Current Status and Challenges of US-Guided Radiofrequency Ablation of Thyroid Nodules in the Long Term: A Systematic Review
Abstract
Simple Summary
Abstract
1. Introduction
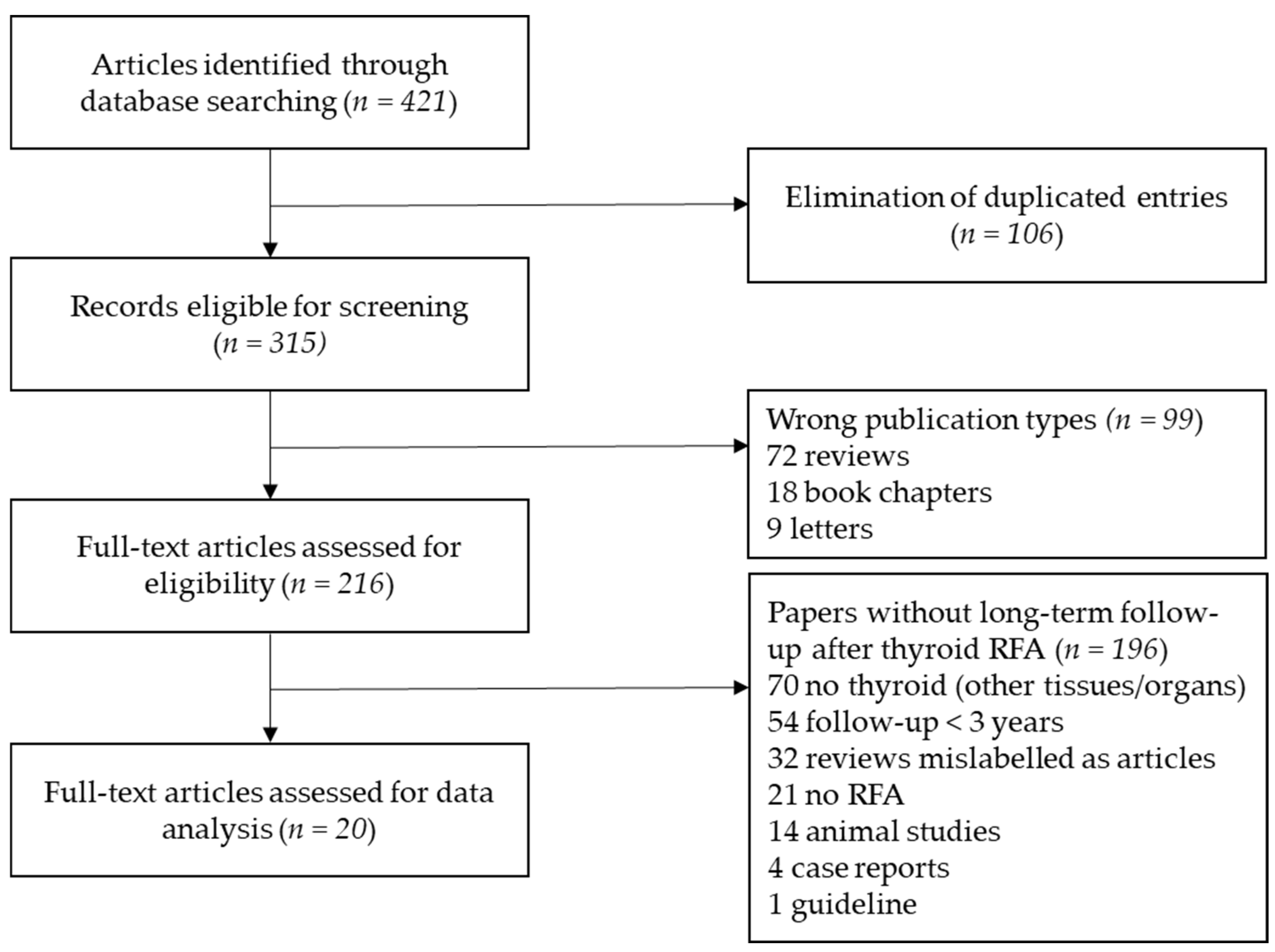
2. Materials and Methods
3. Results
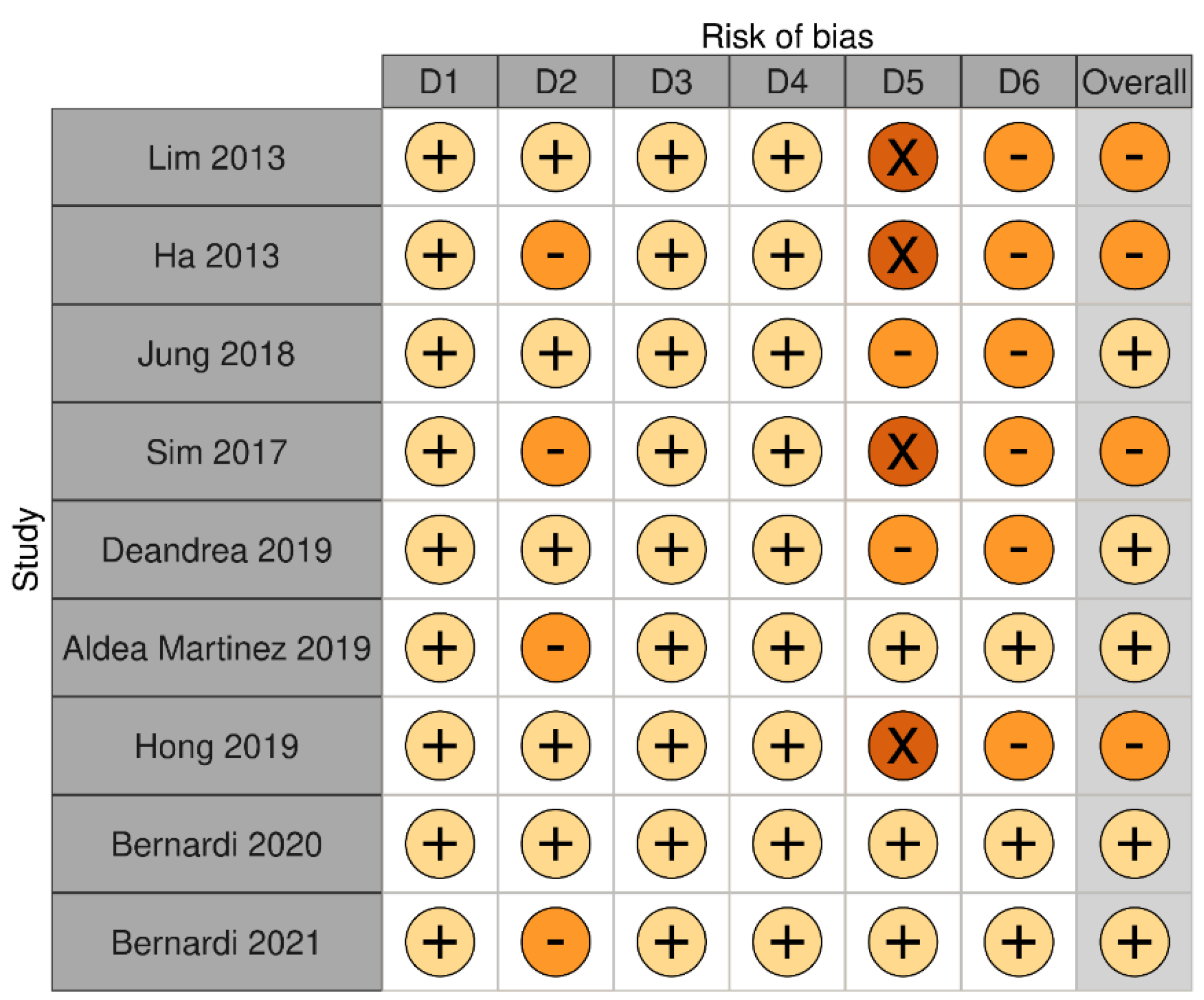
3.1. Long-Term Outcomes of RFA on Benign Thyroid Nodules
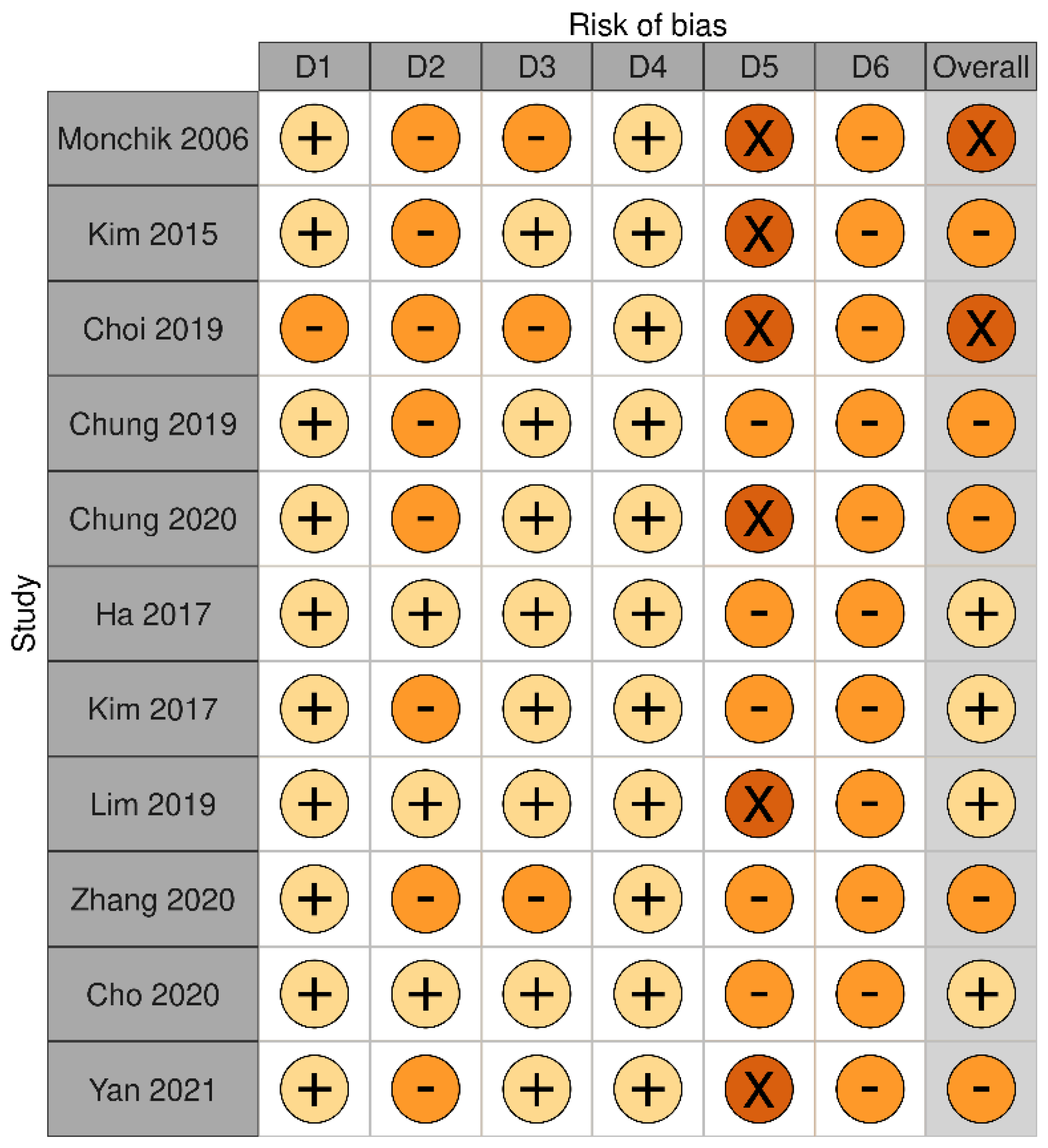
3.2. Long-Term Outcomes of RFA on Malignant Thyroid Nodules
4. Discussion
4.1. The Use of RFA to Treat Benign Thyroid Nodules
4.2. The Use of RFA to Treat Differentiated Thyroid Cancer
4.3. What Do the Guidelines State about the Use of RFA
4.4. Strenghts and Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Trimboli, P. Ultrasound: The Extension of Our Hands to Improve the Management of Thyroid Patients. Cancers 2021, 13, 567. [Google Scholar] [CrossRef] [PubMed]
- Mauri, G.; Sconfienza, L.M. Image-guided thermal ablation might be a way to compensate for image deriving cancer overdiagnosis. Int. J. Hyperth. 2017, 33, 489–490. [Google Scholar] [CrossRef]
- Rangel, L.; Volpi, L.M.; Stabenow, E.; Steck, J.H.; Volpi, E.; Russell, J.O.; Tufano, R.P. Radiofrequency for benign and malign thyroid lesions. World J. Otorhinolaryngol. Head Neck Surg. 2020, 6, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Baek, J.H.; Lim, H.K.; Ahn, H.S.; Baek, S.M.; Choi, Y.J.; Choi, Y.J.; Chung, S.R.; Ha, E.J.; Hahn, S.Y.; et al. 2017 Thyroid Radiofrequency Ablation Guideline: Korean Society of Thyroid Radiology. Korean J. Radiol. 2018, 19, 632–655. [Google Scholar] [CrossRef]
- Papini, E.; Pacella, C.M.; Solbiati, L.A.; Achille, G.; Barbaro, D.; Bernardi, S.; Cantisani, V.; Cesareo, R.; Chiti, A.; Cozzaglio, L.; et al. Minimally-invasive treatments for benign thyroid nodules: A Delphi-based consensus statement from the Italian minimally-invasive treatments of the thyroid (MITT) group. Int. J. Hyperth. 2019, 36, 376–382. [Google Scholar] [CrossRef]
- Papini, E.; Monpeyssen, H.; Frasoldati, A.; Hegedus, L. 2020 European Thyroid Association Clinical Practice Guideline for the Use of Image-Guided Ablation in Benign Thyroid Nodules. Eur. Thyr. J. 2020, 9, 172–185. [Google Scholar] [CrossRef] [PubMed]
- Monzani, F.; Caraccio, N.; Basolo, F.; Iacconi, P.; LiVolsi, V.; Miccoli, P. Surgical and pathological changes after percutaneous ethanol injection therapy of thyroid nodules. Thyroid 2000, 10, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Muller, T.; Bojunga, J.; Dong, Y.; Mauri, G.; Radzina, M.; Dighe, M.; Cui, X.W.; Grunwald, F.; Schuler, A.; et al. Statement and Recommendations on Interventional Ultrasound as a Thyroid Diagnostic and Treatment Procedure. Ultrasound Med. Biol. 2018, 44, 14–36. [Google Scholar] [CrossRef]
- Hegedus, L.; Frasoldati, A.; Negro, R.; Papini, E. European Thyroid Association Survey on Use of Minimally Invasive Techniques for Thyroid Nodules. Eur. Thyr. J. 2020, 9, 194–204. [Google Scholar] [CrossRef]
- Bernardi, S.; Dobrinja, C.; Fabris, B.; Bazzocchi, G.; Sabato, N.; Ulcigrai, V.; Giacca, M.; Barro, E.; De Manzini, N.; Stacul, F. Radiofrequency ablation compared to surgery for the treatment of benign thyroid nodules. Int. J. Endocrinol. 2014, 2014, 934595. [Google Scholar] [CrossRef]
- Bernardi, S.; Stacul, F.; Zecchin, M.; Dobrinja, C.; Zanconati, F.; Fabris, B. Radiofrequency ablation for benign thyroid nodules. J. Endocrinol. Investig. 2016, 39, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- Cesareo, R.; Pasqualini, V.; Simeoni, C.; Sacchi, M.; Saralli, E.; Campagna, G.; Cianni, R. Prospective study of effectiveness of ultrasound-guided radiofrequency ablation versus control group in patients affected by benign thyroid nodules. J. Clin. Endocrinol. Metab. 2015, 100, 460–466. [Google Scholar] [CrossRef]
- Deandrea, M.; Sung, J.Y.; Limone, P.; Mormile, A.; Garino, F.; Ragazzoni, F.; Kim, K.S.; Lee, D.; Baek, J.H. Efficacy and Safety of Radiofrequency Ablation Versus Observation for Nonfunctioning Benign Thyroid Nodules: A Randomized Controlled International Collaborative Trial. Thyroid 2015, 25, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Baek, J.H.; Sung, J.Y.; Min, H.S.; Kim, K.W.; Hah, J.H.; Park, D.J.; Kim, K.H.; Cho, B.Y.; Na, D.G. Radiofrequency ablation of low-risk small papillary thyroidcarcinoma: Preliminary results for patients ineligible for surgery. Int. J. Hyperth. 2017, 33, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.K.; Cho, S.J.; Baek, J.H.; Lee, K.D.; Son, C.W.; Son, J.M.; Baek, S.M. US-Guided Radiofrequency Ablation for Low-Risk Papillary Thyroid Microcarcinoma: Efficacy and Safety in a Large Population. Korean J. Radiol. 2019, 20, 1653–1661. [Google Scholar] [CrossRef]
- Yan, L.; Lan, Y.; Xiao, J.; Lin, L.; Jiang, B.; Luo, Y. Long-term outcomes of radiofrequency ablation for unifocal low-risk papillary thyroid microcarcinoma: A large cohort study of 414 patients. Eur. Radiol. 2021, 31, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.J.; Baek, S.M.; Lim, H.K.; Lee, K.D.; Son, J.M.; Baek, J.H. Long-Term Follow-Up Results of Ultrasound-Guided Radiofrequency Ablation for Low-Risk Papillary Thyroid Microcarcinoma: More than 5-Year Follow-Up for 84 Tumors. Thyroid 2020, 30, 1745–1751. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Yoo, W.S.; Park, Y.J.; Park, D.J.; Yun, T.J.; Choi, S.H.; Sohn, C.H.; Lee, K.E.; Sung, M.W.; Youn, Y.K.; et al. Efficacy and Safety of Radiofrequency Ablation for Treatment of Locally Recurrent Thyroid Cancers Smaller than 2 cm. Radiology 2015, 276, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Jung, S.L.; Bae, J.S.; Lee, S.H.; Jung, C.K.; Jang, J.; Shin, N.Y.; Choi, H.S.; Ahn, K.J.; Kim, B.S. Comparison of efficacy and complications between radiofrequency ablation and repeat surgery in the treatment of locally recurrent thyroid cancers: A single-center propensity score matching study. Int. J. Hyperth. 2019, 36, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Tufano, R.P.; Russell, J.O.; Zhang, Y.; Zhang, Y.; Qiao, Z.; Luo, Y. Ultrasound-Guided Radiofrequency Ablation Versus Surgery for Low-Risk Papillary Thyroid Microcarcinoma: Results of over 5 Years’ Follow-Up. Thyroid 2020, 30, 408–417. [Google Scholar] [CrossRef]
- Bernardi, S.; Giudici, F.; Cesareo, R.; Antonelli, G.; Cavallaro, M.; Deandrea, M.; Giusti, M.; Mormile, A.; Negro, R.; Palermo, A.; et al. Five-Year Results of Radiofrequency and Laser Ablation of Benign Thyroid Nodules: A Multicenter Study from the Italian Minimally Invasive Treatments of the Thyroid Group. Thyroid 2020, 30, 1759–1770. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, S.; Cavallaro, M.; Colombin, G.; Giudici, F.; Zuolo, G.; Zdjelar, A.; Dobrinja, C.; De Manzini, N.; Zanconati, F.; Cova, M.A.; et al. Initial Ablation Ratio Predicts Volume Reduction and Retreatment after 5 Years from Radiofrequency Ablation of Benign Thyroid Nodules. Front. Endocrinol. 2020, 11, 582550. [Google Scholar] [CrossRef]
- Hegedus, L.; Miyauchi, A.; Tuttle, R.M. Nonsurgical Thermal Ablation of Thyroid Nodules: Not if, but Why, When, and How? Thyroid 2020, 30, 1691–1694. [Google Scholar] [CrossRef]
- Cho, S.J.; Baek, J.H.; Chung, S.R.; Choi, Y.J.; Lee, J.H. Long-Term Results of Thermal Ablation of Benign Thyroid Nodules: A Systematic Review and Meta-Analysis. Endocrinol. Metab. 2020, 35, 339–350. [Google Scholar] [CrossRef]
- Bernardi, S.; Giudici, F.; Barbato, V.; Zanatta, L.; Grillo, A.; Fabris, B. Meta-analysis on the Effect of Mild Primary Hyperparathyroidism and Parathyroidectomy upon Arterial Stiffness. J. Clin. Endocrinol. Metab. 2021, 106, 1832–1843. [Google Scholar] [CrossRef]
- Lim, H.K.; Lee, J.H.; Ha, E.J.; Sung, J.Y.; Kim, J.K.; Baek, J.H. Radiofrequency ablation of benign non-functioning thyroid nodules: 4-year follow-up results for 111 patients. Eur. Radiol. 2013, 23, 1044–1049. [Google Scholar] [CrossRef] [PubMed]
- Ha, E.J.; Baek, J.H.; Lee, J.H.; Sung, J.Y.; Lee, D.; Kim, J.K.; Shong, Y.K. Radiofrequency ablation of benign thyroid nodules does not affect thyroid function in patients with previous lobectomy. Thyroid 2013, 23, 289–293. [Google Scholar] [CrossRef]
- Jung, S.L.; Baek, J.H.; Lee, J.H.; Shong, Y.K.; Sung, J.Y.; Kim, K.S.; Lee, D.; Kim, J.H.; Baek, S.M.; Sim, J.S.; et al. Efficacy and Safety of Radiofrequency Ablation for Benign Thyroid Nodules: A Prospective Multicenter Study. Korean J. Radiol. 2018, 19, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Sim, J.S.; Baek, J.H.; Lee, J.; Cho, W.; Jung, S.I. Radiofrequency ablation of benign thyroid nodules: Depicting early sign of regrowth by calculating vital volume. Int. J. Hyperth. 2017, 33, 905–910. [Google Scholar] [CrossRef]
- Deandrea, M.; Trimboli, P.; Garino, F.; Mormile, A.; Magliona, G.; Ramunni, M.J.; Giovanella, L.; Limone, P.P. Long-Term Efficacy of a Single Session of RFA for Benign Thyroid Nodules: A Longitudinal 5-Year Observational Study. J. Clin. Endocrinol. Metab. 2019, 104, 3751–3756. [Google Scholar] [CrossRef]
- Aldea Martinez, J.; Aldea Viana, L.; Lopez Martinez, J.L.; Ruiz Perez, E. Radiofrequency Ablation of Thyroid Nodules: A Long-Term Prospective Study of 24 Patients. J. Vasc. Interv. Radiol. 2019, 30, 1567–1573. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.J.; Sung, J.Y.; Baek, J.H.; Je, M.S.; Choi, D.W.; Yoo, H.; Yang, S.J.; Nam, S.Y.; Yoo, E.Y. Safety and Efficacy of Radiofrequency Ablation for Nonfunctioning Benign Thyroid Nodules in Children and Adolescents in 14 Patients over a 10-Year Period. J. Vasc. Interv. Radiol. 2019, 30, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, S.; Giudici, F.; Colombin, G.; Cavallaro, M.; Stacul, F.; Fabris, B. Residual vital ratio predicts 5-year volume reduction and retreatment after radiofrequency ablation of benign thyroid nodules but not regrowth. Int. J. Hyperth. 2021, 38, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.M.; Sung, J.Y.; Baek, J.H.; Na, D.G.; Kim, J.H.; Yoo, H.; Lee, D.; Whan Choi, D. Radiofrequency ablation of small follicular neoplasms: Initial clinical outcomes. Int. J. Hyperth. 2017, 33, 931–937. [Google Scholar] [CrossRef]
- Mauri, G.; Pacella, C.M.; Papini, E.; Solbiati, L.; Goldberg, S.N.; Ahmed, M.; Sconfienza, L.M. Image-Guided Thyroid Ablation: Proposal for Standardization of Terminology and Reporting Criteria. Thyroid 2019, 29, 611–618. [Google Scholar] [CrossRef]
- Monchik, J.M.; Donatini, G.; Iannuccilli, J.; Dupuy, D.E. Radiofrequency ablation and percutaneous ethanol injection treatment for recurrent local and distant well-differentiated thyroid carcinoma. Ann. Surg. 2006, 244, 296–304. [Google Scholar] [CrossRef]
- Chung, S.R.; Baek, J.H.; Choi, Y.J.; Lee, J.H. Longer-term outcomes of radiofrequency ablation for locally recurrent papillary thyroid cancer. Eur. Radiol. 2019, 29, 4897–4903. [Google Scholar] [CrossRef]
- Chung, S.R.; Baek, J.H.; Choi, Y.J.; Sung, T.Y.; Song, D.E.; Kim, T.Y.; Lee, J.H. Efficacy of radiofrequency ablation for recurrent thyroid cancer invading the airways. Eur. Radiol. 2021, 31, 2153–2160. [Google Scholar] [CrossRef]
- Dupuy, D.E.; Monchik, J.M.; Decrea, C.; Pisharodi, L. Radiofrequency ablation of regional recurrence from well-differentiated thyroid malignancy. Surgery 2001, 130, 971–977. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Lupo, M.A. Radiofrequency Ablation for Benign Thyroid Nodules--a Look Towards the Future of Interventional Thyroidology. Endocr. Pract. 2015, 21, 972–974. [Google Scholar] [CrossRef]
- Gharib, H.; Papini, E.; Garber, J.R.; Duick, D.S.; Harrell, R.M.; Hegedus, L.; Paschke, R.; Valcavi, R.; Vitti, P.; Nodules, A.A.A.T.F.o.T. American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi Medical Guidelines for Clinical Practice for the Diagnosis and Management of Thyroid Nodules—2016 Update. Endocr. Pract. 2016, 22, 622–639. [Google Scholar] [CrossRef] [PubMed]
- Trimboli, P.; Deandrea, M. Treating thyroid nodules by radiofrequency: Is the delivered energy correlated with the volume reduction rate? A pilot study. Endocrine 2020, 69, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Cesareo, R.; Pacella, C.M.; Pasqualini, V.; Campagna, G.; Iozzino, M.; Gallo, A.; Lauria Pantano, A.; Cianni, R.; Pedone, C.; Pozzilli, P.; et al. Laser Ablation Versus Radiofrequency Ablation for Benign Non-Functioning Thyroid Nodules: Six-Month Results of a Randomized, Parallel, Open-Label, Trial (LARA Trial). Thyroid 2020, 30, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Deandrea, M.; Trimboli, P.; Mormile, A.; Cont, A.T.; Milan, L.; Buffet, C.; Giovanella, L.; Limone, P.P.; Poiree, S.; Leenhardt, L.; et al. Determining an energy threshold for optimal volume reduction of benign thyroid nodules treated by radiofrequency ablation. Eur. Radiol. 2021. [Google Scholar] [CrossRef]
- Sim, J.S.; Baek, J.H.; Cho, W. Initial Ablation Ratio: Quantitative Value Predicting the Therapeutic Success of Thyroid Radiofrequency Ablation. Thyroid 2018, 28, 1443–1449. [Google Scholar] [CrossRef]
- Yan, L.; Luo, Y.; Xie, F.; Zhang, M.; Xiao, J. Residual vital ratio: Predicting regrowth after radiofrequency ablation for benign thyroid nodules. Int. J. Hyperth. 2020, 37, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Sim, J.S.; Baek, J.H. Long-Term Outcomes Following Thermal Ablation of Benign Thyroid Nodules as an Alternative to Surgery: The Importance of Controlling Regrowth. Endocrinol. Metab. 2019, 34, 117–123. [Google Scholar] [CrossRef]
- Negro, R.; Trimboli, P. Thermal ablation for benign, non-functioning thyroid nodules: A clinical review focused on outcomes, technical remarks, and comparisons with surgery. Electromagn. Biol. Med. 2020, 39, 347–355. [Google Scholar] [CrossRef]
- Offi, C.; Garberoglio, S.; Antonelli, G.; Esposito, M.G.; Brancaccio, U.; Misso, C.; D’Ambrosio, E.; Pace, D.; Spiezia, S. The Ablation of Thyroid Nodule’s Afferent Arteries Before Radiofrequency Ablation: Preliminary Data. Front. Endocrinol. 2020, 11, 565000. [Google Scholar] [CrossRef]
- Yan, L.; Luo, Y.; Xiao, J.; Lin, L. Non-enhanced ultrasound is not a satisfactory modality for measuring necrotic ablated volume after radiofrequency ablation of benign thyroid nodules: A comparison with contrast-enhanced ultrasound. Eur. Radio. 2020. [Google Scholar] [CrossRef]
- Schiaffino, S.; Serpi, F.; Rossi, D.; Ferrara, V.; Buonomenna, C.; Alì, M.; Monfardini, L.; Sconfienza, L.M.; Mauri, G. Reproducibility of Ablated Volume Measurement Is Higher with Contrast-Enhanced Ultrasound than with B-Mode Ultrasound after Benign Thyroid Nodule Radiofrequency Ablation—A Preliminary Study. J. Clin. Med. 2020, 9, 1504. [Google Scholar] [CrossRef]
- Colombo, C.; Muzza, M.; Pogliaghi, G.; Palazzo, S.; Vannucchi, G.; Vicentini, L.; Persani, L.; Gazzano, G.; Fugazzola, L. The thyroid risk score (TRS) for nodules with indeterminate cytology. Endocr. Relat. Cancer 2021, 28, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Negro, R.; Trimboli, P. Placing Thermal Ablation for Benign Thyroid Nodules into Context. Eur. Thyr. J. 2020, 9, 169–171. [Google Scholar] [CrossRef] [PubMed]
- Park, K.W.; Shin, J.H.; Han, B.K.; Ko, E.Y.; Chung, J.H. Inoperable symptomatic recurrent thyroid cancers: Preliminary result of radiofrequency ablation. Ann. Surg. Oncol. 2011, 18, 2564–2568. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Miyauchi, A.; Inoue, H.; Fukushima, M.; Kihara, M.; Higashiyama, T.; Tomoda, C.; Takamura, Y.; Kobayashi, K.; Miya, A. An observational trial for papillary thyroid microcarcinoma in Japanese patients. World J. Surg. 2010, 34, 28–35. [Google Scholar] [CrossRef]
- Feldkamp, J.; Grunwald, F.; Luster, M.; Lorenz, K.; Vorlander, C.; Fuhrer, D. Non-Surgical and Non-Radioiodine Techniques for Ablation of Benign Thyroid Nodules: Consensus Statement and Recommendation. Exp. Clin. Endocrinol. Diabetes 2020, 128, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Dobnig, H.; Zechmann, W.; Hermann, M.; Lehner, M.; Heute, D.; Mirzaei, S.; Gessl, A.; Stepan, V.; Hofle, G.; Riss, P.; et al. Radiofrequency ablation of thyroid nodules: “Good Clinical Practice Recommendations” for Austria: An interdisciplinary statement from the following professional associations: Austrian Thyroid Association (OSDG), Austrian Society for Nuclear Medicine and Molecular Imaging (OGNMB), Austrian Society for Endocrinology and Metabolism (OGES), Surgical Endocrinology Working Group (ACE) of the Austrian Surgical Society (OEGCH). Wien Med. Wochenschr. 2020, 170, 6–14. [Google Scholar] [CrossRef]
- Mauri, G.; Hegedüs, L.; Bandula, S.; Cazzato, R.L.; Czarniecka, A.; Dudeck, O.; Fugazzola, L.; Netea-Maier, R.; Russ, G.; Wallin, G.; et al. European Thyroid Association and Cardiovascular and Interventional Radiological Society of Europe 2021 Clinical Practice Guideline for the Use of Minimally Invasive Treatments in Malignant Thyroid Lesions. Eur. Thyr. J. 2021. [Google Scholar] [CrossRef]
| Study | Main Findings |
|---|---|
| Lim 2013 [26] | RFA was effective in reducing nodule volume and nodule-related problems such as symptoms and cosmetic concerns (mean VRR was 93.4% at last follow-up). Regrowth rate was 5.6%. |
| Ha 2013 [27] | RFA reduced nodule volume by 87.2% at last follow-up and it did not affect thyroid function in patients with previous lobectomy. |
| Jung 2018 [28] | Nodule volume was reduced by 80.3% after 1 year (n = 276) and by 95.3% after 5 years (n = 6). Solidity and applied energy predicted final volume reduction. |
| Sim 2017 [29] | RFA reduced nodule volume by 97.9% at last follow-up. Regrowth was observed in 24.1% of the nodules. |
| Deandrea 2019 [30] | The VRR that was found at 1 year (63% in 197/215 patients) was maintained at 5 years (67% in 71/215 patients). The best results were obtained in nodules with baseline volume < 10 mL. A total of 4.1% of nodules regrew. |
| Aldea Martinez 2019 [31] | RFA reduced nodule volume by 76.8% after 3 years (in 24/24 patients). |
| Hong 2019 [32] | RFA reduced nodule volume by 92.1% at last follow-up in children and adolescents with no complications. |
| Bernardi 2020 [21] | After propensity score matching, RFA was associated with greater 5-year VRR (75% vs. 56%) and technique efficacy (82% vs. 66%), as well as lower regrowth (17% vs. 34%) and retreatment rate (14% vs. 32%) as compared to LA. Young age, large volume, low 1-year VRR, and low energy delivered were associated with retreatments. |
| Bernardi 2021 [22] | RFA reduced nodule volume by 79% after 5 years (in 78/78 patients). IAR was significantly associated with technique efficacy, VRR, and the likelihood of retreatment but not with regrowth. IAR cut-off were >49% for technique efficacy and >73% for no retreatment. |
| Study | Design | Patients/Nodules * | Age (yrs) | Sex (F%) | Volume (mL) | Diameter (mm) | RFA (n) | Energy (J/mL) | VRR (%) | Follow-Up (Months) |
|---|---|---|---|---|---|---|---|---|---|---|
| Lim 2013 [26] | Retrospective | 111/126 | 37.9 ± 10.6 (9–69) | 91 | 9.8 ± 8.5 (2–43) | 33 ± 10 (20–60) | 1–6 | 2936 ± 1995 (271–9943) | 93.5 ± 11.7 (17–100) | 49.4 ± 13.6 (36–81) |
| Ha 2013 [27] | Retrospective | 11/14 | 44.2 (30–64) | 100 | 9.7 ± 36.3 (0.9–57.6) | 29 ± 24 (15–60) | n.s | n.s. | 87.2 | 43.7 ± 30.7 (7–92) |
| Jung 2018 [28] | Prospective | 276/276 | 46.3 ± 12.8 (15–79) | 88 | 14.2 ± 13.2 (1.1–80.8) | 38 ± 11 (19–80) | 1–2 | 4161 ± 2993 (656–22,031) | 95.3 ± 4.3 (88.5–100) | 60 |
| Sim 2017 [29] | Retrospective | 52/54 | 44.1 ± 13.2 (20–78) | 91 | 14 ± 12.7 (3.1–56.6) | 38 ± 11 (19–77) | 1–? | n.s. | 97.9 | 39.4 ± 21.7 (13–87) |
| Deandrea 2019 [30] | Retrospective | 215/215 | 66# (60–88) | 85 | 20.9# (15–33) | n.s. | 1 | 2210# (1400–3080) | 66.9# | 60 |
| Aldea Martinez 2019 [31] | Prospective | 24/24 | 50.2 ± 13.6 | 83 | 36.3 ± 59.8 (0.7–231.6) | n.s. | 1–? | 1180 ± 716 | 76.8 ± 15.9 | 36 |
| Hong 2019 [32] | Retrospective | 15/15 | 15.7 ± 2.3 (12–19) | 71 | 14.6 ± 13.3 (1.6–49.8) | 37 ± 11 (20–56) | 1–5 | 3153 ± 2065 (782–7504) | 92.1 ± 11.4 | 36.9 ± 21.7 (6–69) |
| Bernardi 2020 [21] | Retrospective | 216/216 | 57# (17–87) | 75 | 17.2# (0.4–179) | n.s. | 1 | 1398# (176–2410) | 77.1# (−34.5–100) | 60 |
| Bernardi 2021 [33] | Retrospective | 78/82 | 59.5# (18–86) | 76 | 11.3# (0.4–54.6) | 23.5# (17.3–30.1) | 1 | n.s. | 79 | 60 |
| Study | Main Findings |
|---|---|
| DTC Neck Tecurrences | |
| Monchik 2006 [36] | No recurrent disease was detected at the treatment site in 14/16 patients. |
| Kim 2015 [18] | After IPTW adjustement, the 3-year recurrence-free survival rate after RFA was comparable to surgery (92.6% vs. 92.2%). |
| Choi 2019 [19] | After PSM, the recurrence-free survival rate after RFA was comparable to surgery (98% vs. 95%). |
| Chung 2019 [37] | RFA reduced DTC recurrences by 99.5% at 5 years and 91.3% of them disappeared. Local recurrences were seen in 27% of patients. |
| Chung 2021 [38] | RFA reduced nodule volume by 81.2% and made disappear 124/172 recurrences (72.1%) after 48 months. |
| Small Follicular Neoplasm | |
| Ha 2017 [34] | RFA reduced the volume of follicular neoplasms by 99.5% after 5 years. 8 out of 10 lesions (80%) disappeared. |
| Low-Risk Papillary Carcinomas/PTMC | |
| Kim 2017 [14] | RFA reduced the volume of papillary carcinoma by 98.5%. 4 out of 6 lesions (66.7%) disappeared. There were no recurrences. |
| Lim 2019 [15] | RFA led to complete disappearance of 91.4% of PTMC, and the remaining PTMC did not regrow. There were no recurrences. |
| Zhang 2020 [20] | RFA was not inferior to surgery in terms of recurrences (1.1% vs. 1.3%). The surgery group had a higher complication rate and a lower quality of life than the RFA group. |
| Cho 2020 [17] | RFA resulted in complete disappearance of all ablated tumors, with no local tumor progression, no lymph-node or distant metastases. 3 patients developed 4 new cancers (4%). |
| Yan 2021 [16] | VRR was 98%. A total of 88.4% of tumors disappeared. Local tumor progression rate was 3.62%. Recurrence rate was 3.4%. |
| Study | Design | Patients/Nodules * | Age (yrs) | Sex (F%) | Volume (mL) | Diameter (mm) | RFA (n) | E (J/mL) | VRR (%) | Recur-rence | Follow-Up (Months) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| DTC Neck Recurrences | |||||||||||
| Monchik 2006 [36] | Retrospective | 16/16 | 53 (28–84) | 75 | n.s. | 17 (8–40) | 1–6 | n.s. | n.s. | 1/16 (6.25%) | 40.7 (10–68) |
| Kim 2015 [18] | Retrospective (vs. surgery) | 27/36 | 42.4 ± 10.3 | 74 | n.s. | 21.1 ± 1.01 | 1–2 | n.s. | 98.4 ± 6.2 (77–100) | 3/26 (11.5%) | 37.7 ± 10.2 |
| Choi 2019 [19] | Retrospective (vs. surgery) | 96/115 | 47.4 ± 14.1 | 72 | n.s | 10 ± 8 | 1–3 | n.s. | n.s. | 12/96 (12.5%) | 76.9 ± 23 |
| Chung 2019 [37] | Retrospective | 29/46 | 51.8 ± 15 (21–84) | 59 | 0.25 ± 0.4 (0.001–2.3) | 8.4 ± 4.7 (3.1–21) | 1–3 | n.s. | 99.5 ± 2.9 (81–100) | 8/29 (27%) | 80 ± 17.3 (60–114) |
| Chung 2021 [38] | Retrospective | 119/172 | 50.7 ± 16 (14–83) | 72 | 0.4 ± 1.4 (0.001–12.6) | 9 ± 6 (3–41) | 1–5 | n.s. | 81.2 ± 55.7 | n.s. | 47.9 ± 35.4 (6–128) |
| Study | Design | Patients/Nodules * | Age (yrs) | Sex (F%) | Volume (mL) | Diameter (mm) | RFA (n) | E (J/mL) | VRR (%) | Recur-rence | Follow-Up (Months) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Small Follicular Neoplasm | |||||||||||
| Ha 2017 [34] | Retrospective | 10/10 | 45 ± 10.5 (27–74) | 100 | 0.6 ± 0.4 (0.2–1.6) | 14 ± 3 (10–19) | 1–2 | 9245 ± 5409 (3976–19,332) | 99.5 ± 1 (97–100) | 0/10 (0%) | 66.4 ± 5.1 (60–76) |
| Low-Risk Papillary Carcinomas/PTMC | |||||||||||
| Kim 2017 [14] | Retrospective | 6/6 | 72 (64–79) | 66 | 0.3 ± 0.2 (0.05–0.4) | 9.2 (6–13) | 1–2 | n.s. | 98.5 ± 3.3 (92–100) | 0/6 (0%) | 48.5 ± 12.3 (36–65) |
| Lim 2019 [15] | Retrospective | 133/152 | 46 ± 12 (19–79) | 85.7 | 0.03 ± 0.04 (0.001–0.3) | 4.3 ± 1.4 (3–10) | 1–2 | 3169 ± 1423 (600–11,550) | 100 | 0/133 (0%) | 39 ± 25 (6–104) |
| Zhang 2020 [20] | Retrospective (vs surgery) | 94/94 | 45 ± 10.8 | 74.5 | 0.17 ± 0.23 | 6.14 ± 2.54 | 1 | n.s. | n.s. | 1/94 (1.1%) | 64.2 ± 2.8 |
| Cho 2020 [17] | Retrospective | 74/84 | 46 ± 12 | 89 | 0.02 (0.001–0.23) | 4 (3–9.9) | 1–2 | 185,237 (13,088–4,716,379) | 100 | 3/74 (4%) | 72 ± 18 (60–124) |
| Yan 2021 [16] | Retrospective | 414/414 | 43.6 ± 9.8 (18–73) | 78 | 0.09 ± 0.08 (0.001–0.5) | 5.22 ± 1.59 (2–10) | 1–? | n.s. | 98.8 ± 6.4 (50–100) | 15/414 (3.62%) | 42.1 ± 11.9 (24–69) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernardi, S.; Palermo, A.; Grasso, R.F.; Fabris, B.; Stacul, F.; Cesareo, R. Current Status and Challenges of US-Guided Radiofrequency Ablation of Thyroid Nodules in the Long Term: A Systematic Review. Cancers 2021, 13, 2746. https://doi.org/10.3390/cancers13112746
Bernardi S, Palermo A, Grasso RF, Fabris B, Stacul F, Cesareo R. Current Status and Challenges of US-Guided Radiofrequency Ablation of Thyroid Nodules in the Long Term: A Systematic Review. Cancers. 2021; 13(11):2746. https://doi.org/10.3390/cancers13112746
Chicago/Turabian StyleBernardi, Stella, Andrea Palermo, Rosario Francesco Grasso, Bruno Fabris, Fulvio Stacul, and Roberto Cesareo. 2021. "Current Status and Challenges of US-Guided Radiofrequency Ablation of Thyroid Nodules in the Long Term: A Systematic Review" Cancers 13, no. 11: 2746. https://doi.org/10.3390/cancers13112746
APA StyleBernardi, S., Palermo, A., Grasso, R. F., Fabris, B., Stacul, F., & Cesareo, R. (2021). Current Status and Challenges of US-Guided Radiofrequency Ablation of Thyroid Nodules in the Long Term: A Systematic Review. Cancers, 13(11), 2746. https://doi.org/10.3390/cancers13112746






