Tumor-Associated Trypsin Inhibitor (TATI) as a Biomarker of Poor Prognosis in Oropharyngeal Squamous Cell Carcinoma Irrespective of HPV Status
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Tissue Microarrays
2.3. HPV Status Determination
2.4. Determination of TATI Serum Concentrations
2.5. IHC of TATI
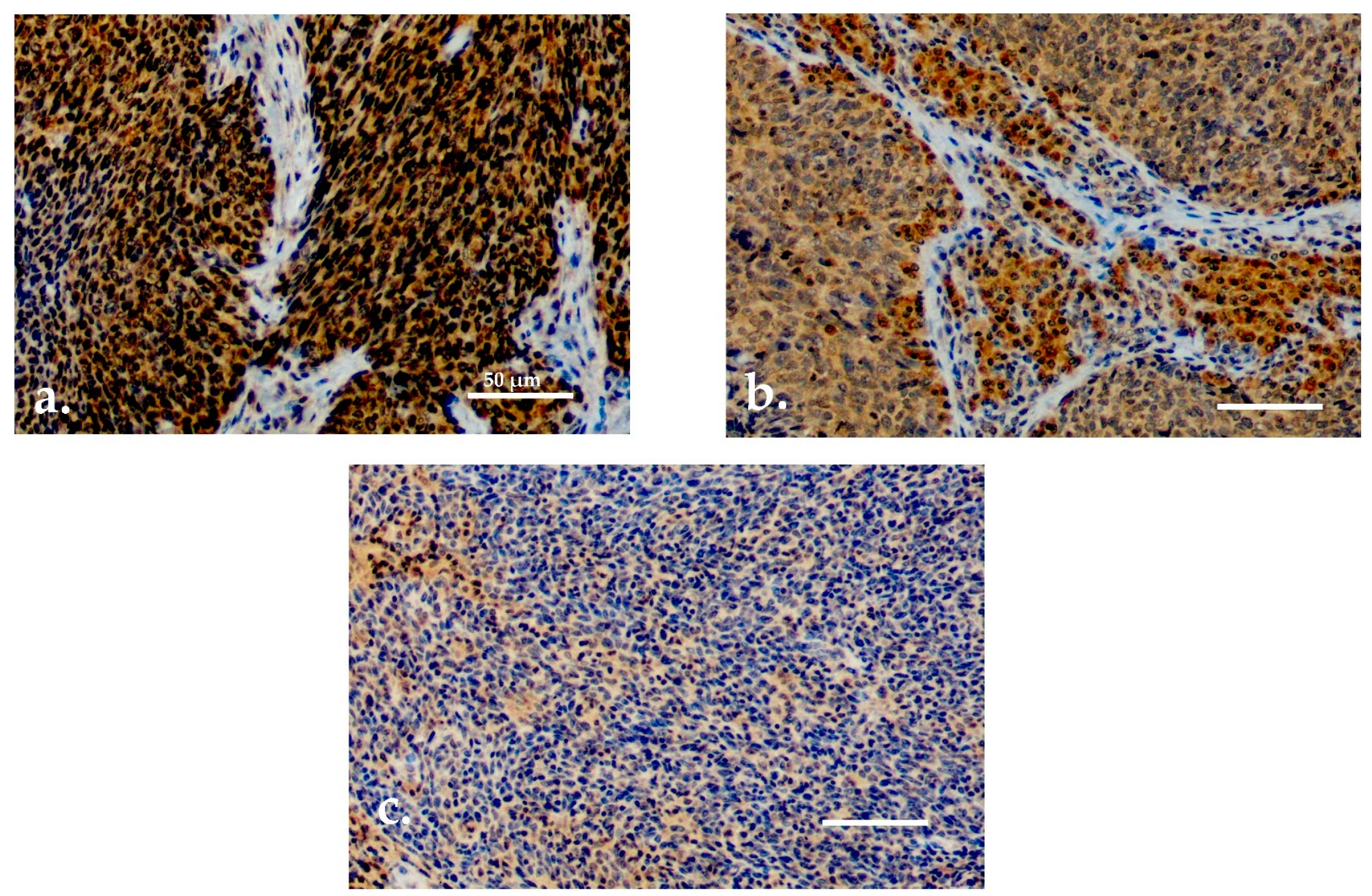
2.6. Sample Scoring
2.7. Data Analysis
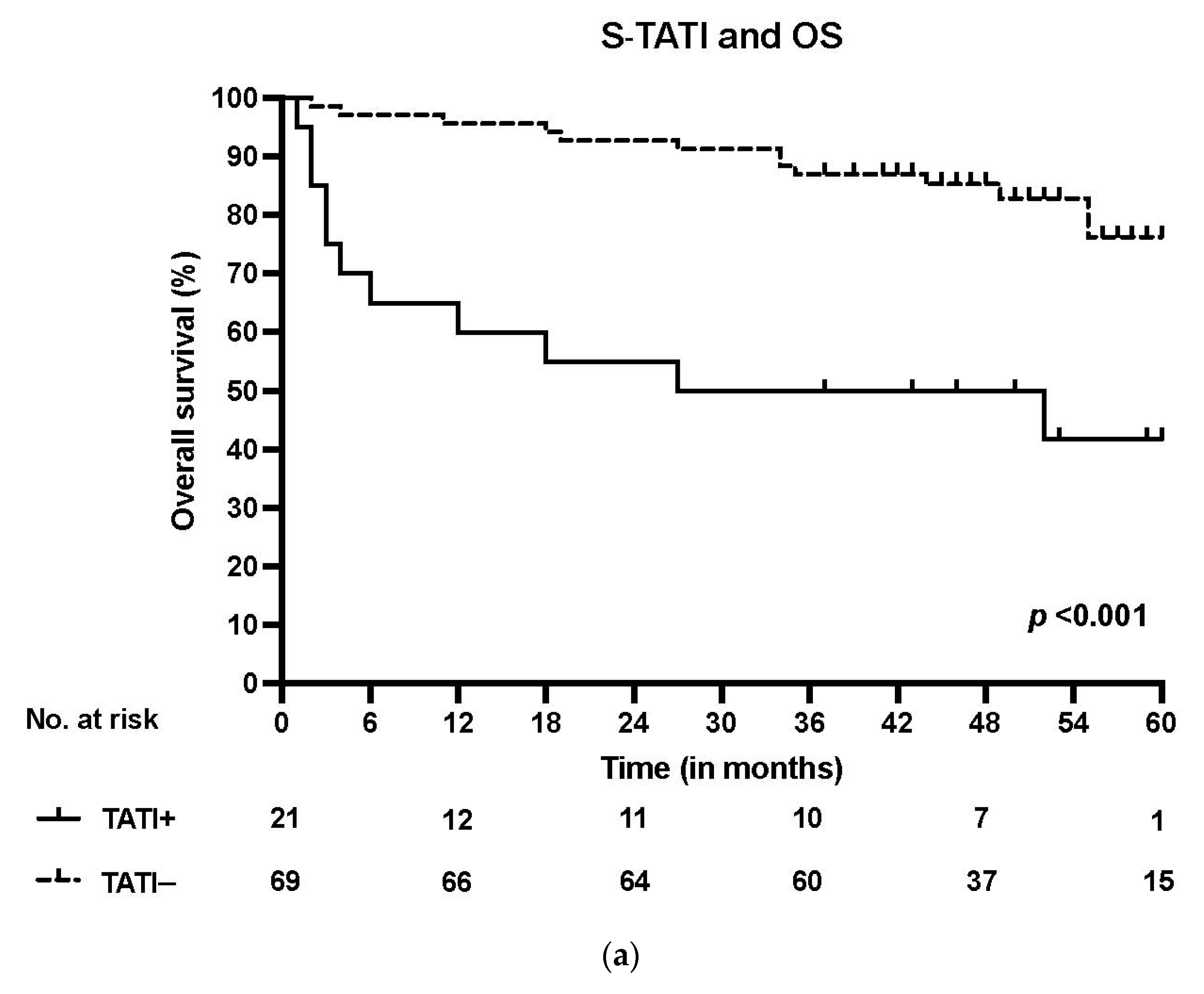
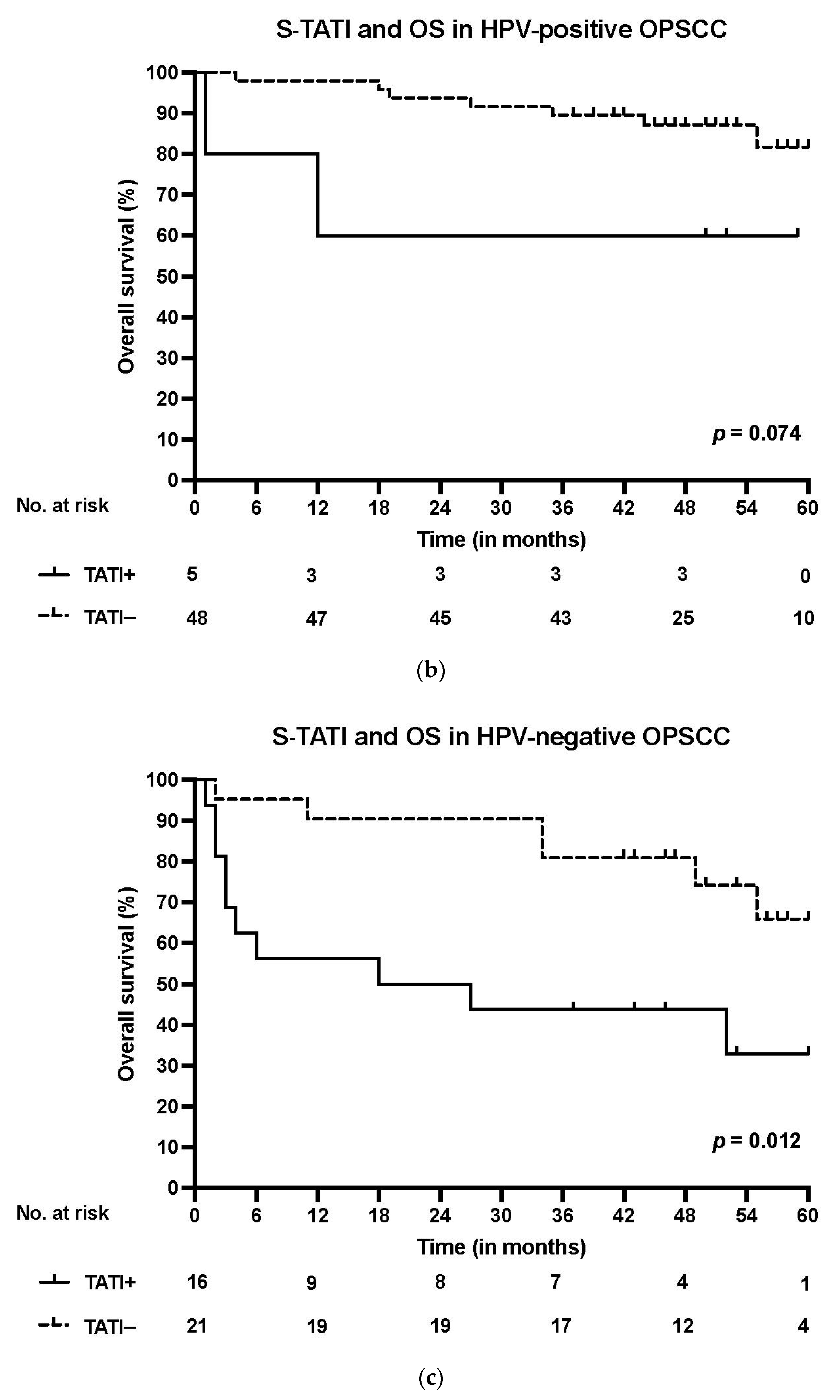
3. Results
3.1. TATI Serum Concentrations and Clinical Characteristics
3.2. TATI Immunoexpression and Clinical Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HIER | Heat-induced epitope retrieval |
| HPV | Human papillomavirus |
| HUS | Helsinki University Hospital |
| IFMA | Immunofluorometric assay |
| Mab | Monoclonal antibody |
| OPSCC | Oropharyngeal squamous cell carcinoma |
| OS | Overall survival |
| SASP | Senescence-associated secretory phenotype |
| S-TATI | Serum concentration of tumor-associated trypsin inhibitor |
| TAT | Tumor-associated trypsin |
| TATI | Tumor-associated trypsin inhibitor |
| TMA | Tissue microarray |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018, GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Warnakulasuriya, S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009, 45, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Lambert, R.; Sauvaget, C.; de Camargo Cancela, M.; Sankaranarayanan, R. Epidemiology of cancer from the oral cavity and oropharynx. Eur. J. Gastroenterol. Hepatol. 2011, 23, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Sinevici, N.; O’sullivan, J. Oral cancer: Deregulated molecular events and their use as biomarkers. Oral Oncol. 2016, 61, 12–18. [Google Scholar] [CrossRef]
- Koneva, L.A.; Zhang, Y.; Virani, S.; Hall, P.B.; McHugh, J.B.; Chepeha, D.B.; Wolf, G.T.; Carey, T.E.; Rozek, L.S.; Sartor, M.A. HPV Integration in HNSCC Correlates with Survival Outcomes, Immune Response Signatures, and Candidate Drivers. Mol. Cancer Res. 2018, 16, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Gooi, Z.; Chan, J.Y.K.; Fakhry, C. The epidemiology of the human papillomavirus related to oropharyngeal head and neck cancer. Laryngoscope 2016, 126, 894–900. [Google Scholar] [CrossRef]
- Pitkäniemi, J.; Malila, N.; Virtanen, A.; Degerlund, H.; Heikkinen, S.; Seppä, K. Syöpä 2018. Tilastoraportti Suomen Syöpätilanteesta; Publication no. 93 of Cancer Society of Finland; Cancer Society of Finland: Helsinki, Finland, 2020; Available online: Https://www.epressi.com/media/userfiles/145405/1588150481/syopa-2018-pa-finska.pdf (accessed on 3 June 2021).
- Chaturvedi, A.K.; Anderson, W.F.; Lortet-Tieulent, J.; Curado, M.P.; Ferlay, J.; Franceschi, S.; Rosenberg, P.S.; Bray, F.; Gillison, M.L. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J. Clin. Oncol. 2013, 31, 4550–4559. [Google Scholar] [CrossRef]
- Gillison, M.L.; D’Souza, G.; Westra, W.; Sugar, E.; Xiao, W.; Begum, S.; Viscidi, L. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J. Natl. Cancer Inst. 2008, 100, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Ramqvist, T.; Dalianis, T. An epidemic of oropharyngeal squamous cell carcinoma (OSCC) due to human papillomavirus (HPV) infection and aspects of treatment and prevention. Anticancer Res. 2011, 31, 1515–1519. [Google Scholar] [PubMed]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tân, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Fakhry, C.; Westra, W.H.; Li, S.; Cmelak, A.; Ridge, J.A.; Pinto, H.; Forastiere, A.; Gillison, M.L. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J. Natl. Cancer Inst. 2008, 100, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Elrefaey, S.; Massaro, M.A.; Chiocca, S.; Chiesa, F.; Ansarin, M. HPV in oropharyngeal cancer: The basics to know in clinical practice. Acta Otorhinolaryngol. Ital. 2014, 34, 299–309. [Google Scholar]
- Sato, F.; Ono, T.; Kawahara, A.; Kawaguchi, T.; Tanaka, H.; Shimamatsu, K.; Kakuma, T.; Akiba, J.; Umeno, H.; Yano, H. Prognostic impact of p16 and PD-L1 expression in patients with oropharyngeal squamous cell carcinoma receiving a definitive treatment. J. Clin. Pathol. 2019, 72, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Adelstein, D.J.; Ismaila, N.; Ku, J.A.; Burtness, B.; Swiecicki, P.L.; Mell, L.; Beitler, J.J.; Gross, N.; Jones, C.U.; Kaufman, M.; et al. Role of Treatment Deintensification in the Management of p16+ Oropharyngeal Cancer: ASCO Provisional Clinical Opinion. J. Clin. Oncol. 2019, 37, 1578–1589. [Google Scholar] [CrossRef] [PubMed]
- Grimminger, C.M.; Danenberg, P.V. Update of prognostic and predictive biomarkers in oropharyngeal squamous cell carcinoma: A review. Eur. Arch. Otorhinolaryngol. 2011, 268, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Vento, S.I.; Jouhi, L.; Mohamed, H.; Haglund, C.; Mäkitie, A.A.; Atula, T.; Hagström, J.; Mäkinen, L.K. MMP-7 expression may influence the rate of distant recurrences and disease-specific survival in HPV-positive oropharyngeal squamous cell carcinoma. Virchows Arc. 2018, 472, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Umbreit, C.; Erben, P.; Faber, A.; Hofheinz, R.-D.; Aderhold, C.; Weiss, C.; Hoermann, K.; Wenzel, A.; Schultz, J.D. MMP9, Cyclin D1 and β-Catenin Are Useful Markers of p16-positive Squamous Cell Carcinoma in Therapeutic EGFR Inhibition In Vitro. Anticancer Res. 2015, 35, 3801–3810. [Google Scholar]
- Plath, M.; Broglie, M.A.; Förbs, D.; Stoeckli, S.J.; Jochum, W. Prognostic significance of cell cycle-associated proteins p16, pRB, cyclin D1 and p53 in resected oropharyngeal carcinoma. J. Otolaryngol. Head Neck Surg. 2018, 47, 53. [Google Scholar] [CrossRef]
- Lang Kuhs, K.A.; Wood, C.B.; Wiggleton, J.; Aulino, J.M.; Latimer, B.; Smith, D.K.; Bender, N.; Rohde, S.; Mannion, K.; Kim, Y.; et al. Transcervical sonography and human papillomavirus 16 E6 antibodies are sensitive for the detection of oropharyngeal cancer. Cancer 2020, 126, 2658–2665. [Google Scholar] [CrossRef]
- Ren, J.; Xu, W.; Su, J.; Ren, X.; Cheng, D.; Chen, Z.; Bender, N.; Mirshams, M.; Habbous, S.; De Almeida, J.R.; et al. Multiple imputation and clinico-serological models to predict human papillomavirus status in oropharyngeal carcinoma: An alternative when tissue is unavailable. Int. J. Cancer 2020, 146, 2166–2174. [Google Scholar] [CrossRef] [PubMed]
- Rivera, C.; Oliveira, A.K.; Costa, R.A.P.; De Rossi, T.; Paes Leme, A.F. Prognostic biomarkers in oral squamous cell carcinoma: A systematic review. Oral Oncol. 2017, 72, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Almangush, A.; Heikkinen, I.; Mäkitie, A.A.; Coletta, R.D.; Läärä, E.; Leivo, I.; Salo, T. Prognostic biomarkers for oral tongue squamous cell carcinoma: A systematic review and meta-analysis. Br. J. Cancer 2017, 117, 856–866. [Google Scholar] [CrossRef] [PubMed]
- Itkonen, O.; Stenman, U.-H. TATI as a biomarker. Clin. Chim. Acta 2014, 431, 260–269. [Google Scholar] [CrossRef]
- Räsänen, K.; Itkonen, O.; Koistinen, H.; Stenman, U.-H. Emerging Roles of SPINK1 in Cancer. Clin. Chem. 2016, 62, 449–457. [Google Scholar] [CrossRef]
- Eddeland, A.; Ohlsson, K. A radioimmunoassay for measurement of human pancreatic secretory trypsin inhibitor in different body fluids. Hoppe Seylers Z. Physiol. Chem. 1978, 359, 671–675. [Google Scholar] [CrossRef]
- Stenman, U.H.; Huhtala, M.L.; Koistinen, R.; Seppälä, M. Immunochemical demonstration of an ovarian cancer-associated urinary peptide. Int. J. Cancer 1982, 30, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Gaber, A.; Nodin, B.; Hotakainen, K.; Nilsson, E.; Stenman, U.-H.; Bjartell, A.; Birgisson, H.; Jirström, K. Increased serum levels of tumour-associated trypsin inhibitor independently predict a poor prognosis in colorectal cancer patients. BMC Cancer 2010, 10, 498. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Long, Q.; Fu, D.; Zhu, D.; Ji, Y.; Han, L.; Zhang, B.; Xu, Q.; Liu, B.; Li, Y.; et al. Targeting SPINK1 in the damaged tumour microenvironment alleviates therapeutic resistance. Nat. Commun. 2018, 9, 4315. [Google Scholar] [CrossRef] [PubMed]
- Randén-Brady, R.; Carpén, T.; Jouhi, L.; Syrjänen, S.; Haglund, C.; Tarkkanen, J.; Remes, S.; Mäkitie, A.; Mattila, P.S.; Silén, S.; et al. In situ hybridization for high-risk HPV E6/E7 mRNA is a superior method for detecting transcriptionally active HPV in oropharyngeal cancer. Hum. Pathol. 2019, 90, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Carpén, T.; Syrjänen, S.; Jouhi, L.; Randen-Brady, R.; Haglund, C.; Mäkitie, A.; Mattila, P.S.; Hagström, J. Epstein-Barr virus (EBV) and polyomaviruses are detectable in oropharyngeal cancer and EBV may have prognostic impact. Cancer Immunol. Immunother. 2020, 69, 1615–1626. [Google Scholar] [CrossRef] [PubMed]
- Smeets, S.J.; Hesselink, A.T.; Speel, E.-J.M.; Haesevoets, A.; Snijders, P.J.F.; Pawlita, M.; Meijel, C.J.L.M.; Braakhuis, B.J.M.; Leemans, C.R.; Brakenhoff, R.H. A novel algorithm for reliable detection of human papillomavirus in paraffin embedded head and neck cancer specimen. Int. J. Cancer 2007, 121, 2465–2472. [Google Scholar] [CrossRef]
- Carpén, T.; Sjöblom, A.; Lundberg, M.; Haglund, C.; Markkola, A.; Syrjänen, S.; Tarkkanen, J.; Mäkitie, A.; Hagström, J.; Mattila, P.S. Presenting symptoms and clinical findings in HPV-positive and HPV-negative oropharyngeal cancer patients. Acta Oto-Laryngol. 2018, 138, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Osman, S.; Turpeinen, U.; Itkonen, O.; Stenman, U.H. Optimization of a time-resolved immunofluorometric assay for tumor-associated trypsin inhibitor (TATI) using the streptavidin-biotin system. J. Immunol. Methods 1993, 161, 97–106. [Google Scholar] [CrossRef]
- Lyytinen, I.; Lempinen, M.; Nordin, A.; Mäkisalo, H.; Stenman, U.-H.; Isoniemi, H. Prognostic significance of tumor-associated trypsin inhibitor (TATI) and human chorionic gonadotropin-β (hCGβ) in patients with hepatocellular carcinoma. Scand. J. Gastroenterol. 2013, 48, 1066–1073. [Google Scholar] [CrossRef]
- Ravela, S.; Valmu, L.; Domanskyy, M.; Koistinen, H.; Kylanpaa, L.; Lindstrom, O.; Stenman, J.; Hämäläinen, E.; Stenman, U.-H.; Itkonen, O. An immunocapture-LC-MS-based assay for serum SPINK1 allows simultaneous quantification and detection of SPINK1 variants. Anal. Bioanal. Chem. 2018, 410, 1679–1688. [Google Scholar] [CrossRef]
- Paju, A.; Hotakainen, K.; Cao, Y.; Laurila, T.; Gadaleanu, V.; Hemminki, A.; Stenman, U.H.; Bjartell, A. Increased expression of tumor-associated trypsin inhibitor, TATI, in prostate cancer and in androgen-independent 22Rv1 cells. Eur. Urol. 2007, 52, 1670–1679. [Google Scholar] [CrossRef]
- Kelloniemi, E.; Rintala, E.; Finne, P.; Stenman, U.-H. Tumor-associated trypsin inhibitor as a prognostic factor during follow-up of bladder cancer. Urology 2003, 62, 249–253. [Google Scholar] [CrossRef]
- Tornberg, S.V.; Nisen, H.; Järvinen, P.; Järvinen, R.; Kilpeläinen, T.P.; Taari, K.; Stenman, U.H.; Visapää, H. Serum tumour associated trypsin inhibitor, as a biomarker for survival in renal cell carcinoma. Scand. J. Urol. 2020, 54, 413–419. [Google Scholar] [CrossRef]
- Kozakiewicz, B.; Chądzyńska, M.; Dmoch-Gajzlerska, E.; Stefaniak, M. Monitoring the treatment outcome in endometrial cancer patients by CEA and TATI. Tumor Biol. 2016, 37, 9367–9374. [Google Scholar] [CrossRef] [PubMed]
- Järvisalo, J.; Hakama, M.; Knekt, P.; Stenman, U.H.; Leino, A.; Teppo, L.; Maatela, J.; Aromaa, A. Serum tumor markers CEA, CA 50, TATI, and NSE in lung cancer screening. Cancer 1993, 71, 1982–1988. [Google Scholar] [CrossRef]
- Zou, W.-B.; Tang, X.-Y.; Zhou, D.-Z.; Qian, Y.-Y.; Hu, L.-H.; Yu, F.-F.; Yu, D.; Wu, H.; Deng, S.-J.; Lin, J.-H. SPINK1, PRSS1, CTRC, and CFTR Genotypes Influence Disease Onset and Clinical Outcomes in Chronic Pancreatitis. Clin. Transl. Gastroenterol. 2018, 9, 204. [Google Scholar] [CrossRef]
- Gaber, A.; Johansson, M.; Stenman, U.-H.; Hotakainen, K.; Pontén, F.; Glimelius, B.; Jirström, B.K.; Birgisson, H. High expression of tumour-associated trypsin inhibitor correlates with liver metastasis and poor prognosis in colorectal cancer. Br. J. Cancer 2009, 100, 1540–1548. [Google Scholar] [CrossRef]
- Wiksten, J.-P.; Lundin, J.; Nordling, S.; Kokkola, A.; Stenman, U.-H.; Haglund, C. High tissue expression of tumour-associated trypsin inhibitor (TATI) associates with a more favourable prognosis in gastric cancer. Histopathology 2005, 46, 380–388. [Google Scholar] [CrossRef]
- Tiwari, R.; Manzar, N.; Bhatia, V.; Yadav, A.; Nengroo, M.A.; Datta, D.; Carskadon, S.; Gupta, N.; Sigouros, M.; Khani, F.; et al. Androgen deprivation upregulates SPINK1 expression and potentiates cellular plasticity in prostate cancer. Nat. Commun. 2020, 11, 384. [Google Scholar] [CrossRef] [PubMed]
- Soreide, K.; Janssen, E.A.; Körner, H.; Baak, J.P.A. Trypsin in colorectal cancer: Molecular biological mechanisms of proliferation, invasion, and metastasis. J. Pathol. 2006, 209, 147–156. [Google Scholar] [CrossRef]
- Nyberg, P.; Moilanen, M.; Paju, A.; Sarin, A.; Stenman, U.-H.; Sorsa, T.; Salo, T. MMP-9 activation by tumor trypsin-2 enhances in vivo invasion of human tongue carcinoma cells. J. Dent. Res. 2002, 81, 831–835. [Google Scholar] [CrossRef]
- Kuilman, T.; Michaloglou, C.; Mooi, W.J.; Peeper, D.S. The essence of senescence. Genes Dev. 2010, 24, 2463–2479. [Google Scholar] [CrossRef] [PubMed]
- Prime, S.S.; Cirillo, N.; Hassona, Y.; Lambert, D.W.; Paterson, I.C.; Mellone, M.; Thomas, G.J.; James, E.N.L.; Parkinson, E.K. Fibroblast activation and senescence in oral cancer. J. Oral Pathol. Med. 2017, 46, 82–88. [Google Scholar] [CrossRef]
- Lukkonen, A.; Lintula, S.; von Boguslawski, K.; Carpén, O.; Ljungberg, B.; Landberg, G.; Stenman, U.H. Tumor-associated trypsin inhibitor in normal and malignant renal tissue and in serum of renal-cell carcinoma patients. Int. J. Cancer 1999, 83, 486–490. [Google Scholar] [CrossRef]
| Variable | S-TATI− | % | S-TATI+ | % | p-Value | Missing/% (n = 90) |
|---|---|---|---|---|---|---|
| Number of patients | 69 | 76.7 | 21 | 23.3 | ||
| Age | 60.8 | 65.1 | 0.054 | |||
| Gender | ||||||
| Male | 52 | 78.8 | 14 | 21.2 | ||
| Female | 17 | 70.8 | 7 | 29.2 | 0.4 | |
| Smoking | ||||||
| Never | 27 | 96.4 | 1 | 3.6 | ||
| Former | 25 | 83.3 | 5 | 16.7 | ||
| Current | 17 | 53.1 | 15 | 46.9 | <0.001 ** | |
| Heavy alcohol use | 16/17.8 | |||||
| Never | 36 | 83.7 | 7 | 16.3 | ||
| Former | 6 | 60.0 | 4 | 40.0 | ||
| Current | 13 | 61.9 | 8 | 38.1 | 0.09 | |
| T class | ||||||
| T1-T2 | 46 | 51.1 | 12 | 13.3 | ||
| T3-T4 | 23 | 25.6 | 9 | 10.0 | 0.4 | |
| N class | ||||||
| N0–N1 | 59 | 79.7 | 15 | 20.3 | ||
| N2–N3 | 10 | 62.5 | 6 | 37.4 | 0.2 | |
| Stage | ||||||
| I-II | 52 | 82.5 | 11 | 17.5 | ||
| III-IV | 17 | 63.0 | 10 | 37.0 | 0.04 * | |
| Grade | ||||||
| I | 1 | 33.3 | 2 | 66.7 | ||
| II | 8 | 53.3 | 7 | 46.7 | ||
| III | 60 | 83.3 | 12 | 16.7 | 0.009 * | |
| Localization | ||||||
| Tonsil | 48 | 90.6 | 5 | 9.4 | ||
| Base of tongue | 17 | 77.3 | 5 | 22.7 | ||
| Soft palate | 3 | 30.0 | 7 | 70.0 | ||
| Posterior wall of oropharynx | 1 | 20.0 | 4 | 80.0 | <0.001 ** | |
| HPV | ||||||
| HPV− | 21 | 56.8 | 16 | 43.2 | ||
| HPV+ | 48 | 90.6 | 5 | 9.4 | <0.001 ** |
| Variable | OS | DSS | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Age | 1.08 | 1.03–1.13 | 0.004 * | 1.07 | 1.01–1.14 | 0.018 * |
| Smoking | 0.034 * | 0.205 | ||||
| Ex-smoker versus never | 1.20 | 0.27–5.40 | 0.816 | 0.62 | 0.10–3.98 | 0.615 |
| Current smoker versus never | 4.14 | 1.22–14.06 | 0.023 * | 2.43 | 0.64–9.18 | 0.192 |
| Stage III–IVversusStage I–II | 1.71 | 0.70–4.20 | 0.243 | 2.19 | 0.72–6.64 | 0.168 |
| HPV-versus HPV+ | 1.01 | 0.36–2.83 | 0.988 | 0.96 | 0.27–3.43 | 0.955 |
| S-TATI | 2.47 | 1.26–4.84 | 0.009 * | 2.54 | 1.07–6.02 | 0.034 * |
| Variable | TATI in Tumor 0–1 | % | TATI in Tumor 2–3 | % | p-Value | Missing/% (n = 77) |
|---|---|---|---|---|---|---|
| Number of patients | 20 | 26.0 | 57 | 63.3 | ||
| Age | 64.4 | 61.1 | 0.2 | |||
| Gender | ||||||
| Male | 15 | 25.4 | 44 | 74.6 | ||
| Female | 5 | 27.8 | 13 | 72.2 | 0.8 | |
| Smoking | ||||||
| Never | 8 | 33.3 | 16 | 66.7 | ||
| Former | 7 | 28.0 | 18 | 72.0 | ||
| Current | 5 | 17.9 | 23 | 82.1 | 0.4 | |
| Heavy alcohol use | 14/18.2 | |||||
| Never | 14 | 35.9 | 25 | 64.1 | ||
| Former | 1 | 16.7 | 5 | 83.3 | ||
| Current | 4 | 22.2 | 14 | 77.8 | 0.4 | |
| T class | ||||||
| T1–T2 | 11 | 22.4 | 38 | 77.6 | ||
| T3–T4 | 9 | 32.1 | 19 | 67.9 | 0.4 | |
| N class | ||||||
| N0–N1 | 12 | 19.0 | 51 | 81.0 | ||
| N2–N3 | 8 | 57.1 | 6 | 42.9 | 0.006 * | |
| Stage | ||||||
| I–II | 9 | 17.0 | 44 | 83.0 | ||
| III–IV | 11 | 45.8 | 13 | 54.1 | 0.007 * | |
| Grade | ||||||
| I | 0 | 0.0 | 2 | 100.0 | ||
| II | 3 | 23.0 | 10 | 77.0 | ||
| III | 17 | 27.4 | 45 | 72.6 | 0.7 | |
| Localization | ||||||
| Tonsil | 10 | 21.7 | 36 | 78.3 | ||
| Base of tongue | 6 | 35.3 | 11 | 64.7 | ||
| Soft palate | 3 | 33.3 | 6 | 66.7 | ||
| Posterior wall of oropharynx | 1 | 20.0 | 4 | 80.0 | 0.7 | |
| HPV | ||||||
| HPV− | 9 | 29.0 | 22 | 71.0 | ||
| HPV+ | 11 | 23.9 | 35 | 76.1 | 0.6 |
| Variable | TATI in Lymphocytes 0–1 | % | TATI in Lymphocytes 2–3 | % | p-Value | Missing/% (n = 76) |
|---|---|---|---|---|---|---|
| Number of patients | 30 | 39.5 | 46 | 60.5 | ||
| Age | 62.6 | 61.3 | 0.6 | |||
| Gender | ||||||
| Male | 23 | 39.7 | 35 | 60.3 | ||
| Female | 7 | 38.9 | 11 | 61.1 | 0.9 | |
| Smoking | ||||||
| Never | 10 | 43.5 | 13 | 56.5 | ||
| Former | 5 | 20.0 | 20 | 80.0 | ||
| Current | 15 | 53.6 | 13 | 46.4 | 0.04 * | |
| Heavy alcohol use | 14/18.4 | |||||
| Never | 17 | 44.7 | 21 | 55.3 | ||
| Former | 1 | 16.7 | 5 | 83.3 | ||
| Current | 9 | 50.0 | 9 | 50.0 | 0.4 | |
| T Class | ||||||
| T1–T2 | 14 | 28.6 | 35 | 71.4 | ||
| T3–T4 | 16 | 59.3 | 11 | 40.7 | 0.009 * | |
| N Class | ||||||
| N0–N1 | 23 | 36.5 | 40 | 63.5 | ||
| N2–N3 | 7 | 53.8 | 6 | 46.2 | 0.2 | |
| Stage | ||||||
| I–II | 16 | 30.2 | 37 | 69.8 | ||
| III–IV | 14 | 60.9 | 9 | 39.1 | 0.01 * | |
| Grade | ||||||
| I | 1 | 50.0 | 1 | 50.0 | ||
| II | 6 | 46.2 | 7 | 53.8 | ||
| III | 23 | 37.7 | 38 | 62.3 | 0.8 | |
| Localization | ||||||
| Tonsil | 15 | 32.6 | 31 | 67.4 | ||
| Base of tongue | 6 | 37.5 | 10 | 62.5 | ||
| Soft palate | 6 | 66.7 | 3 | 33.3 | ||
| Posterior wall of oropharynx | 3 | 60.0 | 2 | 40.0 | 0.2 | |
| HPV | ||||||
| HPV− | 18 | 60.0 | 12 | 40.0 | ||
| HPV+ | 12 | 33.3 | 34 | 66.6 | 0.003 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sjöblom, A.; Stenman, U.-H.; Hagström, J.; Jouhi, L.; Haglund, C.; Syrjänen, S.; Mattila, P.; Mäkitie, A.; Carpén, T. Tumor-Associated Trypsin Inhibitor (TATI) as a Biomarker of Poor Prognosis in Oropharyngeal Squamous Cell Carcinoma Irrespective of HPV Status. Cancers 2021, 13, 2811. https://doi.org/10.3390/cancers13112811
Sjöblom A, Stenman U-H, Hagström J, Jouhi L, Haglund C, Syrjänen S, Mattila P, Mäkitie A, Carpén T. Tumor-Associated Trypsin Inhibitor (TATI) as a Biomarker of Poor Prognosis in Oropharyngeal Squamous Cell Carcinoma Irrespective of HPV Status. Cancers. 2021; 13(11):2811. https://doi.org/10.3390/cancers13112811
Chicago/Turabian StyleSjöblom, Anni, Ulf-Håkan Stenman, Jaana Hagström, Lauri Jouhi, Caj Haglund, Stina Syrjänen, Petri Mattila, Antti Mäkitie, and Timo Carpén. 2021. "Tumor-Associated Trypsin Inhibitor (TATI) as a Biomarker of Poor Prognosis in Oropharyngeal Squamous Cell Carcinoma Irrespective of HPV Status" Cancers 13, no. 11: 2811. https://doi.org/10.3390/cancers13112811
APA StyleSjöblom, A., Stenman, U.-H., Hagström, J., Jouhi, L., Haglund, C., Syrjänen, S., Mattila, P., Mäkitie, A., & Carpén, T. (2021). Tumor-Associated Trypsin Inhibitor (TATI) as a Biomarker of Poor Prognosis in Oropharyngeal Squamous Cell Carcinoma Irrespective of HPV Status. Cancers, 13(11), 2811. https://doi.org/10.3390/cancers13112811







