.; Levenback, C.F.; Ramirez, P.T. Parametrial involvement in radical hysterectomy specimens for women with early-stage cervical cancer. Obstet. Gynecol. 2009, 114, 93–99. [Google Scholar] [CrossRef]
- He, F.; Du, J.; Chen, X.; He, L. Assessment of Parametrial Involvement in Early Stages Cervical Cancer with Preoperative Magnetic Resonance Imaging. Int. J. Gynecol. Cancer 2018, 28, 1758–1765. [Google Scholar] [CrossRef]
- Uppot, R.N.; Sahani, D.V.; Hahn, P.F.; Kalra, M.K.; Saini, S.S.; Mueller, P.R. Effect of obesity on image quality: Fifteen-year longitudinal study for evaluation of dictated radiology reports. Radiology 2006, 240, 435–439. [Google Scholar] [CrossRef]
- Bourgioti, C.; Chatoupis, K.; Rodolakis, A.; Antoniou, A.; Tzavara, C.; Koutoulidis, V.; Moulopoulos, L.A. Incremental prognostic value of MRI in the staging of early cervical cancer: A prospective study and review of the literature. Clin. Imaging 2016, 40, 72–78. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 12 June 2021).
- McNemar, Q. Note on the sampling error of the difference between correlated proportions or percentages. Psychometrika 1947, 12, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, W. Does McNemar’s test compare the sensitivities and specificities of two diagnostic tests? Stat. Methods Med. Res. 2017, 26, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Moskowitz, C.S.; Pepe, M.S. Comparing the predictive values of diagnostic tests: Sample size and analysis for paired study designs. Clin. Trials 2006, 3, 272–279. [Google Scholar] [CrossRef] [PubMed]


| Parameter | n | % | |
|---|---|---|---|
| Number of patients | 400 | 100 | |
| Median age, in years (IQR) | 46 | 37–55.5 | |
| Median BMI, in kg/m2 (IQR) | 23 | 21–27 | |
| Histological type | Squamous cell carcinoma | 302 | 75.5 |
| Adenocarcinoma | 75 | 18.75 | |
| Adenosquamous carcinoma | 19 | 4.75 | |
| Neuroendocrine carcinoma | 2 | 0.5 | |
| Other | 2 | 0.5 | |
| FIGO stage | IB1 | 174 | 43.5 |
| IB2 | 36 | 9 | |
| IIA1 | 16 | 4 | |
| IIA2 | 17 | 4.25 | |
| IIB | 136 | 34 | |
| IIIA | 2 | 0.5 | |
| IIIB | 12 | 3 | |
| IVA | 7 | 1.75 | |
| Median tumor size, cm (IQR) | 3.7 | 2.7–4.9 | |
| pT-stage | pT1a | 1 | 0.25 |
| pT1b1 | 159 | 39.75 | |
| pT1b2 | 56 | 14 | |
| pT2a1 | 12 | 3 | |
| pT2a2 | 7 | 1.75 | |
| pT2b | 155 | 38.75 | |
| pT3b | 2 | 0.5 | |
| pT4 | 8 | 2 | |
| MRI stage (cT) | cT0 | 51 | 12.75 |
| cT1b1 | 123 | 30.75 | |
| cT1b2 | 17 | 4.25 | |
| cT2a | 52 | 13 | |
| cT2b | 138 | 34.5 | |
| cT3a | 2 | 0.5 | |
| cT3b | 1 | 0.25 | |
| cT4 | 16 | 4 | |
| Grading | G1 | 58 | 14.5 |
| G2 | 187 | 46.75 | |
| G3 | 150 | 37.5 | |
| G4 | 1 | 0.25 | |
| Unknown | 4 | 1 | |
| Lymphovascular involvement | Yes | 276 | 69 |
| No | 121 | 30.25 | |
| Unknown | 3 | 0.75 | |
| Blood vessel involvement | Yes | 62 | 15.5 |
| No | 336 | 84 | |
| Unknown | 2 | 0.5 | |
| Pelvic lymph node metastasis | pN0 | 247 | 61.75 |
| pN1 | 153 | 38.25 | |
| Paraaortic lymph node metastasis | pM0 | 364 | 91 |
| pM1 | 36 | 9 | |
| Measure | MRI | aEUA | OR | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | Lower 95% CI | Upper 95% CI | Estimate | Lower 95%-CI | Upper 95% CI | Estimate | Lower 95%-CI | Upper 95%-CI | p-Value | |
| Sensitivity | 68.5 | 61.4 | 75.6 | 77.0 | 70.5 | 83.4 | 1.93 * | 1.01 | 3.88 | 0.048 * |
| Specificity | 81.4 | 76.3 | 86.3 | 87.2 | 83.0 | 91.5 | 2.08 * | 1.04 | 4.38 | 0.038 * |
| PPV | 72.0 | 64.9 | 79.0 | 80.9 | 74.7 | 87.0 | 1.12 ** | 1.03 | 1.22 | 0.007 ** |
| NPV | 78.6 | 73.4 | 83.8 | 84.4 | 79.8 | 88.9 | 1.07 ** | 1.02 | 1.13 | 0.012 ** |
| Accuracy | 76.0 | 71.5 | 80.1 | 83.0 | 79.0 | 86.6 | 2.0 | 1.25 | 3.27 | 0.003 * |
| Tumor Size (MRI) | <2.5 cm (n = 77) | ≥2.5 cm (n = 220) | OR | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | Lower 95%CI | Upper 95%CI | Estimate | Lower 95%CI | Upper 95%CI | Estimate | Lower 95%CI | Upper 95%CI | ||
| Sensitivity | 60 | 29.64 | 90.36 | 70.37 | 62.67 | 78.07 | 1.59 | 0.31 | 7.14 | 0.4917 |
| Specificity | 92.54 | 86.24 | 98.83 | 58.82 | 48.36 | 69.29 | 0.12 | 0.03 | 0.33 | <0.0001 |
| PPV | 54.55 | 25.12 | 83.97 | 73.08 | 65.45 | 80.7 | 2.22 | 0.5 | 10.0 | 0.29 |
| NPV | 93.94 | 88.18 | 99.7 | 55.56 | 45.29 | 65.82 | 0.08 | 0.02 | 0.25 | <0.0001 |
| Accuracy | 89.47 | 80.31 | 95.34 | 65.91 | 59.24 | 72.15 | 0.26 | 0.11 | 0.55 | <0.001 |
| BMI | <30 kg/m2 (n = 337) | ≥30 kg/m2 (n = 55) | OR | p-Value | ||||||
| Estimate | Lower 95%CI | Upper 95%CI | Estimate | Lower 95%CI | Upper 95%CI | Estimate | Lower 95%CI | Upper 95%CI | ||
| Sensitivity | 66.9 | 59.16 | 74.64 | 80.95 | 64.16 | 97.75 | 2.08 | 0.63 | 9.09 | 0.2201 |
| Specificity | 81.01 | 75.52 | 86.53 | 79.41 | 65.82 | 93.0 | 0.90 | 0.35 | 2.65 | 0.8154 |
| PPV | 71.97 | 64.31 | 79.63 | 70.83 | 52.65 | 89.02 | 0.94 | 0.34 | 2.96 | 1.0 |
| NPV | 77.07 | 71.32 | 82.83 | 87.1 | 75.3 | 99.0 | 2 | 0.65 | 8.27 | 0.25 |
| Accuracy | 75.07 | 70.10 | 79.60 | 80.0 | 67.03 | 89.57 | 1.33 | 0.64 | 2.99 | 0.4997 |
| Clinical Parametrial Status | Negative (n = 243) | Positive (n = 157) | OR | p-Vlaue | ||||||
| Estimate | Lower 95%CI | Upper 95%CI | Estimate | Lower 95%CI | Upper 95%CI | Estimate | Lower 95%CI | Upper 95%CI | ||
| Sensitivity | 39.47 | 23.93 | 55.01 | 77.17 | 69.86 | 84.47 | 5.0 | 2.22 | 12.5 | <0.0001 |
| Specificity | 86.83 | 82.2 | 91.46 | 43.33 | 25.60 | 61.07 | 0.12 | 0.05 | 0.29 | <0.0001 |
| PPV | 35.71 | 21.22 | 50.21 | 85.22 | 78.73 | 91.70 | 12.5 | 5.26 | 33.3 | <0.0001 |
| NPV | 88.56 | 84.16 | 92.96 | 30.95 | 16.97 | 44.93 | 0.06 | 0.02 | 0.14 | <0.0001 |
| Accuracy | 79.42 | 73.79 | 84.33 | 70.70 | 62.92 | 77.68 | 0.63 | 0.38 | 1.03 | 0.054 |
| Tumor Size (MRI) | <2.5 cm (n = 77) | ≥2.5 cm (n = 220) | OR | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | Lower 95%CI | Upper 95%CI | Estimate | Lower 95%CI | Upper 95%CI | Estimate | Lower 95%CI | Upper 95%CI | ||
| Sensitivity | 70.0 | 41.6 | 98.4 | 77.78 | 70.76 | 84.79 | 0.67 | 0.14 | 4.25 | 0.6954 |
| Specificity | 89.56 | 82.23 | 96.88 | 80.0 | 71.5 | 88.5 | 0.47 | 0.15 | 1.29 | 0.1223 |
| PPV | 50.0 | 23.81 | 76.19 | 86.07 | 79.92 | 92.21 | 0.16 | 0.04 | 0.63 | 0.003 |
| NPV | 95.24 | 90.0 | 100 | 69.39 | 60.26 | 78.51 | 0.16 | 0.02 | 0.4 | <0.0001 |
| Accuracy | 87.01 | 77.41 | 93.59 | 78.64 | 72.62 | 83.86 | 0.55 | 0.23 | 1.19 | 0.1307 |
| BMI | <30 kg/m2 (n = 243) | ≥30 kg/m2 (n = 157) | OR | p-Value | ||||||
| Estimate | Lower 95%CI | Upper 95%CI | Estimate | Lower 95%CI | Upper 95%CI | Estimate | Lower 95%CI | Upper 95%CI | ||
| Sensitivity | 75.35 | 68.26 | 82.44 | 85.71 | 70.75 | 100 | 0.51 | 0.09 | 1.91 | 0.4105 |
| Specificity | 86.67 | 81.9 | 91.44 | 88.24 | 77.41 | 99.07 | 1.15 | 0.36 | 4.87 | 1.0 |
| PPV | 80.45 | 73.71 | 87.19 | 81.82 | 65.7 | 97.94 | 0.91 | 0.21 | 3.11 | 1.0 |
| NPV | 82.84 | 77.67 | 88.02 | 90.91 | 81.1 | 100 | 2.1 | 0.59 | 11.16 | 0.31 |
| Accuracy | 81.90 | 77.37 | 85.86 | 87.27 | 75.52 | 94.73 | 1.51 | 0.64 | 4.17 | 0.4422 |
| MRI Parametrial Status | Negative (n = 243) | Positive (n = 157) | OR | p-Vlaue | ||||||
| Estimate | Lower 95%CI | Upper 95%CI | Estimate | Lower 95%CI | Upper 95%CI | Estimate | Lower 95%CI | Upper 95%CI | ||
| Sensitivity | 55.77 | 42.27 | 69.27 | 86.73 | 80.47 | 92.98 | 0.2 | 0.08 | 0.45 | <0.0001 |
| Specificity | 93.19 | 89.62 | 96.77 | 61.36 | 46.98 | 75.75 | 0.12 | 0.05 | 0.29 | <0.0001 |
| PPV | 69.05 | 55.07 | 83.03 | 85.22 | 78.73 | 91.70 | 0.39 | 0.16 | 0.98 | 0.04 |
| NPV | 88.56 | 84.16 | 92.96 | 64.29 | 49.79 | 78.78 | 0.23 | 0.1 | 0.55 | 0.0003 |
| Accuracy | 85.19 | 80.08 | 89.40 | 79.62 | 72.46 | 85.62 | 0.68 | 0.39 | 1.20 | 0.1729 |
| Univariable Regression Modeling | ||||||
|---|---|---|---|---|---|---|
| MRI | ||||||
| Parameter | n | Estimate | Standard error | z-value | OR | p |
| Size (<2.5 cm vs. ≥2.5 cm) | 296 | −1.501 | 0.3997 | −3.755 | 0.22 | 0.000173 |
| BMI (<30 kg/m2 vs. ≥30 kg/m2) | 392 | 0.2717 | 0.3601 | 0.755 | 1.31 | 0.45 |
| Parametrial status (clinical exam, negative vs. positive) | 400 | 2.0164 | 0.2868 | 7.032 | 7.511 | <0.0001 |
| aEUA | ||||||
| Parameter | n | Estimate | Standard error | z-value | OR | p |
| Size (<2.5 cm vs. ≥2.5 cm) | 296 | −0.5839 | 0.3771 | −1.548 | 0.56 | 0.122 |
| BMI (<30 kg/m2 vs. ≥30 kg/m2) | 392 | 0.2648 | 0.4078 | 0.649 | 1.3 | 0.516 |
| Parametrial status (MRI, negative vs. positive) | 400 | 2.0164 | 0.2868 | 7.032 | 7.511 | <0.0001 |
| Multivariable Regression Modeling | ||||||
| MRI | ||||||
| Parameter | n | Estimate | Standard error | z-value | OR | p |
| Size (<2.5 cm vs. ≥2.5 cm) | 296 | −1.4885 | 0.4188 | −3.554 | 0.23 | 0.00038 |
| BMI (<30 kg/m2 vs. ≥30 kg/m2) | 0.2475 | 0.3995 | 0.619 | 1.28 | 0.53565 | |
| Parametrial status (clinical exam, negative vs. positive) | 1.7899 | 0.3287 | 5.445 | 5.99 | <0.0001 | |
| aEUA | ||||||
| Parameter | n | Estimate | Standard error | z-value | OR | p |
| Size (<2.5 cm vs. ≥2.5 cm) | 296 | −0.07903 | 0.41051 | −0.193 | 0.92 | 0.847 |
| BMI (<30 kg/m2 vs. ≥30 kg/m2) | 0.51993 | 0.48890 | 1063 | 1.68 | 0.288 | |
| Parametrial status (MRI, negative vs. positive) | 1.7899 | 0.3287 | 5.445 | 5.99 | <0.0001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sodeikat, P.; Lia, M.; Martin, M.; Horn, L.-C.; Höckel, M.; Aktas, B.; Wolf, B. The Importance of Clinical Examination under General Anesthesia: Improving Parametrial Assessment in Cervical Cancer Patients. Cancers 2021, 13, 2961. https://doi.org/10.3390/cancers13122961
Sodeikat P, Lia M, Martin M, Horn L-C, Höckel M, Aktas B, Wolf B. The Importance of Clinical Examination under General Anesthesia: Improving Parametrial Assessment in Cervical Cancer Patients. Cancers. 2021; 13(12):2961. https://doi.org/10.3390/cancers13122961
Chicago/Turabian StyleSodeikat, Paulina, Massimiliano Lia, Mireille Martin, Lars-Christian Horn, Michael Höckel, Bahriye Aktas, and Benjamin Wolf. 2021. "The Importance of Clinical Examination under General Anesthesia: Improving Parametrial Assessment in Cervical Cancer Patients" Cancers 13, no. 12: 2961. https://doi.org/10.3390/cancers13122961
APA StyleSodeikat, P., Lia, M., Martin, M., Horn, L.-C., Höckel, M., Aktas, B., & Wolf, B. (2021). The Importance of Clinical Examination under General Anesthesia: Improving Parametrial Assessment in Cervical Cancer Patients. Cancers, 13(12), 2961. https://doi.org/10.3390/cancers13122961






