Simple Summary
The use of checkpoint inhibitors has changed the treatment landscape for gastroesophageal cancer in the third-line setting. However, success rates in earlier treatment lines are highly variable across trials. Herein, we compare the efficacy and safety of the different anti-PD-1/PD-L1 regimens with or without chemotherapy.
Abstract
Background: The use of checkpoint inhibitors has changed the treatment landscape for gastroesophageal cancer in the third-line setting. However, success rates in earlier treatment lines are highly variable across trials. Herein, we compare the efficacy and safety of the different anti-PD-1/PD-L1 regimens with or without chemotherapy; Methods: We performed a network meta-analysis (NMA) of anti-PD-1/PD-L1 monotherapy or combined with chemotherapy (chemoimmunotherapy) for gastroesophageal cancers without ERBB2 overexpression; Results: The first-line NMA included four trials (N = 3817), showing that chemoimmunotherapy improved OS and PFS without significant safety difference: Nivolumab-chemotherapy, OS (HR: 0.83 [95% CI, 0.75–0.92]), PFS (HR 0.68 [95% CI, 0.57–0.81]), Pembrolizumab-chemotherapy: OS (HR 0.77 [95% CI, 0.67–0.88]), PFS (HR: 0.72 [95% CI, 0.60–0.85]. Pembrolizumab monotherapy was the safest first-line treatment, SAE (OR 0.02 [95% CI, 0.00–0.2]) but showed no survival benefit. The second-line NMA encompassed four trials (N = 2087), showing that anti-PD-1 significantly improved safety but not survival: camrelizumab, SAE (OR 0.37; [95% CI, 0.24–0.56]); nivolumab, SAE (OR 0.13, [95% CI, 0.08–0.2]) pembrolizumab, SAE (OR 0.4; [95% CI, 0.30–0.53]); Conclusions: chemoimmunotherapy improves OS and PFS in previously untreated gastroesophageal cancers. Anti-PD-1 monotherapies improve safety in refractory disease, with no significant survival benefit.
1. Introduction
Gastric and esophageal cancers are the third and sixth leading causes of cancer mortality worldwide, with an estimated 768,793 and 544,076 deaths in 2020, respectively [1]. The diagnosis usually occurs in patients with locally advanced unresectable or metastatic disease, when treatment options are limited and with no curative intent. Chemotherapy remains the primary way to improve survival and quality of life in patients with gastroesophageal cancer. For those without overexpression of ERBB2 (previously, HER2), the first-line treatment is usually a choice of a platinum-fluoropyrimidine doublet, resulting in median survival of one year [2,3,4,5]. In the second-line setting, a taxane (docetaxel, paclitaxel) or irinotecan can improve survival in patients with good performance status [6,7,8]. However, the median overall survival is only six months, with a more significant benefit in patients that progressed 3–6 months after first-line chemotherapy. [7] Ramucirumab, an anti-VEGFR, showed similar survival benefits to chemotherapy as a single agent [9], while improving overall survival from 5.9 to 8.5 months when combined with paclitaxel as a second-line treatment [10].
Immune checkpoint inhibitors (ICI) targeting the programmed death-1 (PD-1)/programmed cell death ligand-1 (PD-L1) pathways are more established treatment options for patients with gastroesophageal cancer that progressed after two or more chemotherapy lines [11,12]. However, immunotherapy had not significantly improved survival in earlier therapy lines until recently [13,14,15,16,17]. Preliminary results from the KEYNOTE-590 and CheckMate 649 presented at the 2020 European Society for Medical Oncology (ESMO) annual meeting showed combinations of anti-PD-1 drugs with chemotherapy (chemoimmunotherapy) might be more effective than chemotherapy alone [18,19].
This study aimed to compare the efficacy and safety of PD-1/PD-L1 inhibitors for patients with advanced ERBB2 negative gastric and esophageal cancers. We performed a comprehensive analysis of the current data published from phase III randomized clinical trials (RCT) to inform decision making and enable the development of optimal first- and second-line treatment strategies for those patients.
2. Materials and Methods
We performed our study under the extension for network meta-analysis from the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) [20,21]. We created a prospective protocol and uploaded it to PROSPERO (CRD42020221822).
2.1. Eligibility Criteria
We considered eligible all randomized clinical trials comparing PD-1/PD-L1 inhibitors or anti–PD-L1, as single agents or combined with chemotherapy versus chemotherapy alone, in patients with esophageal, gastric, and gastroesophageal junction tumors, in the frontline or second-line treatments. We considered ineligible trials in phases 1 or 2 and trials that compared PD-1/PD-L1 inhibitors with other immunotherapies. When we found multiple references for the same study, we favored the latest and most complete report.
2.2. Data Sources and Extraction
We performed an extensive database search (PubMed, Embase, Cochrane Central, Web of Science, Medline, Scopus, and ClinicalTrials.gov) for entries from 1 January 2010 to 23 November 2020. We also reviewed abstracts from the American Society of Clinical Oncology and the ESMO libraries until 21 November 2020. A detailed search strategy is available in Table A1.
We uploaded titles and abstracts to Rayyan QCRI, a web-based platform for systematic review management. [22] Three authors independently performed the screening. Data from the included trials was performed by two authors, in tandem, and using a pre-piloted spreadsheet containing trial identification, baseline patient characteristics (including PD-L1 expression status), treatments, and outcomes. We resolved discrepancies by consensus. The efficacy outcomes of interest were overall survival (OS) and progression-free survival (PFS). The safety outcome of interest was the incidence of serious adverse events (SAEs), characterized as treatment-related adverse events (TRAEs) grade 3 to 5.
2.3. Risk of Bias Assessment
We used the Cochrane Collaboration’s tool (version 2.0), which includes five domains (randomization process, deviation from intended interventions, missing outcome data, measurement of the outcome, and selection of reported results) and results in judgments of “low risk of bias”, “some concerns”, or “high risk of bias” [23]. Two authors independently applied the tool to each included trial. Any inconsistencies were solved by a discussion and between the authors.
2.4. Statistical Analysis
We performed a network meta-analysis with a frequentist approach and a random-effects model using the package ‘netmeta’ for R statistical software (version 4.0.3, R Project for Statistical Computing) [24]. We used multivariate normal distribution and random-effects models to account for between-arm correlation in multi-arm trials inside the frequentist network [25]. We generated forest plots for back-transformed network estimates. We assessed heterogeneity between and within designs using Cochran’s Q statistics and quantified using I2 statistics. I2 can be used to describe the proportion of the variability in effect estimates due to heterogeneity within three thresholds 25% (low), 50% (moderate) and, 75% (high) [26,27]. We expressed OS and PFS outcomes as hazard ratios (HR) with the respective 95% confidence interval (95% CI) and SAEs as odds ratios (OR) with the respective 95% CI.
3. Results
3.1. Study Selection
We found a total of 2386 unique entries. After excluding duplicates, we screened titles and abstracts for 1000 records. We assessed 149 full-text publications, including 12 trial registrations (Figure A1). We included eight trials in the quantitative synthesis: four in the first-line setting and four in the second-line setting.
3.2. First-Line Treatments
3.2.1. Study Characteristics
The four trials in the first-line setting involved 3817 patients. ATTRACTION-4 and KEYNOTE-649 evaluated nivolumab + chemotherapy (Nivo-Chemo). KEYNOTE-590 evaluated Pembrolizumab + chemotherapy (Pembro-Chemo). KEYNOTE-062 had three-arms, comparing Pembrolizumab monotherapy (Pembro) or Pembro-Chemo with chemotherapy alone (Table 1). KEYNOTE-062 included only patients with PD-L1 combined positive score (CPS) ≥ 1. Further details PD-L1 expression subgroups in each included trial can be found in the Table A2.

Table 1.
Characteristics of Trials Included in the Network Meta-Analysis of first-line treatments.
3.2.2. Network Meta-Analysis
We found that the combination of anti-PD-1 with chemotherapy improves survival. Pembro-Chemo showed similar OS benefit (HR, 0.77; 95% CI, 0.67–0.88) to Nivo-Chemo (HR, 0.83; 95% CI, 0.75–0.92). PFS was also comparable with Nivo-Chemo (HR, 0.68; 95% CI, 0.57–0.81) and Pembro-Chemo (HR, 0.72; 95% CI, 0.60–0.85). Pembro monotherapy did not improve survival, OS (HR, 0.91; 95% CI, 0.71–1.16), PFS (HR, 1.62; 95% CI, 1.23–2.14) but showed a markedly better safety profile than chemotherapy, SAE (OR, 0.02; 95% CI, 0.00–0.2). We found no significant safety difference in SAE from Nivo-Chemo (OR 0.54; 0; 95% CI, 0.1–2.92) or Pembro-Chemo SAE (OR, 1.31; 0; 95% CI, 0.23–7.35), compared with chemotherapy (Figure 1A).
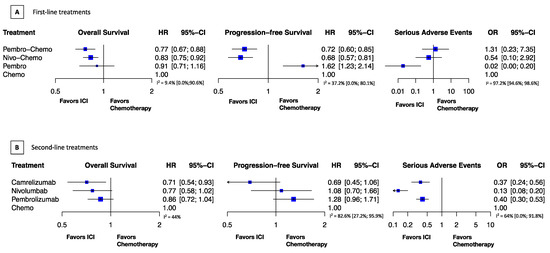
Figure 1.
Relative treatment effects. HR indicates hazard ratio; (A). First-line treatments; (B). Second-line treatments; OR, odds ratio; CI, confidence interval. Treatment abbreviations: Chemo indicates chemotherapy; Pembro, pembrolizumab; Pembro-Chemo, pembrolizumab plus chemotherapy; Nivo-Chemo, nivolumab plus chemotherapy.
3.2.3. Risk of Bias
All four trials had a low risk of bias for OS. We considered CheckMate 649 a high risk of bias for PFS and SAEs, mostly related to missing outcome data. The remaining studies had a low risk of bias for PFS and SAEs (Table 2).

Table 2.
Risk of bias assessment, first-line trials.
3.3. Second-Line Treatments
3.3.1. Study Characteristics
The studies in the second-line setting involved 2087 individuals. All trials (KEYNOTE-061, ATTRACTION-3, KEYNOTE-181, and ESCORT) compared chemotherapy with pembrolizumab, nivolumab, or camrelilzumab. The predominant tumor site was esophageal (Table 3). All studies had subgroups according to PD-L1. All publications had OS, PFS, and SAEs data available (Table A3).

Table 3.
Characteristics of Trials Included in the Network Meta-Analysis of second-line treatments.
3.3.2. Network Meta-Analysis
Camrelizumab showed a greater survival benefit compared to chemotherapy, OS (HR 0.71; 95% CI, 0.54–0.93), PFS (HR 0.69; 95% CI, 0.45–1.06); followed by nivolumab, OS (HR 0.77; 95% CI, 0.58–1.02), PFS (HR 1.08; 95% CI, 0.77–1.66) and pembrolizumab, OS (HR 0.86; 95% CI, 0.72–1.04), PFS (HR 1.28; 95% CI, 0.96–1.71). Anti-PD-1 drugs significantly improved safety, nivolumab had the lowest chance of serious adverse events (OR, 0.13; 95% CI, 0.08–0.2), followed by camrelizumab (OR, 0.37; 95% CI, 0.24–0.56) and pembrolizumab (OR, 0.4; 95% CI, 0.30–0.53) (Figure 1B).
3.3.3. Risk of Bias
The four trials had a low risk of bias for OS and PFS. For SAE, KEYNOTE-061 raised some concerns due to missing outcome data. The remaining studies had a low risk of bias for PFS (Table 4).

Table 4.
Risk of bias assessment, second-line trials.
3.4. Subgroup Analysis: PD-L1 Expression
Published data from PD-L1 expression subgroups across trials was not consistent. There were variable cut-off values, so it was not statistically meaningful to add those subgroups in our network meta-analysis. To evaluate PD-L1 expression as a predictor of response to ICIs, we pooled the available OS HR from chemoimmunotherapy subgroups according to PD-L1 CPS. In the first-line setting, patients that overexpress PD-L1 had better OS, CPS ≥ 10 (HR 0.75; 95% CI, 0.65–0.87), CPS ≥ 5 (HR 0.71; 95% CI, 0.61–0.83); those with CPS ≥ 1, OS (HR 0.79; 95% CI, 0.71–0.89) had similar response to all randomized patients, OS (HR 0.80; 95% CI, 0.72–0.89) (Figure 2). In the second-line setting, we included all the single-agent PD-1 inhibitors: patients with PD-L1 CPS ≥ 1 had better OS (HR 0.71; 95% CI, 0.71–0.87) in comparison to all patients, OS (HR 0.83; 95% CI, 0.74–0.94), but patients with PD-L1 CPS ≥ 10 had a significant difference in OS (HR 1.19; 95% CI, 0.71–0.87) (Figure 3).
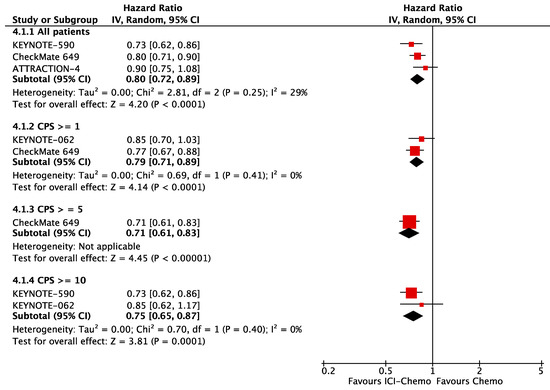
Figure 2.
OS according to PD-L1 expression in the first-line setting.
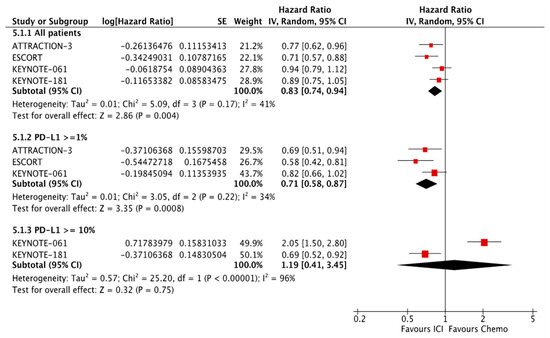
Figure 3.
OS according to PD-L1 expression in the second-line setting.
4. Discussion
The present study offers valuable insight on recent advances involving the use of PD-1 inhibitors in patients with advanced gastroesophageal cancers that do not overexpress ERBB2. For previously untreated patients, chemoimmunotherapy was the best strategy. Both Nivo-Chemo and Pembro-Chemo showed significantly better OS and PFS with no significant difference in SAEs. Conversely, pembrolizumab monotherapy was markedly safer than ICI-Chemo but did not improve OS and had the worst PFS.
For patients that progressed after one line of chemotherapy, our final selection encompassed three different anti-PD-1 drugs. Camrelizumab showed the best OS, followed by nivolumab and pembrolizumab. We did not observe the same benefit for PFS. We found that camrelizumab might improve PFS, but nivolumab and pembrolizumab might worsen PFS compared to chemotherapy. Importantly, PD-1 inhibitors were significantly safer than chemotherapy as second-line treatments. Nivolumab likely has the best profile, followed by camrelizumab and pembrolizumab.
A growing body of evidence shows that drugs such as cisplatin, oxaliplatin, and paclitaxel can up-regulate PD-L1 expression in tumor and immune cells, therefore blocking the chemotherapy effectiveness but opening an opportunity to the use of PD-1/PD-L1 inhibitors [29,30,31]. Other studies point that cytotoxic therapies can turn ‘cold’ tumors into ‘hot’ tumors by making them abundantly infiltrated by CD8+ T cells and dendritic cells, making them more susceptible to ICIs [32,33,34]. The impact of these changes in the tumor microenvironment in clinical effectiveness is yet to be proven [35]. However, they help explain why chemoimmunotherapy led to better survival outcomes than pembrolizumab alone in previously untreated patients, ref. [16] while single-agent PD-1 inhibitors provided better OS benefit in patients that progressed after chemotherapy [11,12].
The use of chemoimmunotherapy for a shorter period, followed by treatment with immunotherapy only, can lead to earlier disease control with more extended survival benefits and lower SAE rates. The CheckMate 9LA has recently demonstrated the benefit of such a strategy in patients with lung cancer [31]. Ongoing phase II trials such as “Blinded for peer review” (nivolumab with or without ipilimumab) and “Blinded for peer review” (avelumab) will help to identify optimal dosing and administration schedules of immunogenic chemotherapy for gastroesophageal cancers.
Several trials in our analysis did not achieve their primary endpoints, which can be related to heterogeneity inside the cohorts [13,14,15,16]. For instance, in KEYNOTE-062, all patients had CPS ≥1, and neither Pembro alone nor Pembro-Chemo significantly improved survival. In the subset of patients with CPS ≥ 10, Pembro prolonged OS (median 17.4 months versus 10.8 months; HR 0.69; 95% CI, 0.49–0.97), however no statistical test was applied to this difference [16]. Hence, identifying which tumors will respond to immune checkpoint inhibitors is paramount. Our analysis shows that PD-L1 CPS was not a robust predictor of efficacy, as the OS benefit from chemoimmunotherapy was similar OS across subgroups, which can be related to inconsistencies in PD-L1 assessment methods and cutoff values. Hopefully, the final results from ATTRACTION-4 and the ongoing KEYNOTE-859 will help consolidate the role of PD-L1 CPS in selecting patients for chemoimmunotherapy in the first-line setting. However, there is a need for alternative biomarkers.
5. Limitations
Our study’s first limitation comes to its nature as a network meta-analysis where we derived most of our conclusions from indirect comparisons. We used trial-level data rather than patient-level data, which could lower the power of our analysis.
Second, the trials had several differences in baseline characteristics that could affect the generalizability of the results. In the first-line, gastric and gastroesophageal adenocarcinomas were the predominant type, while in the second-line, esophageal (adenocarcinoma and squamous cell carcinoma) were the most frequent.
Third, the PFS and SAE data from CheckMate 649 included in our analysis in the first-line setting refers only to the subgroup of patients with PD-L1 CPS ≥ 5, which raises concerns for publication bias. We obtained most of the data in the first line from conference abstracts, and hopefully, further peer-reviewed publications will provide more detailed data from all patients included in each study.
6. Conclusions
Chemoimmunotherapy is the best first-line treatment for HER2 negative, advanced gastro-esophageal cancers. Nivo-Chemo and Pembro-Chemo improved OS and PFS similarly. Pembro did not improve survival but was significantly less toxic and should be considered as a first-line option. In the second-line setting, anti-PD-1 drugs might prolong survival, but camrelizumab was the only one to improve OS significantly. All anti-PD-1 drugs were significantly less toxic than chemotherapy for patients with refractory disease. The association of higher levels of PD-L1 expression with better outcomes remains unclear and would be better assessed in further analyses.
Author Contributions
This work has been conceptualized, written, reviewed and edited by all the authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
Gilberto Lopes declares honoraria from Boehringer Ingelheim and consulting or advisory role for Pfizer, AstraZeneca and research Funding for Merck Sharp & Dohme (Inst), EMD Serono (Inst), AstraZeneca (Inst), AstraZeneca, Blueprint Medicines (Inst), Tesaro (Inst), Bavarian Nordic (Inst), Novartis (Inst), G1 Therapeutics (Inst), Adaptimmune (Inst), Bristol Myers Squibb (Inst), GlaxoSmithKline (Inst), AbbVie (Inst), Rgenix (Inst), Pfizer (Inst), Roche (Inst), Genentech (Inst), Eli Lilly (Inst), Janssen (Inst) and travel, accommodations, expenses from Boehringer Ingelheim, Pfizer, E.R. Squibb Sons, Janssen. Pedro Aguiar Jr declares honoraria from Boehringer Ingelheim and Merck Sharp & Dohme. Benjamin Haaland declares consulting or advisory role for Astra Zeneca, Prometics Life Sciences, Value Analytics, and the National Kidney Foundation and travel expenses from Flatiron Health. Laercio Lopes da Silva, Eduardo Edelman Saul, and Robin Park declare no conflict of interest.
Appendix A

Table A1.
Database search strategy.
Table A1.
Database search strategy.
| Database | Keywords | Results |
|---|---|---|
| PubMed | ||
| #1 | pembrolizumab OR keytruda OR MK-3475 OR ‘SCH 900475’ OR nivolumab OR NIVO OR opdivo OR BMS-936558 OR MDX-1106 OR ONO-4538 OR atezolizumab OR tecentriq OR ‘MPDL 3280A’ OR RG7446 OR durvalumab OR imfinzi OR MEDI-4736 OR MEDI4736 OR avelumab OR bavencio OR MSB-0010718C OR MSB0010718C OR camrelizumab OR ‘SHR 1210’ | 9694 |
| #2 | ‘programmed death-1’ OR pd-1 OR pd1 OR ‘programmed death ligand-1’ OR pd-l1 OR pdl1 OR ‘checkpoint inhibitor’ OR ‘checkpoint blockade’ | 46,549 |
| #3 | esophageal OR oesophageal OR esophagus OR gastric OR stomach OR ‘gastro-esophageal junction’ OR oesophagogastric OR oesophagastric OR esophagogastric OR esophago-gastric OR gastroesophageal OR gastro-oesophageal | 560,223 |
| #4 | cancer OR carcinoma OR adenocarcinoma OR ‘squamous cell carcinoma’ OR ‘squamous-cell carcinoma’ | 4,296,021 |
| #5 | #1 OR #2 | 49,382 |
| #6 | #3 AND #4 | 224,313 |
| #7 | #5 AND #6 | 1646 |
| #8 | (((“randomized controlled trial”[pt] OR “controlled clinical trial”[pt] OR “clinical trials as topic”[mesh] OR “random allocation”[mesh] OR “double-blind method”[mesh] OR “single-blind method”[mesh] OR “clinical trial”[pt] OR “research design”[mesh:noexp] OR “comparative study”[pt] OR “evaluation studies”[pt] OR “follow-up studies”[mesh] OR “prospective studies”[mesh] OR “clinical trial”[tw] OR ((singl*[tw] OR doubl*[tw] OR trebl*[tw]) AND (mask*[tw] OR blind*[tw])) OR placebo*[tw] OR random*[tw] OR “control”[tw] OR “controls”[tw] OR prospectiv*[tw] OR volunteer*[tw]))) | 8,009,931 |
| #9 | #7 AND #8 AND 2010/01/01[PDAT]:2020/11/23[PDAT] | 412 |
| Embase | ||
| #1 | ‘pembrolizumab’/exp OR pembrolizumab OR ‘keytruda’/exp OR keytruda OR ‘mk 3475’/exp OR ‘mk 3475’ OR ‘sch 900475’/exp OR ‘sch 900475’ OR ‘nivolumab’/exp OR nivolumab OR nivo OR ‘opdivo’/exp OR opdivo OR ‘bms 936558’/exp OR ‘bms 936558’ OR ‘mdx 1106’/exp OR ‘mdx 1106’ OR ‘ono 4538’/exp OR ‘ono 4538’ OR ‘atezolizumab’/exp OR atezolizumab OR ‘tecentriq’/exp OR tecentriq OR ‘mpdl 3280a’/exp OR ‘mpdl 3280a’ OR ‘rg7446’/exp OR rg7446 OR ‘durvalumab’/exp OR durvalumab OR ‘imfinzi’/exp OR imfinzi OR ‘medi 4736’/exp OR ‘medi 4736’ OR ‘medi4736’/exp OR medi4736 OR ‘avelumab’/exp OR avelumab OR ‘bavencio’/exp OR bavencio OR ‘msb 0010718c’/exp OR ‘msb 0010718c’ OR ‘msb0010718c’/exp OR msb0010718c OR camrelizumab OR ‘shr 1210’ | 32,910 |
| #2 | ‘programmed death-1’ OR ‘pd 1’ OR pd1 OR ‘programmed death ligand-1’ OR ‘pd l1’ OR pdl1 OR ‘checkpoint inhibitor’ OR ‘checkpoint blockade’ | 68,631 |
| #3 | #1 OR #2 | 86,155 |
| #4 | esophageal OR oesophageal OR esophagus OR gastric OR stomach OR ‘gastro-esophageal junction’ OR oesophagogastric OR oesophagastric OR esophagogastric OR ‘esophago gastric’ OR gastroesophageal OR ‘gastro oesophageal’ | 848,125 |
| #5 | cancer OR carcinoma OR adenocarcinoma OR ‘squamous cell carcinoma’ OR ‘squamous-cell carcinoma’ | 4,739,285 |
| #6 | #4 AND #5 | 307,418 |
| #7 | #3 AND #6 | 4023 |
| #8 | ‘randomized controlled trial’ OR ‘controlled clinical trial’ OR ‘random*’ OR groups | 4,414,728 |
| #9 | ‘clinical trials as topic’ OR trial | 2,210,774 |
| #10 | #8 AND #9 | 1,286,636 |
| #11 | #7 AND #10 AND [english]/lim AND (2010–2020)/py | 501 |
| Cochrane Central | ||
| #1 | pembrolizumab OR keytruda OR MK-3475 OR ‘SCH 900475′ OR nivolumab OR NIVO OR opdivo OR BMS-936558 OR MDX-1106 OR ONO-4538 OR avelumab OR bavencio OR MSB-0010718C OR MSB0010718C OR ‘programmed death-1’ OR ‘pd-1’ OR ‘pd1’ OR ‘programmed death ligand-1’ OR ‘pd-l1’ OR ‘pdl1’ OR ‘checkpoint inhibitor’ OR ‘checkpoint blockade | 5427 |
| #2 | oesophageal OR esophagus OR esophageal OR gastric OR stomach OR ‘gastro-esophageal junction’ OR oesophagogastric OR oesophagastric OR esophagogastric OR esophago-gastric OR gastroesophageal OR gastro-oesophageal | 45,933 |
| #3 | cancer OR carcinoma OR adenocarcinoma OR ‘squamous cell carcinoma’ OR ‘squamous-cell carcinoma’ | 192,826 |
| #4 | #2 AND #3 | 14,291 |
| #5 | #1 AND #4 | 342 |
| #6 | (‘clinical trials as topic’ OR trial) | 1,253,558 |
| #7 | (‘randomized controlled trial’ OR ‘controlled clinical trial’ OR ‘random*’ OR groups) | 1,352,768 |
| #8 | #5 AND #6 AND #7 | 283 |
| #6 | Library publication date from Jan 2010 to Nov 2020 | 283 |
| Web of Science | ||
| (“esophageal cancer” OR “esophageal carcinoma” OR “esophageal squamous cell carcinoma” OR “gastric cancer” OR “gastric carcinoma” OR “stomach cancer” OR “gastric adenocarcinoma” OR “gastro-esophageal junction carcinoma” OR “gastro-esophageal junction cancer”) AND TOPIC: (“programmed death-1” OR “pd-1” OR “pd1” OR “programmed death ligand-1” OR “pd-l1” OR “pdl1” OR “checkpoint inhibitor” OR “checkpoint blockade”) AND TOPIC: (“randomized clinical trial” OR “controlled clinical trial” OR “survival rate” OR “mortality” OR “progression-free survival” OR “treatment outcome”)Refined by: LANGUAGES: (ENGLISH)Timespan: All years. Indexes: SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, BKCI-S, BKCI-SSH, ESCI, CCR-EXPANDED, IC. | 116 | |
| Medline | ||
| #1 | (“esophageal cancer” or “esophageal carcinoma” or “esophageal squamous cell carcinoma” or “gastric cancer” or “gastric carcinoma” or “stomach cancer” or “gastric adenocarcinoma” or “gastro-esophageal junction carcinoma” or “gastro-esophageal junction cancer”).mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] | 91,242 |
| #2 | (pembrolizumab or nivolumab or atezolizumab or bevacizumab or Avelumab or durvalumab).mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] | 21,133 |
| #3 | (“programmed cell death 1 receptor” or “pd-l1”).mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] | 12,555 |
| #4 | #2 OR #3 | 31,129 |
| #5 | #1 AND #4 | 511 |
| #6 | limit 5 to (humans and yr = “2010-Current” and english) | 420 |
| SCOPUS | ||
| ( TITLE-ABS-KEY ( “esophageal cancer” OR “esophageal carcinoma” OR “esophageal squamous cell carcinoma” OR “gastric cancer” OR “gastric carcinoma” OR “stomach cancer” OR “gastric adenocarcinoma” OR “gastro-esophageal junction carcinoma” OR “gastro-esophageal junction cancer”)) AND ((TITLE-ABS-KEY ( pembrolizumab OR nivolumab OR atezolizumab OR avelumab OR camrelizumab)) OR ( TITLE-ABS-KEY (“programmed cell death 1 receptor” OR “pd-l1”))) AND (LIMIT-TO ( DOCTYPE, “ar”) OR LIMIT-TO ( DOCTYPE, “re”) OR LIMIT-TO ( DOCTYPE, “cp”)) AND ( LIMIT-TO ( LANGUAGE, “English”)) AND ( LIMIT-TO ( EXACTKEYWORD, “Humans”)) | 642 | |
| ClinicalTrials.gov | ||
| ‘Gastric Cancer’ OR ‘Esophageal Cancer’|‘Immunotherapy’ OR ‘immune checkpoint inhibitor’ OR ‘PD-L1′OR ‘PD-1′ | Phase 3 (12 records) | ||
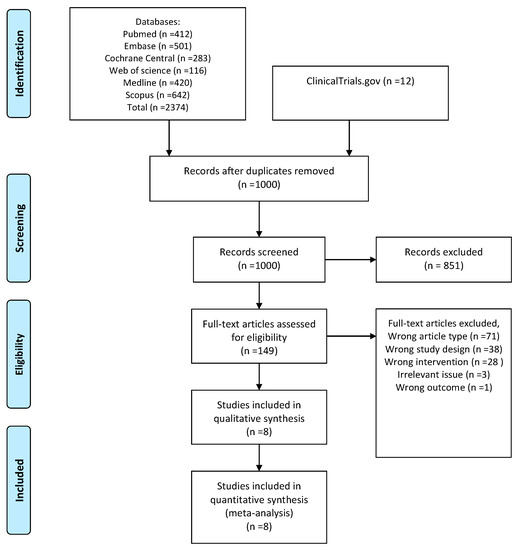
Figure A1.
Flowchart of the study selection process.

Table A2.
Outcomes according to subgroups in the first-line setting.
Table A2.
Outcomes according to subgroups in the first-line setting.
| Trial ID | Tumor Type | PD-L1 Status | N | Arm A | Arm B | HR OS (95%, CI) | HR PFS (95%, CI) | SAE A | SAE B |
|---|---|---|---|---|---|---|---|---|---|
| ATTRACTION-4 | G/GEJ | All | 724 | Nivo-Chemo | Chemo | 0.9 (0.75–1.08) | 0.68 (0.51–0.90 c) | 0.579 | 0.492 |
| KEYNOTE-590 | ESCC | CPS ≥ 10 | NA | Pembro-Chemo | Chemo | 0.57 (0.43–0.75) | NA | NA | NA |
| ESCC | All | 547 | Pembro-Chemo | Chemo | 0.72 (0.60–0.88) | 0.65 (0.54–0.78) | NA | NA | |
| ESCC/EGJ | CPS ≥ 10 | NA | Pembro-Chemo | Chemo | 0.62 (0.49–0.78) | 0.51 (0.41–0.65) | NA | NA | |
| ESCC/EGJ | All | 749 | Pembro-Chemo | Chemo | 0.73 (0.62–0.86) | 0.65 (0.55–0.76) | 0.72 | 0.68 | |
| CheckMate 649 | G/GEJ/EAC | CPS ≥ 1 | 1296 | Nivo-Chemo | Chemo | 0.77 (0.64–0.92 a) | NA | NA | |
| G/GEJ/EAC | CPS ≥ 5 | 955 | Nivo-Chemo | Chemo | 0.71 (0.59–0.86 b) | 0.68 (0.56–0.81 d) | 0.59 | 0.44 | |
| G/GEJ/EAC | All | 1581 | Nivo-Chemo | Chemo | 0.8 (0.68–0.94 a) | NA | NA | ||
| KEYNOTE-062 | G/GEJ | CPS ≥ 1 | 506 | Pembro | Chemo | 0.91 (0.74–1.10) | 1.66 (1.37–2.01) | 0.169 | 0.693 |
| G/GEJ | CPS ≥ 10 | 182 | Pembro | Chemo | 0.69 (0.49–0.97) | 1.1 (0.79–1.51) | NA | NA | |
| G/GEJ | CPS ≥ 1 | 507 | Pembro-Chemo | Chemo | 0.85 (0.70–1.03) | 0.84 (0.70–1.02) | 0.732 | 0.693 | |
| G/GEJ | CPS ≥ 10 | 189 | Pembro-Chemo | Chemo | 0.85 (0.62–1.17) | 0.73 (0.53–1) | NA | NA |
a 99.3% CI, b 98.4% CI, c 98,51% CI, d 98% CI. ESCC = esophageal squamous cell carcinoma; G = gastric; GEJ = gastroesophageal junction; EAC = esophageal adenocarcinoma.

Table A3.
Outcomes according to subgroups in the second-line setting.
Table A3.
Outcomes according to subgroups in the second-line setting.
| Trial ID | Tumor Type | PD-L1 Status | N | Arm A | Arm B | HR OS (95% CI) | HR PFS (95% CI) | SAE A | SAE B |
|---|---|---|---|---|---|---|---|---|---|
| KEYNOTE-061 | G/GEJ | CPS ≥ 1 | 395 | Pembrolizumab | Chemo | 0.82 (0.66–1.03) | 1.27 (1.03–1.57) | NA | NA |
| G/GEJ | All | 592 | Pembrolizumab | Chemo | 0.94 (0.79–1.12) | 1.49 (1.25–1.77) | 42 (0.14) | 96 (0.35) | |
| G/GEJ | CPS ≥ 10 | 197 | Pembrolizumab | Chemo | 2.05 (1.5–2.79) | NA | NA | NA | |
| ATTRACTION-3 | ESCC | All | 419 | Nivolumab | Chemo | 0.77 (0.62−0·96) | 1.08 (0.87–1.34) | 38 (0.18) | 133 (0.65) |
| ESCC | PD-L1 ≥ 1% | 203 | Nivolumab | Chemo | 0.69 (0.51−0·94) | NA | 38 (0.18) | 133 (0.65) | |
| KEYNOTE-181 | EAC/ESCC | All | 628 | Pembrolizumab | Chemo | 0.89 (0.75–1.05) | 1.11 (0.94–1.31) | 57 (0.182) | 121 (0.49) |
| EAC/ESCC | CPS ≥ 10 | 222 | Pembrolizumab | Chemo | 0.69 (0.52–0.93) | 0.73 (0.54–0.97) | NA | NA | |
| ESCC | All | 401 | Pembrolizumab | Chemo | 0.78 (0.63–0.96) | 0.92 (0.75–1.13) | NA | NA | |
| ESCORT | ESCC | All | 448 | Camrelizumab | Chemo | 0.71(0.57–0.87) | 0.69 (0.56–0.86) | 44 (0.193) | 87 (0.395) |
| ESCC | CPS ≥ 1% | 191 | Camrelizumab | Chemo | 0.58 (0.42–0.81) | NA | 44 (0.193) | 87 (0.395) |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021. [Google Scholar] [CrossRef]
- Smyth, E.C.; Verheij, M.; Allum, W.; Cunningham, D.; Cervantes, A.; Arnold, D.; on behalf of the ESMO Guidelines Committee. Gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, v38–v49. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Moiseyenko, V.M.; Tjulandin, S.; Majlis, A.; Constenla, M.; Boni, C.; Rodrigues, A.; Fodor, M.; Chao, Y.; Voznyi, E.; et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: A report of the V325 Study Group. J. Clin. Oncol. 2006, 24, 4991–4997. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, D.; Starling, N.; Rao, S.; Iveson, T.; Nicolson, M.; Coxon, F.; Middleton, G.; Daniel, F.; Oates, J.; Norman, A.R.; et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N. Engl. J. Med. 2008, 358, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Ajani, J.A.; D'Amico, T.A.; Bentrem, D.J.; Chao, J.; Corvera, C.; Das, P.; Denlinger, C.S.; Enzinger, P.C.; Fanta, P.; Farjah, F.; et al. Esophageal and Esophagogastric Junction Cancers, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. JNCCN 2019, 17, 855–858. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Lee, S.I.; Lim, D.H.; Park, K.W.; Oh, S.Y.; Kwon, H.C.; Hwang, I.G.; Lee, S.C.; Nam, E.; Shin, D.B.; et al. Salvage chemotherapy for pretreated gastric cancer: A randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J. Clin. Oncol. 2012, 30, 1513–1518. [Google Scholar] [CrossRef]
- Ford, H.E.; Marshall, A.; Bridgewater, J.A.; Janowitz, T.; Coxon, F.Y.; Wadsley, J.; Mansoor, W.; Fyfe, D.; Madhusudan, S.; Middleton, G.W.; et al. Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): An open-label, phase 3 randomised controlled trial. Lancet Oncol. 2014, 15, 78–86. [Google Scholar] [CrossRef]
- Thuss-Patience, P.C.; Kretzschmar, A.; Bichev, D.; Deist, T.; Hinke, A.; Breithaupt, K.; Dogan, Y.; Gebauer, B.; Schumacher, G.; Reichardt, P. Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer--a randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Eur. J. Cancer 2011, 47, 2306–2314. [Google Scholar] [CrossRef]
- Fuchs, C.S.; Tomasek, J.; Yong, C.J.; Dumitru, F.; Passalacqua, R.; Goswami, C.; Safran, H.; Dos Santos, L.V.; Aprile, G.; Ferry, D.R.; et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): An international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014, 383, 31–39. [Google Scholar] [CrossRef]
- Wilke, H.; Muro, K.; Van Cutsem, E.; Oh, S.C.; Bodoky, G.; Shimada, Y.; Hironaka, S.; Sugimoto, N.; Lipatov, O.; Kim, T.Y.; et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): A double-blind, randomised phase 3 trial. Lancet Oncol. 2014, 15, 1224–1235. [Google Scholar] [CrossRef]
- Kang, Y.K.; Boku, N.; Satoh, T.; Ryu, M.H.; Chao, Y.; Kato, K.; Chung, H.C.; Chen, J.S.; Muro, K.; Kang, W.K.; et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017. [Google Scholar] [CrossRef]
- Fuchs, C.S.; Doi, T.; Jang, R.W.; Muro, K.; Satoh, T.; Machado, M.; Sun, W.; Jalal, S.I.; Shah, M.A.; Metges, J.-P.; et al. Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol. 2018, 4, e180013. [Google Scholar] [CrossRef] [PubMed]
- Shitara, K.; Ozguroglu, M.; Bang, Y.-J.; Di Bartolomeo, M.; Mandala, M.; Ryu, M.-H.; Fornaro, L.; Olesinski, T.; Caglevic, C.; Chung, H.C.; et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): A randomised, open-label, controlled, phase 3 trial. Lancet 2018, 392, 123–133. [Google Scholar] [CrossRef]
- Kato, K.; Cho, B.C.; Takahashi, M.; Okada, M.; Lin, C.Y.; Chin, K.; Kadowaki, S.; Ahn, M.J.; Hamamoto, Y.; Doki, Y.; et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 1506–1517. [Google Scholar] [CrossRef]
- Kojima, T.; Shah, M.A.; Muro, K.; Francois, E.; Adenis, A.; Hsu, C.H.; Doi, T.; Moriwaki, T.; Kim, S.B.; Lee, S.H.; et al. Randomized Phase III KEYNOTE-181 Study of Pembrolizumab Versus Chemotherapy in Advanced Esophageal Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, JCO2001888. [Google Scholar] [CrossRef]
- Shitara, K.; Van Cutsem, E.; Bang, Y.J.; Fuchs, C.; Wyrwicz, L.; Lee, K.W.; Kudaba, I.; Garrido, M.; Chung, H.C.; Lee, J.; et al. Efficacy and Safety of Pembrolizumab or Pembrolizumab plus Chemotherapy vs Chemotherapy Alone for Patients with First-line, Advanced Gastric Cancer: The KEYNOTE-062 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xu, J.; Chen, Y.; Zhuang, W.; Zhang, Y.; Chen, Z.; Chen, J.; Zhang, H.; Niu, Z.; Fan, Q.; et al. Camrelizumab versus investigator's choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): A multicentre, randomised, open-label, phase 3 study. Lancet. Oncol. 2020, 21, 832–842. [Google Scholar] [CrossRef]
- Moehler, M.; Shitara, K.; Garrido, M.; Salman, P.; Shen, L.; Wyrwicz, L.; Yamaguchi, K.; Skoczylas, T.; Campos Bragagnoli, A.; Liu, T.; et al. Nivolumab (nivo) plus chemotherapy (chemo) versus chemo as first-line (1L) treatment for advanced gastric cancer/gastroesophageal junction cancer (GC/GEJC)/esophageal adenocarcinoma (EAC): First results of the CheckMate 649 study. Ann. Oncol. 2020, 31, S1191. [Google Scholar] [CrossRef]
- Kato, K.; Sun, J.M.; Shah, M.A.; Enzinger, P.C.; Adenis, A.; Doi, T.; Kojima, T.; Metges, J.P.; Li, Z.; Kim, S.B.; et al. Pembrolizumab plus chemotherapy versus chemotherapy as first-line therapy in patients with advanced esophageal cancer: The phase 3 KEYNOTE-590 study. Ann. Oncol. 2020, 31, S1192–S1193. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Harrer, M. Doing Meta-Analysis in R: A Hands-on Guide. 2019. Available online: https://zenodo.org/badge/latestdoi/152492192 (accessed on 25 November 2020).
- Rücker, G.; Cates, C.J.; Schwarzer, G. Methods for including information from multi-arm trials in pairwise meta-analysis. Res. Synth. Methods 2017, 8, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Boku, N.; Ryu, M.H.; Oh, D.Y.; Oh, S.C.; Chung, H.C.; Lee, K.W.; Omori, T.; Shitara, K.; Sakuramoto, S.; Chung, I.J.; et al. Nivolumab plus chemotherapy versus chemotherapy alone in patients with previously untreated advanced or recurrent gastric/gastroesophageal junction (G/GEJ) cancer: ATTRACTION-4 (ONO-4538-37) study. Ann. Oncol. 2020, 31, S1192. [Google Scholar] [CrossRef]
- Fournel, L.; Wu, Z.; Stadler, N.; Damotte, D.; Lococo, F.; Boulle, G.; Ségal-Bendirdjian, E.; Bobbio, A.; Icard, P.; Trédaniel, J.; et al. Cisplatin increases PD-L1 expression and optimizes immune check-point blockade in non-small cell lung cancer. Cancer Lett. 2019, 464, 5–14. [Google Scholar] [CrossRef]
- Lacour, M.; Hiltbrunner, S.; Lee, S.Y.; Soltermann, A.; Rushing, E.J.; Soldini, D.; Weder, W.; Curioni-Fontecedro, A. Adjuvant Chemotherapy Increases Programmed Death-Ligand 1 (PD-L1) Expression in Non-small Cell Lung Cancer Recurrence. Clin Lung Cancer 2019, 20, 391–396. [Google Scholar] [CrossRef]
- Park, S.J.; Ye, W.; Xiao, R.; Silvin, C.; Padget, M.; Hodge, J.W.; Van Waes, C.; Schmitt, N.C. Cisplatin and oxaliplatin induce similar immunogenic changes in preclinical models of head and neck cancer. Oral Oncol. 2019, 95, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, E.; Yamashita, K.; Tanaka, T.; Sawada, R.; Sugita, Y.; Arimoto, A.; Fujita, M.; Takiguchi, G.; Matsuda, T.; Oshikiri, T.; et al. Neoadjuvant Chemotherapy Increases PD-L1 Expression and CD8. Anticancer Res. 2019, 39, 4539–4548. [Google Scholar] [CrossRef] [PubMed]
- Pfirschke, C.; Engblom, C.; Rickelt, S.; Cortez-Retamozo, V.; Garris, C.; Pucci, F.; Yamazaki, T.; Poirier-Colame, V.; Newton, A.; Redouane, Y.; et al. Immunogenic Chemotherapy Sensitizes Tumors to Checkpoint Blockade Therapy. Immunity 2016, 44, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Sakai, H.; Takeda, M.; Sakai, K.; Nakamura, Y.; Ito, A.; Hayashi, H.; Tanaka, K.; Nishio, K.; Nakagawa, K. Impact of cytotoxic chemotherapy on PD-L1 expression in patients with non-small cell lung cancer negative for EGFR mutation and ALK fusion. Lung Cancer 2019, 127, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Humeau, J.; Buqué, A.; Zitvogel, L.; Kroemer, G. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2020, 17, 725–741. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).