Indolent T-Cell Lymphoproliferative Disorders of the Gastrointestinal Tract (iTLPD-GI): A Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Updated Clinical and Pathological Findings on iTLPD-GI
3.1. Epidemiology and Aetiology
3.2. Clinical Features and Course
3.3. Pathology
3.4. Molecular Genetics
3.5. Prognosis and Treatment
4. Immunophenotypical Profile of iTLPD-GI
4.1. Pan-T-Cell Markers
4.2. TFH Markers
4.3. Cytotoxic Markers
4.4. Clonality Markers
4.5. CD103
4.6. CD30
4.7. T-Bet and GATA3
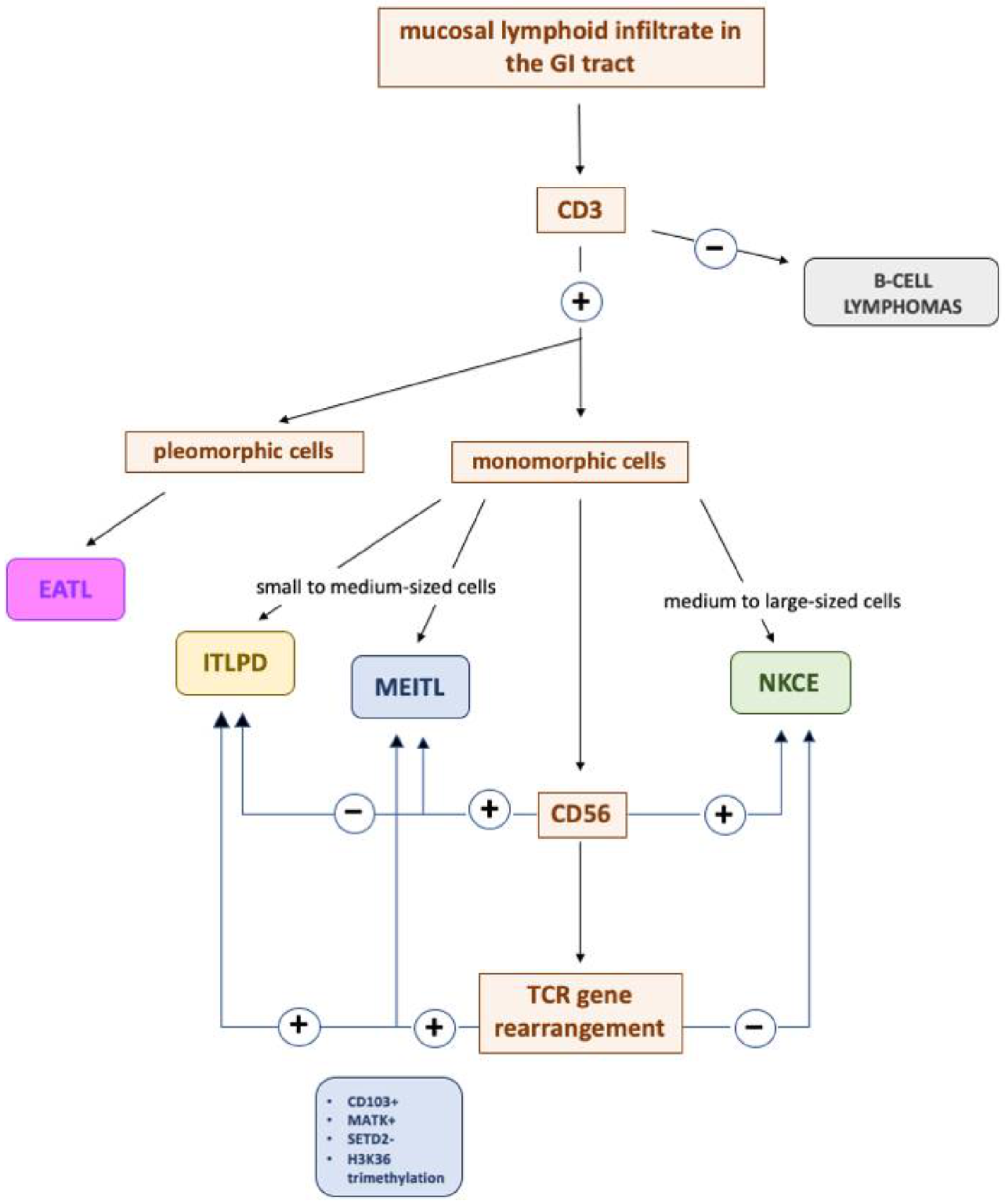
5. Differential Diagnosis
5.1. iTLPD-GI vs. MEITL
5.2. iTLPD-GI vs. EATL
5.3. iTLPD-GI vs. NKCE
5.4. iTLPD-GI vs. ENKTL
5.5. iTLPD-GI vs. B-Cell Lymphomas
5.6. iTLPD-GI vs. IBD
5.7. iTLPD-GI vs. CD
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chan, J.K.C.; Fukuyama, M. Haematolymphoid tumours of the digestive system. In WHO Classification of Tumours of the Digestive System, 5th ed.; WHO Classification of Tumours Editorial Board, Ed.; IARC: Lyon, France, 2019; pp. 373–432. [Google Scholar]
- Jaffe, E.S.; Chott, A.; Ott, G.; Chan, J.K.C.; Bhagat, G.; Tan, S.Y.; Stein, H.; Isaacson, P.G. Intestinal T-cell lymphoma. In WHO Classification of Tumours Haematopoietic and Lymphoid Tissues, Revised, 4th ed.; WHO Classification of Tumours Editorial Board, Ed.; IARC: Lyon, France, 2017; pp. 372–380. [Google Scholar]
- Carbonnel, F.; Lavergne, A.; Messing, B.; Tsapis, A.; Berger, R.; Galian, A.; Nemeth, J.; Brouet, J.C.; Rambaud, J.C. Extensive small intestinal T-cell lymphoma of low-grade malignancy associated with a new chromosomal translocation. Cancer 1994, 73, 1286–1291. [Google Scholar] [CrossRef]
- Egawa, N.; Fukayama, M.; Kawaguchi, K.; Hishima, T.; Hayashi, Y.; Funata, N.; Ibuka, T.; Koike, M.; Miyashita, H.; Tajima, T. Relapsing oral and colonic ulcers with monoclonal T-cell infiltration. A low grade mucosal T-lymphoproliferative disease of the digestive tract. Cancer 1995, 75, 1728–1733. [Google Scholar] [CrossRef]
- Tsutsumi, Y.; Inada, K.; Morita, K.; Suzuki, T. T-cell lymphomas diffusely involving the intestine: Report of two rare cases. Jpn. J. Clin. Oncol. 1996, 26, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, K.; Fuchigami, T.; Nakamura, S.; Daimaru, Y.; Ohshima, K.; Sakai, Y.; Ichimaru, T. Primary gastrointestinal T-cell lymphoma resembling multiple lymphomatous polyposis. Gastroenterology 1996, 111, 778–782. [Google Scholar] [CrossRef]
- Carbonnel, F.; d’Almagne, H.; Lavergne, A.; Matuchansky, C.; Brouet, J.C.; Sigaux, F.; Beaugerie, L.; Nemeth, J.; Coffin, B.; Cosnes, J.; et al. The clinicopathological features of extensive small intestinal CD4 T cell infiltration. Gut 1999, 45, 662–667. [Google Scholar] [CrossRef]
- Ranheim, E.A.; Jones, C.; Zehnder, J.L.; Warnke, R.; Yuen, A. Spontaneously relapsing clonal, mucosal cytotoxic T-cell lymphoproliferative disorder: Case report and review of the literature. Am. J. Surg. Pathol. 2000, 24, 296–301. [Google Scholar] [CrossRef]
- Isomoto, H.; Maeda, T.; Akashi, T.; Tsuchiya, T.; Kawaguchi, Y.; Sawayama, Y.; Koida, S.; Ohnita, K.; Kohno, S.; Tomonaga, M. Multiple lymphomatous polyposis of the colon originating from T-cells: A case report. Dig. Liver Dis. 2004, 36, 218–221. [Google Scholar] [CrossRef]
- Zivny, J.; Banner, B.F.; Agrawal, S.; Pihan, G.; Barnard, G.F. CD4+ T-cell lymphoproliferative disorder of the gut clinically mimicking celiac sprue. Dig. Dis. Sci. 2004, 49, 551–555. [Google Scholar] [CrossRef]
- Svrcek, M.; Garderet, L.; Sebbagh, V.; Rosenzwaig, M.; Parc, Y.; Lagrange, M.; Bennis, M.; Lavergne-Slove, A.; Fléjou, J.F.; Fabiani, B. Small intestinal CD4+ T-cell lymphoma: A rare distinctive clinicopathological entity associated with prolonged survival. Virchows Arch. 2007, 451, 1091–1093. [Google Scholar] [CrossRef]
- Perry, A.M.; Warnke, R.A.; Hu, Q.; Gaulard, P.; Copie-Bergman, C.; Alkan, S.; Wang, H.Y.; Cheng, J.X.; Bacon, C.M.; Delabie, J.; et al. Indolent T-cell lymphoproliferative disease of the gastrointestinal tract. Blood 2013, 122, 3599–3606. [Google Scholar] [CrossRef]
- Leventaki, V.; Manning, J.T., Jr.; Luthra, R.; Mehta, P.; Oki, Y.; Romaguera, J.E.; Medeiros, L.J.; Vega, F. Indolent peripheral T-cell lymphoma involving the gastrointestinal tract. Hum. Pathol. 2014, 45, 421–426. [Google Scholar] [CrossRef]
- Malamut, G.; Meresse, B.; Kaltenbach, S.; Derrieux, C.; Verkarre, V.; Macintyre, E.; Ruskone-Fourmestraux, A.; Fabiani, B.; Radford-Weiss, I.; Brousse, N.; et al. Small intestinal CD4+ T-cell lymphoma is a heterogenous entity with common pathology features. Clin. Gastroenterol. Hepatol. 2014, 12, 599–608. [Google Scholar] [CrossRef]
- Sena Teixeira Mendes, L.; Attygalle, A.D.; Cunningham, D.; Benson, M.; Andreyev, J.; Gonzales-de-Castro, D.; Wotherspoon, A. CD4−positive small T-cell lymphoma of the intestine presenting with severe bile-acid malabsorption: A supportive symptom control approach. Br. J. Haematol. 2014, 167, 265–269. [Google Scholar] [CrossRef][Green Version]
- Margolskee, E.; Jobanputra, V.; Lewis, S.K.; Alobeid, B.; Green, P.H.R.; Bhagat, G. Indolent small intestinal CD4+ T-cell lymphoma is a distinct entity with unique biologic and clinical features. PLoS ONE 2013, 8, e68343. [Google Scholar] [CrossRef]
- Sharma, A.; Oishi, N.; Boddicker, R.L.; Hu, G.; Benson, H.K.; Ketterling, R.P.; Greipp, P.T.; Knutson, D.L.; Kloft-Nelson, S.M.; He, R.; et al. Recurrent STAT3-JAK2 fusions in indolent T-cell lymphoproliferative disorder of the gastrointestinal tract. Blood 2018, 131, 2262–2266. [Google Scholar] [CrossRef]
- Edison, N.; Belhanes-Peled, H.; Eitan, Y.; Guthmann, Y.; Yeremenko, Y.; Raffeld, M.; Elmalah, I.; Trougouboff, P. Indolent T-cell lymphoproliferative disease of the gastrointestinal tract after treatment with adalimumab in resistant Crohn’s colitis. Hum. Pathol. 2016, 57, 45–50. [Google Scholar] [CrossRef]
- Liang, S.; Bogun, Y. A case of indolent T-cell lymphoproliferative disorder of the gastrointestinal tract. Pathology 2018, 50, S79. [Google Scholar] [CrossRef]
- Wang, X.; Ng, C.S.; Chen, C.; Yu, G.; Yin, W. An unusual case report of indolent T-cell lymphoproliferative disorder with aberrant CD20 expression involving the gastrointestinal tract and bone marrow. Diagn. Pathol. 2018, 13, 82. [Google Scholar] [CrossRef]
- Soderquist, C.R.; Patel, N.; Murty, V.V.; Betman, S.; Aggarwal, N.; Young, K.H.; Xerri, L.; Leeman-Neill, R.; Lewis, S.K.; Green, P.H.; et al. Genetic and phenotypic characterization of indolent T-cell lymphoproliferative disorders of the gastrointestinal tract. Haematologica 2020, 105, 1895–1906. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- Soderquist, C.R.; Bhagat, G. Gastrointestinal T- and NK-cell lymphomas and indolent lymphoproliferative disorders. Sem. Diagn. Pathol. 2020, 37, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Montes-Moreno, S.; King, R.L.; Oschlies, I.; Ponzoni, M.; Goodlad, J.R.; Dotlic, S.; Traverse-Glehen, A.; Ott, G.; Ferry, J.A.; Calaminici, M. Update on lymphoproliferative disorders of the gastrointestinal tract: Disease spectrum from indolent lymphoproliferations to aggressive lymphomas. Virchows Arch. 2020, 476, 667–681. [Google Scholar] [CrossRef] [PubMed]
- Perry, A.M.; Bailey, N.G.; Bonnett, M.; Jaffe, E.S.; Chan, W.C. Disease progression in a patient with indolent T-cell lymphoproliferative disease of the gastrointestinal tract. Int. J. Surg. Pathol. 2019, 27, 102–107. [Google Scholar] [CrossRef]
- Jassim, S.H.; Smith, L.B. New/Revised Entities in Gastrointestinal Lymphoproliferative Disorders. Surg. Pathol. Clin. 2019, 12, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Kohri, M.; Tsukasaki, K.; Akuzawa, Y.; Tanae, K.; Takahashi, N.; Saeki, T.; Okamura, D.; Ishikawa, M.; Maeda, T.; Kawai, N.; et al. Peripheral T-cell lymphoma with gastrointestinal involvement and indolent T-lymphoproliferative disorders of the gastrointestinal tract. Leuk. Res. 2020, 91, 106336. [Google Scholar] [CrossRef] [PubMed]
- Zanelli, M.; Zizzo, M.; Sanguedolce, F.; Martino, G.; Soriano, A.; Ricci, S.; Castro Ruiz, C.; Annessi, V.; Ascani, S. Indolent T-cell lymphoproliferative disorder of the gastrointestinal tract: A tricky diagnosis of a gastric case. BMC Gastroenterol. 2020, 20, 336. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Wen, Z.; Su, X.; Xiao, S.; Wang, Y. Indolent T-cell lymphoproliferative disease with synchronous diffuse large B-cell lymphoma. Medicine 2019, 98, e15323. [Google Scholar] [CrossRef]
- Laâbi, Y.; Gras, M.P.; Carbonnel, F.; Brouet, J.C.; Berger, R.; Larsen, C.J.; Tsapis, A. A new gene, BCM, on chromosome 16 is fused to the interleukin 2 gene by a t(4;16)(q26;p13) translocation in a malignant T cell lymphoma. EMBO J. 1992, 11, 3897–3904. [Google Scholar] [CrossRef]
- Liau, N.P.D.; Laktyushin, A.; Lucet, I.S.; Murphy, J.M.; Yao, S.; Whitlock, E.; Callaghan, K.; Nicola, N.A.; Kershaw, N.J.; Babon, J.J. The molecular basis of JAK/STAT inhibition by SOCS1. Nat. Commun. 2018, 9, 1558. [Google Scholar] [CrossRef]
- Jaffe, E.S. T-cell and NK-cell neoplasms of the gastrointestinal tract—recurrent themes, but clinical and biological distinctions exist. Haematologica 2020, 105, 1760–1762. [Google Scholar] [CrossRef]
- Rodríguez Pinilla, S.M.; Roncador, G.; Rodríguez-Peralto, J.L.; Mollejo, M.; García, J.F.; Montes-Moreno, S.; Camacho, F.I.; Ortiz, P.; Limeres-González, M.A.; Torres, A.; et al. Primary cutaneous CD4+ small/medium-sized pleomorphic T-cell lymphoma expresses follicular T-cell markers. Am. J. Surg. Pathol. 2009, 33, 81–90. [Google Scholar] [CrossRef]
- Xiao, W.; Gupta, G.K.; Yao, J.; Jang, Y.J.; Xi, L.; Baik, J.; Sigler, A.; Kumar, A.; Moskowitz, A.J.; Arcila, M.E.; et al. Recurrent somatic JAK3 mutations in NK-cell enteropathy. Blood 2019, 134, 986–991. [Google Scholar] [CrossRef]
- Cannons, J.L.; Lu, K.T.; Schwartzberg, P.L. T follicular helper cell diversity and plasticity. Trends Immunol. 2013, 34, 200–207. [Google Scholar] [CrossRef]
- Matnani, R.; Ganapathi, K.A.; Lewis, S.K.; Green, P.H.; Alobeid, B.; Bhagat, G. Indolent T- and NK-cell lymphoproliferative disorders of the gastrointestinal tract: A review and update. Hematol. Oncol. 2017, 35, 3–16. [Google Scholar] [CrossRef]
- Petrella, T.; Maubec, E.; Cornillet-Lefebvre, P.; Willemze, R.; Pluot, M.; Durlach, A.; Marinho, E.; Benhamou, J.L.; Jansen, P.; Robson, A.; et al. Indolent CD8−positive lymphoid proliferation of the ear: A distinct primary cutaneous T-cell lymphoma? Am. J. Surg. Pathol. 2007, 31, 1887–1892. [Google Scholar] [CrossRef]
- Beltraminelli, H.; Mullegger, R.; Cerroni, L. Indolent CD8+ lymphoid proliferation of the ear: A phenotypic variant of the small-medium pleomorphic cutaneous T-cell lymphoma? J. Cutan. Pathol. 2010, 37, 81–84. [Google Scholar] [CrossRef]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef]
- Dewar, R.; Andea, A.A.; Guitart, J.; Arber, D.A.; Weiss, L.M. Best Practices in Diagnostic Immunohistochemistry—Workup of Cutaneous Lymphoid Lesions in the Diagnosis of Primary Cutaneous Lymphoma. Arch. Pathol. Lab. Med. 2015, 139, 338–350. [Google Scholar] [CrossRef]
- Lehmann, J.; Huehn, J.; de la Rosa, M.; Maszyna, F.; Kretschmer, U.; Krenn, V.; Brunner, M.; Scheffold, A.; Hamann, A. Expression of the integrin alpha Ebeta 7 identifies unique subsets of CD25+ as well as CD25- regulatory T cells. Proc. Natl. Acad. Sci. USA 2002, 99, 13031–13036. [Google Scholar] [CrossRef]
- Aziz, S.; Fackler, O.T.; Meyerhans, A.; Müller-Lantzsch, N.; Zeitz, M.; Schneider, T. Replication of M-tropic HIV-1 in activated human intestinal lamina propria lymphocytes is the main reason for increased virus load in the intestinal mucosa. J. Acquir. Immune Defic. Syndr. 2005, 38, 23–30. [Google Scholar] [CrossRef]
- Farstad, I.N.; Halstensen, T.S.; Lien, B.; Kilshaw, P.J.; Lazarovits, A.I.; Brandtzaeg, P. Distribution of 7 integrins in human intestinal mucosa and organized gut-associated lymphoid tissue. Immunology 1996, 89, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Micklem, K.J.; Dong, Y.; Willis, A.; Pulford, K.A.; Visser, L.; Dürkop, H.; Poppema, S.; Stein, H.; Mason, D.Y. HML-1 antigen on mucosa-associated T cells, activated cells, and hairy leukemic cells is a new integrin containing the beta 7 subunit. Am. J. Pathol. 1991, 139, 1297–1301. [Google Scholar] [PubMed]
- Shaw, S.K.; Brenner, M.B. The 7 integrins in mucosal homing and retention. Semin. Immunol. 1995, 7, 335–342. [Google Scholar] [CrossRef]
- Peine, M.; Rausch, S.; Helmstetter, C.; Fröhlich, A.; Hegazy, A.N.; Kühl, A.A.; Grevelding, C.G.; Höfer, T.; Hartmann, S.; Löhning, M. Stable T-bet(+)GATA-3(+) Th1/Th2 hybrid cells arise in vivo, can develop directly from naive precursors, and limit immunopathologic inflammation. PLoS. Biol. 2013, 11, e1001633. [Google Scholar] [CrossRef]
- Hegazy, A.N.; Peine, M.; Helmstetter, C.; Panse, I.; Fröhlich, A.; Bergthaler, A.; Flatz, L.; Pinschewer, D.D.; Radbruch, A.; Löhning, M. Interferons direct Th2 cell reprogramming to generate a stable GATA-3(+)T-bet(+) cell subset with combined Th2 and Th1 cell functions. Immunity 2010, 32, 116–128. [Google Scholar] [CrossRef]
- Tai, T.S.; Pai, S.Y.; Hao, I.C. GATA-3 Regulates the Homeostasis and Activation of CD8+ T Cells. J. Immunol. 2013, 190, 428–437. [Google Scholar] [CrossRef]
- Wang, T.; Feldman, A.L.; Wada, D.A.; Lu, Y.; Polk, A.; Briski, R.; Ristow, K.; Habermann, T.M.; Thomas, D.; Ziesmer, S.C. GATA-3 expression identifies a high-risk subset of PTCL, NOS with distinct molecular and clinical features. Blood 2014, 123, 3007–3015. [Google Scholar] [CrossRef]
- Manso, R.; Bellas, C.; Martín-Acosta, P.; Mollejo, M.; Menárguez, J.; Rojo, F.; Llamas, P.; Piris, M.A.; Rodríguez-Pinilla, S.M. C-MYC is related to GATA3 expression and associated with poor prognosis in nodal peripheral T-cell lymphomas. Haematologica 2016, 101, e336–e338. [Google Scholar] [CrossRef][Green Version]
- Van Vliet, C.; Spagnolo, D.V. T- and NK-cell lymphoproliferative disorders of the gastrointestinal tract: Review and update. Pathology 2020, 52, 128–141. [Google Scholar] [CrossRef]
- Swerdlow, S.H.; Jaffe, E.S.; Brousset, P.; Chan, J.K.; de Leval, L.; Gaulard, P.; Harris, N.L.; Pileri, S.; Weiss, L.M.; International Lymphoma Study Group. Cytotoxic T-cell and NK-cell lymphomas: Current questions and controversies. Am. J. Surg. Pathol. 2014, 38, e60–e71. [Google Scholar] [CrossRef]
- Tse, E.; Gill, H.; Loong, F.; Kim, S.J.; Ng, S.B.; Tang, T.; Ko, Y.H.; Chng, W.J.; Lim, S.T.; Kim, W.S. Type II enteropathy-associated T-cell lymphoma: A multicenter analysis from the Asia Lymphoma Study Group. Am. J. Hematol. 2012, 87, 663–668. [Google Scholar] [CrossRef]
- Foukas, P.G.; de Leval, L. Recent advances in intestinal lymphomas. Histopathology 2015, 66, 112–136. [Google Scholar] [CrossRef]
- Kabul, S.; Uğraş, N.; Yerci, Ö.; Öztürk, E. Perforation of the small intestine caused by enteropathy-associated T cell lymphoma. Turk. J. Surg. 2018, 34, 253–255. [Google Scholar] [CrossRef]
- Skinnider, B.F. Lymphoproliferative Disorders of the Gastrointestinal Tract. Arch. Pathol. Lab. Med. 2018, 142, 44–52. [Google Scholar] [CrossRef]
- Weindorf, S.C.; Smith, L.B.; Owens, S.R. Update on Gastrointestinal Lymphomas. Arch. Pathol. Lab. Med. 2018, 142, 1347–1351. [Google Scholar] [CrossRef]
- Roberti, A.; Dobay, M.P.; Bisig, B.; Vallois, D.; Boéchat, C.; Lanitis, E.; Bouchindhomme, B.; Parrens, M.C.; Bossard, C.; Quintanilla-Martinez, L.; et al. Type II enteropathy-associated T-cell lymphoma features a unique genomic profile with highly recurrent SETD2 alterations. Nat. Commun. 2016, 7, 12602. [Google Scholar] [CrossRef]
- Cheminant, M.; Bruneau, J.; Malamut, G.; Sibon, D.; Guegan, N.; van Gils, T.; Cording, S.; Trinquand, A.; Verkarre, V.; Lhermitte, L.; et al. CELAC network. NKp46 is a diagnostic biomarker and may be a therapeutic target in gastrointestinal T-cell lymphoproliferative diseases: A CELAC study. Gut 2019, 68, 1396–1405. [Google Scholar] [CrossRef]
- Delabie, J.; Holte, H.; Vose, J.M.; Ullrich, F.; Jaffe, E.S.; Savage, K.J.; Connors, J.M.; Rimsza, L.; Harris, N.L.; Müller-Hermelink, K.; et al. Enteropathy-associated T-cell lymphoma: Clinical and histological findings from the international peripheral T-cell lymphoma project. Blood 2011, 118, 148–155. [Google Scholar] [CrossRef]
- Nagaishi, T.; Yamada, D.; Suzuki, K.; Fukuyo, R.; Saito, E.; Fukuda, M.; Watabe, T.; Tsugawa, N.; Takeuchi, K.; Yamamoto, K.; et al. Indolent T cell lymphoproliferative disorder with villous atrophy in small intestine diagnosed by single-balloon enteroscopy. Clin. J. Gastroenterol. 2019, 12, 434–440. [Google Scholar] [CrossRef]
- Malamut, G.; Chandesris, O.; Verkarre, V.; Meresse, B.; Callens, C.; Macintyre, E.; Bouhnik, Y.; Gornet, J.M.; Allez, M.; Jian, R.; et al. Enteropathy associated T cell lymphoma in celiac disease: A large retrospective study. Dig. Liver Dis. 2013, 45, 377–384. [Google Scholar] [CrossRef]
- Xia, D.; Morgan, E.A.; Berger, D.; Pinkus, G.S.; Ferry, J.A.; Zukerberg, L.R. NK-Cell Enteropathy and Similar Indolent Lymphoproliferative Disorders: A Case Series with Literature Review. Am. J. Clin. Pathol. 2019, 151, 75–85. [Google Scholar]
- Derrieux, C.; Trinquand, A.; Bruneau, J.; Verkarre, V.; Lhermitte, L.; Alcantara, M.; Villarese, P.; Meresse, B.; Sibon, D.; Hermine, O.; et al. A Single-Tube, EuroClonality-Inspired, TRG Clonality Multiplex PCR Aids Management of Patients with Enteropathic Diseases, including from Formaldehyde-Fixed, Paraffin-Embedded Tissues. J. Mol. Diagn. 2019, 21, 111–122. [Google Scholar] [CrossRef]
- Zanelli, M.; Negro, A.; Santi, R.; Bisagni, A.; Ragazzi, M.; Ascani, S.; De Marco, L. Letter: Sprue-like enteropathy associated with angiotensin II receptor blockers other than olmesartan. Aliment. Pharmacol. Ther. 2017, 46, 471–473. [Google Scholar] [CrossRef]
- Negro, A.; De Marco, L.; Cesario, V.; Santi, R.; Boni, M.C.; Zanelli, M. A case of Moderate Sprue-Like Enteropathy Associated with Telmisartan. J. Clin. Med. Res. 2017, 9, 1022–1025. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanguedolce, F.; Zanelli, M.; Zizzo, M.; Luminari, S.; Martino, G.; Soriano, A.; Ricci, L.; Caprera, C.; Ascani, S. Indolent T-Cell Lymphoproliferative Disorders of the Gastrointestinal Tract (iTLPD-GI): A Review. Cancers 2021, 13, 2790. https://doi.org/10.3390/cancers13112790
Sanguedolce F, Zanelli M, Zizzo M, Luminari S, Martino G, Soriano A, Ricci L, Caprera C, Ascani S. Indolent T-Cell Lymphoproliferative Disorders of the Gastrointestinal Tract (iTLPD-GI): A Review. Cancers. 2021; 13(11):2790. https://doi.org/10.3390/cancers13112790
Chicago/Turabian StyleSanguedolce, Francesca, Magda Zanelli, Maurizio Zizzo, Stefano Luminari, Giovanni Martino, Alessandra Soriano, Linda Ricci, Cecilia Caprera, and Stefano Ascani. 2021. "Indolent T-Cell Lymphoproliferative Disorders of the Gastrointestinal Tract (iTLPD-GI): A Review" Cancers 13, no. 11: 2790. https://doi.org/10.3390/cancers13112790
APA StyleSanguedolce, F., Zanelli, M., Zizzo, M., Luminari, S., Martino, G., Soriano, A., Ricci, L., Caprera, C., & Ascani, S. (2021). Indolent T-Cell Lymphoproliferative Disorders of the Gastrointestinal Tract (iTLPD-GI): A Review. Cancers, 13(11), 2790. https://doi.org/10.3390/cancers13112790





