Improved Tumor-Targeting with Peptidomimetic Analogs of Minigastrin 177Lu-PP-F11N
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of the NMGs
2.2. (Radio) Metal Labeling, and Evaluation In Vitro
2.3. In Vivo Stability
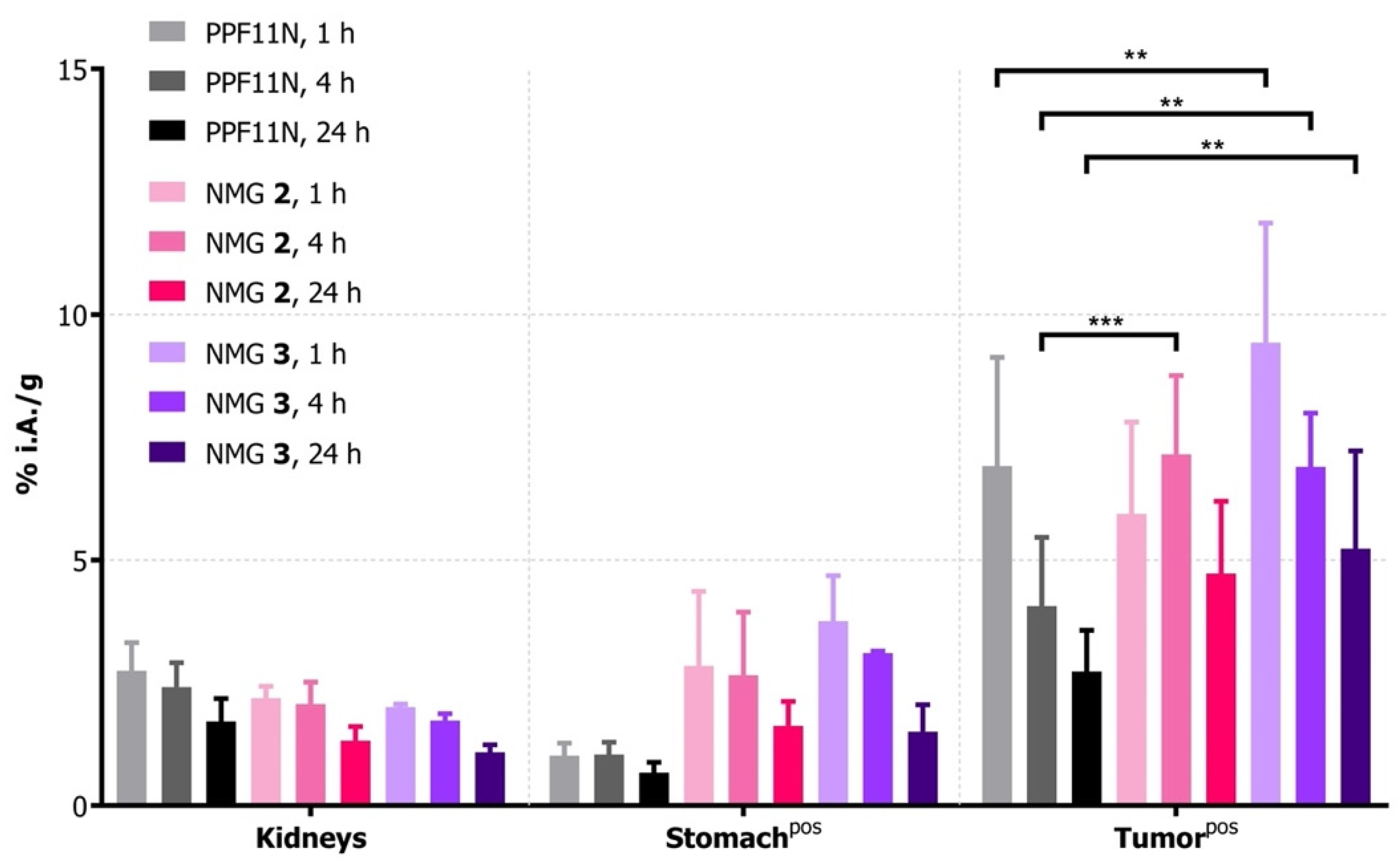
2.4. Biodistribution
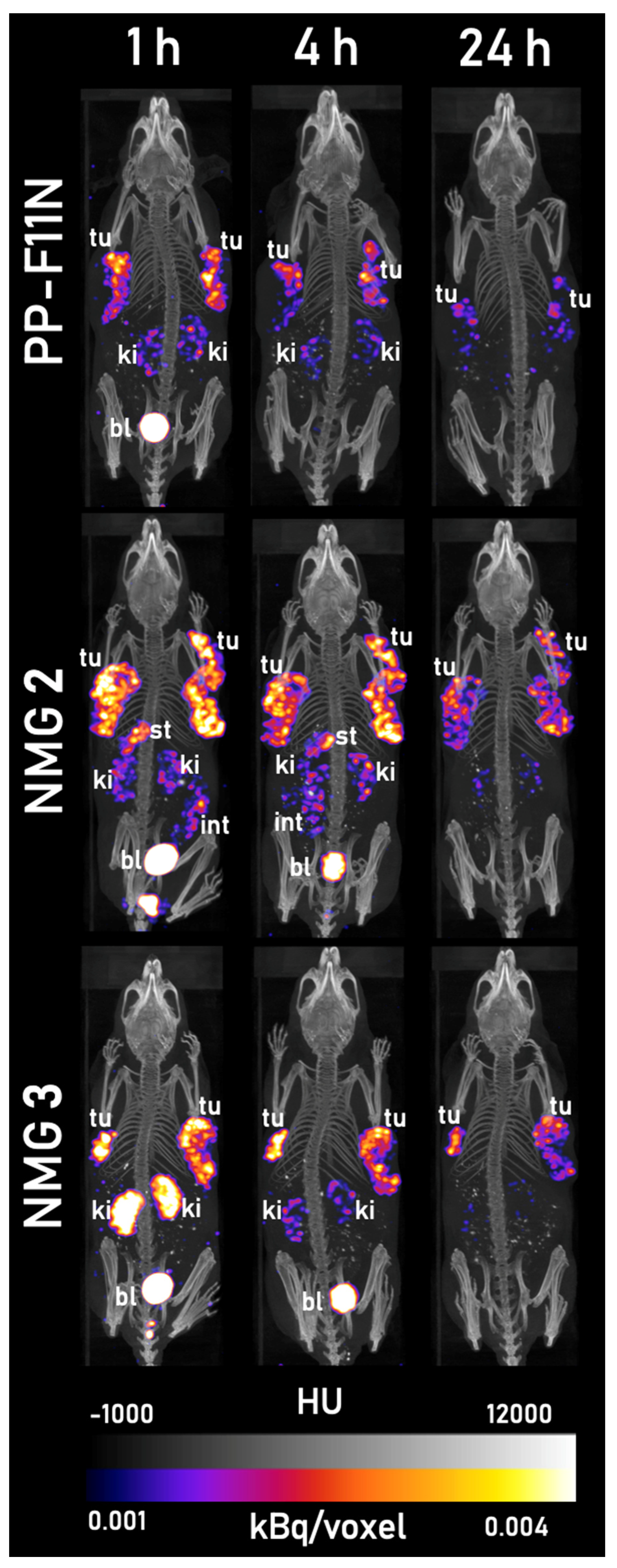
2.5. SPECT/CT Imaging
3. Results
3.1. Synthesis and Radio-labeling
3.2. Evaluation In Vitro
3.3. Evaluation In Vivo
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reubi, J.C.; Waser, B. Unexpected High Incidence of Cholecystokinin-B/Gastrin Receptors in Human Medullary Thyroid Carcinomas. Int. J. Cancer 1996, 67, 644–647. [Google Scholar] [CrossRef]
- Reubi, J.C.; Schaer, J.-C.; Waser, B. Cholecystokinin(CCK)-A and CCK-B/Gastrin Receptors in Human Tumors. Cancer Res. 1997, 57, 1377–1386. [Google Scholar] [PubMed]
- Biersack, H.-J.; Grünwald, F. Thyroid Cancer; Springer: Berlin/Heidelberg, Germany, 2001; pp. 251–273. [Google Scholar]
- Kalemkerian, G.P.; Akerley, W.; Bogner, P.; Borghaei, H.; Chow, L.Q.; Downey, R.J.; Gandhi, L.; Ganti, A.K.P.; Govindan, R.; Grecula, J.C.; et al. Small Cell Lung Cancer. J. Natl. Compr. Cancer Netw. 2013, 11, 78–98. [Google Scholar] [CrossRef] [PubMed]
- Roman, S.; Lin, R.; Sosa, J.A. Prognosis of Medullary Thyroid Carcinoma. Cancer 2006, 107, 2134–2142. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, Z.; Wang, Q. Emerging Therapies for Small Cell Lung Cancer. J. Hematol. Oncol. 2019, 12, 47. [Google Scholar] [CrossRef]
- Kaloudi, A.; Nock, B.A.; Krenning, E.P.; Maina, T.; De Jong, M. Radiolabeled Gastrin/CCK Analogs in Tumor Diagnosis: Towards Higher Stability and Improved Tumor Targeting. Q. J. Nucl. Med. Mol. Imaging 2015, 59, 287–302. [Google Scholar]
- Clinical Trial NCT02088645, S.-N. 177Lu-PP-F11N for Receptor Targeted Therapy and Imaging of Metastatic Thyroid Cancer. (Lumed). Available online: https://clinicaltrials.gov/ct2/show/NCT02088645?term=8.%09NCT02088645&draw=2&rank=1 (accessed on 11 April 2021).
- Clinical Trial NCT03246659, S.-N. Radiolabelled CCK-2/Gastrin Receptor Analogue for Personalized Theranostic Strategy in Advanced MTC (GRAN-T-MTC). Available online: https://clinicaltrials.gov/ct2/show/NCT03246659?term=NCT03246659&draw=2&rank=1 (accessed on 11 April 2021).
- Rottenburger, C.; Nicolas, G.P.; McDougall, L.; Kaul, F.; Cachovan, M.; Vija, A.H.; Schibli, R.; Geistlich, S.; Schumann, A.; Rau, T.; et al. Cholecystokinin-2 Receptor Agonist 177Lu-PP-F11N for Radionuclide Therapy of Medullary Thyroid Carcinoma—Results of the Lumed Phase 0a Study. J. Nucl. Med. 2020, 61, 520–526. [Google Scholar] [CrossRef] [PubMed]
- von Guggenberg, E.; Klingler, M.; Garnusze, P.; Mikołajczak, R.; Janota, B.; Hubalewska-Dydejczyk, A.; Kieć-Klimczak, M.; Przybylik-Mazurek, E.; Virgolini, I. Gallium-68 Labelled Minigastrin Analogue for High Sensitivity PET Imaging of Cholecystokinin-2 Receptor Expressing Tumours. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, S268. [Google Scholar]
- Behr, T.M.; Béhé, M.P. Cholecystokinin-B/Gastrin Receptor-Targeting Peptides for Staging and Therapy of Medullary Thyroid Cancer and other Cholecystokinin-B Receptor-Expressing Malignancies. Semin. Nucl. Med. 2002, 32, 97–109. [Google Scholar] [CrossRef]
- Vegt, E.; de Jong, M.; Wetzels, J.F.M.; Masereeuw, R.; Melis, M.; Oyen, W.J.G.; Gotthardt, M.; Boerman, O.C. Renal Toxicity of Radiolabeled Peptides and Antibody Fragments: Mechanisms, Impact on Radionuclide Therapy, and Strategies for Prevention. J. Nucl. Med. 2010, 51, 1049–1058. [Google Scholar] [CrossRef]
- Kaloudi, A.; Nock, B.A.; Lymperis, E.; Krenning, E.P.; de Jong, M.; Maina, T. Improving the In Vivo Profile of Minigastrin Radiotracers: A Comparative Study Involving the Neutral Endopeptidase Inhibitor Phosphoramidon. Cancer Biother. Radiopharm. 2016, 31, 20–28. [Google Scholar] [CrossRef]
- Kaloudi, A.; Nock, B.A.; Lymperis, E.; Valkema, R.; Krenning, E.P.; de Jong, M.; Maina, T. Impact of Clinically Tested NEP/ACE Inhibitors on Tumor Uptake of [111In-DOTA]MG11—First Estimates for Clinical Translation. Eur. J. Nucl. Med. Mol. Imaging Res. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Grob, N.M.; Häussinger, D.; Deupi, X.; Schibli, R.; Behe, M.; Mindt, T.L. Triazolo-Peptidomimetics: Novel Radiolabeled Minigastrin Analogs for Improved Tumor Targeting. J. Med. Chem. 2020, 63, 4484–4495. [Google Scholar] [CrossRef]
- Grob, N.M.; Schmid, S.; Schibli, R.; Behe, M.; Mindt, T.L. Design of Radiolabeled Analogs of Minigastrin by Multiple Amide-to-Triazole Substitutions. J. Med. Chem. 2020, 63, 4496–4505. [Google Scholar] [CrossRef] [PubMed]
- Mascarin, A.; Valverde, I.E.; Vomstein, S.; Mindt, T.L. 1,2,3-Triazole Stabilized Neurotensin-Based Radiopeptidomimetics for Improved Tumor Targeting. Bioconjug. Chem. 2015, 26, 2143–2152. [Google Scholar] [CrossRef] [PubMed]
- Valverde, I.E.; Bauman, A.; Kluba, C.A.; Vomstein, S.; Walter, M.A.; Mindt, T.L. 1,2,3-Triazoles as Amide Bond Mimics: Triazole Scan Yields Protease-Resistant Peptidomimetics for Tumor Targeting. Angew. Chem. Int. Ed. 2013, 52, 8957–8960. [Google Scholar] [CrossRef]
- Valverde, I.E.; Vomstein, S.; Fischer, C.A.; Mascarin, A.; Mindt, T.L. Probing the Backbone Function of Tumor Targeting Peptides by an Amide-to-Triazole Substitution Strategy. J. Med. Chem. 2015, 58, 7475–7484. [Google Scholar] [CrossRef] [PubMed]
- Behe, M.; Schibli, R. Mini-Gastrin Analogue, in Particular for Use in CCK2 Receptor Positive Tumour Diagnosis and/or Treatment. WO2015067473 A1, 14 May 2015. [Google Scholar]
- Kolenc Peitl, P.; Tamma, M.; Kroselj, M.; Braun, F.; Waser, B.; Reubi, J.C.; Sollner Dolenc, M.; Maecke, H.R.; Mansi, R. Stereochemistry of Amino Acid Spacers Determines the Pharmacokinetics of 111In–DOTA–Minigastrin Analogues for Targeting the CCK2/Gastrin Receptor. Bioconjug. Chem. 2015, 26, 1113–1119. [Google Scholar] [CrossRef]
- Aloj, L.; Caracò, C.; Panico, M.; Zannetti, A.; Del Vecchio, S.; Tesauro, D.; De Luca, S.; Arra, C.; Pedone, C.; Morelli, G.; et al. In Vitro and In Vivo Evaluation of 111In-DTPAGlu-G-CCK8 for Cholecystokinin-B Receptor Imaging. J. Nucl. Med. 2004, 45, 485–494. [Google Scholar] [PubMed]
- Ritler, A.; Shoshan, M.S.; Deupi, X.; Wilhelm, P.; Schibli, R.; Wennemers, H.; Béhé, M. Elucidating the Structure–Activity Relationship of the Pentaglutamic Acid Sequence of Minigastrin with Cholecystokinin Receptor Subtype 2. Bioconjug. Chem. 2019, 30, 657–666. [Google Scholar] [CrossRef]
- Klingler, M.; Summer, D.; Rangger, C.; Haubner, R.; Foster, J.; Sosabowski, J.; Virgolini, I.; Decristoforo, C.; von Guggenberg, E. DOTA-MGS5, a New Cholecystokinin-2 Receptor Targeting Peptide Analog with Optimized Targeting Profile for Theranostic Use. J. Nucl. Med. 2019, 60, 1010–1016. [Google Scholar] [CrossRef] [PubMed]
- Maina, T.; Konijnenberg, M.W.; KolencPeitl, P.; Garnuszek, P.; Nock, B.A.; Kaloudi, A.; Kroselj, M.; Zaletel, K.; Maecke, H.; Mansi, R.; et al. Preclinical Pharmacokinetics, Biodistribution, Radiation Dosimetry and Toxicity Studies Required for Regulatory Approval of a Phase I Clinical Trial with 111In-CP04 in Medullary Thyroid Carcinoma Patients. Eur. J. Pharm. Sci. 2016, 91, 236–242. [Google Scholar] [CrossRef]
- Grob, N.M.; Schibli, R.; Béhé, M.; Valverde, I.E.; Mindt, T.L. 1,5-Disubstituted 1,2,3-Triazoles as Amide Bond Isosteres Yield Novel Tumor-Targeting Minigastrin Analogs. ACS Med. Chem. Lett. 2021, 12, 585–592. [Google Scholar] [CrossRef]
- Ocak, M.; Helbok, A.; Rangger, C.; Peitl, P.; Nock, B.; Morelli, G.; Eek, A.; Sosabowski, J.; Breeman, W.P.; Reubi, J.; et al. Comparison of Biological Stability and Metabolism of CCK2 Receptor Targeting Peptides, a Collaborative Project under COST BM0607. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1426–1435. [Google Scholar] [CrossRef] [PubMed]
- Nock, B.A.; Maina, T.; Krenning, E.P.; de Jong, M. “To Serve and Protect”: Enzyme Inhibitors as Radiopeptide Escorts Promote Tumor Targeting. J. Nucl. Med. 2014, 55, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Sauter, A.W.; Mansi, R.; Hassiepen, U.; Muller, L.; Panigada, T.; Wiehr, S.; Wild, A.-M.; Geistlich, S.; Béhé, M.; Rottenburger, C.; et al. Targeting of the Cholecystokinin-2 Receptor with the Minigastrin Analog 177Lu-DOTA-PP-F11N: Does the Use of Protease Inhibitors Further Improve In Vivo Distribution? J. Nucl. Med. 2019, 60, 393–399. [Google Scholar] [CrossRef]
- Brandt, M.; Cardinale, J.; Giammei, C.; Guarrochena, X.; Happl, B.; Jouini, N.; Mindt, T.L. Mini-review: Targeted radiopharmaceuticals incorporating reversible, low molecular weight albumin binders. Nucl. Med. Biol. 2019, 70, 46–52. [Google Scholar] [CrossRef]
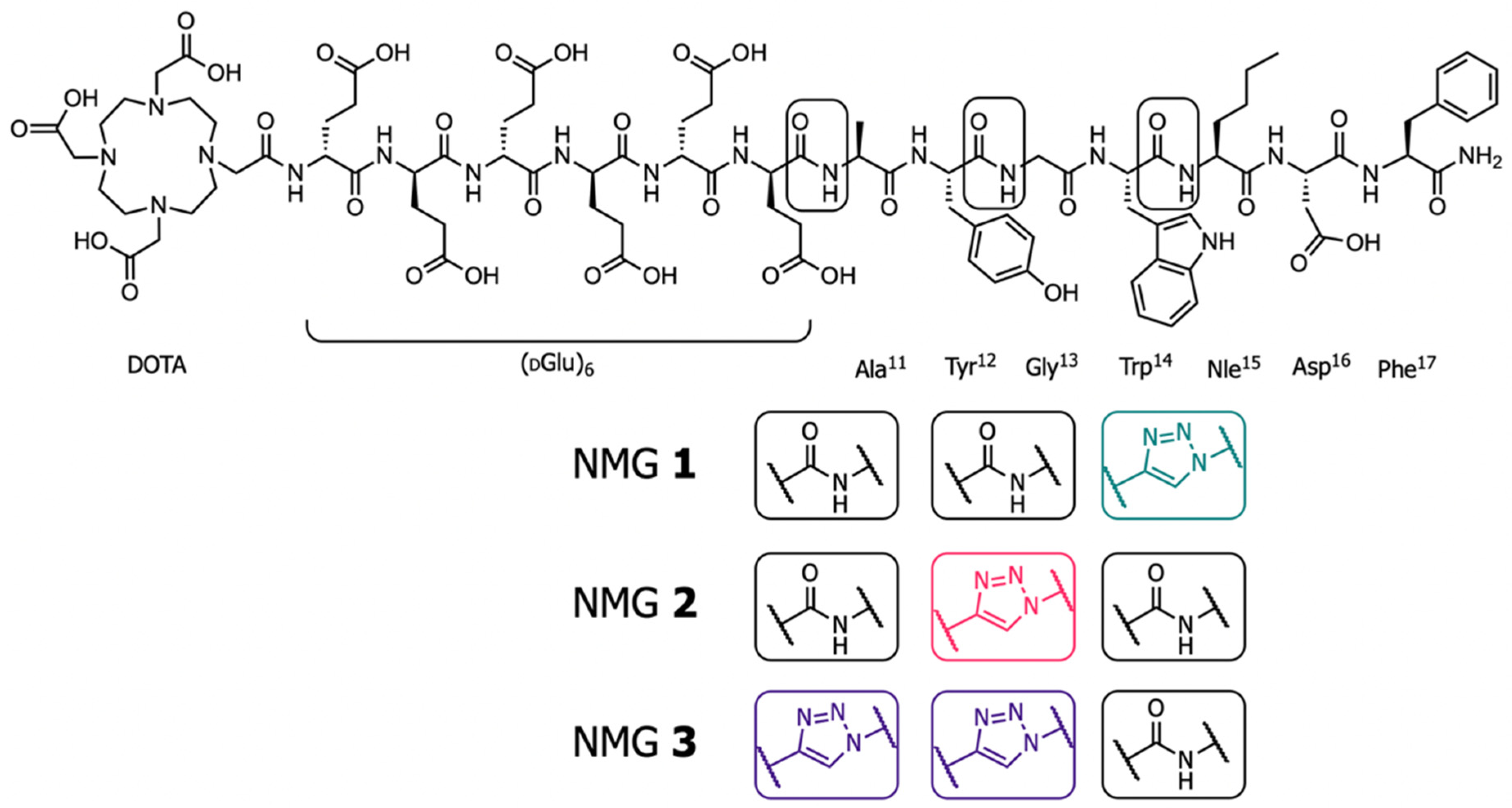
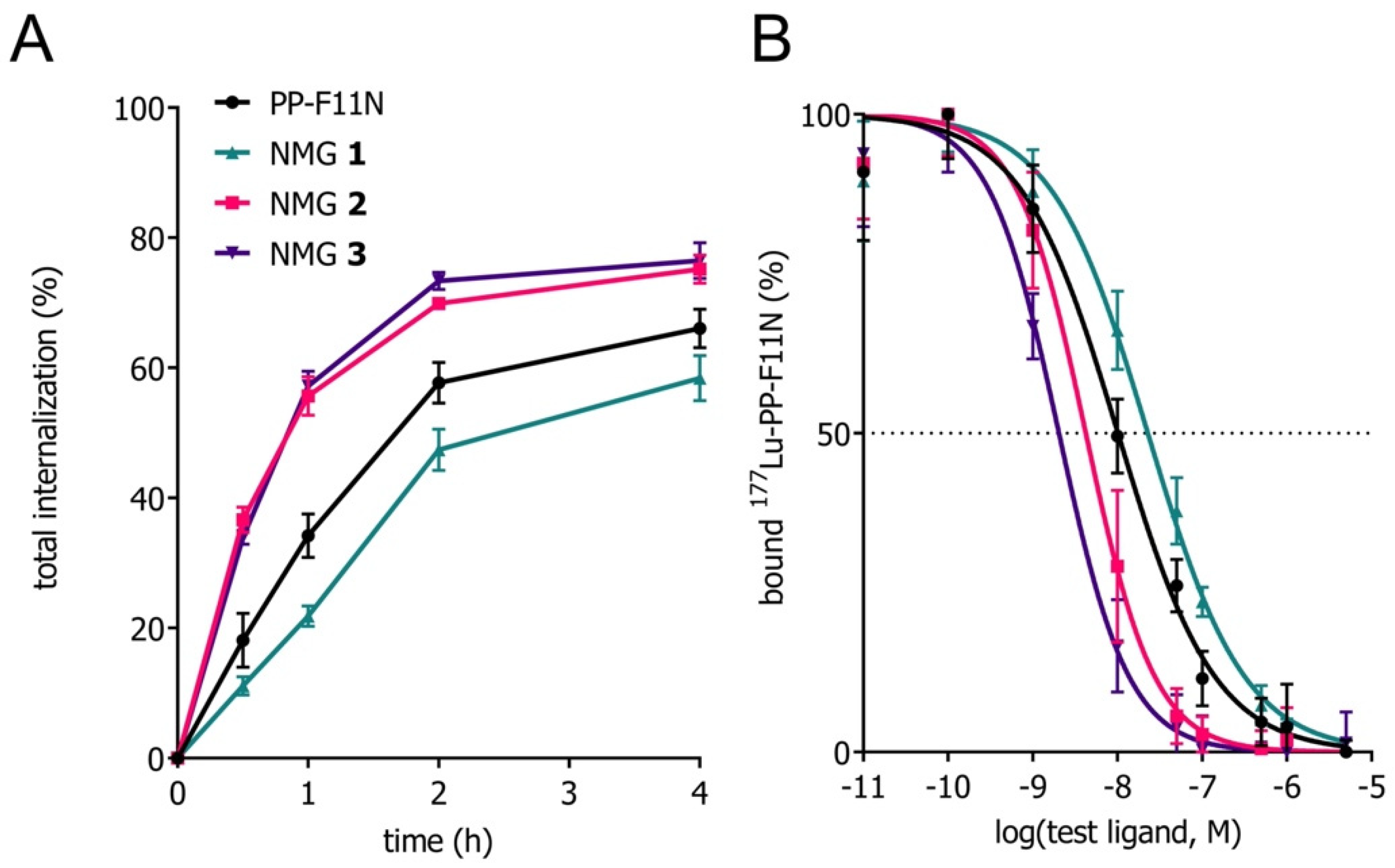

| Compound | Sequence | IC50 (95% CI, nM) a,c | Internalization 4 h Total (%) b,d,f | Intact Compound In Vitro (%), 24 h, Blood Plasma b,d,g | Intact Compound In Vivo (%), 10 min b,d,g |
|---|---|---|---|---|---|
| PP-F11N e | DOTA-(DGlu)6-Ala-Tyr-Gly-Trp-Nle-Asp-Phe-NH2 | 10.1 | 66.0 ± 2.9 | 98.1 ± 0.9 | 43.1 ± 2.7 |
| (8.7–11.8) | |||||
| NMG 1 | DOTA-(DGlu)6-Ala-Tyr-Gly-Trp-Ψ[Tz]-Nle-Asp-Phe-NH2 | 22.8 | 58.4 ± 3.5 | 98.3 ± 0.9 | 52.0 ± 0.2 |
| (19.5–26.6) | |||||
| NMG 2 | DOTA-(DGlu)6-Ala-Tyr-Ψ[Tz]-Gly-Trp-Nle-Asp-Phe-NH2 | 4.2 | 75.2 ± 2.2 | 96.3 ± 0.1 | 21.3 ± 3.0 |
| (3.6–5.0) | |||||
| NMG 3 | DOTA-(DGlu)6 -Ψ[Tz]-Ala-Tyr-Ψ[Tz]-Gly-Trp-Nle-Asp-Phe-NH2 | 2.0 | 76.5 ± 2.8 | 99.2 ± 0.8 | 39.3 ± 3.5 |
| (1.7–2.4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grob, N.M.; Schibli, R.; Béhé, M.; Mindt, T.L. Improved Tumor-Targeting with Peptidomimetic Analogs of Minigastrin 177Lu-PP-F11N. Cancers 2021, 13, 2629. https://doi.org/10.3390/cancers13112629
Grob NM, Schibli R, Béhé M, Mindt TL. Improved Tumor-Targeting with Peptidomimetic Analogs of Minigastrin 177Lu-PP-F11N. Cancers. 2021; 13(11):2629. https://doi.org/10.3390/cancers13112629
Chicago/Turabian StyleGrob, Nathalie M., Roger Schibli, Martin Béhé, and Thomas L. Mindt. 2021. "Improved Tumor-Targeting with Peptidomimetic Analogs of Minigastrin 177Lu-PP-F11N" Cancers 13, no. 11: 2629. https://doi.org/10.3390/cancers13112629
APA StyleGrob, N. M., Schibli, R., Béhé, M., & Mindt, T. L. (2021). Improved Tumor-Targeting with Peptidomimetic Analogs of Minigastrin 177Lu-PP-F11N. Cancers, 13(11), 2629. https://doi.org/10.3390/cancers13112629









