Uni-, Bi- or Trifocal Hepatocellular Carcinoma in Western Patients: Recurrence and Survival after Percutaneous Thermal Ablation
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Ethics
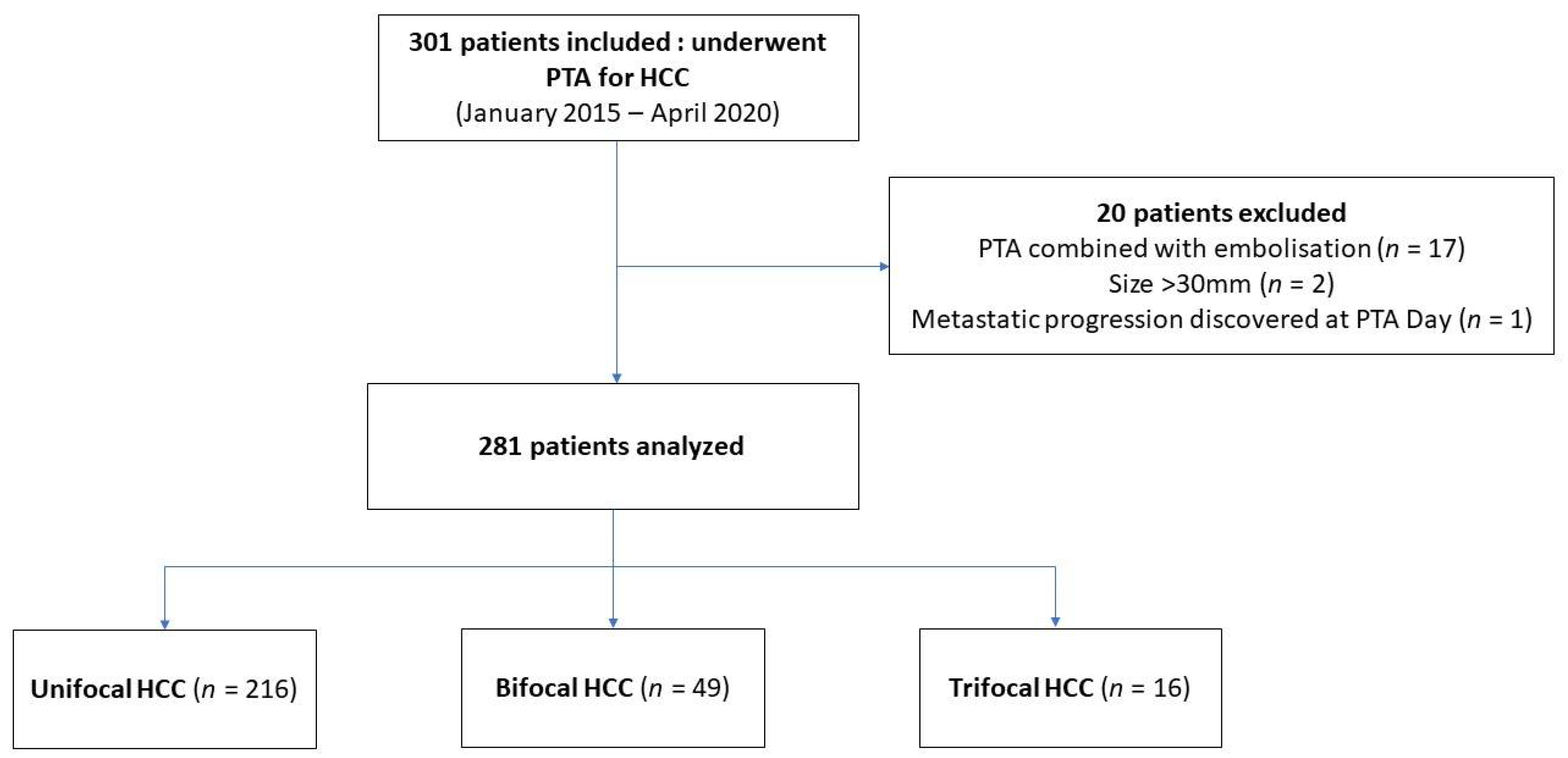
2.2. Patients and Tumor Data
2.3. Percutaneous Thermal Ablation
2.4. Follow-Up and Outcomes
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Follow-Up and Events
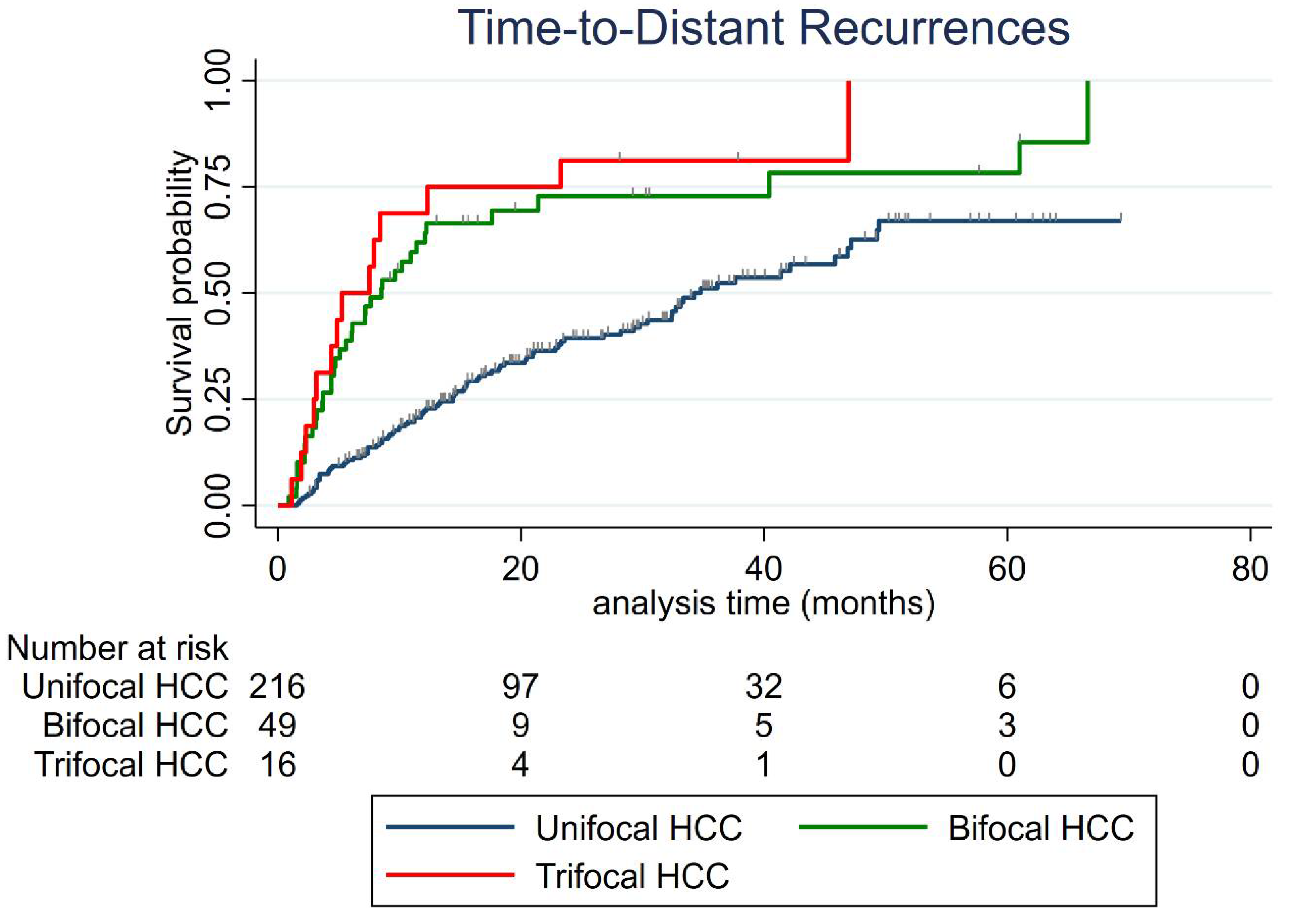
3.3. Distant Recurrence
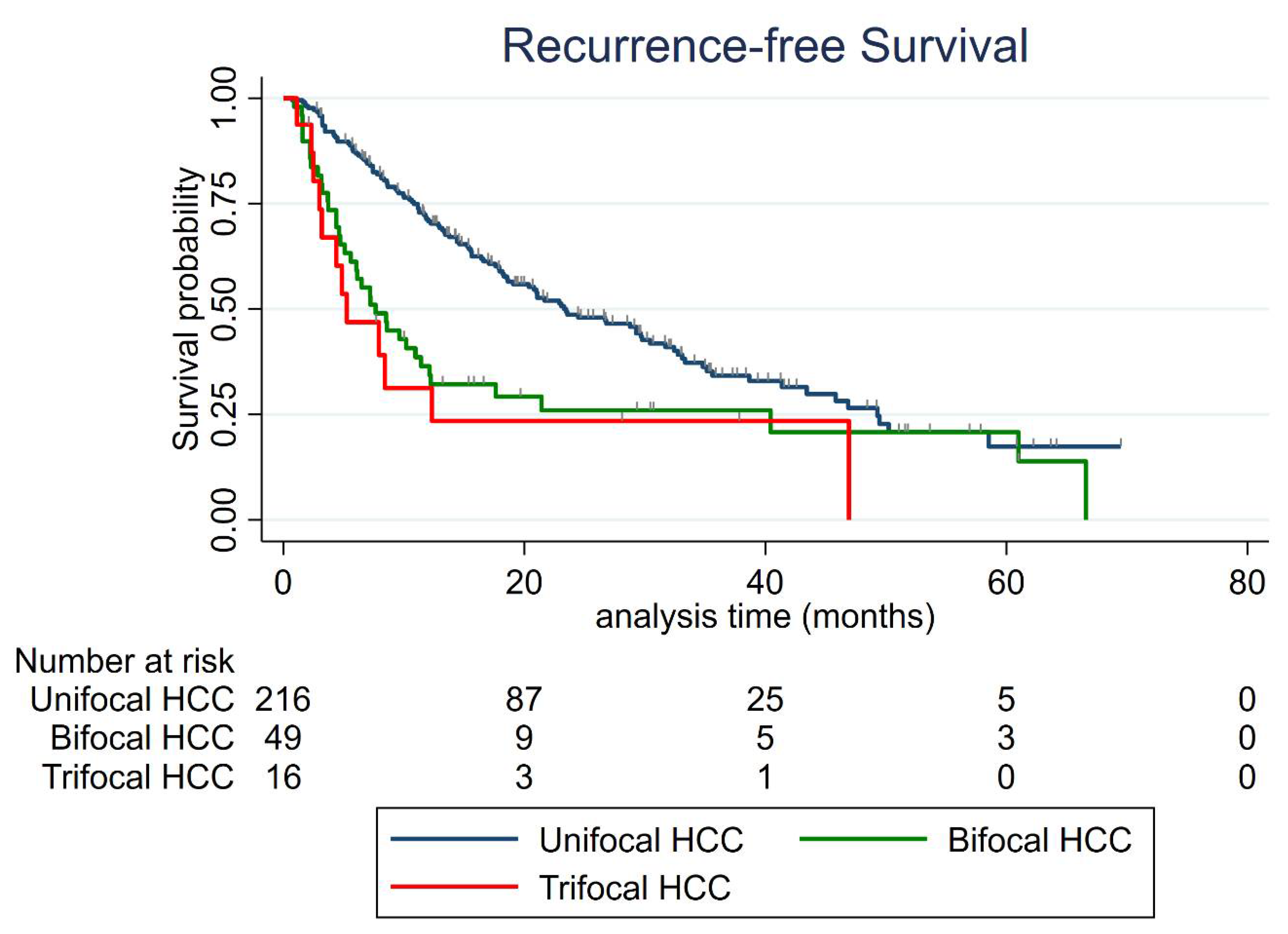
3.4. Recurrence-Free Survival (RFS)
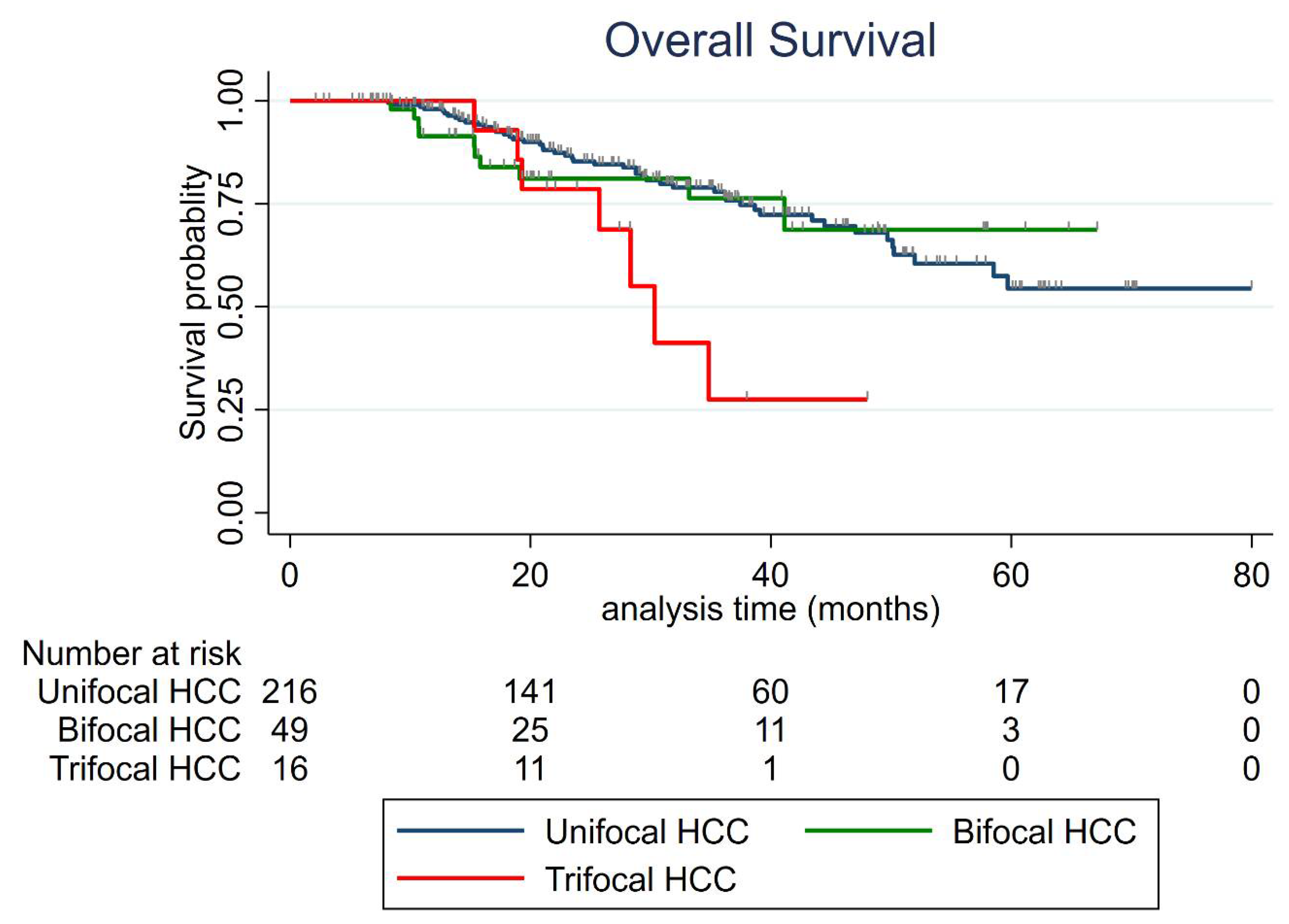
3.5. Overall Survival (OS)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PTA | Percutaneous Thermal Ablation |
| HCC | Hepatocellular Carcinoma |
| BCLC | Barcelona Clinic Liver Cancer |
| US | Ultrasonography |
| RFS | Recurrence-Free Survival |
| AFP | Alpha-Fetoprotein |
| OS | Overall Survival |
| MELD | Model for End-Stage Liver Disease |
| NAFLD | Non-Alcoholic Fatty Liver Disease |
| ECOG | Eastern Cooperative Oncology Group |
| AASLD | American Association for the Study of Liver Disease |
| EASL | European Association for the Study of the Liver |
| BMI | Body Mass Index |
| ALBI | Albumin–Bilirubin |
| MRI | Magnetic Resonance Imaging |
| ASA | American Society of Anesthesiologists |
| CT | Computed Tomography |
| SIR | Society of Interventional Radiology |
| LTP | Local tumor progression |
| SD | Standard Deviation |
| IQR | Interquartile Range |
| AIC | Akaike Information Criterion |
| TACE | Transarterial Chemoembolization |
| OR | Odds Ratio |
| CI | Confidence Interval |
| HR | Hazard Ratio |
| NASH | Non-Alcoholic Steatohepatitis |
References
- Galle, P.R.; Forner, A.; Llovet, J.M.; Mazzaferro, V.; Piscaglia, F.; Raoul, J.-L.; Schirmacher, P.; Vilgrain, V. EASL Clinical Practice Guidelines: Management of Hepatocellular Carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular Carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD Guidelines for the Treatment of Hepatocellular Carcinoma: Heimbach et al. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef]
- Shiina, S.; Tateishi, R.; Arano, T.; Uchino, K.; Enooku, K.; Nakagawa, H.; Asaoka, Y.; Sato, T.; Masuzaki, R.; Kondo, Y.; et al. Radiofrequency Ablation for Hepatocellular Carcinoma: 10-Year Outcome and Prognostic Factors. Am. J. Gastroenterol. 2012, 107, 569–577. [Google Scholar] [CrossRef] [PubMed]
- N’Kontchou, G.; Mahamoudi, A.; Aout, M.; Ganne-Carrié, N.; Grando, V.; Coderc, E.; Vicaut, E.; Trinchet, J.C.; Sellier, N.; Beaugrand, M.; et al. Radiofrequency Ablation of Hepatocellular Carcinoma: Long-Term Results and Prognostic Factors in 235 Western Patients with Cirrhosis. Hepatology 2009, 50, 1475–1483. [Google Scholar] [CrossRef] [PubMed]
- Lencioni, R.; Cioni, D.; Crocetti, L.; Franchini, C.; Pina, C.D.; Lera, J.; Bartolozzi, C. Early-Stage Hepatocellular Carcinoma in Patients with Cirrhosis: Long-Term Results of Percutaneous Image-Guided Radiofrequency Ablation. Radiology 2005, 234, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Lee, J.M.; Lee, J.Y.; Kim, S.H.; Yoon, J.H.; Kim, Y.J.; Han, J.K.; Choi, B.I. Radiofrequency Ablation of Hepatocellular Carcinoma as First-Line Treatment: Long-Term Results and Prognostic Factors in 162 Patients with Cirrhosis. Vasc. Interv. Radiol. 2014, 270, 10. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Ravetta, V.; Rosa, L.; Ghittoni, G.; Viera, F.T.; Garbagnati, F.; Silini, E.M.; Dionigi, P.; Calliada, F.; Quaretti, P.; et al. Repeated Radiofrequency Ablation for Management of Patients with Cirrhosis with Small Hepatocellular Carcinomas: A Long-Term Cohort Study. Hepatol. Baltim. Md. 2011, 53, 136–147. [Google Scholar] [CrossRef]
- Kim, Y.; Lim, H.K.; Rhim, H.; Lee, M.W.; Choi, D.; Lee, W.J.; Paik, S.W.; Koh, K.C.; Lee, J.H.; Choi, M.S.; et al. Ten-Year Outcomes of Percutaneous Radiofrequency Ablation as First-Line Therapy of Early Hepatocellular Carcinoma: Analysis of Prognostic Factors. J. Hepatol. 2013, 58, 89–97. [Google Scholar] [CrossRef]
- Doyle, A.; Gorgen, A.; Muaddi, H.; Aravinthan, A.D.; Issachar, A.; Mironov, O.; Zhang, W.; Kachura, J.; Beecroft, R.; Cleary, S.P.; et al. Outcomes of Radiofrequency Ablation as First-Line Therapy for Hepatocellular Carcinoma Less than 3 Cm in Potentially Transplantable Patients. J. Hepatol. 2019, 70, 866–873. [Google Scholar] [CrossRef]
- Hermida, M.; Cassinotto, C.; Piron, L.; Aho-Glélé, S.; Guillot, C.; Schembri, V.; Allimant, C.; Jaber, S.; Pageaux, G.-P.; Assenat, E.; et al. Multimodal Percutaneous Thermal Ablation of Small Hepatocellular Carcinoma: Predictive Factors of Recurrence and Survival in Western Patients. Cancers 2020, 12, 313. [Google Scholar] [CrossRef]
- Guiu, B.; Petit, J.-M.; Loffroy, R.; Ben Salem, D.; Aho, S.; Masson, D.; Hillon, P.; Krause, D.; Cercueil, J.-P. Quantification of Liver Fat Content: Comparison of Triple-Echo Chemical Shift Gradient-Echo Imaging and in Vivo Proton MR Spectroscopy. Radiology 2009, 250, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Cardella, J.F.; Kundu, S.; Miller, D.L.; Millward, S.F.; Sacks, D. Society of Interventional Radiology Clinical Practice Guidelines. J. Vasc. Interv. Radiol. 2009, 20, S189–S191. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Yan, J.; Li, X.; Xia, F.; Ma, K.; Wang, S.; Bie, P.; Dong, J. A Randomized Controlled Trial of Radiofrequency Ablation and Surgical Resection in the Treatment of Small Hepatocellular Carcinoma. J. Hepatol. 2012, 57, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Takuma, Y.; Shota, I.; Miyatake, H.; Uematsu, S.; Okamoto, R.; Araki, Y.; Takabatake, H.; Morimoto, Y.; Yamamoto, H. Nomograms to Predict the Disease-Free Survival and Overall Survival after Radiofrequency Ablation for Hepatocellular Carcinoma. Intern. Med. 2018, 57, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.-T.; Wang, C.-C.; Lu, L.-G.; Zhang, W.-D.; Zhang, F.-J.; Shi, F.; Li, C.-X. Hepatocellular Carcinoma: Clinical Study of Long-Term Survival and Choice of Treatment Modalities. World J. Gastroenterol. 2013, 19, 3649–3657. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, L.; Yan, L.; Yang, J.; Li, B.; Wen, T.; Zeng, Y.; Wang, W.; Xu, M. Radiofrequency Ablation for HCC Patients with Multifocal Tumours Meeting the Milan Criteria: A Single-Centre Experience. Dig. Liver Dis. 2016, 48, 1485–1491. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, X.-Y.; Lu, X.; Zhai, B. Laparoscopic Microwave Ablation of Hepatocellular Carcinoma at Liver Surface: Technique Effectiveness and Long-Term Outcomes. Technol. Cancer Res. Treat. 2019, 18. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Lu, S.-N.; Hung, C.-H.; Wang, J.-H.; Chen, C.-H.; Yen, Y.-H.; Kuo, Y.-H.; Kee, K.-M. Predicting Outcomes for Recurrent Hepatocellular Carcinoma within Milan Criteria after Complete Radiofrequency Ablation. PLoS ONE 2020, 15, e0242113. [Google Scholar] [CrossRef]
- Yan, K.; Chen, M.H.; Yang, W.; Wang, Y.B.; Gao, W.; Hao, C.Y.; Xing, B.C.; Huang, X.F. Radiofrequency Ablation of Hepatocellular Carcinoma: Long-Term Outcome and Prognostic Factors. Eur. J. Radiol. 2008, 67, 336–347. [Google Scholar] [CrossRef]
- Brar, G.; Greten, T.F.; Graubard, B.I.; McNeel, T.S.; Petrick, J.L.; McGlynn, K.A.; Altekruse, S.F. Hepatocellular Carcinoma Survival by Etiology: A SEER-Medicare Database Analysis. Hepatol. Commun. 2020, 4, 1541–1551. [Google Scholar] [CrossRef]
- Asaoka, Y.; Tateishi, R.; Nakagomi, R.; Kondo, M.; Fujiwara, N.; Minami, T.; Sato, M.; Uchino, K.; Enooku, K.; Nakagawa, H.; et al. Frequency of and Predictive Factors for Vascular Invasion after Radiofrequency Ablation for Hepatocellular Carcinoma. PLoS ONE 2014, 9, e111662. [Google Scholar] [CrossRef]
- Kim, P.N.; Choi, D.; Rhim, H.; Rha, S.E.; Hong, H.P.; Lee, J.; Choi, J.-I.; Kim, J.W.; Seo, J.W.; Lee, E.J.; et al. Planning Ultrasound for Percutaneous Radiofrequency Ablation to Treat Small (≤3 cm) Hepatocellular Carcinomas Detected on Computed Tomography or Magnetic Resonance Imaging: A Multicenter Prospective Study to Assess Factors Affecting Ultrasound Visibility. J. Vasc. Interv. Radiol. 2012, 23, 627–634. [Google Scholar] [CrossRef]
- Llovet, J.M.; De Baere, T.; Kulik, L.; Haber, P.K.; Greten, T.F.; Meyer, T.; Lencioni, R. Locoregional Therapies in the Era of Molecular and Immune Treatments for Hepatocellular Carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 293–313. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, A.; Roccarina, D.; Thorburn, D.; Davidson, B.R.; Tsochatzis, E.; Gurusamy, K.S. Management of People with Early- or Very Early-Stage Hepatocellular Carcinoma: An Attempted Network Meta-Analysis. Cochrane Database Syst. Rev. 2017, 3, CD011650. [Google Scholar] [CrossRef]
- Centonze, L.; Di Sandro, S.; Lauterio, A.; De Carlis, R.; Frassoni, S.; Rampoldi, A.; Tuscano, B.; Bagnardi, V.; Vanzulli, A.; De Carlis, L. Surgical Resection vs. Percutaneous Ablation for Single Hepatocellular Carcinoma: Exploring the Impact of Li-RADS Classification on Oncological Outcomes. Cancers 2021, 13, 1671. [Google Scholar] [CrossRef]
- Sempokuya, T.; Wong, L.L. Ten-Year Survival and Recurrence of Hepatocellular Cancer. Hepatoma Res. 2019, 5. [Google Scholar] [CrossRef] [PubMed]
- Imamura, H.; Matsuyama, Y.; Tanaka, E.; Ohkubo, T.; Hasegawa, K.; Miyagawa, S.; Sugawara, Y.; Minagawa, M.; Takayama, T.; Kawasaki, S.; et al. Risk Factors Contributing to Early and Late Phase Intrahepatic Recurrence of Hepatocellular Carcinoma after Hepatectomy. J. Hepatol. 2003, 38, 200–207. [Google Scholar] [CrossRef]
- Tabrizian, P.; Jibara, G.; Shrager, B.; Schwartz, M.; Roayaie, S. Recurrence of Hepatocellular Cancer after Resection: Patterns, Treatments, and Prognosis. Ann. Surg. 2015, 261, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Fong, Y.; Fortner, J.; Sun, R.L.; Brennan, M.F.; Blumgart, L.H. Clinical Score for Predicting Recurrence After Hepatic Resection for Metastatic Colorectal Cancer: Analysis of 1001 Consecutive Cases. Ann. Surg. 1999, 230, 309. [Google Scholar] [CrossRef]
- Petrowsky, H.; Gonen, M.; Jarnagin, W.; Lorenz, M.; DeMatteo, R.; Heinrich, S.; Encke, A.; Blumgart, L.; Fong, Y. Second Liver Resections Are Safe and Effective Treatment for Recurrent Hepatic Metastases from Colorectal Cancer. Ann Surg 2002, 235, 9. [Google Scholar] [CrossRef]
- Aufhauser, D.D.; Sadot, E.; Murken, D.R.; Eddinger, K.; Hoteit, M.; Abt, P.L.; Goldberg, D.S.; DeMatteo, R.P.; Levine, M.H. Incidence of Occult Intrahepatic Metastasis in Hepatocellular Carcinoma Treated With Transplantation Corresponds to Early Recurrence Rates After Partial Hepatectomy. Ann. Surg. 2018, 267, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular Carcinoma. Nat. Rev. Dis. Primer 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Villanueva, A.; Marrero, J.A.; Schwartz, M.; Meyer, T.; Galle, P.R.; Lencioni, R.; Greten, T.F.; Kudo, M.; Mandrekar, S.J.; et al. Trial Design and Endpoints in Hepatocellular Carcinoma: AASLD Consensus Conference. Hepatol. Baltim. Md 2021, 73 (Suppl. 1), 158–191. [Google Scholar] [CrossRef]
- Bolondi, L.; Burroughs, A.; Dufour, J.-F.; Galle, P.R.; Mazzaferro, V.; Piscaglia, F.; Raoul, J.L.; Sangro, B. Heterogeneity of Patients with Intermediate (BCLC B) Hepatocellular Carcinoma: Proposal for a Subclassification to Facilitate Treatment Decisions. Semin. Liver Dis. 2012, 32, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Golfieri, R.; Bargellini, I.; Spreafico, C.; Trevisani, F. Patients with Barcelona Clinic Liver Cancer Stages B and C Hepatocellular Carcinoma: Time for a Subclassification. Liver Cancer 2019, 8, 78–91. [Google Scholar] [CrossRef]
- Takayasu, K.; Arii, S.; Kudo, M.; Ichida, T.; Matsui, O.; Izumi, N.; Matsuyama, Y.; Sakamoto, M.; Nakashima, O.; Ku, Y.; et al. Superselective Transarterial Chemoembolization for Hepatocellular Carcinoma. Validation of Treatment Algorithm Proposed by Japanese Guidelines. J. Hepatol. 2012, 56, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Maeda, N.; Osuga, K.; Mikami, K.; Higashihara, H.; Onishi, H.; Nakaya, Y.; Tatsumi, M.; Hori, M.; Kim, T.; Tomoda, K.; et al. Angiographic Evaluation of Hepatic Arterial Damage after Transarterial Chemoembolization for Hepatocellular Carcinoma. Radiat. Med. 2008, 26, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Suh, C.H.; Shin, J.H.; Yoon, H.M.; Yoon, H.-K.; Ko, G.-Y.; Gwon, D.-I.; Kim, J.-H.; Sung, K.-B. Angiographic Evaluation of Hepatic Arterial Injury after Cisplatin and Gelfoam–Based Transcatheter Arterial Chemoembolization for Hepatocellular Carcinoma in a 205 Patient Cohort during a 6-Year Follow-Up. Br. J. Radiol. 2014, 87, 20140054. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- Hiroishi, K.; Eguchi, J.; Baba, T.; Shimazaki, T.; Ishii, S.; Hiraide, A.; Sakaki, M.; Doi, H.; Uozumi, S.; Omori, R.; et al. Strong CD8(+) T-Cell Responses against Tumor-Associated Antigens Prolong the Recurrence-Free Interval after Tumor Treatment in Patients with Hepatocellular Carcinoma. J. Gastroenterol. 2010, 45, 451–458. [Google Scholar] [CrossRef]
- Duffy, A.G.; Ulahannan, S.V.; Makorova-Rusher, O.; Rahma, O.; Wedemeyer, H.; Pratt, D.; Davis, J.L.; Hughes, M.S.; Heller, T.; ElGindi, M.; et al. Tremelimumab in Combination with Ablation in Patients with Advanced Hepatocellular Carcinoma. J. Hepatol. 2017, 66, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Zerbini, A.; Pilli, M.; Penna, A.; Pelosi, G.; Schianchi, C.; Molinari, A.; Schivazappa, S.; Zibera, C.; Fagnoni, F.F.; Ferrari, C.; et al. Radiofrequency Thermal Ablation of Hepatocellular Carcinoma Liver Nodules Can Activate and Enhance Tumor-Specific T-Cell Responses. Cancer Res. 2006, 66, 1139–1146. [Google Scholar] [CrossRef] [PubMed]
- Mizukoshi, E.; Yamashita, T.; Arai, K.; Sunagozaka, H.; Ueda, T.; Arihara, F.; Kagaya, T.; Yamashita, T.; Fushimi, K.; Kaneko, S. Enhancement of Tumor-Associated Antigen-Specific T Cell Responses by Radiofrequency Ablation of Hepatocellular Carcinoma. Hepatol. Baltim. Md. 2013, 57, 1448–1457. [Google Scholar] [CrossRef] [PubMed]
- Hermida, M.; Preel, A.; Assenat, E.; Piron, L.; Cassinotto, C.; Ursic-Bedoya, J.; Guillot, C.; Herrero, A.; Panaro, F.; Pageaux, G.; et al. Small Steatotic HCC: A Radiological Variant Associated With Improved Outcome After Ablation. Hepatol. Commun. 2021, 5, 689–700. [Google Scholar] [CrossRef]
- Nault, J.-C.; Martin, Y.; Caruso, S.; Hirsch, T.Z.; Bayard, Q.; Calderaro, J.; Charpy, C.; Copie-Bergman, C.; Ziol, M.; Bioulac-Sage, P.; et al. Clinical Impact of Genomic Diversity From Early to Advanced Hepatocellular Carcinoma. Hepatol. Baltim. Md. 2020, 71, 164–182. [Google Scholar] [CrossRef]
| Variables | Global | Unifocal | Bifocal | Trifocal | p-Value |
| Patients | 281 | 216 | 49 | 16 | |
| Age (median (IQR) years) | 65 (59–72) | 66 (60–73) | 63 (57–69) | 58.5 (52.5–65.5) | 0.082 |
| Sex (n, %) | |||||
| Male | 225 (80.07) | 170 (78.70) | 42 (85.71) | 13 (81.25) | 0.568 |
| Female | 56 (19.93) | 46 (21.30) | 7 (14.29) | 3 (18.75) | |
| ASA score (n, %) | 0.253 | ||||
| 1–2 | 146 (51.96) | 110 (50.92) | 30 (61.22) | 6 (37.50) | |
| 3–4 | 135 (48.04) | 106 (49.08) | 19 (38.78) | 10 (62.50) | |
| Diabetes (n, %) | 0.534 | ||||
| No | 170 (60.50) | 124 (57.41) | 33 (67.35) | 13 (81.25) | |
| Yes | 111 (39.50) | 92 (42.59) | 16 (32.65) | 3 (18.75) | |
| Metformin treatment (n, %) | 52 (18.51) | 43 (19.91) | 7 (14.29) | 2 (12.50) | 0.627 |
| Statin treatment (n, %) | 49 (17.44) | 40 (18.52) | 8 (16.33) | 1 (6.25) | 0.648 |
| BMI (median (IQR) kg/m2) | 27 (24–30) | 27 (24–30) | 27 (24–31) | 27 (24–29.5) | 0.399 |
| Prior treatment for HCC (n, %) | 0.264 | ||||
| Naïve patient | 138 (49.11) | 106 (49.07) | 27 (55.10) | 5 (31.25) | |
| Yes | 143 (50.89) | 110 (50.93) | 22 (44.90) | 11 (68.75) | |
| PTA (in medical history) | 58 (20.64) | 49 (22.69) | 6 (12.24) | 3 (18.75) | |
| Liver disease | |||||
| Cirrhosis (n, %) | 1.000 | ||||
| No | 23 (8.19) | 18 (8.33) | 4 (8.16) | 1 (6.25) | |
| Yes | 258 (91.81) | 198 (91.67) | 45 (91.84) | 15 (93.75) | |
| Causes for hepatopathy (n, %) | 0.23 | ||||
| Alcohol | 115 (40.9) | 83 (38.4) | 25 (51) | 7 (43.8) | |
| Viral hepatitis or mixed | 113 (40.2) | 88 (40.7) | 19 (38.8) | 6 (37.5) | |
| NASH | 41 (14.6) | 36 (16.7) | 2 (4.1) | 3 (18.7) | |
| Hemochromatosis and others | 12 (4.3) | 9 (4.2) | 3 (6.1) | 0 (0) | |
| Steatosis (n, %) | 0.430 | ||||
| Absent | 176 (64.23) | 138 (65.71) | 30 (62.50) | 8 (50.00) | |
| Present | 98 (35.77) | 72 (34.29) | 18 (37.50) | 8 (50.00) | |
| MR quantification (median (IQR) %) | 3 (2–6) | 3 (2–6) | 3 (2–6) | 5 (3–10) | 0.547 |
| Child-Pugh class | 0.511 | ||||
| A5 | 235 (83.6) | 183 (84.7) | 39 (79.6) | 13 (81.2) | |
| A6 | 39 (13.9) | 27 (12.5) | 9 (18.4) | 3 (18.8) | |
| B7 | 7 (2.5) | 7 (3.2) | 0 (0.0) | 0 (0.0) | |
| MELD score (median (IQR)) | 8 (7–10) | 8 (7–10) | 10 (7–13) | 8.5 (8–10) | 0.144 |
| MELD score > 9 | 95 (33.81) | 68 (31.48) | 20 (40.82) | 7 (43.75) | 0.300 |
| Laboratory data (median (IQR)) | |||||
| AFP (ng/mL) | 5.2 (7.7) | 4.7 (6.3) | 7.6 (20.8) | 7.7 (12.4) | 0.094 |
| Total bilirubin (µmol/l) | 11 (10.2) | 11 (9.6) | 12 (9) | 12 (13.1) | 0.368 |
| Albumin (g/l) | 41 (6) | 41 (6) | 40 (6) | 41.5 (6.5) | 0.707 |
| Prothrombin activity (%) | 85 (23) | 86 (22) | 78 (23) | 84 (23.5) | 0.102 |
| Platelet count (×10/mm3) | 124 (91) | 132 (96) | 100.5 (116.5) | 100.5 (116.5) | 0.231 |
| Platelet count ≤ 90000/ mm3 (n, %) | 96 (34.16) | 66 (30.56) | 22 (44.90) | 8 (50.00) | 0.063 |
| Neutrophiles (×10/mm3) | 3.28 (1.63) | 3.32 (1.59) | 3.16 (1.85) | 3.65 (2.51) | 0.813 |
| Lymphocytes (×10/mm3) | 1.42 (0.91) | 1.41 (0.92) | 1.42 (0.95) | 1.65 (1.19) | 0.879 |
| Monocytes (×10/mm3) | 0.51 (0.27) | 0.52 (0.28) | 0.49 (0.22) | 0.56 (0.24) | 0.812 |
| Creatinine (µmol/L) | 75 (28) | 76.5 (29) | 71 (18.40) | 66.5 (26) | 0.057 |
| ALBI score | 0.839 | ||||
| 1 | 179 (66.54) | 139 (67.48) | 30 (63.83) | 10 (62.50) | |
| 2 | 90 (33.46) | 67 (32.52) | 17 (36.17) | 6 (37.50) | |
| HCC | |||||
| Size of the largest nodule (median (IQR) mm) | 16 (13–20) | 15 (12–20) | 17 (13–20) | 17 (16–21) | 0.099 |
| Tumor size < 20 mm (n, %) | 197 (70.11) | 154 (71.30) | 32 (65.31) | 11 (68.75) | 0.696 |
| At least one biospy-proven nodule (n, %) | 55 (19.6) | 42 (19.4) | 10 (20.4) | 3 (18.8) | 0.96 |
| Subcaspular location (n, %) | 105 (37.4) | 77 (35.7) | 23 (46.9) | 5 (31.3) | 0.31 |
| Dome location (n, %) | 70 (24.9) | 60 (27.8) | 7 (14.3) | 3 (18.8) | 0.11 |
| Peri-vascular tumor (n, %) | 64 (22.8) | 58 (26.8) | 5 (10.2) | 1 (6.3) | 0.01 |
| Steatotic HCC (n, %) | 57 (22.27) | 51 (25.63) | 4 (9.30) | 2 (14.29) | 0.046 |
| PTA | |||||
| PTA modality (n, %) | 0.857 | ||||
| Radiofrequency | 122 (43.42) | 92 (42.59) | 23 (46.94) | 7 (43.75) | |
| Microwave | 159 (56.58) | 124 (57.41) | 26 (53.06) | 9 (56.25) | |
| Imaging guidance (n, %) | 0.369 | ||||
| Ultrasonography guidance | 147 (52.31) | 119 (55.09) | 21 (42.86) | 7 (43.75) | |
| CT guidance | 130 (46.26) | 93 (43.06) | 28 (57.14) | 9 (56.25) |
| Cumulative Distant Recurrence Rate Per Year | Unifocal | Bifocal | Trifocal |
|---|---|---|---|
| 6-month | 10.7% (95% CI: 7.3–15.7) | 38.8% (95% CI: 26.8–53.8) | 50% (95% CI: 29–75.5) |
| 1-year | 21.8% (95% CI: 16.8–28.1%) | 61.9% (95% CI: 48.5–75.4%) | 68.8% (95% CI: 46.4–88.6%) |
| 2-year | 39.4% (95% CI: 32.6–47.1%) | 72.9% (95% CI: 58.9–85.2%) | 81.3% (95% CI: 59.8–95.4%) |
| 3-year | 51.1% (95% CI: 43–59.7%) | 72.9% (95% CI: 58.9–85.2%) | 81.3% (95% CI: 59.8–95.4%) |
| Characteristics of the 1st Distant Recurrence | Unifocal | Bifocal | Trifocal | p Value |
|---|---|---|---|---|
| Tumor number recurrence ≤ 3 | 64/93 (68.8%) | 29/37 (78.4%) | 9/14 (64.3%) | 0.5 |
| Portal vein invasion or extra-hepatic metastasis | 13/93 (14%) | 5/37 (13.5%) | 2/14 (14.3%) | 0.82 |
| Size of the largest nodule (mm) | 14 (11–18) | 14 (12–19) | 13 (10–16) | 0.59 |
| Alpha-foetoprotein (ng/mL) | 4.7 (3–10.3) | 7.7 (4–31.9) | 6.3 (4–13.1) | 0.49 |
| Non-Curative treatment * | 42.4% | 36.2% | 38.5% | 0.71 |
| Univariate Analysis | Multivariate Analysis | Bootstrapping (200 Replications) | ||||
|---|---|---|---|---|---|---|
| Variables | Hazard Ratio (95% CI) | p Value | Hazard Ratio (95% CI) | p Value | Hazard Ratio (95% CI) | p Value |
| Patients | ||||||
| Age | 1.0 (0.99–1.02) | 0.65 | ||||
| Sex female vs. male | 0.82 (0.58–1.17) | 0.28 | ||||
| Body Mass Index | 0.99 (0.96–1.02) | 0.36 | ||||
| ASA (>2 vs. ≤2) | 0.92 (0.68–1.23) | 0.56 | ||||
| Diabetes | 0.90 (0.67–1.22) | 0.52 | ||||
| Metformin treatment | 0.79 (0.51–1.22) | 0.29 | ||||
| Statin treatment | 1.05 (0.71–1.56) | 0.79 | ||||
| Treatment-naïve patient | 0.48 (0.36–0.66) | <0.001 | 0.46 (0.32–0.65) | <0.001 | 0.46 (0.31–0.67) | <0.001 |
| Cirrhosis | 1.23 (0.67–2.28) | 0.50 | ||||
| Child-Pugh (B vs. A) | 1.63 (0.79–3.36) | 0.19 | ||||
| Cause of liver disease(vs. alcohol) | ||||||
| Viral hepatitis or mixed | 0.82 (0.56–1.18) | 0.28 | ||||
| Hemochromatosis and others | 0.82 (0.38–1.76) | 0.61 | ||||
| NASH | 0.52 (0.32–0.84) | 0.007 | 0.67 (0.37–1.22) | 0.190 | 0.67 (0.34–1.32) | 0.245 |
| Steatosis | 1.09 (0.80–1.49) | 0.59 | ||||
| Laboratory Data | ||||||
| AFP ≥ 100 vs. <100 ng/mL | 3.27 (1.63–6.55) | 0.001 | 3.31 (1.87–5.87) | <0.001 | 3.31 (1.70–6.45) | <0.001 |
| AFP (per unit) | 1.0 (1.0–1.0) | <0.001 | ||||
| Prothrombin time | 1.0 (0.98–1.01) | 0.56 | ||||
| Platelet count | 1.0 (1.0–1.0) | 0.81 | ||||
| Albumin | 0.97 (0.94–1.00) | 0.057 | ||||
| Bilirubin | 1.02 (1.0–1.03) | 0.11 | ||||
| Creatinine | 1.0 (1.0–1.0) | 0.97 | ||||
| MELD (>9 vs. ≤9) | 1.17 (0.86–1.59) | 0.33 | ||||
| ALBI score 2 vs. 1 | 1.33 (0.97–1.82) | 0.07 | ||||
| HCC | ||||||
| Bifocal HCC (vs. unifocal) | 1.98 (1.27–3.06) | 0.002 | 2.46 (1.60–3.77) | <0.001 | 2.46 (1.53–3.96) | <0.001 |
| Trifocal HCC (vs. unifocal) | 2.50 (1.19–5.23) | 0.015 | 2.70 (1.16–6.29) | 0.021 | 2.70 (1.02–7.08) | 0.044 |
| Tumor size < 2 vs. ≥2 cm | 0.99 (0.73–1.35) | 0.968 | ||||
| Steatotic HCC | 0.59 (0.39–0.88) | 0.011 | 0.81 (0.52–1.26) | 0.35 | 0.81 (0.51–1.28) | 0.364 |
| PTA | ||||||
| PTA modality: MWA vs. RF | 1.1 (0.81–1.48) | 0.54 | ||||
| PTA imaging guidance: US vs. CT | 0.96 (0.71–1.30) | 0.81 | ||||
| Harrell’s C statistic: 0.69 AIC: 1256.97 | ||||||
| Overall Survival Rate Per Year | Unifocal | Bifocal | Trifocal |
|---|---|---|---|
| 1-year | 98% (95%CI: 94.8–99.2%) | 91.4% (95%CI: 78.7–96.7%) | 100% (95 CI: not evaluable) |
| 2-year | 85.3% (95%CI: 78.9–89.9%) | 81.1% (95%CI: 65.6–90.1%) | 78.6% (95%CI: 47.3–92.5%) |
| 3-year | 77.9% (95%CI: 70.3–83.9%) | 76.3% (95%CI: 58.1–87.5%) | 27.5% (95%CI: 4.4–58.6%) |
| Variables | Univariate Analysis | Multivariate Analysis | Bootstrapping (200 Replications) | |||
|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | p Value | Hazard Ratio (95% CI) | p Value | Hazard Ratio (95% CI) | p Value | |
| Patients | ||||||
| Age | 1.01 (0.99–1.04) | 0.25 | ||||
| Sex female vs. male | 0.67 (0.35–1.29) | 0.23 | ||||
| Body Mass Index | 1.02 (0.97–1.07) | 0.48 | ||||
| ASA (>2 vs. ≤2) | 1.79 (1.10–2.95) | 0.020 | 1.14 (0.76–2.78) | 0.25 | 1.46 (0.67–3.18) | 0.34 |
| Diabetes | 1.53 (0.94–2.48) | 0.09 | ||||
| Metformin treatment | 1.0 (0.49–2.0) | 1.0 | ||||
| Statin treatment | 1.44 (0.83–2.49) | 0.190 | ||||
| Treatment-naïve patient | 0.59 (0.36–0.98) | 0.041 | 0.42 (0.22–0.79) | 0.007 | 0.42 (0.2–0.85) | 0.017 |
| Local recurrence | 1.12 (0.65–1.93) | 0.67 | ||||
| Distant recurrence | 1.50 (0.90–2.52) | 0.12 | ||||
| Non-Transplantable Recurrence | 4.78 (2.66–8.58) | <0.001 | ||||
| Cirrhosis | 1.0 (0.46–2.1) | 0.99 | ||||
| Child-Pugh (B vs. A) | 2.24 (0.47–10.62) | 0.31 | ||||
| Cause of liver disease(vs. alcohol) | ||||||
| Viral hepatitis or mixed | 0.85 (0.47–1.53) | 0.59 | ||||
| Hemochromatosis and others | 0.24 (0.03–1.7) | 0.15 | ||||
| NASH | 0.71 (0.33–1.52) | 0.38 | ||||
| Steatosis | 1.08 (0.65–1.8) | 0.76 | ||||
| AFP ≥ 100 vs. <100 ng/mL | 4.36 (2.12–8.96) | <0.001 | 3.03 (1.33–6.91) | 0.008 | 3.03 (1.1–8.37) | 0.032 |
| AFP (per unit) | 1.002 (1.001–1.003) | <0.001 | ||||
| Prothrombin time | 0.97 (0.96–0.99) | <0.001 | ||||
| Albumin | 0.93 (0.88–0.98) | 0.006 | ||||
| Bilirubin | 1.04 (1.00–1.07) | 0.026 | ||||
| Creatinine | 1.01 (1.00–1.01) | 0.001 | ||||
| MELD (>9 vs. ≤9) | 2.28 (1.40–3.73) | 0.001 | 2.84 (1.54–5.26) | 0.001 | 2.84 (1.46–5.53) | 0.002 |
| ALBI score 2 vs. 1 | 1.48 (0.88–2.46) | 0.14 | ||||
| HCC | ||||||
| Bifocal HCC (vs. unifocal) | 1.1 (0.53–2.25) | 0.80 | 1.60 (0.69–3.72) | 0.27 | 1.60 (0.66–3.92) | 0.30 |
| Trifocal HCC (vs. unifocal) | 2.75 (1.34–5.63) | 0.006 | 3.30 (1.36–8.02) | 0.008 | 3.31 (1.15–9.49) | 0.026 |
| Tumor size <2 cm (vs. ≥2 cm) | 1.07 (0.64–1.79) | 0.790 | ||||
| Steatotic HCC | 0.18 (0.056–0.56) | 0.003 | 0.12 (0.01–0.89) | 0.038 | 0.12 (3.2 ^−18–4.3^15) | 0.912 |
| PTA | ||||||
| PTA modality: MWA vs. RF | 1.34 (0.82–2.2) | 0.24 | ||||
| PTA imaging guidance US vs. CT | 0.75 (0.48–1.24) | 0.26 | ||||
| Harrell’s C statistic: 0.79 AIC: 356.36 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Preel, A.; Hermida, M.; Allimant, C.; Assenat, E.; Guillot, C.; Gozzo, C.; Aho-Glele, S.; Pageaux, G.-P.; Cassinotto, C.; Guiu, B. Uni-, Bi- or Trifocal Hepatocellular Carcinoma in Western Patients: Recurrence and Survival after Percutaneous Thermal Ablation. Cancers 2021, 13, 2700. https://doi.org/10.3390/cancers13112700
Preel A, Hermida M, Allimant C, Assenat E, Guillot C, Gozzo C, Aho-Glele S, Pageaux G-P, Cassinotto C, Guiu B. Uni-, Bi- or Trifocal Hepatocellular Carcinoma in Western Patients: Recurrence and Survival after Percutaneous Thermal Ablation. Cancers. 2021; 13(11):2700. https://doi.org/10.3390/cancers13112700
Chicago/Turabian StylePreel, Ancelin, Margaux Hermida, Carole Allimant, Eric Assenat, Chloé Guillot, Cecilia Gozzo, Serge Aho-Glele, Georges-Philippe Pageaux, Christophe Cassinotto, and Boris Guiu. 2021. "Uni-, Bi- or Trifocal Hepatocellular Carcinoma in Western Patients: Recurrence and Survival after Percutaneous Thermal Ablation" Cancers 13, no. 11: 2700. https://doi.org/10.3390/cancers13112700
APA StylePreel, A., Hermida, M., Allimant, C., Assenat, E., Guillot, C., Gozzo, C., Aho-Glele, S., Pageaux, G.-P., Cassinotto, C., & Guiu, B. (2021). Uni-, Bi- or Trifocal Hepatocellular Carcinoma in Western Patients: Recurrence and Survival after Percutaneous Thermal Ablation. Cancers, 13(11), 2700. https://doi.org/10.3390/cancers13112700







