Newly Obtained Apple Pectin as an Adjunct to Irinotecan Therapy of Colorectal Cancer Reducing E. coli Adherence and β-Glucuronidase Activity
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Pectin Isolation
2.3. Cell Culture
2.4. Cell Viability Assay
2.5. Isobolographic Analysis
2.6. Flow Cytometry Analysis
2.7. Caspase-3 Activation Assay
2.8. Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. E. coli Strains
2.10. Adherence Assay
2.11. E. coli Proliferation
2.12. β-Glucuronidase (GUS) Activity Assay
2.13. Statistical Analysis
3. Results
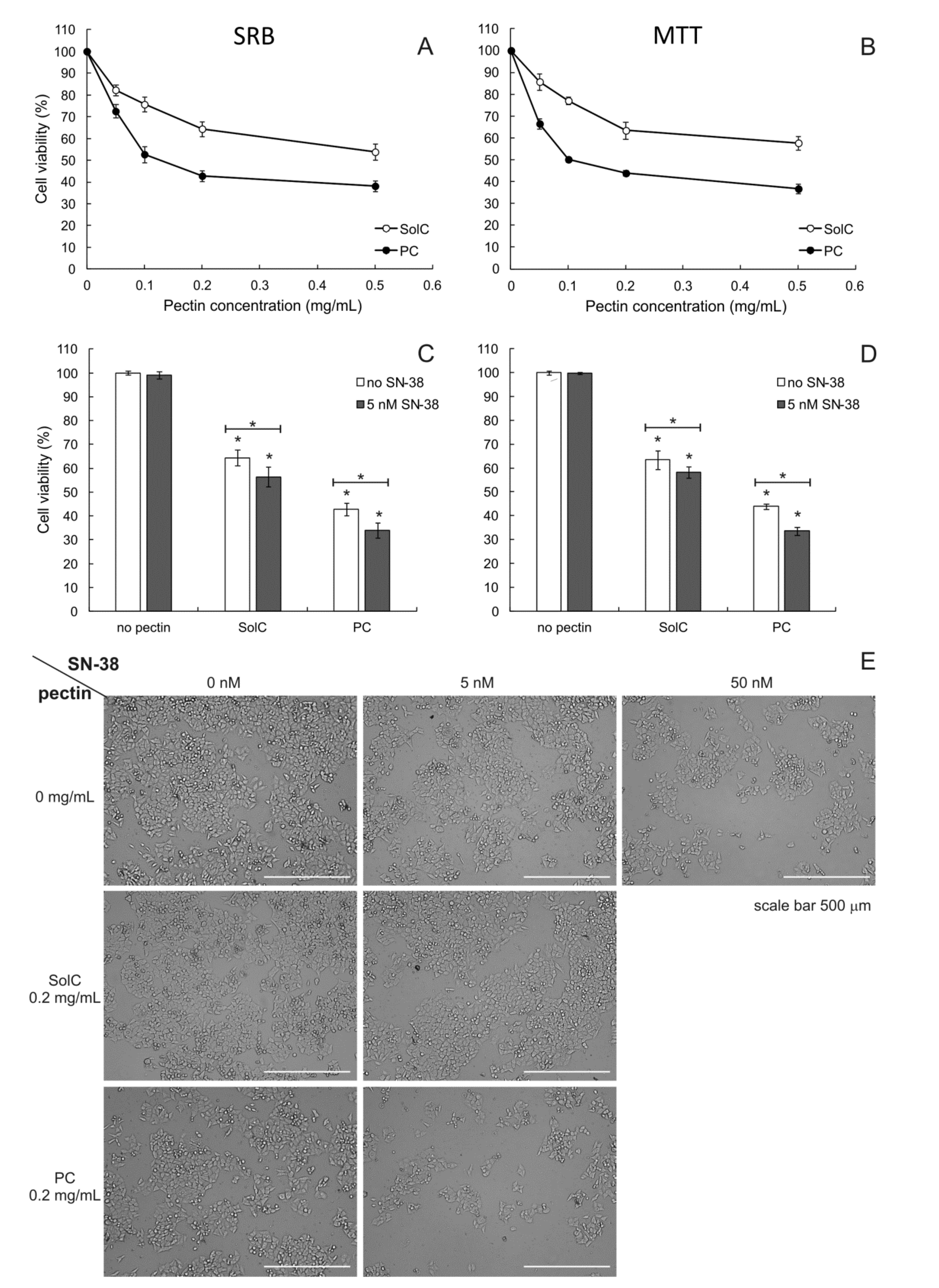
3.1. Anticancer Activity of Pectins
3.1.1. Cytotoxicity of Pectins
3.1.2. Cytotoxicity of Pectins Combined with SN-38
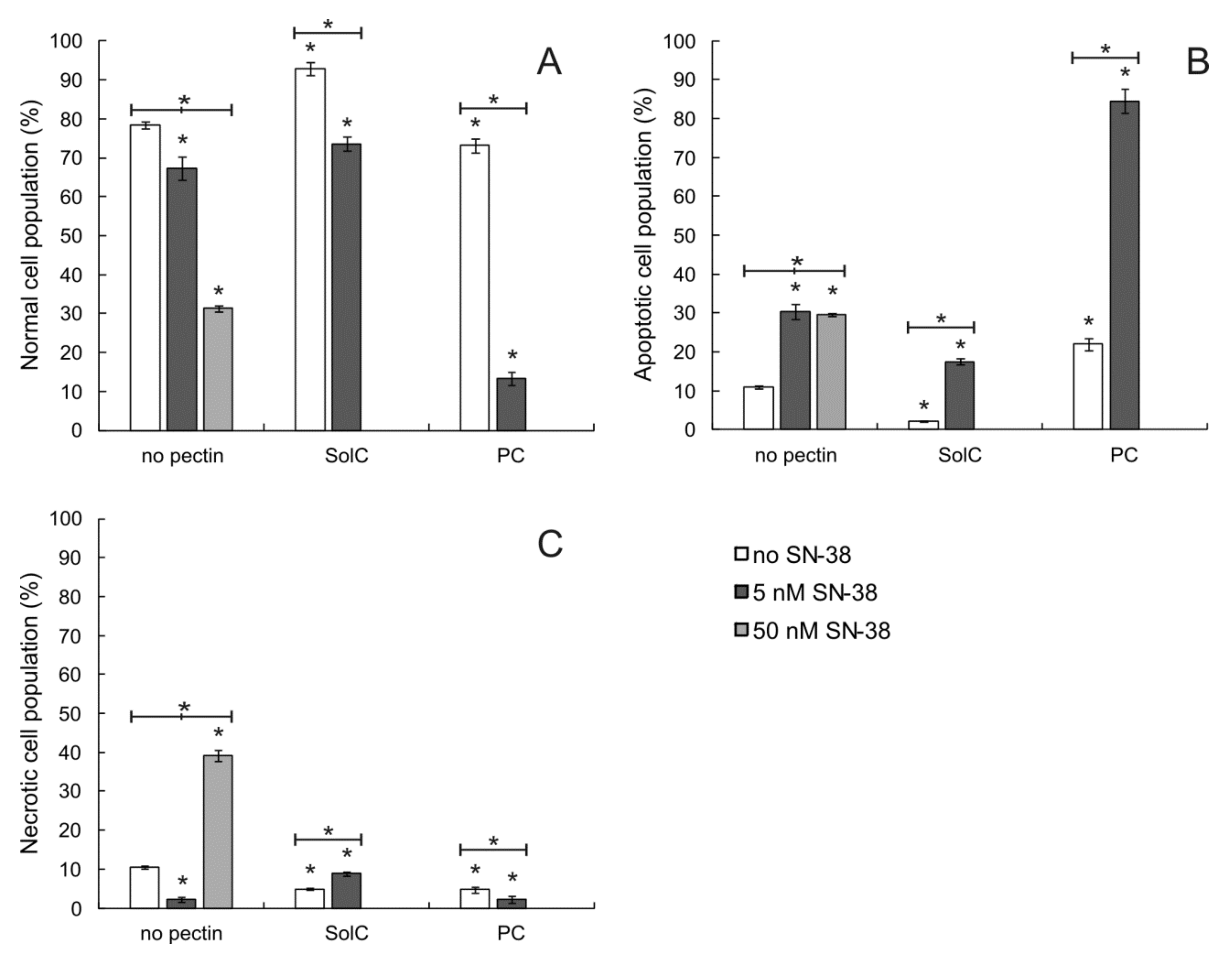
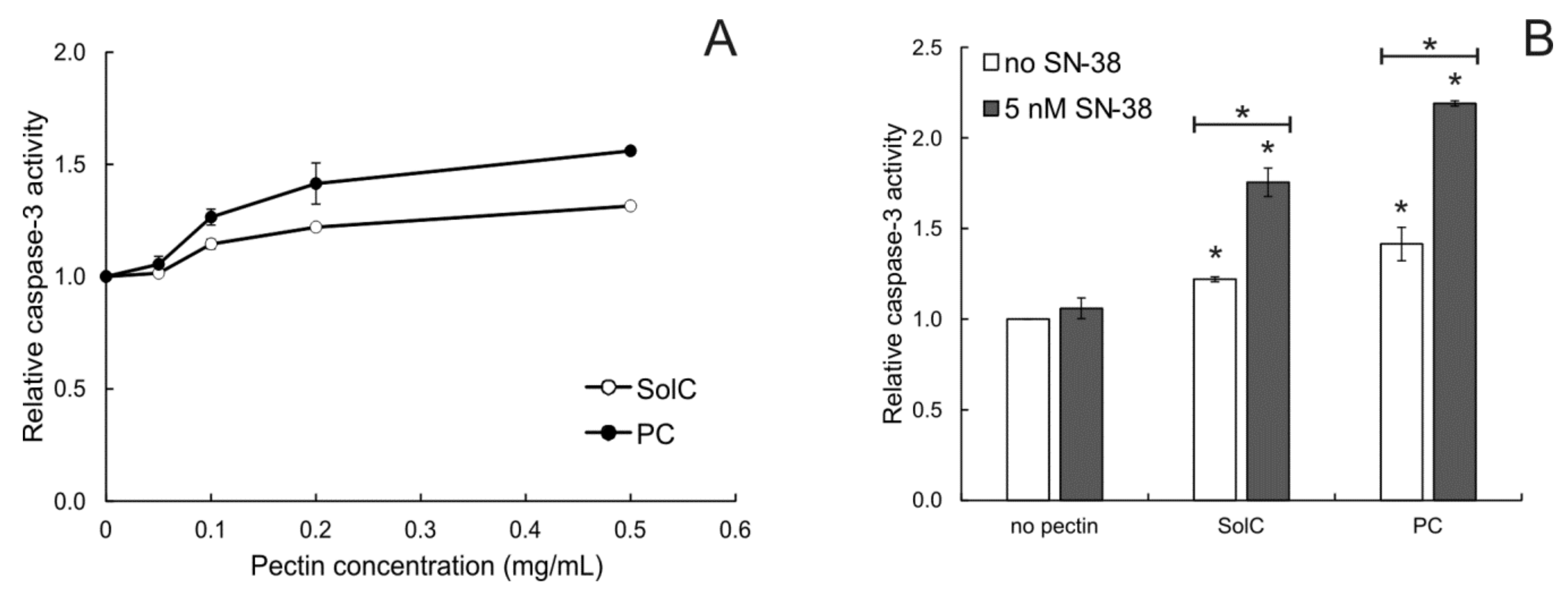
3.2. Proapoptotic Activity of Pectins
3.2.1. Annexin V/PI Double Staining Assay
3.2.2. Caspase-3 Activation
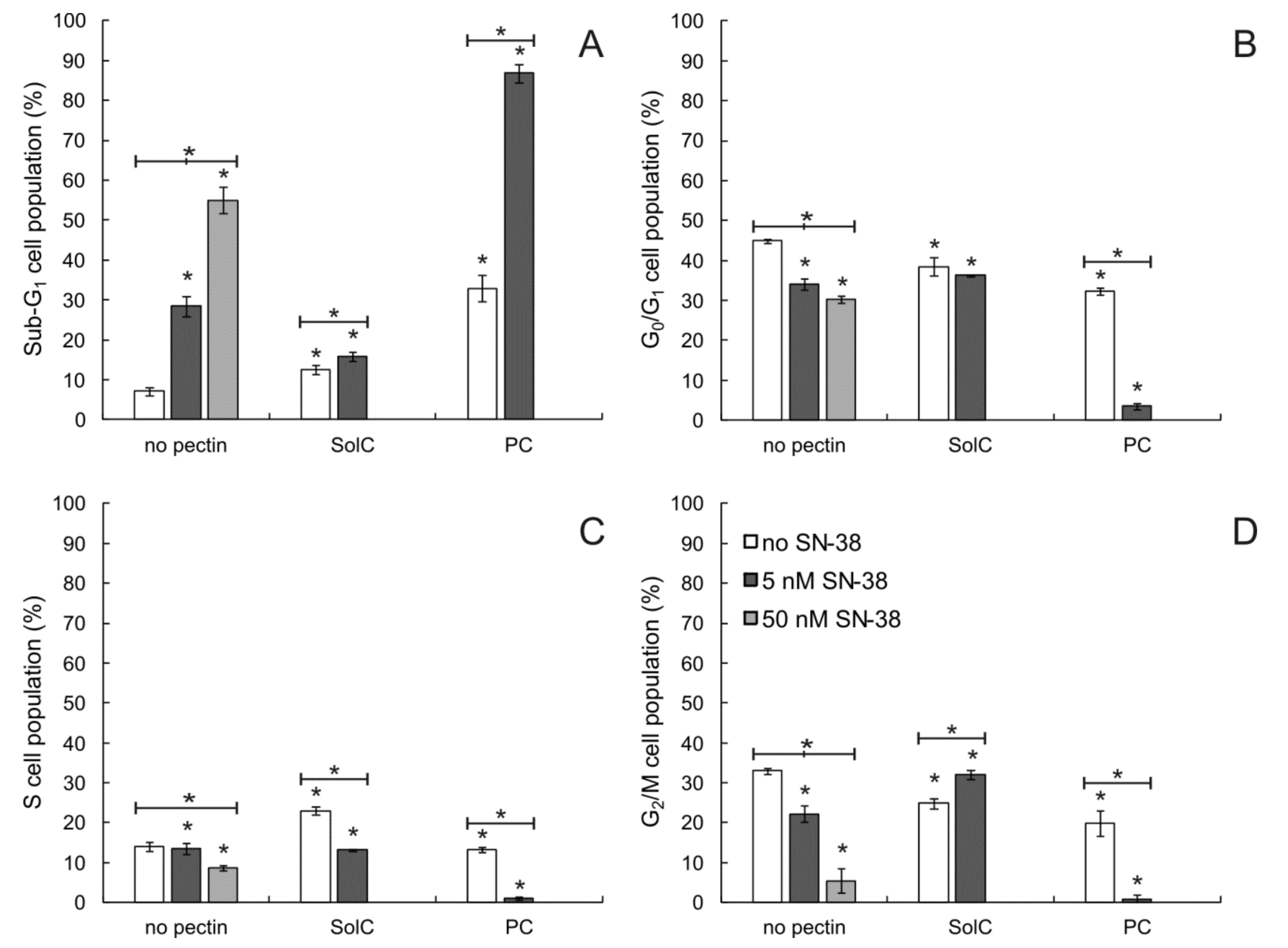
3.3. The Influence of Pectins on Cell Cycle
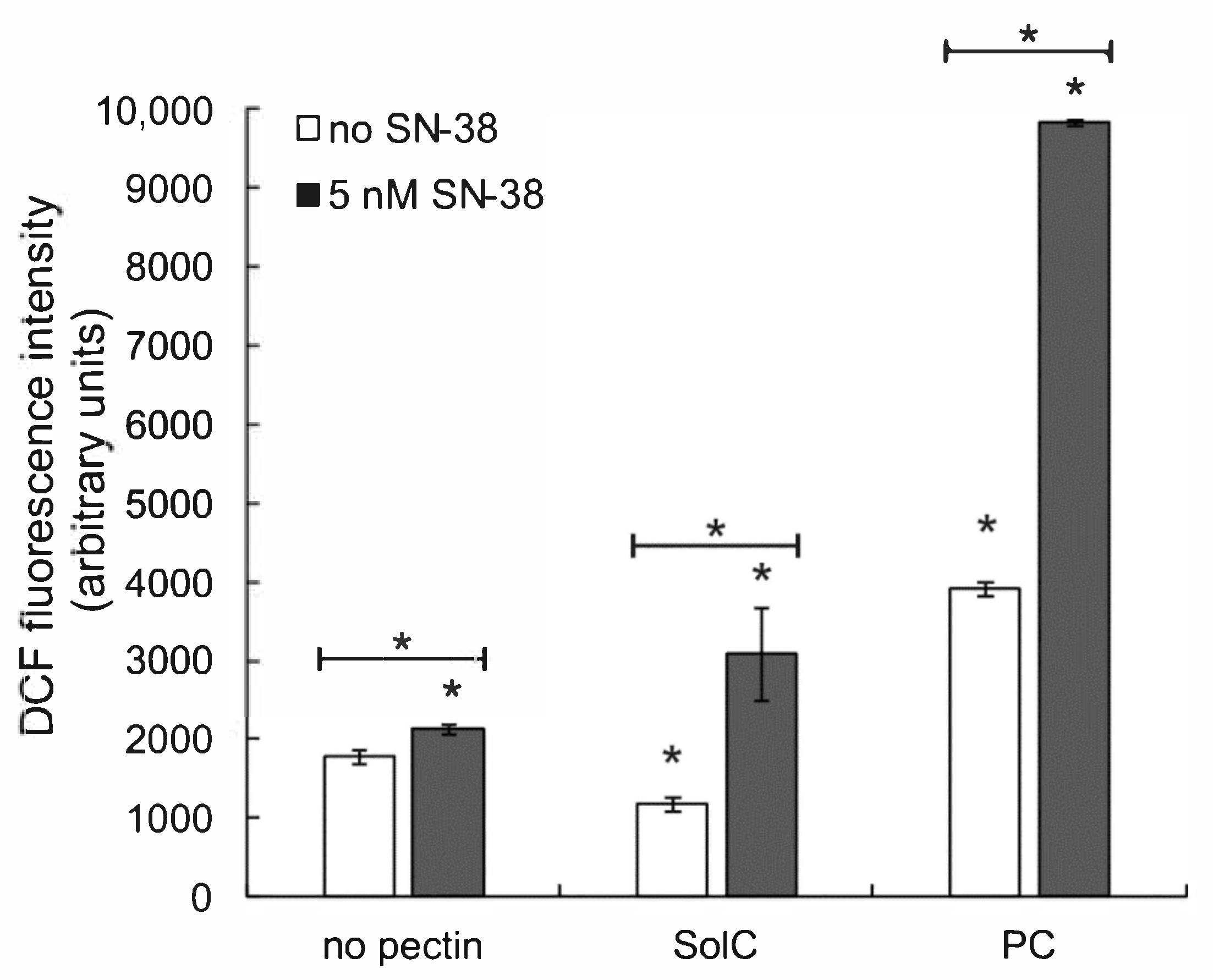
3.4. The Effect of Pectins on Reactive Oxygen Species (ROS) Production
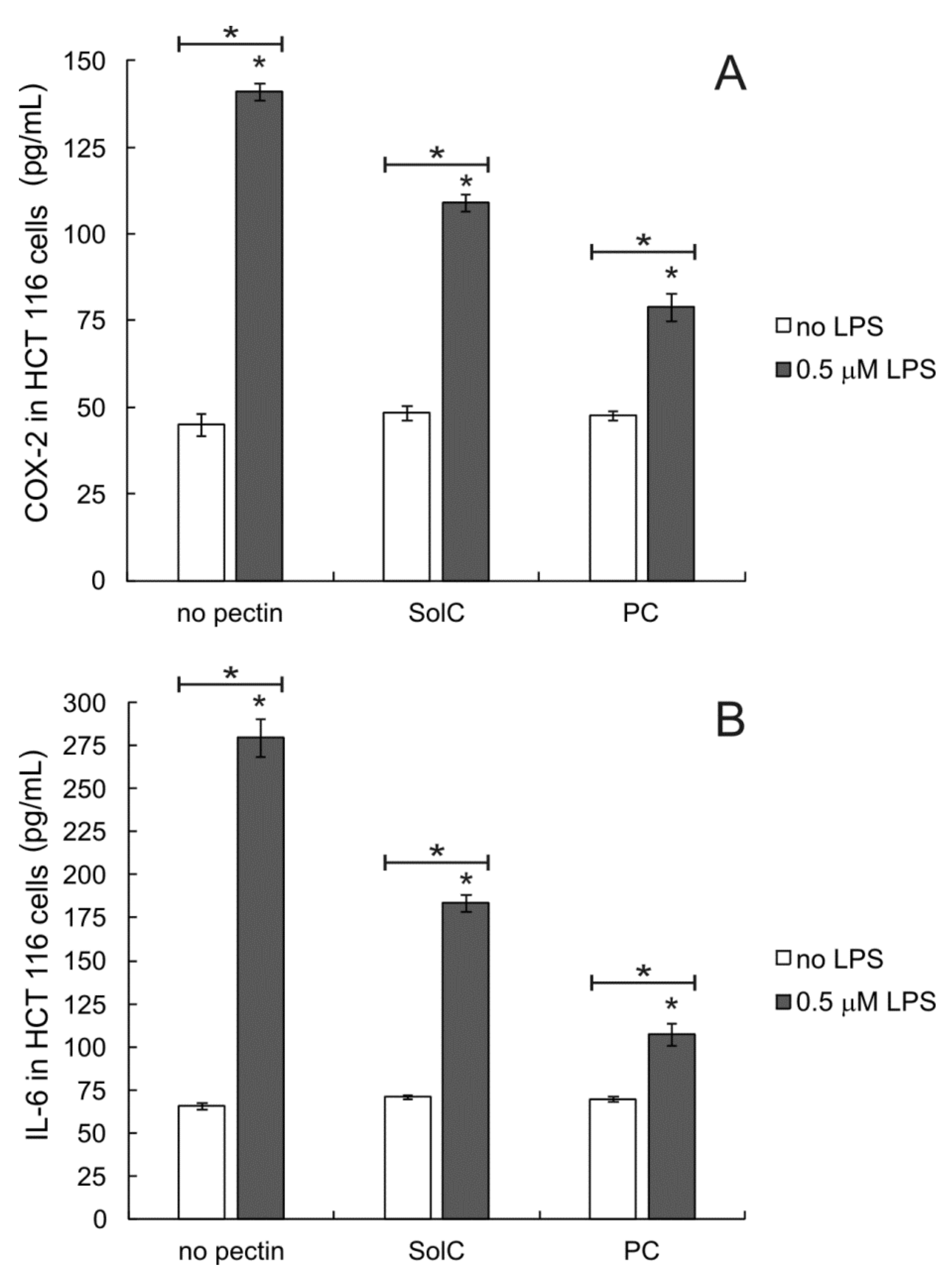
3.5. Anti-Inflammatory Activity of Pectins
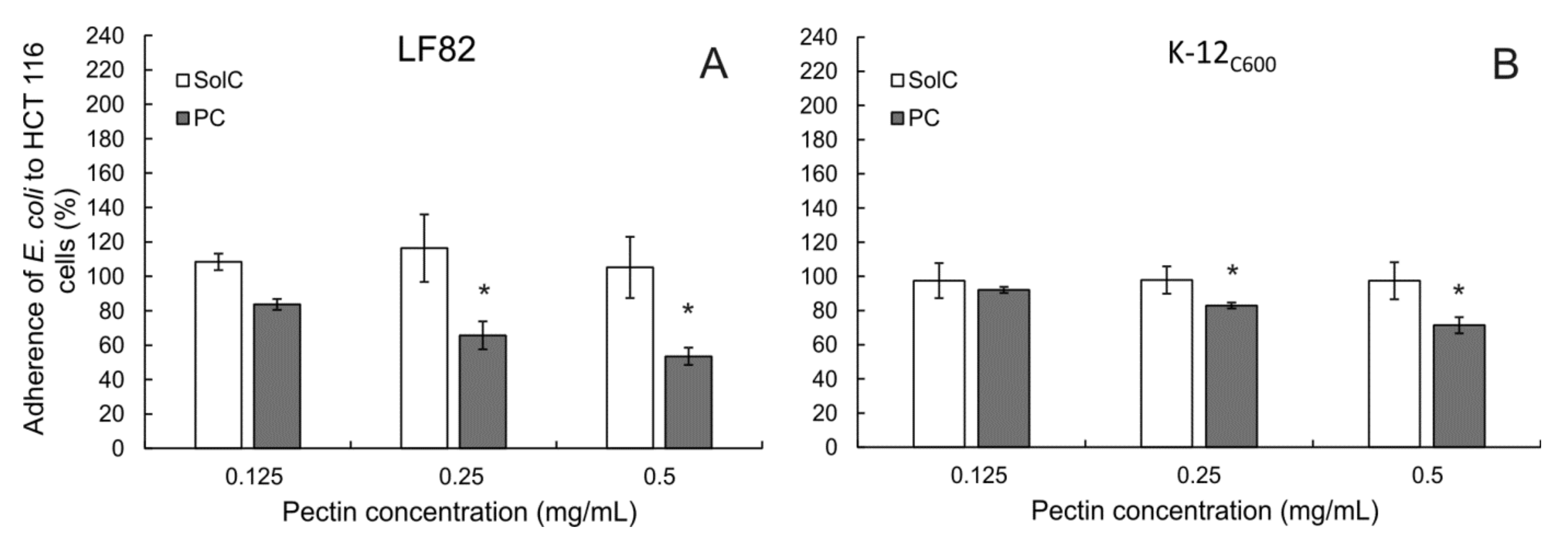
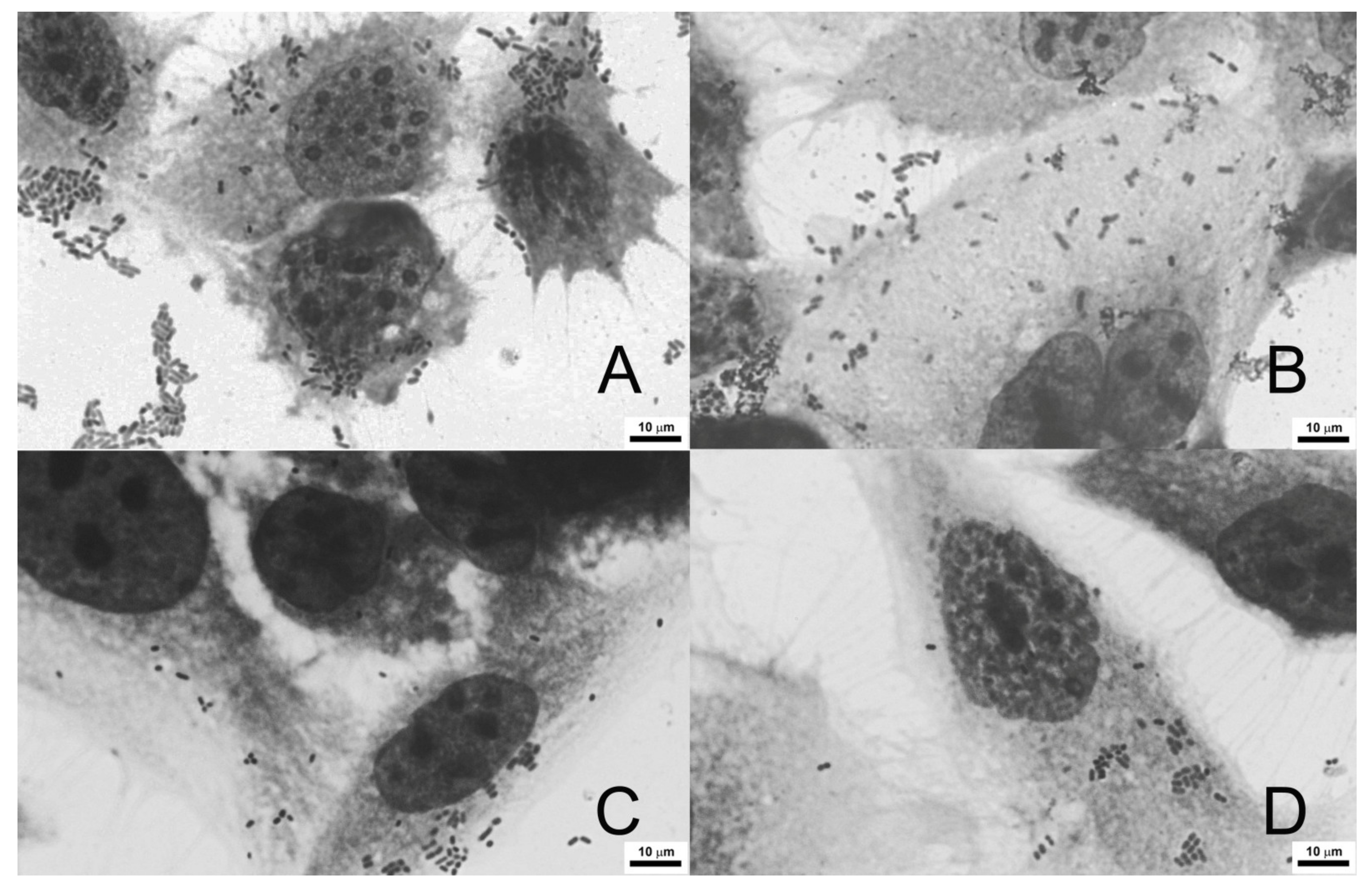
3.6. Adherence of E. coli to Colon Cancer Cells
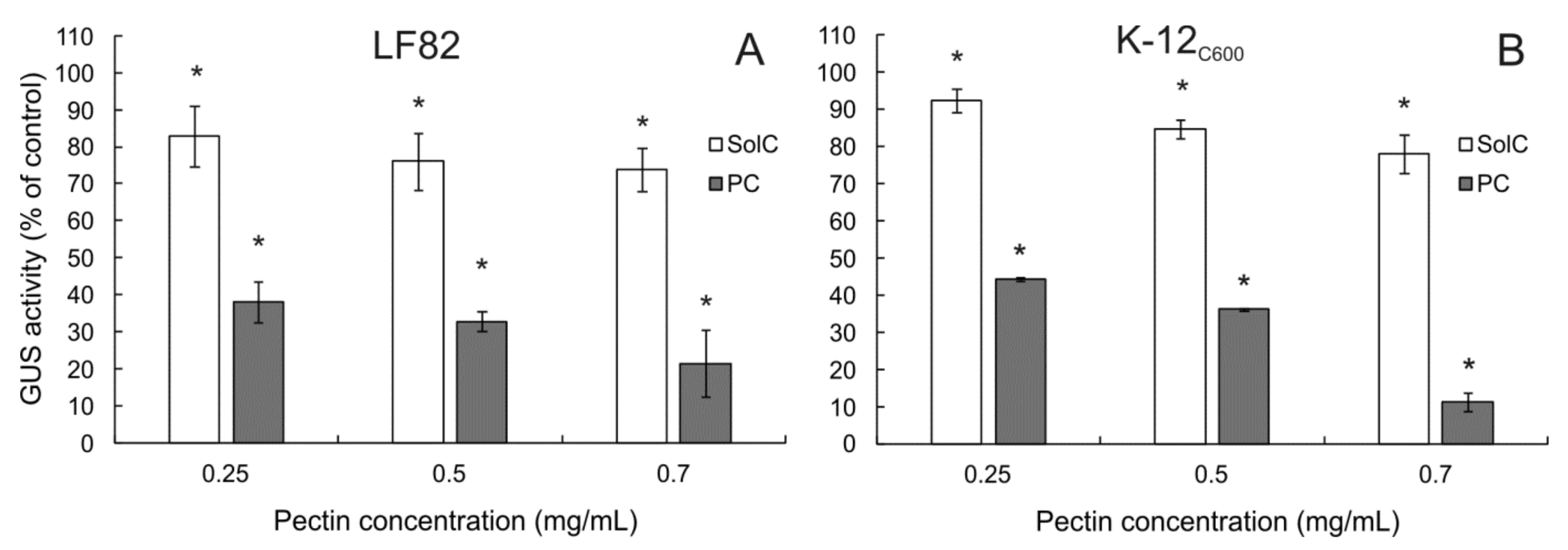
3.7. The Effect of Pectins on β-Glucuronidase (GUS) Activity
4. Discussion
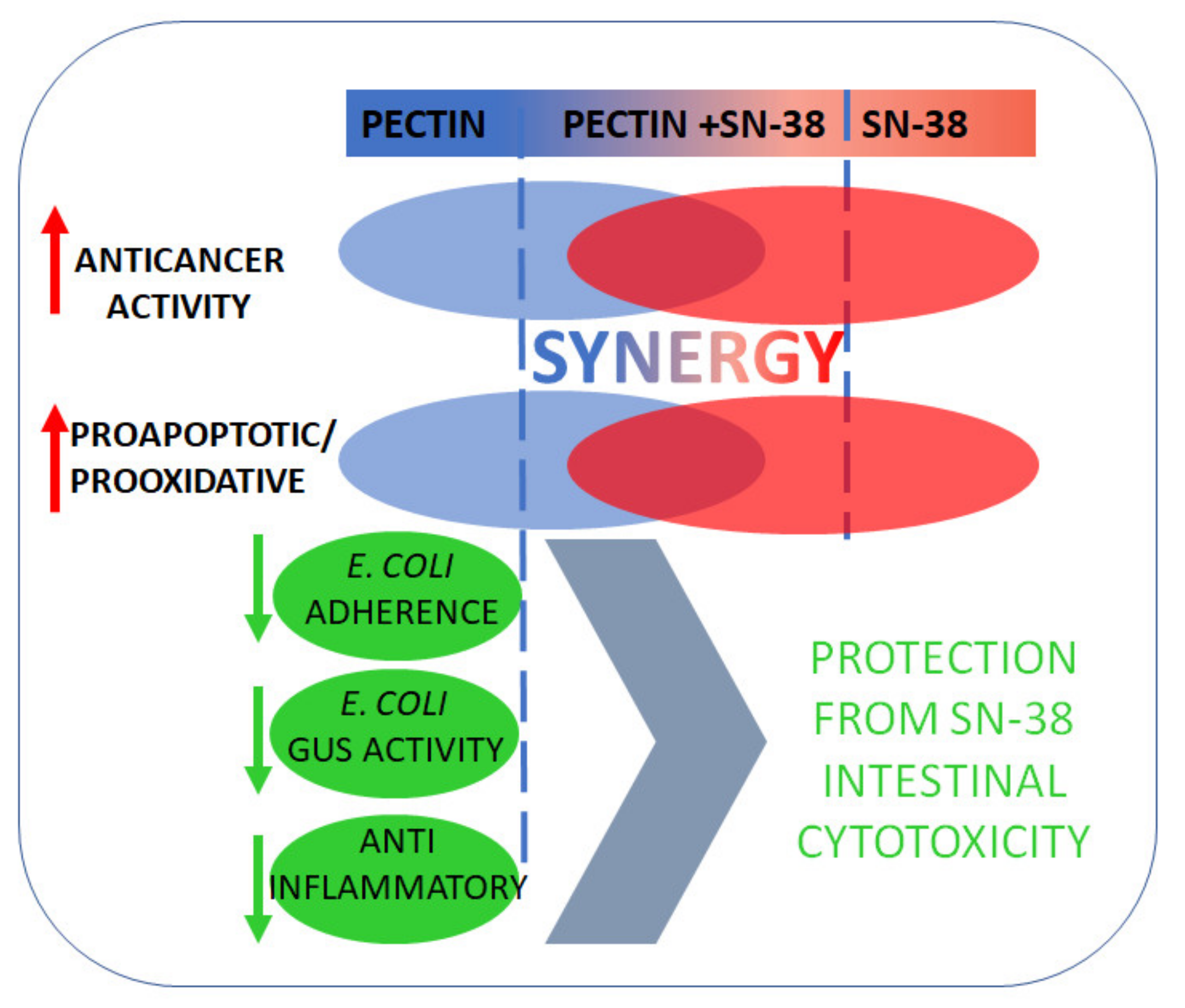
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cancer Today. Available online: https://gco.iarc.fr/today/fact-sheets-cancers (accessed on 14 March 2021).
- Recio-Boiles, A.; Cagir, B. Colon Cancer. In Stat Pearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Fantini, M.C.; Guadagni, I. From inflammation to colitis-associated colorectal cancer in inflammatory bowel disease: Pathogenesis and impact of current therapies. Dig. Liver Dis. 2021, 53, 558–565. [Google Scholar] [CrossRef]
- Peddareddigari, V.G.; Wang, D.; Dubois, R.N. The tumor microenvironment in colorectal carcinogenesis. Cancer Microenviron. 2010, 3, 149–166. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Cai, X.; Zhang, J.; Wang, W.; Sheng, Q.; Hua, H.; Zhou, X. Role of gut microbiota in the development and treatment of colorectal cancer. Digestion 2019, 100, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Heo, G.; Lee, Y.; Im, E. Interplay between the gut microbiota and inflammatory mediators in the development of colorectal cancer. Cancers 2021, 13, 734. [Google Scholar] [CrossRef] [PubMed]
- Hibberd, A.A.; Lyra, A.; Ouwehand, A.C.; Rolny, P.; Lindegren, H.; Cedgard, L.; Wettergren, Y. Intestinal microbiota is altered in patients with colon cancer and modified by probiotic intervention. BMJ Open Gastroenterol. 2017, 4, e000145. [Google Scholar] [CrossRef]
- Karwowska, Z.; Szemraj, J.; Karwowski, B. Microbiota alterations in gastrointestinal cancers. Appl. Sci. 2020, 10, 585. [Google Scholar] [CrossRef]
- Lehouritis, P.; Cummins, J.; Stanton, M.; Murphy, C.T.; McCarthy, F.O.; Reid, G.; Urbaniak, C.; Byrne, W.L.; Tangney, M. Local bacteria affect the efficacy of chemotherapeutic drugs. Sci. Rep. 2015, 5, 14554. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.L.; Wilson, I.D.; Teare, J.; Marchesi, J.R.; Nicholson, J.K.; Kinross, J.M. Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 356–365. [Google Scholar] [CrossRef]
- Kanemitsu, Y.; Shitara, K.; Mizusawa, J.; Hamaguchi, T.; Shida, D.; Komori, K.; Ikeda, S.; Ojima, H.; Ike, H.; Shiomi, A.; et al. Primary tumor resection plus chemotherapy versus chemotherapy alone for colorectal cancer patients with asymptomatic, synchro-nous unresectable metastases (JCOG1007; iPACS): A randomized clinical trial. J. Clin. Oncol. 2021, 39, 1098–1107. [Google Scholar] [CrossRef]
- Rivory, L.P. Irinotecan (CPT-11): A brief overview. Clin. Exp. Pharmacol. Physiol. 1996, 23, 1000–1004. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; van Krieken, J.H.; Aderka, D.; Aranda Aguilar, E.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef]
- Yoshino, T.; Arnold, D.; Taniguchi, H.; Pentheroudakis, G.; Yamazaki, K.; Xu, R.H.; Kim, T.W.; Ismail, F.; Tan, I.B.; Yeh, K.H.; et al. Pan-Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: A JSMO-ESMO Initiative Endorsed by CSCO, KACO, MOS, SSO and TOS. Ann. Oncol. 2018, 29, 44–70. [Google Scholar] [CrossRef]
- Gibson, R.J.; Stringer, A.M. Chemotherapy-induced diarrhoea. Curr. Opin. Support. Palliat. 2009, 3, 31–35. [Google Scholar] [CrossRef]
- Stein, A.; Voigt, W.; Jordan, K. Review: Chemotherapy-induced diarrhea: Pathophysiology, frequency and guideline-based management. Ther. Adv. Med. Oncol. 2010, 2, 51–63. [Google Scholar] [CrossRef]
- Guthrie, L.; Gupta, S.; Daily, J.; Kelly, L. Human microbiome signatures of differential colorectal cancer drug metabolism. NPJ Biofilms Microbiomes 2017, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.T.; Chen, K.C.; Cheng, C.M.; Cheng, T.L.; Tao, M.H.; Roffler, S.R. impediments to enhancement of CPT-11 anticancer activity by E. coli directed beta-glucuronidase therapy. PLoS ONE 2015, 10, e0118028. [Google Scholar] [CrossRef]
- Willats, W.G.T.; Mccartney, L.; Mackie, W.; Knox, J.P. Pectin: Cell biology and prospects for functional analysis. Plant. Mol. Biol. 2001, 47, 9–27. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.A.; Binhamad, H.A.S. Isolation and characterisation of pectin. In Pectin: Technological and Physiological Properties; Kontogiorgos, V., Ed.; Springer Nature: Basingstoke, UK, 2020; pp. 61–82. [Google Scholar] [CrossRef]
- Eliaz, I.; Raz, A. Pleiotropic effects of modified citrus pectin. Nutrients 2019, 11, 2619. [Google Scholar] [CrossRef]
- Beukema, M.; Faas, M.M.; de Vos, P. The effects of different dietary fiber pectin structures on the gastrointestinal immune barrier: Impact via gut microbiota and direct effects on immune cells. Exp. Mol. Med. 2020, 52, 1364–1376. [Google Scholar] [CrossRef]
- Wikiera, A.; Mika, M.; Starzyńska-Janiszewska, A.; Stodolak, B. Endo-Xylanase and endo-cellulase-assisted extraction of pectin from apple pomace. Carbohydr. Polym. 2016, 142, 199–205. [Google Scholar] [CrossRef]
- Wikiera, A.; Grabacka, M.; Byczyński, Ł.; Stodolak, B.; Mika, M. Enzymatically extracted apple pectin possesses antioxidant and antitumor activity. Molecules 2021, 26, 1434. [Google Scholar] [CrossRef] [PubMed]
- Hossein, G.; Halvaei, S.; Heidarian, Y.; Dehghani-Ghobadi, Z.; Hassani, M.; Hosseini, H.; Naderi, N.; Sheikh Hassani, S. Pectasol-C modified citrus pectin targets galectin-3-induced STAT3 activation and synergize paclitaxel cytotoxic effect on ovarian cancer spheroids. Cancer Med. 2019, 8, 4315–4329. [Google Scholar] [CrossRef] [PubMed]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; Mcmahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef]
- Palko-Labuz, A.; Sroda-Pomianek, K.; Uryga, A.; Kostrzewa-Suslow, E.; Michalak, K. Anticancer activity of baicalein and luteolin studied in colorectal adenocarcinoma LoVo cells and in drug-resistant LoVo/Dx cells. Biomed. Pharmacother. 2017, 88, 232–241. [Google Scholar] [CrossRef]
- Chou, T.C.; Martin, N. CompuSyn for Drug Combinations: PC Software and User’s Guide: A Computer Program for Quantitation of Synergism and Antagonism In Drug Combinations, and the Determination of IC50 and ED50 and LD50 Values; ComboSyn Inc.: Paramus, NJ, USA, 2005. [Google Scholar]
- Aich, S.; Delbaere, L.T.J.; Chen, R. Continuous spectrophotometric assay for β-glucuronidase. Biotechniques 2001, 30, 846–850. [Google Scholar] [CrossRef]
- Leclere, L.; van Cutsem, P.; Michiels, C. Anti-cancer activities of pH- or heat-modified pectin. Front. Pharmacol. 2013, 4, 128. [Google Scholar] [CrossRef]
- Cheng, H.; Li, S.; Fan, Y.; Gao, X.; Hao, M.; Wang, J.; Zhang, X.; Tai, G.; Zhou, Y. comparative studies of the antiproliferative effects of ginseng polysaccharides on HT-29 human colon cancer cells. Med. Oncol. 2011, 28, 175–181. [Google Scholar] [CrossRef]
- Cheng, H.; Zhang, Z.; Leng, J.; Liu, D.; Hao, M.; Gao, X.; Tai, G.; Zhou, Y. The inhibitory effects and mechanisms of rhamnogalacturonan I pectin from potato on HT-29 colon cancer cell proliferation and cell cycle progression. Int. J. Food Sci. Nutr. 2013, 64, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, E.G.; Colquhoun, I.J.; Chau, H.K.; Hotchkiss, A.T.; Waldron, K.W.; Morris, V.J.; Belshaw, N.J. Modified sugar beet pectin induces apoptosis of colon cancer cells via an interaction with the neutral sugar side-chains. Carbohydr. Polym. 2016, 136, 923–929. [Google Scholar] [CrossRef]
- Naqash, F.; Masoodi, F.A.; Rather, S.A.; Wani, S.M.; Gani, A. Emerging concepts in the nutraceutical and functional properties of pectin—A review. Carbohydr. Polym. 2017, 168, 227–239. [Google Scholar] [CrossRef]
- Motwani, M.; Jung, C.; Shah, M.A.; Schwartz, G.K.; Sirotnak, F.M.; She, Y.; Gonen, M. Augmentation of apoptosis and tumor regression by flavopiridol in the presence of CPT-11 in HCT116 colon cancer monolayers and xenografts. Clin. Cancer Res. 2001, 7, 4209–4219. [Google Scholar] [PubMed]
- Hossein, G.; Keshavarz, M.; Ahmadi, S.; Naderi, N. Synergistic effects of PectaSol-C modified citrus pectin an inhibitor of galectin-3 and paclitaxel on apoptosis of human SKOV-3 ovarian cancer cells. Asian Pac. J. Cancer Prev. 2013, 14, 7561–7568. [Google Scholar] [CrossRef]
- Tehranian, N.; Sepehri, H.; Mehdipour, P.; Biramijamal, F.; Hossein-Nezhad, A.; Sarrafnejad, A.; Hajizadeh, E. Combination effect of PectaSol and doxorubicin on viability, cell cycle arrest and apoptosis in DU-145 and LNCaP prostate cancer cell lines. Cell. Biol. Int. 2012, 36, 601–610. [Google Scholar] [CrossRef]
- Jackson, C.L.; Dreaden, T.M.; Theobald, L.K.; Tran, N.M.; Beal, T.L.; Eid, M.; Gao, M.Y.; Shirley, R.B.; Stoffel, M.T.; Kumar, M.V.; et al. Pectin induces apoptosis in human prostate cancer cells: Correlation of apoptotic function with pectin structure. Glycobiology 2007, 17, 805–819. [Google Scholar] [CrossRef]
- Yan, J.; Katz, A. PectaSol-C modified citrus pectin induces apoptosis and inhibition of proliferation in human and mouse androgen-dependent and-independent prostate cancer cells. Integr. Cancer Ther. 2010, 9, 197–203. [Google Scholar] [CrossRef]
- Olano-Martin, E.; Rimbach, G.H.; Gibson, G.R.; Rastall, R.A. Pectin and pectic-oligosaccharides induce apoptosis in in vitro human colonic adenocarcinoma cells. Anticancer Res. 2003, 23, 341–346. [Google Scholar]
- Ogutu, F.O.; Mu, T.H.; Sun, H.; Zhang, M. Ultrasonic modified sweet potato pectin induces apoptosis like cell death in colon cancer (HT-29) cell line. Nutr. Cancer 2018, 70, 136–145. [Google Scholar] [CrossRef]
- Fang, T.; Liu, D.; Ning, H.M.; Liu, D.; Sun, J.Y.; Huang, X.J.; Dong, Y.; Geng, M.Y.; Yun, S.F.; Yan, J.; et al. Modified citrus pectin inhibited bladder tumor growth through downregulation of galectin-3. Acta Pharmacol. Sin. 2018, 39, 1885–1893. [Google Scholar] [CrossRef]
- Avivi-Green, C.; Madar, Z.; Schwartz, B. Pectin-enriched diet affects distribution and expression of apoptosis-cascade proteins in colonic crypts of dimethylhydrazine-treated rats. Int. J. Mol. Med. 2000, 6, 689–698. [Google Scholar] [CrossRef]
- Dumic, J.; Dabelic, S.; Flogel, M. Galectin-3: An open-ended story. Biochim. Biophys. Acta 2006, 1760, 616–635. [Google Scholar] [CrossRef]
- Newlaczyl, A.U.; Yu, L.G. Galectin-3—A jack-of-all-trades in cancer. Cancer Lett. 2011, 313, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Minzanova, S.; Mironov, V.; Arkhipova, D.; Khabibullina, A.; Mironova, L.; Zakirova, Y.; Milyukov, V. Biological activity and pharmacological application of pectic polysaccharides: A review. Polymers 2018, 10, 1407. [Google Scholar] [CrossRef]
- Conti, S.; Vexler, A.; Hagoel, L.; Kalich-Philosoph, L.; Corn, B.W.; Honig, N.; Shtraus, N.; Meir, Y.; Ron, I.; Eliaz, I.; et al. Modified citrus pectin as a potential sensitizer for radiotherapy in prostate cancer. Integr. Cancer Ther. 2018, 17, 1225–1234. [Google Scholar] [CrossRef]
- Salehi, F.; Behboudi, H.; Kavoosi, G.; Ardestani, S.K. Oxidative DNA damage induced by ROS-modulating agents with the ability to target DNA: A comparison of the biological characteristics of citrus pectin and apple pectin. Sci. Rep. 2018, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Davoodi, H.; Hashemi, S.R.; Seow, H.F. Increased NFκ-B activity in HCT116 colorectal cancer cell line harboring TLR4 Asp299Gly variant. Iran. J. Allergy Asthma Immunol. 2012, 11, 121–132. [Google Scholar]
- Chung, Y.H.; Kim, D. Enhanced TLR4 expression on colon cancer cells after chemotherapy promotes cell survival and epithelial-mesenchymal transition through phosphorylation of GSK3β. Anticancer Res. 2016, 36, 3383–3394. [Google Scholar] [PubMed]
- Lu, Y.C.; Yeh, W.C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef]
- Luo, C.; Zhang, H. The Role of proinflammatory pathways in the pathogenesis of colitis-associated colorectal cancer. Mediat. Inflamm. 2017, 2017, 5126048. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Sheu, M.T.; Chen, T.F.; Wang, Y.C.; Hou, W.C.; Liu, D.Z.; Chung, T.C.; Liang, Y.C. Suppression of endotoxin-induced proinflammatory responses by citrus pectin through blocking LPS signaling pathways. Biochem. Pharmacol. 2006, 72, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fan, L.; Sun, Y.; Zhang, D.; Yue, Z.; Niu, Y.; Meng, J.; Yang, T.; Liu, W.; Mei, Q. An apple oligogalactan suppresses endotoxin-induced cyclooxygenase-2 expression by inhibition of LPS pathways. Int. J. Biol. Macromol. 2013, 61, 75–81. [Google Scholar] [CrossRef]
- Zhang, D.; Li, Y.H.; Mi, M.; Jiang, F.L.; Yue, Z.G.; Sun, Y.; Fan, L.; Meng, J.; Zhang, X.; Liu, L.; et al. Modified apple polysaccharides suppress the migration and invasion of colorectal cancer cells induced by lipopolysaccharide. Nutr. Res. 2013, 33, 839–848. [Google Scholar] [CrossRef]
- Ishisono, K.; Yabe, T.; Kitaguchi, K. Citrus pectin attenuates endotoxin shock via suppression of Toll-Like Receptor signaling in Peyer’s patch myeloid cells. J. Nutr. Biochem. 2017, 50, 38–45. [Google Scholar] [CrossRef]
- Sabater, C.; Molina-Tijeras, J.A.; Vezza, T.; Corzo, N.; Montilla, A.; Utrilla, P. Intestinal anti-inflammatory effects of artichoke pectin and modified pectin fractions in the dextran sulfate sodium model of mice colitis. artificial neural network modelling of inflammatory markers. Food Funct. 2019, 10, 7793–7805. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Chen, W.; Liu, Q.; Yang, G.; Li, K. Pectin Oligosaccharides ameliorate colon cancer by regulating oxidative stress- and inflammation-activated signaling pathways. Front. Immunol. 2018, 9, 1504. [Google Scholar] [CrossRef] [PubMed]
- Cochet, F.; Peri, F. The role of carbohydrates in the lipopolysaccharide (LPS)/Toll-Like Receptor 4 (TLR4) signalling. Int. J. Mol. Sci. 2017, 18, 2318. [Google Scholar] [CrossRef] [PubMed]
- Mazgaeen, L.; Gurung, P. Recent advances in lipopolysaccharide recognition systems. Int. J. Mol. Sci. 2020, 21, 379. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.W.; Colombo, G.; Sonvico, F. Pectin matrix as oral drug delivery vehicle for colon cancer treatment. AAPS PharmSciTech. 2011, 12, 201–214. [Google Scholar] [CrossRef]
- Ferrario, C.; Statello, R.; Carnevali, L.; Mancabelli, L.; Milani, C.; Mangifesta, M.; Duranti, S.; Lugli, G.A.; Lodge, J.B.; Viappiani, S.; et al. How to feed the mammalian gut microbiota: Bacterial and metabolic modulation by dietary fibers. Front. Microbiol. 2017, 8, 1749. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Mou, H.; Luo, B.; Jiang, X. Inhibition of adhesion of intestinal pathogens (Escherichia coli, Vibrio cholerae, Campylobacter jejuni, and Salmonella typhimurium) by common oligosaccharides. Foodborne Pathog. Dis. 2015, 12, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Rhoades, J.; Manderson, K.; Wells, A.; Hotchkiss, A.T., Jr.; Gibson, G.R.; Formentin, K.; Beer, M.; Rastall, R.A. Oligosaccharide-mediated inhibition of the adhesion of pathogenic Escherichia coli strains to human gut epithelial cells in vitro. J. Food Prot. 2008, 71, 2272–2277. [Google Scholar] [CrossRef]
- Di, R.; Vakkalanka, M.S.; Onumpai, C.; Chau, H.K.; White, A.; Rastall, R.A.; Yam, K.; Hotchkiss, A.T., Jr. Pectic oligosaccharide structure-function relationships: Prebiotics, inhibitors of Escherichia coli O157:H7 adhesion and reduction of Shiga toxin cytotoxicity in HT29 cells. Food Chem. 2017, 227, 245–254. [Google Scholar] [CrossRef]
- Ganan, M.; Collins, M.; Rastall, R.; Hotchkiss, A.T.; Chau, H.K.; Carrascosa, A.V.; Martinez-Rodriguez, A.J. Inhibition by pectic oligosaccharides of the invasion of undifferentiated and differentiated Caco-2 cells by Campylobacter jejuni. Int. J. Food Microbiol. 2010, 137, 181–185. [Google Scholar] [CrossRef]
- Wilkowska, A.; Nowak, A.; Antczak-Chrobot, A.; Motyl, I.; Czyżowska, A.; Paliwoda, A. Structurally different pectic oligosaccharides produced from apple pomace and their biological activity in vitro. Foods 2019, 8, 365. [Google Scholar] [CrossRef]
- Lee, J.H.; Shim, J.S.; Lee, J.S.; Kim, M.K.; Chung, M.S.; Kim, K.H. Pectin-like acidic polysaccharide from Panax ginseng with selective antiadhesive activity against pathogenic bacteria. Carbohydr. Res. 2006, 341, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.B.; Dieleman, L.A.; Ketabi, A.; Bibova, I.; Sawyer, M.B.; Xue, H.; Field, C.J.; Baracos, V.E.; Ganzle, M.G. Irinotecan (CPT-11) chemotherapy alters intestinal microbiota in tumour bearing rats. PLoS ONE 2012, 7, e39764. [Google Scholar] [CrossRef]
- Dashnyam, P.; Mudududdla, R.; Hsieh, T.J.; Lin, T.C.; Lin, H.Y.; Chen, P.Y.; Hsu, C.Y.; Lin, C.H. β-Glucuronidases of opportunistic bacteria are the major contributors to xenobiotic-induced toxicity in the gut. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wallace, B.D.; Wang, H.; Lane, K.T.; Scott, J.E.; Orans, J.; Koo, J.S.; Venkatesh, M.; Jobin, C.; Yeh, L.A.; Mani, S.; et al. Alleviating cancer drug toxicity by inhibiting a bacterial enzyme. Science 2010, 330, 831–835. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.P.; Pellock, S.J.; Biernat, K.A.; Walton, W.G.; Wallace, B.D.; Creekmore, B.C.; Letertre, M.M.; Swann, J.R.; Wilson, I.D.; Roques, J.R.; et al. Targeted inhibition of gut bacterial β-glucuronidase activity enhances anticancer drug efficacy. Proc. Natl. Acad. Sci. USA 2020, 117, 7374–7381. [Google Scholar] [CrossRef]
- Ross, J.K.; Leklem, J.E. The effect of dietary citrus pectin on the excretion of human fecal neutral and acid steroids and the activity of 7alpha-dehydroxylase and beta-glucuronidase. Am. J. Clin. Nutr. 1981, 34, 2068–2077. [Google Scholar] [CrossRef]
- Borisenkov, M.F.; Bakutova, L.A.; Latkin, D.S.; Golovchenko, V.V.; Vityazev, F.V. Interaction of microbial β-glucuronidase with vegetable pectins. J. Agric. Food Chem. 2011, 59, 9922–9926. [Google Scholar] [CrossRef]
- Bauer, H.G.; Asp, N.G.; Dahlqvist, A.; Fredlund, P.E.; Nyman, M.; Oste, R. Effect of two kinds of pectin and guar gum on 1,2-dimethylhydrazine initiation of colon tumors and on fecal beta-glucuronidase activity in the rat. Cancer Res. 1981, 41, 2518–2523. [Google Scholar] [PubMed]
- Ohno, K.; Narushima, S.; Takeuchi, S.; Itoh, K.; Itoh, T.; Hioki, K.; Nomura, T. Effect of bacterial metabolism in the intestine on colorectal tumors induced by 1,2-dimethylhydrazine in transgenic mice harboring human prototype c-Ha-ras genes. J. Exp. Clin. Cancer Res. 2001, 20, 51–56. [Google Scholar] [PubMed]
- Chen, H.L.; Lin, Y.M.; Wang, Y.C. Comparative effects of cellulose and soluble fibers (pectin, konjac glucomannan, inulin) on fecal water toxicity toward Caco-2 cells, fecal bacteria enzymes, bile acid, and short-chain fatty acids. J. Agric. Food Chem. 2010, 58, 10277–10281. [Google Scholar] [CrossRef] [PubMed]
- Lindop, R.; Tasman-Jones, C.; Thomsen, L.L.; Lee, S.P. Cellulose and pectin alter intestinal beta-glucuronidase (EC 3.2.1.31) in the rat. Br. J. Nutr. 1985, 54, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Ohkami, H.; Tazawa, K.; Yamashita, I.; Shimizu, T.; Murai, K.; Kobashi, K.; Fujimaki, M. Effects of apple pectin on fecal bacterial enzymes in azoxymethane-induced rat colon carcinogenesis. Jpn. J. Cancer Res. 1995, 86, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Tazawa, K.; Okami, H.; Yamashita, I.; Ohnishi, Y.; Kobashi, K.; Fujimaki, M. Effects of apple pectin on fecal enzyme activities and prostaglandin E2 levels in azoxymethane-induced rat colon carcinogenesis. In Food Factors for Cancer Prevention; Ohigashi, H., Osawa, T., Terao, J., Watanabe, S., Yoshikawa, T., Eds.; Springer: Tokyo, Japan, 1997; pp. 178–181. [Google Scholar] [CrossRef]
- Rao, C.V.; Chou, D.; Simi, B.; Ku, H.; Reddy, B.S. Prevention of colonic aberrant crypt foci and modulation of large bowel microbial activity by dietary coffee fiber, inulin and pectin. Carcinogenesis 1998, 19, 1815–1819. [Google Scholar] [CrossRef] [PubMed]
| Concentration (mg/mL) | Ratio | Combination Index (CI) | |
|---|---|---|---|
| SN-38 | SolC | ||
| 1.96 × 10−6 | 0.2 | 102,000:1 | 1000 |
| SN-38 | PC | ||
| 0.96 x 10−6 | 0.1 | 104,400:1 | 0.822 |
| 1.96 x 10−6 | 0.2 | 102,000:1 | 0.779 |
| 3.92 x 10−6 | 0.5 | 127,550:1 | 0.792 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palko-Łabuz, A.; Maksymowicz, J.; Sobieszczańska, B.; Wikiera, A.; Skonieczna, M.; Wesołowska, O.; Środa-Pomianek, K. Newly Obtained Apple Pectin as an Adjunct to Irinotecan Therapy of Colorectal Cancer Reducing E. coli Adherence and β-Glucuronidase Activity. Cancers 2021, 13, 2952. https://doi.org/10.3390/cancers13122952
Palko-Łabuz A, Maksymowicz J, Sobieszczańska B, Wikiera A, Skonieczna M, Wesołowska O, Środa-Pomianek K. Newly Obtained Apple Pectin as an Adjunct to Irinotecan Therapy of Colorectal Cancer Reducing E. coli Adherence and β-Glucuronidase Activity. Cancers. 2021; 13(12):2952. https://doi.org/10.3390/cancers13122952
Chicago/Turabian StylePalko-Łabuz, Anna, Jerzy Maksymowicz, Beata Sobieszczańska, Agnieszka Wikiera, Magdalena Skonieczna, Olga Wesołowska, and Kamila Środa-Pomianek. 2021. "Newly Obtained Apple Pectin as an Adjunct to Irinotecan Therapy of Colorectal Cancer Reducing E. coli Adherence and β-Glucuronidase Activity" Cancers 13, no. 12: 2952. https://doi.org/10.3390/cancers13122952
APA StylePalko-Łabuz, A., Maksymowicz, J., Sobieszczańska, B., Wikiera, A., Skonieczna, M., Wesołowska, O., & Środa-Pomianek, K. (2021). Newly Obtained Apple Pectin as an Adjunct to Irinotecan Therapy of Colorectal Cancer Reducing E. coli Adherence and β-Glucuronidase Activity. Cancers, 13(12), 2952. https://doi.org/10.3390/cancers13122952








