Accurate Prognosis Prediction of Pancreatic Ductal Adenocarcinoma Using Integrated Clinico-Genomic Data of Endoscopic Ultrasound-Guided Fine Needle Biopsy
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
2.1. Baseline Characteristics
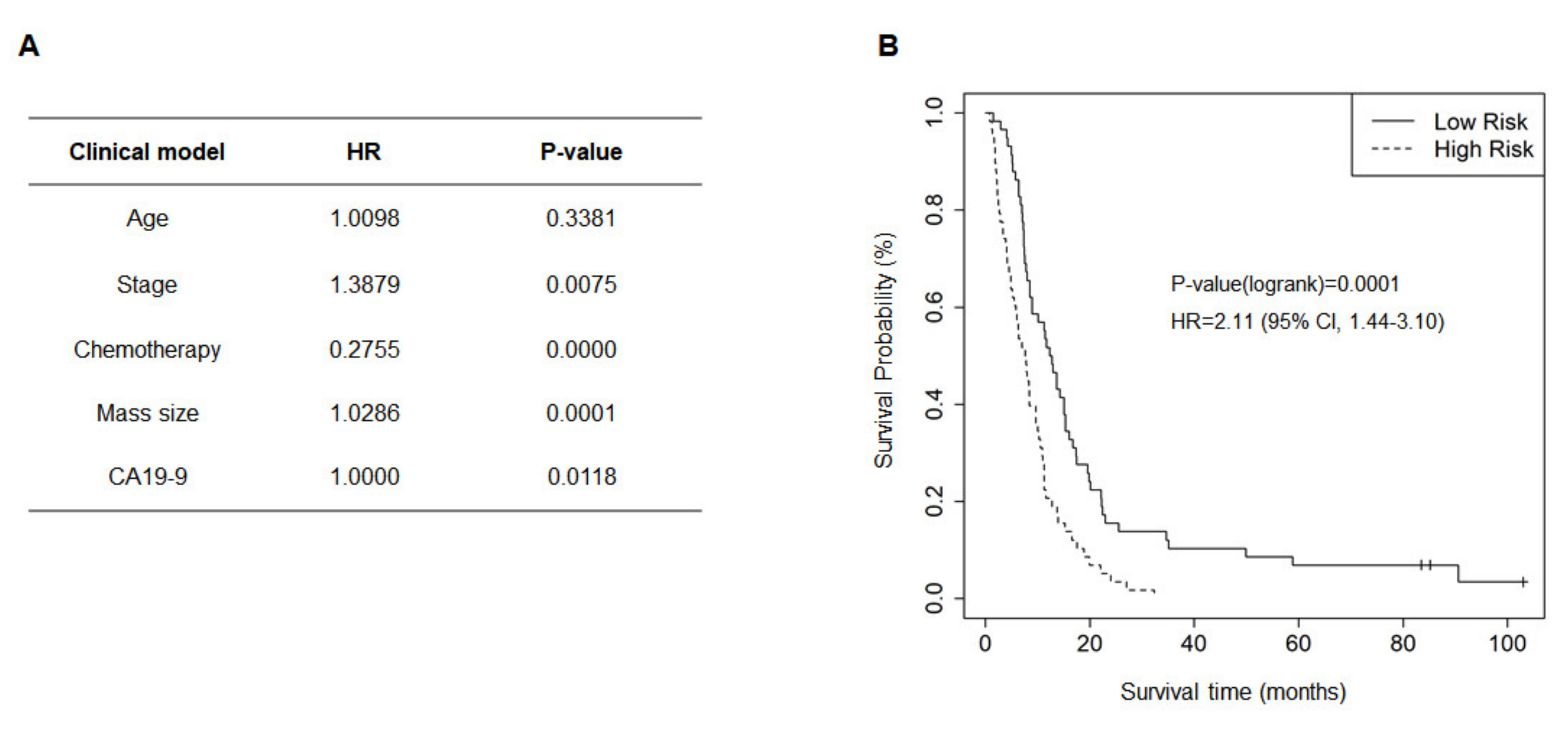
2.2. Clinical Prognostic Factors Affecting OS and Clinical Model
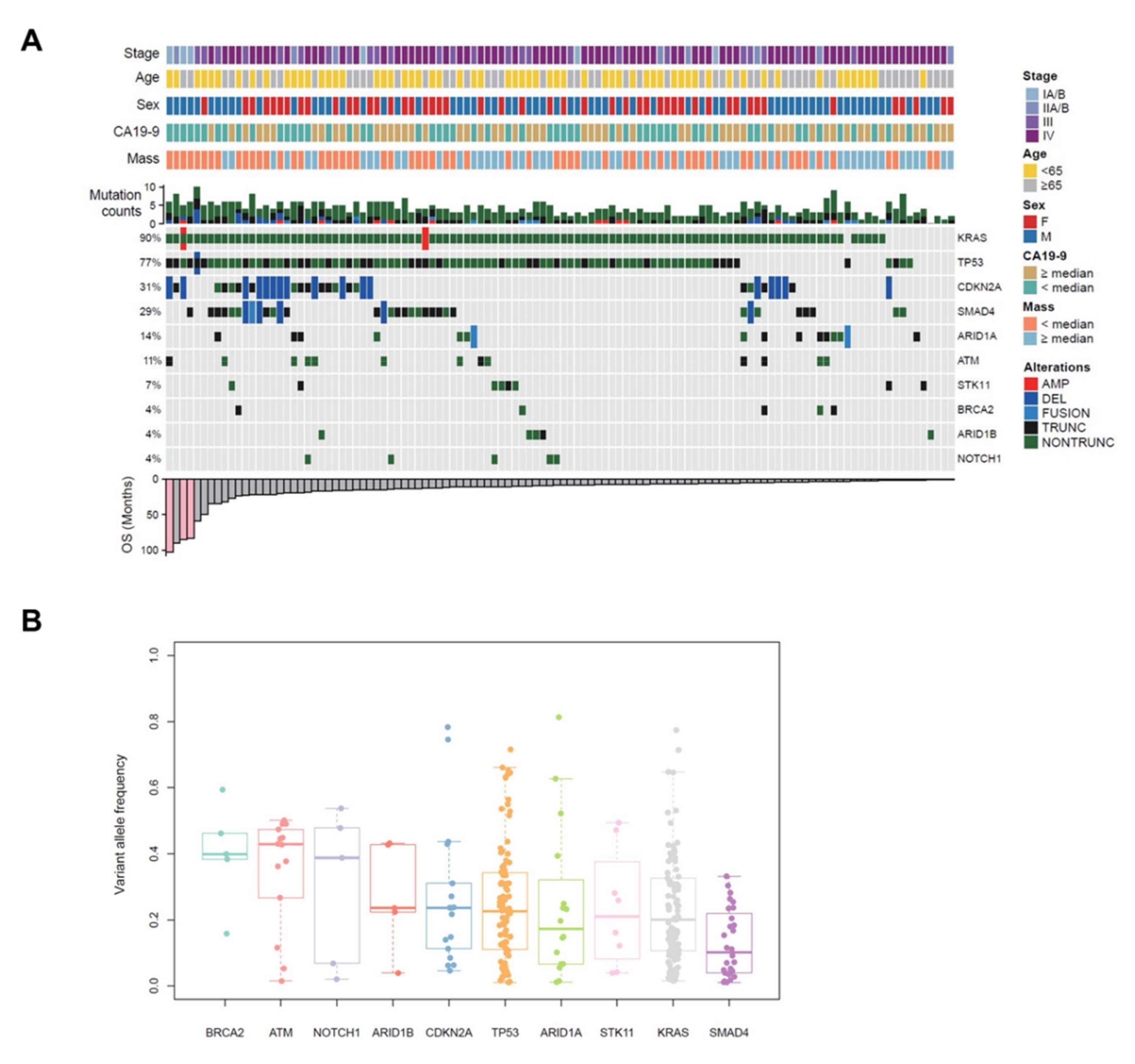
2.3. Characteristics of Genetic Alterations in EUS-FNB Samples
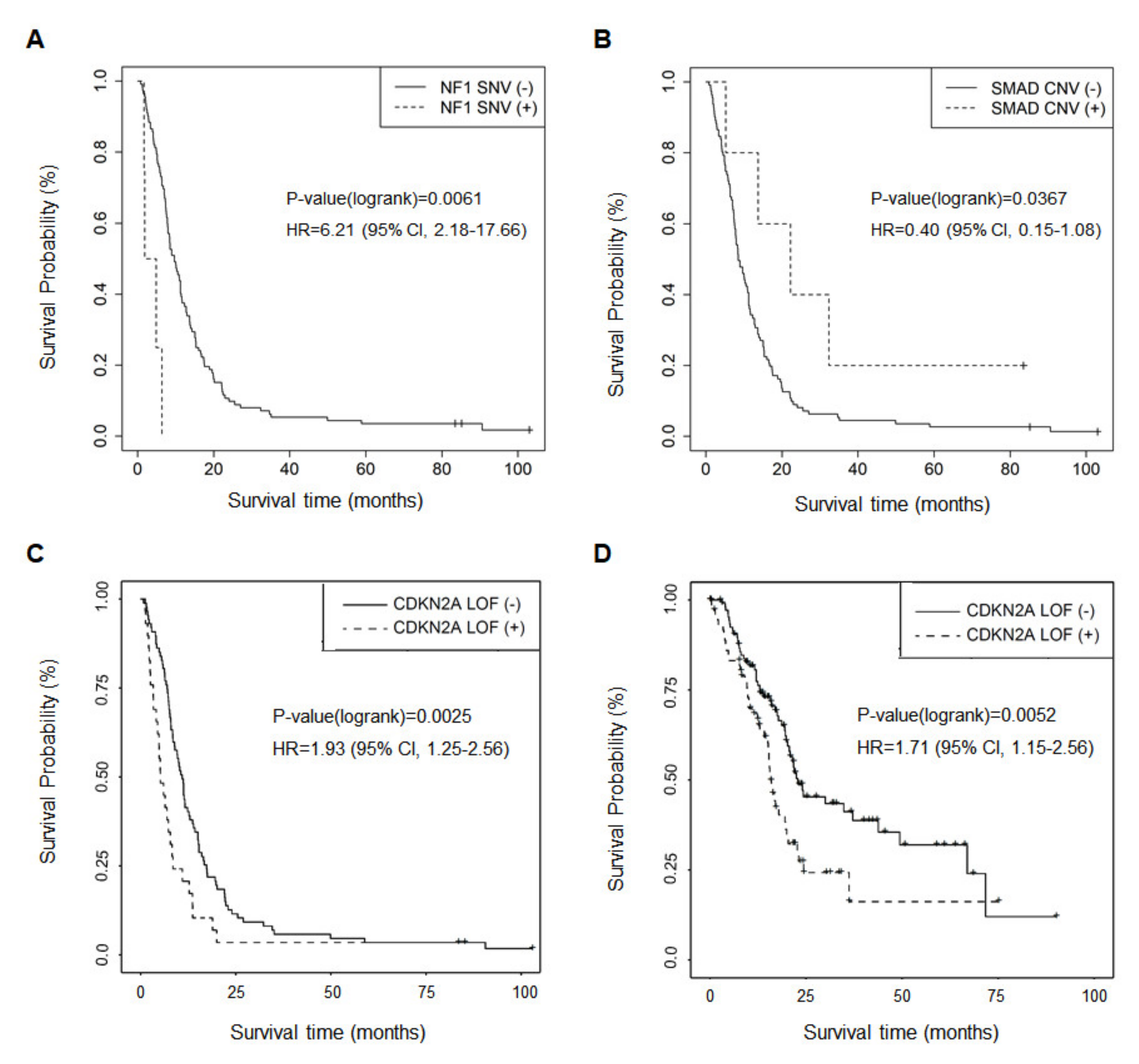
2.4. Genetic Alterations Associated with OS and Metastasis
2.5. Development of Clinico-Genomic Model
3. Discussion
4. Materials and Methods
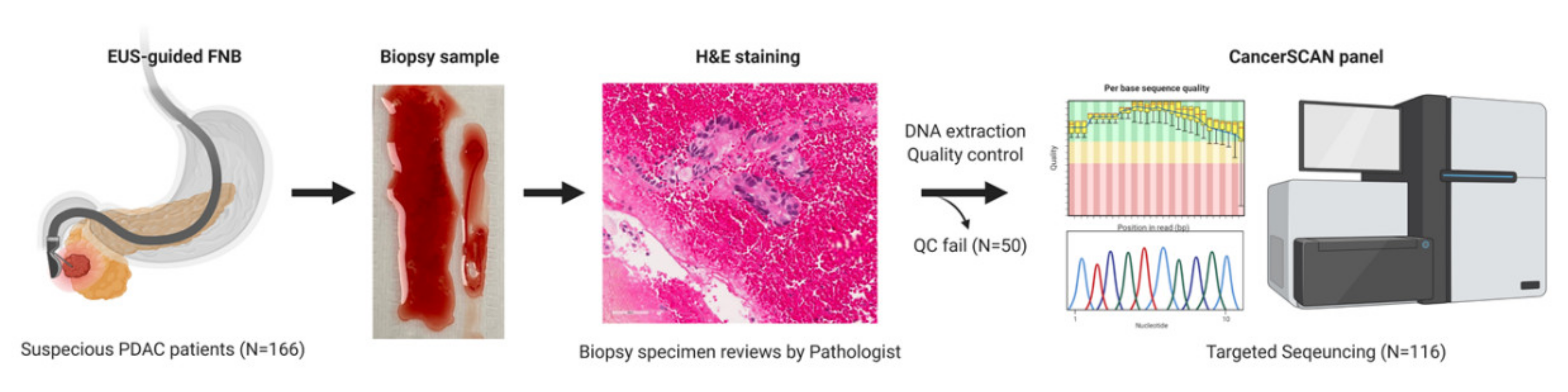
4.1. Patients
4.2. EUS-FNB Procedure
4.3. Targeted Deep Sequencing
4.4. Clinical and Clinico-Genomic Model
4.5. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. Ca A Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Jung, K.W.; Won, Y.J.; Kong, H.J.; Oh, C.M.; Cho, H.; Lee, D.H.; Lee, K.H. Cancer statistics in Korea: Incidence, mortality, survival, and prevalence in 2012. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2015, 47, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, M. Pancreatic cancer. N. Engl. J. Med. 2010, 362, 1605–1617. [Google Scholar] [CrossRef]
- Adham, M.; Jaeck, D.; Le Borgne, J.; Oussoultzouglou, E.; Chenard-Neu, M.P.; Mosnier, J.F.; Scoazec, J.Y.; Mornex, F.; Partensky, C. Long-term survival (5–20 years) after pancreatectomy for pancreatic ductal adenocarcinoma: A series of 30 patients collected from 3 institutions. Pancreas 2008, 37, 352–357. [Google Scholar] [CrossRef]
- Meldrum, C.; Doyle, M.A.; Tothill, R.W. Next-generation sequencing for cancer diagnostics: A practical perspective. Clin. Biochem. Rev. 2011, 32, 177–195. [Google Scholar] [PubMed]
- Jones, S.; Zhang, X.; Parsons, D.W.; Lin, J.C.; Leary, R.J.; Angenendt, P.; Mankoo, P.; Carter, H.; Kamiyama, H.; Jimeno, A.; et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 2008, 321, 1801–1806. [Google Scholar] [CrossRef] [PubMed]
- Waddell, N.; Pajic, M.; Patch, A.M.; Chang, D.K.; Kassahn, K.S.; Bailey, P.; Johns, A.L.; Miller, D.; Nones, K.; Quek, K.; et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 2015, 518, 495–501. [Google Scholar] [CrossRef]
- Chu, G.C.; Kimmelman, A.C.; Hezel, A.F.; DePinho, R.A. Stromal biology of pancreatic cancer. J. Cell. Biochem. 2007, 101, 887–907. [Google Scholar] [CrossRef] [PubMed]
- De Biase, D.; Visani, M.; Baccarini, P.; Polifemo, A.M.; Maimone, A.; Fornelli, A.; Giuliani, A.; Zanini, N.; Fabbri, C.; Pession, A.; et al. Next generation sequencing improves the accuracy of KRAS mutation analysis in endoscopic ultrasound fine needle aspiration pancreatic lesions. PLoS ONE 2014, 9, e87651. [Google Scholar] [CrossRef]
- Bailey, P.; Chang, D.K.; Nones, K.; Johns, A.L.; Patch, A.M.; Gingras, M.C.; Miller, D.K.; Christ, A.N.; Bruxner, T.J.; Quinn, M.C.; et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 2016, 531, 47–52. [Google Scholar] [CrossRef]
- Witkiewicz, A.K.; McMillan, E.A.; Balaji, U.; Baek, G.; Lin, W.C.; Mansour, J.; Mollaee, M.; Wagner, K.U.; Koduru, P.; Yopp, A.; et al. Whole-exome sequencing of pancreatic cancer defines genetic diversity and therapeutic targets. Nat. Commun. 2015, 6, 6744. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network. Integrated Genomic Characterization of Pancreatic Ductal Adenocarcinoma. Cancer Cell 2017, 32, 185–203. [Google Scholar] [CrossRef] [PubMed]
- Goswami, R.S.; Luthra, R.; Singh, R.R.; Patel, K.P.; Routbort, M.J.; Aldape, K.D.; Yao, H.; Dang, H.D.; Barkoh, B.A.; Manekia, J.; et al. Identification of Factors Affecting the Success of Next-Generation Sequencing Testing in Solid Tumors. Am. J. Clin. Pathol. 2016, 145, 222–237. [Google Scholar] [CrossRef]
- Imaoka, H.; Sasaki, M.; Hashimoto, Y.; Watanabe, K.; Ikeda, M. New Era of Endoscopic Ultrasound-Guided Tissue Acquisition: Next-Generation Sequencing by Endoscopic Ultrasound-Guided Sampling for Pancreatic Cancer. J. Clin. Med. 2019, 8, 1173. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, F.C.; Kerr, S.E.; Kipp, B.R.; Voss, J.S.; Minot, D.M.; Tu, Z.J.; Henry, M.R.; Graham, R.P.; Vasmatzis, G.; Cheville, J.C.; et al. Targeted next generation sequencing of endoscopic ultrasound acquired cytology from ampullary and pancreatic adenocarcinoma has the potential to aid patient stratification for optimal therapy selection. Oncotarget 2016, 7, 54526–54536. [Google Scholar] [CrossRef]
- Elhanafi, S.; Mahmud, N.; Vergara, N.; Kochman, M.L.; Das, K.K.; Ginsberg, G.G.; Rajala, M.; Chandrasekhara, V. Comparison of endoscopic ultrasound tissue acquisition methods for genomic analysis of pancreatic cancer. J. Gastroenterol. Hepatol. 2019, 34, 907–913. [Google Scholar] [CrossRef]
- Young, G.; Wang, K.; He, J.; Otto, G.; Hawryluk, M.; Zwirco, Z.; Brennan, T.; Nahas, M.; Donahue, A.; Yelensky, R.; et al. Clinical next-generation sequencing successfully applied to fine-needle aspirations of pulmonary and pancreatic neoplasms. Cancer Cytopathol. 2013, 121, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Bilici, A. Prognostic factors related with survival in patients with pancreatic adenocarcinoma. World J. Gastroenterol. WJG 2014, 20, 10802–10812. [Google Scholar] [CrossRef]
- Dischinger, P.S.; Tovar, E.A.; Essenburg, C.J.; Madaj, Z.B.; Gardner, E.E.; Callaghan, M.E.; Turner, A.N.; Challa, A.K.; Kempston, T.; Eagleson, B.; et al. NF1 deficiency correlates with estrogen receptor signaling and diminished survival in breast cancer. NPJ Breast Cancer 2018, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Rogers, A.; Wang, L.M.; Karavitaki, N.; Grossman, A.B. Neurofibromatosis Type 1 and pancreatic islet cell tumours: An association which should be recognized. QJM 2015, 108, 573–576. [Google Scholar] [CrossRef]
- Tascilar, M.; Skinner, H.G.; Rosty, C.; Sohn, T.; Wilentz, R.E.; Offerhaus, G.J.; Adsay, V.; Abrams, R.A.; Cameron, J.L.; Kern, S.E.; et al. The SMAD4 protein and prognosis of pancreatic ductal adenocarcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2001, 7, 4115–4121. [Google Scholar]
- Blackford, A.; Serrano, O.K.; Wolfgang, C.L.; Parmigiani, G.; Jones, S.; Zhang, X.; Parsons, D.W.; Lin, J.C.; Leary, R.J.; Eshleman, J.R.; et al. SMAD4 gene mutations are associated with poor prognosis in pancreatic cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009, 15, 4674–4679. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.; Shao, Y.W.; Lin, P.; Cai, X.; Wang, B.; Ding, Y.; Ma, X.; Wu, X.; Xia, Y.; Zhu, D.; et al. SMAD4 and NF1 mutations as potential biomarkers for poor prognosis to cetuximab-based therapy in Chinese metastatic colorectal cancer patients. BMC Cancer 2018, 18, 479. [Google Scholar] [CrossRef]
- Schutte, M.; Hruban, R.H.; Geradts, J.; Maynard, R.; Hilgers, W.; Rabindran, S.K.; Moskaluk, C.A.; Hahn, S.A.; Schwarte-Waldhoff, I.; Schmiegel, W.; et al. Abrogation of the Rb/p16 tumor-suppressive pathway in virtually all pancreatic carcinomas. Cancer Res. 1997, 57, 3126–3130. [Google Scholar]
- Salo-Mullen, E.E.; O’Reilly, E.M.; Kelsen, D.P.; Ashraf, A.M.; Lowery, M.A.; Yu, K.H.; Reidy, D.L.; Epstein, A.S.; Lincoln, A.; Saldia, A.; et al. Identification of germline genetic mutations in patients with pancreatic cancer. Cancer 2015, 121, 4382–4388. [Google Scholar] [CrossRef]
- Jiao, L.; Zhu, J.; Hassan, M.M.; Evans, D.B.; Abbruzzese, J.L.; Li, D. K-ras mutation and p16 and preproenkephalin promoter hypermethylation in plasma DNA of pancreatic cancer patients: In relation to cigarette smoking. Pancreas 2007, 34, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Choi, B.Y.; Lee, M.H.; Bode, A.M.; Dong, Z. Implications of Genetic and Epigenetic Alterations of CDKN2A (p16(INK4a)) in Cancer. EBioMedicine 2016, 8, 30–39. [Google Scholar] [CrossRef]
- Connor, A.A.; Denroche, R.E.; Jang, G.H.; Lemire, M.; Zhang, A.; Chan-Seng-Yue, M.; Wilson, G.; Grant, R.C.; Merico, D.; Lungu, I.; et al. Integration of Genomic and Transcriptional Features in Pancreatic Cancer Reveals Increased Cell Cycle Progression in Metastases. Cancer Cell 2019, 35, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Makohon-Moore, A.P.; Zhang, M.; Reiter, J.G.; Bozic, I.; Allen, B.; Kundu, D.; Chatterjee, K.; Wong, F.; Jiao, Y.; Kohutek, Z.A.; et al. Limited heterogeneity of known driver gene mutations among the metastases of individual patients with pancreatic cancer. Nat. Genet. 2017, 49, 358–366. [Google Scholar] [CrossRef]
- Brar, G.; Blais, E.M.; Joseph Bender, R.; Brody, J.R.; Sohal, D.; Madhavan, S.; Picozzi, V.J.; Hendifar, A.E.; Chung, V.M.; Halverson, D.; et al. Multi-omic molecular comparison of primary versus metastatic pancreatic tumours. Br. J. Cancer 2019, 121, 264–270. [Google Scholar] [CrossRef]
- Friess, H.; Guo, X.Z.; Tempia-Caliera, A.A.; Fukuda, A.; Martignoni, M.E.; Zimmermann, A.; Korc, M.; Büchler, M.W. Differential expression of metastasis-associated genes in papilla of vater and pancreatic cancer correlates with disease stage. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2001, 19, 2422–2432. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Friess, H.; Graber, H.U.; Kashiwagi, M.; Zimmermann, A.; Korc, M.; Büchler, M.W. KAI1 expression is up-regulated in early pancreatic cancer and decreased in the presence of metastases. Cancer Res. 1996, 56, 4876–4880. [Google Scholar] [PubMed]
- Stratford, J.K.; Bentrem, D.J.; Anderson, J.M.; Fan, C.; Volmar, K.A.; Marron, J.S.; Routh, E.D.; Caskey, L.S.; Samuel, J.C.; Der, C.J.; et al. A six-gene signature predicts survival of patients with localized pancreatic ductal adenocarcinoma. PLoS Med. 2010, 7, e1000307. [Google Scholar] [CrossRef] [PubMed]
- Golan, T.; Kanji, Z.S.; Epelbaum, R.; Devaud, N.; Dagan, E.; Holter, S.; Aderka, D.; Paluch-Shimon, S.; Kaufman, B.; Gershoni-Baruch, R.; et al. Overall survival and clinical characteristics of pancreatic cancer in BRCA mutation carriers. Br. J. Cancer 2014, 111, 1132–1138. [Google Scholar] [CrossRef]
- James, E.; Waldron-Lynch, M.G.; Saif, M.W. Prolonged survival in a patient with BRCA2 associated metastatic pancreatic cancer after exposure to camptothecin: A case report and review of literature. Anticancer Drugs 2009, 20, 634–638. [Google Scholar] [CrossRef]
- Petrovics, G.; Price, D.K.; Lou, H.; Chen, Y.; Garland, L.; Bass, S.; Jones, K.; Kohaar, I.; Ali, A.; Ravindranath, L.; et al. Increased frequency of germline BRCA2 mutations associates with prostate cancer metastasis in a racially diverse patient population. Prostate Cancer Prostatic Dis. 2019, 22, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.T.; Choi, Y.L.; Yun, J.W.; Kim, N.K.D.; Kim, S.Y.; Jeon, H.J.; Nam, J.Y.; Lee, C.; Ryu, D.; Kim, S.C.; et al. Prevalence and detection of low-allele-fraction variants in clinical cancer samples. Nat. Commun. 2017, 8, 1377. [Google Scholar] [CrossRef] [PubMed]


| Variable | n = 116 |
|---|---|
| Age, years (median ± SD) | 64.0 ± 10.4 |
| Gender | |
| Male | 67 (57.8) |
| Female | 49 (42.2) |
| BMI (median ± SD) | 22.1 ± 3.1 |
| Performance status (ECOG) | |
| 0 | 76 (65.5) |
| 1 | 36 (31.0) |
| 2 | 3 (2.6) |
| 3 | 1 (0.9) |
| CEA (ng/mL) (median ± SD) | 2.8 ± 34.3 (13 missing) |
| CA19-9 (U/mL) (median ± SD) | 393.0 ± 27295.3 |
| Smoking, n (%) | |
| Never | 78 (67.2) |
| Former | 22 (19.0) |
| Current smoker | 16 (13.8) |
| Treatment, n (%) | |
| Operation | |
| No | 103 (88.8%) |
| operation with curative intention | 13 (11.2%) |
| Chemotherapy | |
| No | 24 (20.7%) |
| Yes | 92 (79.3%) |
| Tumor mass size (cm) (median ± SD) | 4.0 ± 1.6 |
| Tumor location, n (%) | |
| Head | 49 (42.2) |
| Body | 41 (35.4) |
| Tail | 26 (22.4) |
| Stage (AJCC 8th edition), n (%) | |
| IA/B | 1 (0.9) |
| IIA | 6 (5.2) |
| IIB | 8 (6.9) |
| III | 26 (22.4) |
| IV | 75 (64.6) |
| Metastasis, n (%) | |
| None | 41 (35.3) |
| Liver | 45 (38.8) |
| Other sites except liver | 30 (25.9) |
| Mutaion | None (n = 41) | Liver (n = 45) | Other Sites (n = 30) | p-Value |
|---|---|---|---|---|
| BRCA2 mutation | 0.0162 | |||
| No | 41 | 40 | 30 | |
| Point mutation | 0 | 5 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.K.; Kim, H.; Son, D.-S.; Kim, N.K.D.; Sung, Y.K.; Cho, M.; Lee, C.; Noh, D.H.; Lee, S.-H.; Lee, K.T.; et al. Accurate Prognosis Prediction of Pancreatic Ductal Adenocarcinoma Using Integrated Clinico-Genomic Data of Endoscopic Ultrasound-Guided Fine Needle Biopsy. Cancers 2021, 13, 2791. https://doi.org/10.3390/cancers13112791
Park JK, Kim H, Son D-S, Kim NKD, Sung YK, Cho M, Lee C, Noh DH, Lee S-H, Lee KT, et al. Accurate Prognosis Prediction of Pancreatic Ductal Adenocarcinoma Using Integrated Clinico-Genomic Data of Endoscopic Ultrasound-Guided Fine Needle Biopsy. Cancers. 2021; 13(11):2791. https://doi.org/10.3390/cancers13112791
Chicago/Turabian StylePark, Joo Kyung, Hyemin Kim, Dae-Soon Son, Nayoung K. D. Kim, Young Kyung Sung, Minseob Cho, Chung Lee, Dong Hyo Noh, Se-Hoon Lee, Kyu Taek Lee, and et al. 2021. "Accurate Prognosis Prediction of Pancreatic Ductal Adenocarcinoma Using Integrated Clinico-Genomic Data of Endoscopic Ultrasound-Guided Fine Needle Biopsy" Cancers 13, no. 11: 2791. https://doi.org/10.3390/cancers13112791
APA StylePark, J. K., Kim, H., Son, D.-S., Kim, N. K. D., Sung, Y. K., Cho, M., Lee, C., Noh, D. H., Lee, S.-H., Lee, K. T., Lee, J. K., Jang, K.-T., Park, W.-Y., & Lee, K. H. (2021). Accurate Prognosis Prediction of Pancreatic Ductal Adenocarcinoma Using Integrated Clinico-Genomic Data of Endoscopic Ultrasound-Guided Fine Needle Biopsy. Cancers, 13(11), 2791. https://doi.org/10.3390/cancers13112791






