Modeling-Based Decision Support System for Radical Prostatectomy Versus External Beam Radiotherapy for Prostate Cancer Incorporating an In Silico Clinical Trial and a Cost–Utility Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
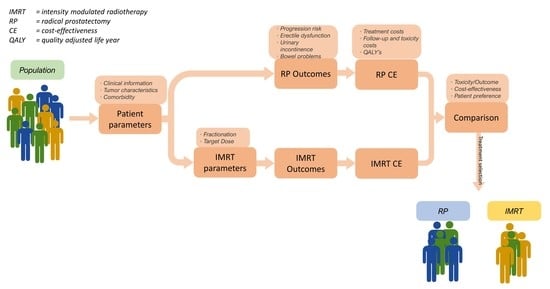
2.1. Decision Support System
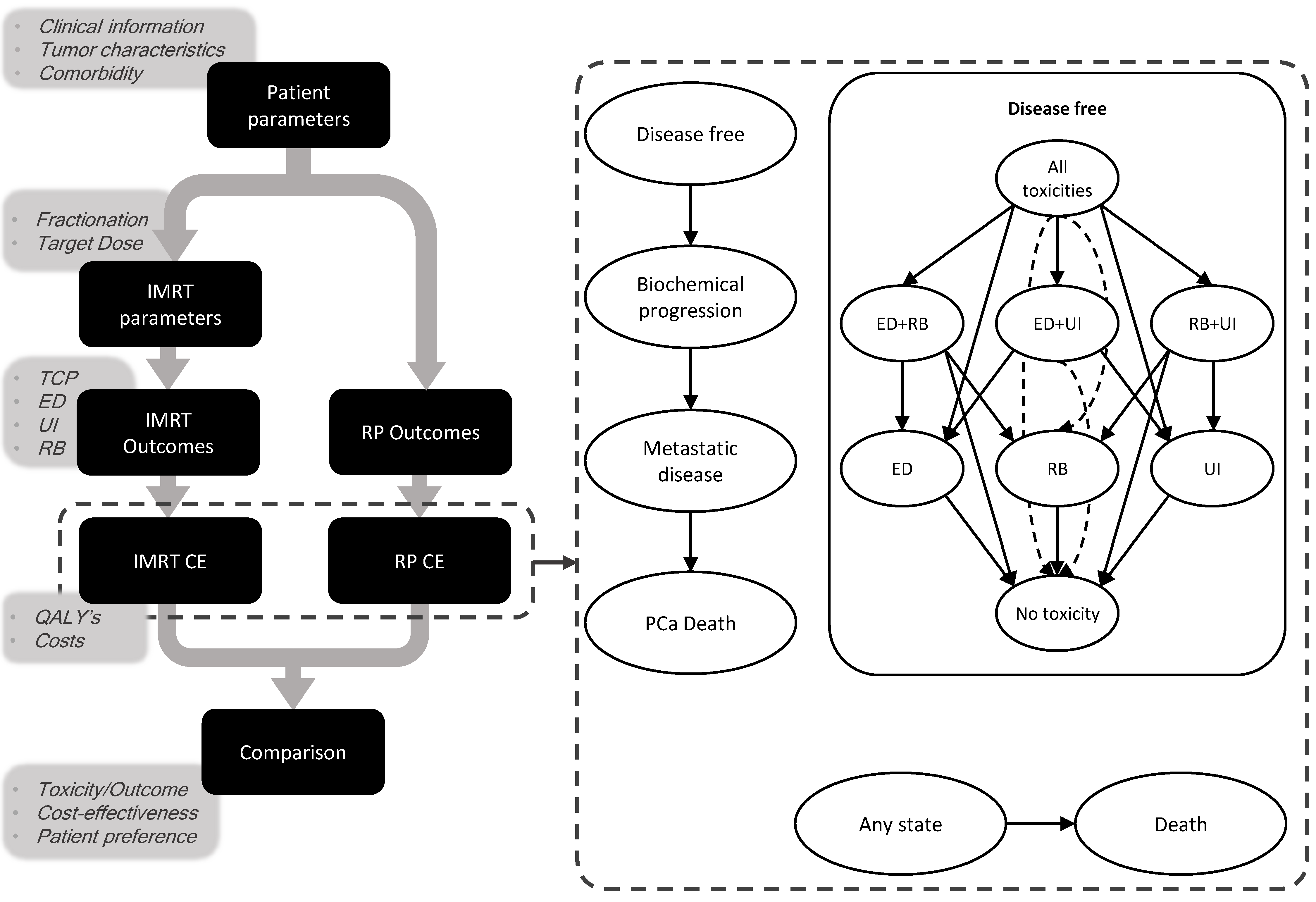
2.1.1. Markov Model
2.1.2. Predictive Models
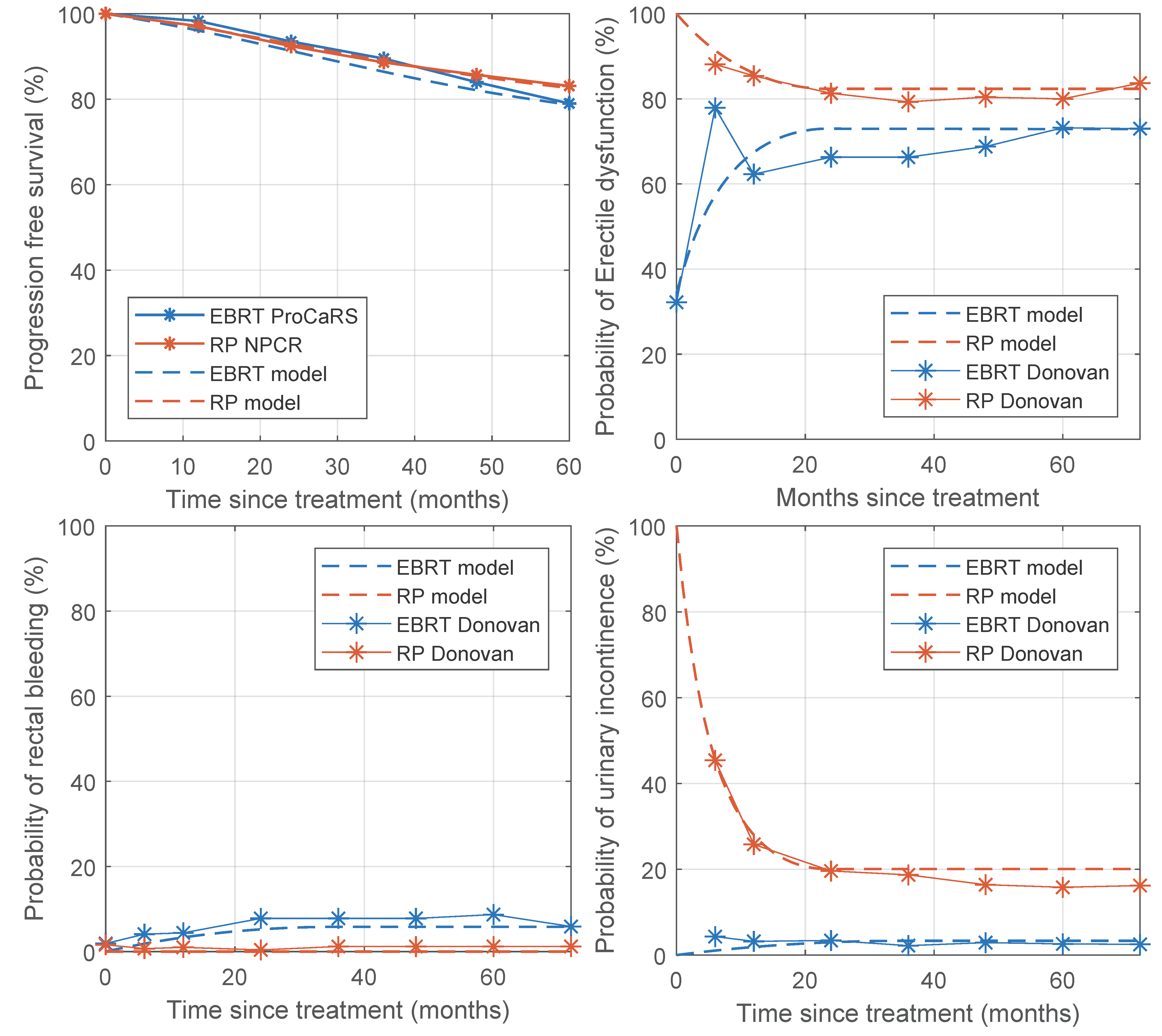
2.2. Validation
2.2.1. Synthetic Dataset
2.2.2. Validation of NTCP and TCP Models
2.2.3. In Silico Trial
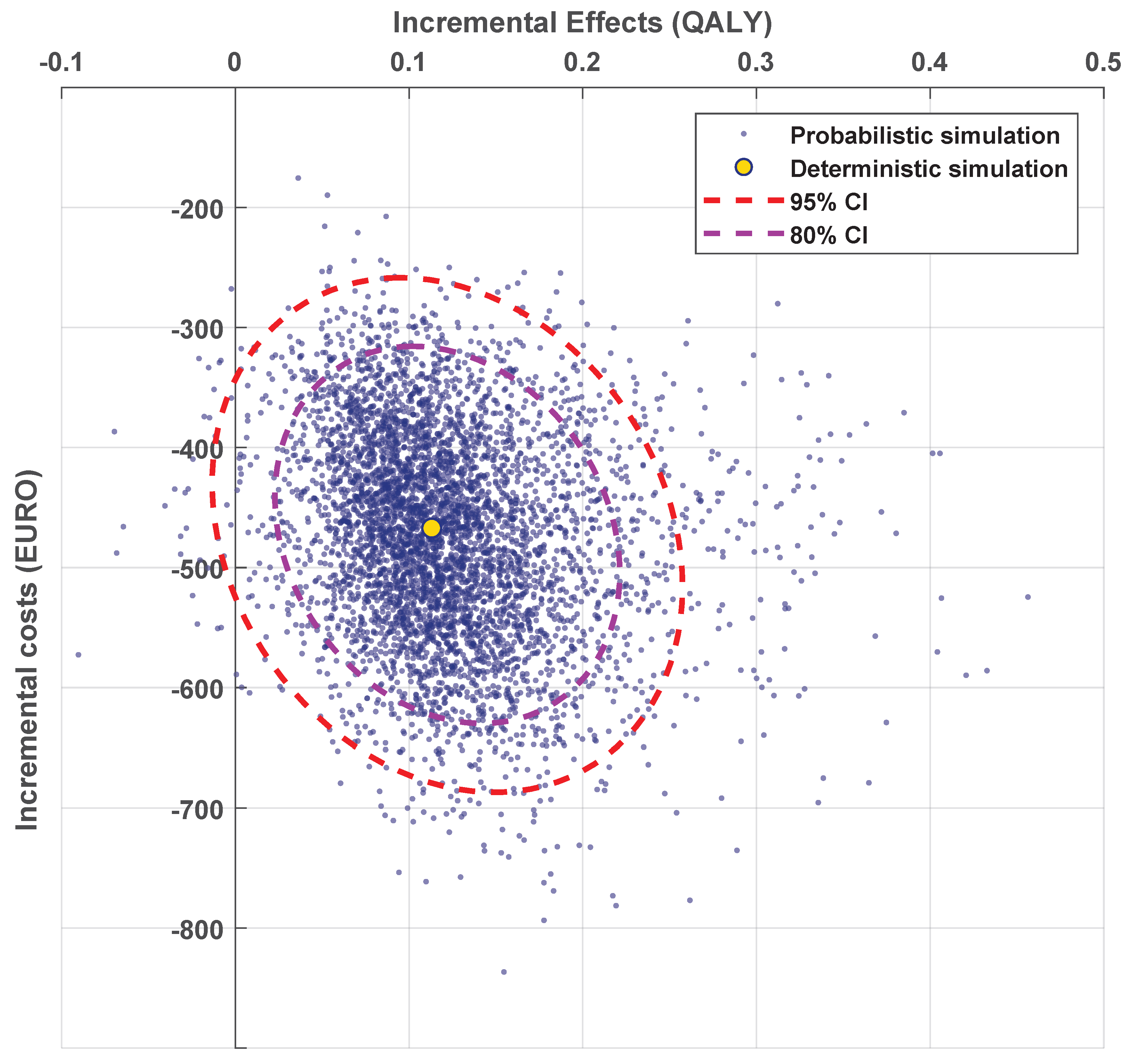
2.3. Cost-Effectiveness Analysis
3. Results
3.1. Synthetic Data Characteristics
3.2. Validation
3.3. In Silico Trial
3.4. Cost-Effectiveness Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- D’Amico, A.V.; Whittington, R.; Malkowicz, S.B.; Schultz, D.; Blank, K.; Broderick, G.A.; Tomaszewski, J.E.; Renshaw, A.A.; Kaplan, I.; Beard, C.J.; et al. Biochemical Outcome after Radical Prostatectomy, External Beam Radiation Therapy, Or Interstitial Radiation Therapy for Clinically Localized Prostate Cancer. JAMA 1998, 280, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Filella, X.; Fernández-Galán, E.; Bonifacio, R.F.; Foj, L. Emerging biomarkers in the diagnosis of prostate cancer. Pharm. Pers. Med. 2018, 11, 83–94. [Google Scholar] [CrossRef]
- Lane, J.A.; Donovan, J.L.; Davis, M.; Walsh, E.; Dedman, D.; Down, L.; Turner, E.L.; Mason, M.D.; Metcalfe, C.; Peters, T.; et al. Active monitoring, radical prostatectomy, or radiotherapy for localised prostate cancer: Study design and diagnostic and baseline results of the ProtecT randomised phase 3 trial. Lancet Oncol. 2014, 15, 1109–1118. [Google Scholar] [CrossRef]
- Heidenreich, A.; Bastian, P.J.; Bellmunt, J.; Bolla, M.; Joniau, S.; van der Kwast, T.; Mason, M.; Matveev, V.; Wiegel, T.; Zattoni, F.; et al. EAU Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent—Update 2013. Eur. Urol. 2014, 65, 124–137. [Google Scholar] [CrossRef]
- Chen, L.; Li, Q.; Wang, Y.; Zhang, Y.; Ma, X. Comparison on efficacy of radical prostatectomy versus external beam radiotherapy for the treatment of localized prostate cancer. Oncotarget 2017, 8, 79854–79863. [Google Scholar] [CrossRef]
- Potosky, A.L.; Davis, W.W.; Hoffman, R.M.; Stanford, J.L.; Stephenson, R.A.; Penson, D.F.; Harlan, L.C. Five-Year Outcomes After Prostatectomy or Radiotherapy for Prostate Cancer: The Prostate Cancer Outcomes Study. J. Natl. Cancer Inst. 2004, 96, 1358–1367. [Google Scholar] [CrossRef]
- Donovan, J.L.; Hamdy, F.C.; Lane, J.A.; Mason, M.; Metcalfe, C.; Walsh, E.; Blazeby, J.M.; Peters, T.; Holding, P.; Bonnington, S.; et al. Patient-Reported Outcomes after Monitoring, Surgery, or Radiotherapy for Prostate Cancer. N. Engl. J. Med. 2016, 375, 1425–1437. [Google Scholar] [CrossRef]
- Van Wijk, Y.; Halilaj, I.; van Limbergen, E.; Walsh, S.; Lutgens, L.; Lambin, P.; Vanneste, B.G.L. Decision Support Systems in Prostate Cancer Treatment: An Overview. BioMed Res. Int. 2019, 2019, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.; De Jong, E.E.; Van Timmeren, J.E.; Ibrahim, A.; Compter, I.; Peerlings, J.; Sanduleanu, S.; Refaee, T.; Keek, S.; Larue, R.T.; et al. Decision Support Systems in Oncology. JCO Clin. Cancer Inform. 2019, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lambin, P.; Zindler, J.; Vanneste, B.G.; Van De Voorde, L.; Eekers, D.; Compter, I.; Panth, K.M.; Peerlings, J.; Larue, R.T.; Deist, T.M.; et al. Decision support systems for personalized and participative radiation oncology. Adv. Drug Deliv. Rev. 2017, 109, 131–153. [Google Scholar] [CrossRef]
- Shariat, S.F.; I Karakiewicz, P.; Margulis, V.; Kattan, M. Inventory of prostate cancer predictive tools. Curr. Opin. Urol. 2008, 18, 279–296. [Google Scholar] [CrossRef]
- Lambin, P.; Zindler, J.; Vanneste, B.; Van De Voorde, L.; Jacobs, M.; Eekers, D.; Peerlings, J.; Reymen, B.; LaRue, R.T.H.M.; Deist, T.M.; et al. Modern clinical research: How rapid learning health care and cohort multiple randomised clinical trials complement traditional evidence based medicine. Acta Oncol. 2015, 54, 1289–1300. [Google Scholar] [CrossRef]
- Lambin, P.; Van Stiphout, R.G.P.M.; Starmans, M.H.W.; Rios-Velazquez, E.; Nalbantov, G.; Aerts, H.J.W.L.; Roelofs, E.; Van Elmpt, W.; Boutros, P.C.; Granone, P.; et al. Predicting outcomes in radiation oncology—multifactorial decision support systems. Nat. Rev. Clin. Oncol. 2012, 10, 27–40. [Google Scholar] [CrossRef]
- Meropol, N.J.; Schrag, D.; Smith, T.J.; Mulvey, T.M.; Jr, R.M.L.; Blum, D.; Ubel, P.A.; Schnipper, L.E. American Society of Clinical Oncology Guidance Statement: The Cost of Cancer Care. J. Clin. Oncol. 2009, 27, 3868–3874. [Google Scholar] [CrossRef] [PubMed]
- Widder, J.; Van Der Schaaf, A.; Lambin, P.; Marijnen, C.A.; Pignol, J.-P.; Rasch, C.R.; Slotman, B.J.; Verheij, M.; Langendijk, J.A. The Quest for Evidence for Proton Therapy: Model-Based Approach and Precision Medicine. Int. J. Radiat. Oncol. 2016, 95, 30–36. [Google Scholar] [CrossRef]
- Stewart, S.T.; Lenert, L.; Bhatnagar, V.; Kaplan, R.M. Utilities For Prostate Cancer Health States in Men Aged 60 and Older. Med. Care 2005, 43, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Heijink, R.; Van Baal, P.; Oppe, M.; Koolman, X.; Westert, G. Decomposing cross-country differences in quality adjusted life expectancy: The impact of value sets. Popul. Health Metrics 2011, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G.M. Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD): The TRIPOD statement. BJOG: Int. J. Obstet. Gynaecol. 2015, 122, 434–443. [Google Scholar] [CrossRef]
- Warner, A.; Pickles, T.; Crook, J.; Martin, A.-G.; Souhami, L.; Catton, C.; Lukka, H.; Rodrigues, G. Development of ProCaRS Clinical Nomograms for Biochemical Failure-free Survival Following Either Low-Dose Rate Brachytherapy or Conventionally Fractionated External Beam Radiation Therapy for Localized Prostate Cancer. Cureus 2015, 7, e276. [Google Scholar] [CrossRef]
- Bjartell, A.; Bottai, M.; Persson, J.; Bratt, O.; Damber, J.-E.; Stattin, P.; Akre, O. Prediction of clinical progression after radical prostatectomy in a nationwide population-based cohort. Scand. J. Urol. 2016, 50, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Schaake, W.; Van Der Schaaf, A.; Van Dijk, L.V.; Bergh, A.C.M.V.D.; Langendijk, J.A. Development of a prediction model for late urinary incontinence, hematuria, pain and voiding frequency among irradiated prostate cancer patients. PLoS ONE 2018, 13, e0197757. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, K.; Kent, M.T.; Vickers, A.J.; Von Bodman, C.; Bernstein, M.; Touijer, K.A.; Coleman, J.A.; Laudone, V.T.; Scardino, P.T.; Eastham, J.A.; et al. Preoperative predictive model of recovery of urinary continence after radical prostatectomy. BJU Int. 2015, 116, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Alemozaffar, M.; Regan, M.M.; Cooperberg, M.R.; Wei, J.T.; Michalski, J.M.; Sandler, H.M.; Hembroff, L.; Sadetsky, N.; Saigal, C.S.; Litwin, M.S.; et al. Prediction of Erectile Function Following Treatment for Prostate Cancer. JAMA 2011, 306, 1205–1214. [Google Scholar] [CrossRef]
- Liu, M.; Moiseenko, V.; Agranovich, A.; Karvat, A.; Kwan, W.; Saleh, Z.H.; Apte, A.A.; Deasy, J.O. Normal Tissue Complication Probability (NTCP) modeling of late rectal bleeding following external beam radiotherapy for prostate cancer: A Test of the QUANTEC-recommended NTCP model. Acta Oncol. 2010, 49, 1040–1044. [Google Scholar] [CrossRef]
- Miralbell, R.; Roberts, S.A.; Zubizarreta, E.; Hendry, J.H. Dose-Fractionation Sensitivity of Prostate Cancer Deduced From Radiotherapy Outcomes of 5,969 Patients in Seven International Institutional Datasets: α/β = 1.4 (0.9–2.2) Gy. Int. J. Radiat. Oncol. 2012, 82, e17–e24. [Google Scholar] [CrossRef]
- Hamdy, F.C.; Donovan, J.L.; Lane, J.A.; Mason, M.; Metcalfe, C.; Holding, P.; Davis, M.; Peters, T.; Turner, E.L.; Martin, R.M.; et al. 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N. Engl. J. Med. 2016, 375, 1415–1424. [Google Scholar] [CrossRef]
- Dearnaley, D.; Syndikus, I.; Mossop, H.; Khoo, V.; Birtle, A.; Bloomfield, D.; Graham, J.; Kirkbride, P.; Logue, J.; Malik, Z.; et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016, 17, 1047–1060. [Google Scholar] [CrossRef]
- The Council for Public Health and Health Care. Sensible and Sustainable Care. Zoetermeer, The Netherlands, 2006. [Google Scholar]
- Attema, A.E.; Brouwer, W.B.F.; Claxton, K. Discounting in Economic Evaluations. Pharmacoeconomics 2018, 36, 745–758. [Google Scholar] [CrossRef]
- Morgan, S.C.; Morton, G.C.; Berlin, A.; Cheung, P.; Chung, P.; Ménard, C.; Pickles, T.; Souhami, L.; Warde, P.R.; Lukka, H.R. Current topics in radiotherapy for genitourinary cancers: Consensus statements of the Genitourinary Radiation Oncologists of Canada. Can. Urol. Assoc. J. 2020, 14, E588–E593. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, G.; Lukka, H.; Warde, P.; Brundage, M.; Souhami, L.; Crook, J.; Cury, F.; Catton, C.; Mok, G.; Martin, A.-G.; et al. The prostate cancer risk stratification (ProCaRS) project: Recursive partitioning risk stratification analysis. Radiother. Oncol. 2013, 109, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Center, R.O. The National Prostate Cancer Register (NPCR) in Sweden 2002–2006; Regional Oncological Center, Uppsala-Örebro: Uppsala, Sweden, 2008. [Google Scholar]
- Hood, L.; Friend, S.H. Predictive, personalized, preventive, participatory (P4) cancer medicine. Nat. Rev. Clin. Oncol. 2011, 8, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Barry, M.J.; Edgman-Levitan, S. Shared decision making—Pinnacle of patient-centered care. N. Engl. J. Med. 2012, 366, 780–781. [Google Scholar] [CrossRef] [PubMed]
- Sferrazza, S.; Accettone, R.; Pampoorickel, K.; Caggianelli, G.; Casciato, S.; Cinque, A.; Matarese, M. Men’s experiences of deciding about treatment for localized prostate cancer: A meta-synthesis. Prof. Inferm. 2019, 72, 272–282. [Google Scholar]
- Van Wijk, Y.; Vanneste, B.G.L.; Walsh, S.; van der Meer, S.; Ramaekers, B.; van Elmpt, W.; Pinkawa, M.; Lambin, P. Development of a virtual spacer to support the decision for the placement of an implantable rectum spacer for prostate cancer radiotherapy: Comparison of dose, toxicity and cost-effectiveness. Radiother. Oncol. 2017, 125, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Van Wijk, Y.; Vanneste, B.G.L.; Jochems, A.; Walsh, S.; Oberije, C.J.; Pinkawa, M.; Ramaekers, B.L.T.; Vega, A.; Lambin, P. Development of an isotoxic decision support system integrating genetic markers of toxicity for the implantation of a rectum spacer. Acta Oncol. 2018, 57, 1499–1505. [Google Scholar] [CrossRef] [PubMed]
- Bergom, C.; West, C.M.; Higginson, D.S.; Abazeed, M.E.; Arun, B.; Bentzen, S.M.; Bernstein, J.L.; Evans, J.D.; Gerber, N.K.; Kerns, S.L.; et al. The Implications of Genetic Testing on Radiation Therapy Decisions: A Guide for Radiation Oncologists. Int. J. Radiat. Oncol. 2019, 105, 698–712. [Google Scholar] [CrossRef]
| Model | TRIPOD | Treatment (n) | Parameters | Outcome | Performance |
|---|---|---|---|---|---|
| Warner et al. 2015 * [20] | 1a + 4 | EBRT (822 + 967) | ADT [months] PSA [ng/mL] Gleason score BED [Gy] | 5-year BFFS | R2 = 0.868 |
| Bjartell et al. 2016 [21] | 3 | RP (3452 + 1762) | T-stage [T1/T2] PSA [ng/mL] Primary Gleason grade Secondary Gleason grade Positive biopsy cores [n] Negative biopsy cores [n] | 5-year RFS ** | C-index = 0.68 |
| Schaake et al. 2018 [22] | 1b | EBRT (243) | Mean Trigone dose [Gy] | Late UI after 3 years | AUC = 0.66 |
| Matsushita et al. 2015 * [23] | 2a | RP (2849) | Age [years] BMI [kg/m2] ASA score [I/II/III/IV] Urethral length [mm] | Recovery from UI after 1 year | AUC = 0.71 |
| Alemozaffar et al. 2011 [24] | 3 | Both (524 + 241) | Age [years] Nerve-sparing [y/n] PSA [ng/mL] ADT [y/n] | Erection recovery after 2 years | AUC = 0.77 for RP, AUC = 0.87 for EBRT |
| Liu et al. 2010 [25] | 2a | EBRT (161) | DVH V75 | Late RB after 3 years | AUC = 0.62 |
| Parameter Name | EBRT Mean (SD) | RP Mean (SD) | p |
|---|---|---|---|
| Age (years) | 63.8 (10.7) | 58.8 (9.1) | <<0.001 |
| PSA (ng/mL) | 8.3 (3.5) | 6.8 (3.5) | <<0.001 |
| T-stage 2 (%) | 48.7 | 24.4 | <<0.001 |
| P. Gleason grade 4 (%) | 27.1 | 3.3 | <<0.001 |
| S. Gleason grade 4 (%) | 14.7 | 20.9 | 0.01 |
| ADT given (%) | 0 | 80.0 | 0.72 |
| Study | Treatment | Outcome | Clinical Trial (%) | Simulation (%) | Difference (%) |
|---|---|---|---|---|---|
| Hamdy 2016 [27] | EBRT [545] | 5 year BDFS | 88.5 | 89.0 | 0.5 |
| RP [553] | 5 year BDFS | 88.1 | 87.0 | 1.1 | |
| CHHiP [28] | EBRT 74 Gy [1065] | 5 year BDFS | 88.3 | 88.4 | 0.1 |
| EBRT 60 Gy [1074] | 5 year BDFS | 90.6 | 89.9 | 0.7 | |
| EBRT 57 Gy [1077] | 5 year BDFS | 85.9 | 87.9 | 2.0 | |
| Donovan 2016 [8] | EBRT [545] | 6 month UI | 5 | 3.3 | 1.7 |
| 6 year UI | 3.5 | 3.3 | 0.2 | ||
| 6 month ED | 77.8 | 57.3 | 20.5 | ||
| 6 year ED | 73.0 | 72.9 | 0.1 | ||
| 6 month RB | 3.8 | 1.9 | 1.9 | ||
| 6 year RB | 5.9 | 5.8 | 0.1 | ||
| RP [553] | 6 month UI | 46 | 45.5 | 0.5 | |
| 6 year UI | 17 | 20.1 | 3.1 | ||
| 6 month ED | 88 | 91.3 | 3.3 | ||
| 6 year ED | 83.5 | 82.4 | 1.1 |
| Outcome Type | RP Mean (SD) | EBRT Mean (SD) | DSS Mean (SD) | RP PTED Mean (SD) | EBRT PTED Mean (SD) | DSS PTED Mean (SD) |
|---|---|---|---|---|---|---|
| 5 year TCP [%] | 84 (7) | 84 (4) | 84 (4) | 84 (7) | 84 (4) | 85 (5) |
| 2 year ED [%] | 93 (6) | 61 (19) | 61 (20) | 100 (0) | 98 (2) | 98 (2) |
| 1 year UI [%] | 70 (16) | 7 (4) | 8 (10) | 70 (16) | 7 (4) | 22 (26) |
| 3 year RB [%] | 0 (0) | 5 (4) | 5 (4) | 0 (0) | 5 (4) | 3 (4) |
| QALY | 4.28 (0.87) | 4.46 (0.86) | 4.46 (0.86) | 4.25 (0.85) | 4.30 (0.83) | 4.32 (0.84) |
| Costs (1000 €) | 15.2 (1.1) | 12.6 (1.0) | 12.7 (1.1) | 15.2 (1.1) | 12.7 (1.0) | 13.3 (1.5) |
| Outcome Type | RP Mean (SD) | EBRT Mean (SD) | p | Random Mean (SD) | DSS Mean (SD) | p |
|---|---|---|---|---|---|---|
| 5 year TCP [%] | 83 (8) | 84 (4) | 0.003 | 83 (7) | 85 (5) | <<0.001 |
| 2 year Erectile Dysfunction [%] | 83 (13) | 59 (2) | <<0.001 | 71 (14) | 67 (20) | <<0.001 |
| 1 year Urinary Incontinence [%] | 27 (18) | 7 (4) | <<0.001 | 16 (9) | 13 (15) | <<0.001 |
| 3 year Rectal Bleeding [%] | 0 (0) | 5 (4) | <<0.001 | 2 (2) | 1 (3) | 0.61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Wijk, Y.; Ramaekers, B.; Vanneste, B.G.L.; Halilaj, I.; Oberije, C.; Chatterjee, A.; Marcelissen, T.; Jochems, A.; Woodruff, H.C.; Lambin, P. Modeling-Based Decision Support System for Radical Prostatectomy Versus External Beam Radiotherapy for Prostate Cancer Incorporating an In Silico Clinical Trial and a Cost–Utility Study. Cancers 2021, 13, 2687. https://doi.org/10.3390/cancers13112687
van Wijk Y, Ramaekers B, Vanneste BGL, Halilaj I, Oberije C, Chatterjee A, Marcelissen T, Jochems A, Woodruff HC, Lambin P. Modeling-Based Decision Support System for Radical Prostatectomy Versus External Beam Radiotherapy for Prostate Cancer Incorporating an In Silico Clinical Trial and a Cost–Utility Study. Cancers. 2021; 13(11):2687. https://doi.org/10.3390/cancers13112687
Chicago/Turabian Stylevan Wijk, Yvonka, Bram Ramaekers, Ben G. L. Vanneste, Iva Halilaj, Cary Oberije, Avishek Chatterjee, Tom Marcelissen, Arthur Jochems, Henry C. Woodruff, and Philippe Lambin. 2021. "Modeling-Based Decision Support System for Radical Prostatectomy Versus External Beam Radiotherapy for Prostate Cancer Incorporating an In Silico Clinical Trial and a Cost–Utility Study" Cancers 13, no. 11: 2687. https://doi.org/10.3390/cancers13112687
APA Stylevan Wijk, Y., Ramaekers, B., Vanneste, B. G. L., Halilaj, I., Oberije, C., Chatterjee, A., Marcelissen, T., Jochems, A., Woodruff, H. C., & Lambin, P. (2021). Modeling-Based Decision Support System for Radical Prostatectomy Versus External Beam Radiotherapy for Prostate Cancer Incorporating an In Silico Clinical Trial and a Cost–Utility Study. Cancers, 13(11), 2687. https://doi.org/10.3390/cancers13112687